Short abstract
Single cell mass cytometry of human mononuclear phagocyte cells reveals myeloid phenotypes, and highlights S100A9 as a key MDSC marker.
Keywords: CyTOF, MDSCs, macrophages, monocyte
Abstract
The monocyte phagocyte system (MPS) includes numerous monocyte, macrophage, and dendritic cell (DC) populations that are heterogeneous, both phenotypically and functionally. In this study, we sought to characterize those diverse MPS phenotypes with mass cytometry (CyTOF). To identify a deep phenotype of monocytes, macrophages, and DCs, a panel was designed to measure 38 identity, activation, and polarization markers, including CD14, CD16, HLA‐DR, CD163, CD206, CD33, CD36, CD32, CD64, CD13, CD11b, CD11c, CD86, and CD274. MPS diversity was characterized for 1) circulating monocytes from healthy donors, 2) monocyte‐derived macrophages further polarized in vitro (i.e., M‐CSF, GM‐CSF, IL‐4, IL‐10, IFN‐γ, or LPS long‐term stimulations), 3) monocyte‐derived DCs, and 4) myeloid‐derived suppressor cells (MDSCs), generated in vitro from bone marrow and/or peripheral blood. Known monocyte subsets were detected in peripheral blood to validate the panel and analysis pipeline. Then, using various culture conditions and stimuli before CyTOF analysis, we constructed a multidimensional framework for the MPS compartment, which was registered against historical M1 or M2 macrophages, monocyte subsets, and DCs. Notably, MDSCs generated in vitro from bone marrow expressed more S100A9 than when generated from peripheral blood. Finally, to test the approach in vivo, peripheral blood from patients with melanoma (n = 5) was characterized and observed to be enriched for MDSCs with a phenotype of CD14+HLA‐DRlowS100A9high (3% of PBMCs in healthy donors, 15.5% in patients with melanoma, P < 0.02). In summary, mass cytometry comprehensively characterized phenotypes of human monocyte, MDSC, macrophage, and DC subpopulations in both in vitro models and patients.
Abbreviations
- AML
acute myeloid leukemia
- CyTOF
cytometry by time‐of‐flight
- DC
dendritic cell
- HD
healthy donor
- M_b
macrophage at baseline
- M‐CSF
macrophage CSF
- MDSC
myeloid‐derived suppressor cell
- MEM
marker enrichment modeling
- MLR
mixed lymphocyte reaction
- MPS
monocyte phagocyte system
- SPADE
spanning‐tree progression analysis of density‐normalized events
- TPP
TNF‐α Pam3 PGE2
- viSNE
visualization of t‐distributed stochastic neighbor embedding
Introduction
The MPS is a complex, cellular compartment that includes phenotypically and functionally heterogeneous cells, including monocyte, macrophage, and DC populations [1]. MPS cells belong to the innate immune system, whose activities can include infection defense, tissue homeostasis, and controlling T cell immunity [2, 3–4].
Phenotypic definitions of myeloid cells vary because of the lack of consistency among markers first identified in mice and humans. For example, although macrophages and MDSCs are typically defined as F4/80high and Gr1+, respectively, in mice [5], in humans EMR1 (the human F4/80 homolog) is expressed on eosinophils instead of macrophages [6], and Gr1 has no human homolog [7]. Furthermore, there are few unique markers of cell identity because most markers of interest (e.g., CD14, CD11b, CD33, HLA‐DR, and CD64) are shared by various myeloid cells, and none are lineage specific. Finally, myeloid cells, particularly monocytes and macrophages, are highly plastic with respect to phenotype and function and depend on various surrounding signals for differentiation/polarization. In the context of cancer or sepsis, an altered myelopoiesis can give rise to suppressive myeloid cells with poor phagocytic activity [8]. Overall, this phenotype complexity is highlighted by the growing literature on monocyte, DC, or macrophage nomenclature [1, 8, 9, 10–11]. In particular, monocytes are classified in 4 phenotypic subsets (CD14+CD16−, CD14+CD16+, CD14dimCD16+Slanlow, and CD14dimCD16+Slanhigh) [10, 12]; however, within those traditional phenotypes, additional functional subsets have been discovered, such as Tie2‐expressing monocytes, involved in angiogenesis, or monocytic‐MDSCs, involved in T cell immune suppression [8, 13]. Moreover, the paradigm of macrophage polarization has dramatically evolved in the past decade from a binary polarization (classically activated [M1, IFN‐γ, or LPS driven] vs. alternatively activated [M2, IL‐4, or IL‐10 driven]) to a much more complicated landscape [11, 14, 15]. Recently, Xue et al. [16] assessed the transcriptional landscape of multiple activated human‐macrophage subpopulations generated by numerous in vitro stimuli. At least 9 clusters were found to recapitulate macrophage‐polarization status, in particular, an already described regulatory macrophage (M_TPP) associated with TNF, prostaglandin E2, and TLR2–ligand stimuli [16, 17–18].
At the protein level, characterization of these heterogeneous cell types has been largely accomplished with “low‐resolution” approaches (e.g., morphologic evaluation and immunohistochemistry), wherein only one or a few proteins were used to identify populations. For example, CD68 and CD163 are frequently proposed to characterize macrophage types [19]. High‐resolution approaches, such as mass cytometry (also known as cytometry by time‐of‐flight or CyTOF), are valuable to better understand their diversity and function and to identify potential targets for novel therapies [2, 15, 20]. CyTOF, combined with high‐dimensional analysis, in particular, viSNE, SPADE, and MEM, are robust methods for identifying numerous and novel subsets from heterogeneous populations [21, 22, 23, 24, 25–26]. Indeed, several studies using CyTOF have explored the immune compartment, including B, T, NK, or myeloid cells, either from peripheral blood or from tissues [21, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37–38]. In particular, Becher et al. [29] developed a myeloid‐dedicated panel to characterize myeloid cells across 8 murine tissues, which revealed previously unidentified populations using unsupervised analysis of CyTOF data [39].
We hypothesized that human MPS complexity would benefit from a high‐dimensional, single‐cell approach [20, 39, 40]. Here, a single mass cytometry panel comprising 38 Abs was combined with high‐dimensional analysis methods to decipher the human MPS compartment in primary samples, including PBMCs from HDs and from patients with melanoma. Results from primary cells were compared with observations from in vitro models of myeloid differentiation using human blood and bone marrow cells exposed to established, polarizing inflammation factors. Unsupervised analysis tools, including viSNE, SPADE, and MEM, were used to create and describe a comprehensive reference framework for the MPS compartment and to characterize an abnormal abundance of MDSCs in the peripheral blood of patients with melanoma.
MATERIALS AND METHODS
Samples and mononuclear cell preparations
Peripheral blood from HDs or from patients with melanoma was obtained in accordance with the Declaration of Helsinki, following protocols approved by Vanderbilt University Medical Center Institutional Review Board. Bone marrow from HDs was obtained under French legal guidelines and fulfilled the requirements of the University Hospital of Rennes Institutional Ethics Committee. Peripheral blood was drawn by venipuncture into heparinized tubes. Bone marrow was obtained by aspiration after sternotomy for cardiac surgery, and cells were kept in sodium‐heparin bags. Mononuclear cells were isolated with Ficoll‐Paque PLUS (GE Healthcare Life Sciences, Uppsala, Sweden) centrifugation. Freshly isolated mononuclear cells were immediately cryopreserved in FBS (Thermo Fisher Scientific, Waltham, MA, USA) containing 12% DMSO (Thermo Fischer Scientific). For in vitro, monocyte‐derived cell experiments, buffy coats from HDs were obtained according to protocols accepted by the institutional review board at the University Hospital of Rennes. After collection, monocytes were purified from PBMCs by elutriation before cryopreservation (plate‐forme DTC; CIC Biotherapie, Nantes, France). Monocytes represented >85% of the cells.
In vitro culture and stimulation
For in vitro differentiations, cells were cultured in 6‐well plates at 2 × 106 cells/ml in a humidified atmosphere at 37°C, 5% CO2 in RPMI 1640 (Mediatech, Manassas, VA, USA) enriched with FCS 10% (Gibco; Thermo Fisher Scientific) and supplemented with 1% PenStrep solution (Gibco; Thermo Fisher Scientific). MDSCs were derived from peripheral blood or bone marrow mononuclear cells. Cells were cultured for 4 d, and activations were performed with GM‐CSF (40 ng/ml; PeproTech, Rocky Hill, NJ, USA) and G‐CSF (40 ng/ml; PeproTech) and, for bone marrow cells, GM‐CSF and IL‐6 (40 ng/ml; PeproTech), as previously described [41, 42]. Immature DCs were generated from monocytes by GM‐CSF and IL‐4 (40 ng/ml; EMD Millipore, Billerica, MA, USA) for 6 d; media were changed at 3 d. Then, for terminal differentiation, TNF‐α (10 ng/ml; EMD Millipore) was added in culture for 2 d. M_b was generated from monocytes by stimulation by M‐CSF (50 ng/ml; Cell Signaling, Danvers, MA, USA) for 3 d, as previously described [16]. Then, M_b were further polarized for 3 d by IL‐4, IL‐10 (10 ng/ml; PeproTech), IL‐6 (10 ng/ml; PeproTech), IFN‐g (10 ng/ml; Cell Signaling), LPS (10 ng/ml; Sigma‐Aldrich, St Louis, MO, USA), or TPP (TNF‐α [10 ng/ml; EMD Millipore]; Pam3CSK4 [100 ng/ml; InvivoGen, San Diego, CA, USA]; prostaglandin E2 [1 μg/ml, Sigma‐Aldrich]). At the end of each conditioning culture, except for DCs, wells were treated with Accutase (Sigma‐Aldrich) prewarmed at 37°C for 30 s before collection, washing, and staining.
Allogeneic, 3‐way, MLR assay
Suppressive capacities of in vitro PBMC‐ and bone marrow–derived MDSCs were determined in an allogeneic, 3‐way, MLR assay. T cells were purified from PBMCs from an HD using the Pan T Cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). DCs and MDSCs were obtained by culture conditions described above. DCs were derived from PBMCs obtained from an allogeneic donor. MDSCs were obtained from 3 donors for PBMCs and 2 for bone marrow samples. After 4 d of in vitro differentiation, CD14+CD33+CD11b+HLA‐DRlow MDSCs from bone marrow and monocytes were sorted using a FACS ARIA cell sorter (BD Biosciences, Franklin Lakes, NJ, USA). For the MLRs, 1 × 105 T cells from 1 donor were seeded in culture medium with 2,000 allogeneic DCs and different MDSC:T ratio (1:8, 1:4, 1:2). The MLR assays were performed over 5 d in round‐bottomed, 96‐well plates to ensure efficient DC/T cell contact. T cell proliferation was measured by thymidine uptake (1 µCi/well) during the last 16 h.
Abs, cell labeling, and mass cytometry analysis
Purified Abs from BioLegend (San Diego, CA, USA) or Immunotech (Marseille, France) were labeled with MaxPar DN3 labeling kits (Fluidigm, San Francisco, CA, USA), titrated, and stored at 4°C in Ab‐stabilization buffer (Candor Bioscience, Wangen, Germany). Abs from Miltenyi Biotech or R&D systems (Minneapolis, MN, USA) were labeled with FITC, PE, or APC (Supplemental Table 1). Abs metal‐tagged were from Fluidigm. Cell labeling and mass‐cytometry analysis were performed as previously described [20, 43]. Briefly, cells were incubated with a viability reagent (cisplatin, 25 μM; Enzo Life Sciences, Farmingdale, NY, USA), as previously described [44]. Then, 3 × 106 cells were washed in PBS (HyClone Laboratories, Logan, UT, USA) containing 1% BSA (Thermo Fisher Scientific) and stained in 50 μl PBS and BSA 1%‐containing Ab cocktail. Cells were stained for 30 min at room temperature using the Abs listed (Supplemental Table 1). Cells were washed twice in PBS and BSA 1% and then fixed with 1.6% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA). Cells were washed once in PBS and permeabilized by resuspending in ice‐cold methanol. After incubating overnight at −20°C, cells were washed twice with PBS and BSA 1% and stained with an iridium DNA intercalator (Fluidigm) for 20 min at room temperature. Finally, cells were washed twice with PBS and twice with diH2O before being resuspended in 1X EQTM Four Element Calibration Beads (Fluidigm) and collected on a CyTOF 1.0 mass cytometer (Fluidigm) at the Vanderbilt Flow Cytometry Shared Resource Center. Events were normalized, as previously described [45].
Data processing and analysis
Data analysis was performed using the workflow already described [46]. Raw median intensity values were transformed to a hyperbolic arcsine (arcsinh) scale with a cofactor of 5. Analysis was performed on Cytobank (Santa Clara, CA, USA) using published techniques, including SPADE, viSNE, and hierarchical clustering [25, 47]. Each file was pregated on singlets and viable cells as defined by cisplatin and iridium gating. The analysis pipeline was as follows: after gating on nucleated cells (iridium+), the labeling was assessed on biaxial plots with CD45+ cells. Then, a viSNE analysis was performed. On the viSNE map, B, T, and NK cells were distinguished, and then the remaining cells were engulfed in an MPS gate and were further clustered with SPADE. Heat maps were performed using the MEM algorithm [24].
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA) using Wilcoxon or Mann‐Whitney tests as appropriate.
RESULTS
CyTOF delineates four monocyte subsets in peripheral blood from HDs
To recapitulate the diversity and heterogeneity of monocyte subsets, a CyTOF panel with 38 parameters was designed (Supplemental Table 1). Based on literature profiling, proteins in that panel were expected to be expressed at different levels for MPS cell types and associate with differentiation, polarization, and activation states. PBMCs from HDs were first tested and the MPS gate defined with the analysis pipeline ( Fig. 1A and B and Supplemental Fig. 1A). To characterize known and expected monocyte subpopulations in peripheral blood (i.e., classic, intermediate, and nonclassic), the analysis was initially defined to seek 30 nodes, representing populations of phenotypically distinct cells. In manual review of the features distinguishing the identified nodes, four groups were apparent. The four phenotypically similar groups of clusters aligned closely with canonical monocyte populations in peripheral blood, namely CD14+CD16−, CD14+CD16+, CD14dimCD16+Slanlow, and CD14dimCD16+Slanhigh. Those subsets comprised 85%, 9%, 3%, and 3% of monocytes, respectively, as expected [12] (Fig. 1C). DC‐population SPADE nodes were recognized within the MPS gate as HLA‐DRhighCD123high (pDC) or HLA‐DRhighCD11chigh (cDC), whereas polynuclear basophils were recognized as HLA‐DRlowCD123+ (Supplemental Fig. 1B). Finally, the relative expression of additional markers across the monocyte subsets, as obtained by mass cytometry, was compared (Fig. 1D). Both Slanhigh and Slanlow subsets of nonclassic monocytes expressed lower level of CD36, CD64, CCR2, and CD14, consistent with previously published data [12, 48]. These observations confirmed that the panel design and analysis strategy captured well‐established monocyte subtypes.
Figure 1.
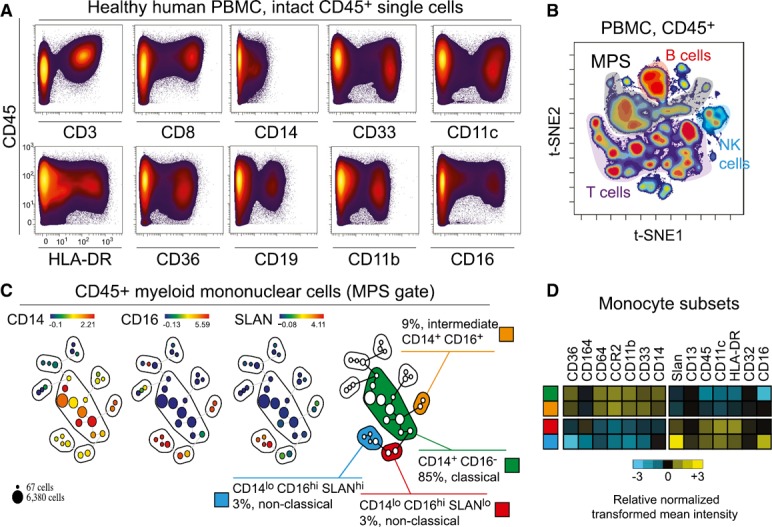
CyTOF panel and workflow analysis delineates four monocyte subsets in peripheral blood. (A) Biaxial plots showing the expression of markers on Ir+CD45+ PBMCs measured by mass cytometry. A representative HD is shown. An arcsinh scale (−5.0 to 104) with a cofactor of 5 was used. (B) By mass cytometry analysis >100,000 Ir+CD45+ cells were defined on a biaxial plot before classification with the viSNE algorithm. MPS (>20,000 cells) was gated as remaining cells after the exclusion of B (CD19+), T (CD3+), and NK (CD3−CD16+CD45RA+) lymphocytes and doublets (see Supplemental Fig. 1). (C) Events in the MPS gate were then parsed with SPADE into 30 nodes using all markers for clustering, except CD19 and CD3. CD14+, CD16+, and Slan+ SPADE groups were observed to match classic (CD14+CD16−), intermediate (CD14+CD16+), nonclassic Slanlow (CD14dimCD16+Slanlow), and nonclassic Slan+ (CD14dimCD16+Slanhigh) monocytes. A representative HD is shown. % represents the frequency among PBMCs. (D) On the 4 monocyte subsets previously described in (B), heat maps show the relative normalized transformed mean intensity for various markers tested by mass cytometry, for a representative HD.
DC‐, MDSC‐, and macrophage‐derived in vitro from monocyte were profiled by CyTOF
Given that CD14 and CD16, the two central markers used to delineate monocyte subsets in the established nomenclature, show a continuous gradient of expression, we hypothesized that a high‐dimensional approach would enhance the characterization of monocytic MDSCs and macrophage polarization subtypes. In vitro–derived DCs, MDSCs, and macrophage subsets (M_b, M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP) from peripheral blood monocytes were characterized as comparison points for in vivo studies ( Fig. 2A ). In vitro subsets were derived according to best practices for characterizing myeloid‐cell polarization [11, 16, 42]. After a SPADE analysis (Fig. 2B), variation of cell abundance under stimulation in each node was summarized (Fig. 2C). Before stimulation, monocytes comprised 98.6% of the MPS. Under appropriate stimulation, DC, MDSC, and M_b were increased from 0.1% to 76, 87, and 78%, respectively, in the MPS gate. After polarization, M_LPS, M_IFN‐g, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP were increased from <10% to 52, 66, 56, 80, 40, and 81%, respectively. Interestingly, some conditions polarized monocytes to >1 main population. For instance, M‐CSF + LPS increased the percentage of cells in both the LPS gate (from 0.9 to 53%) and TPP gate (from 3.2 to 22%) (Fig. 2C). Finally, unclassified cells (i.e., those not included in any gate) were <10% in all conditions. Of note, T cells were increased under IL‐4, IFN‐γ, or IL‐6 treatments (from 4% in the control to approximately 22% after culture).
Figure 2.
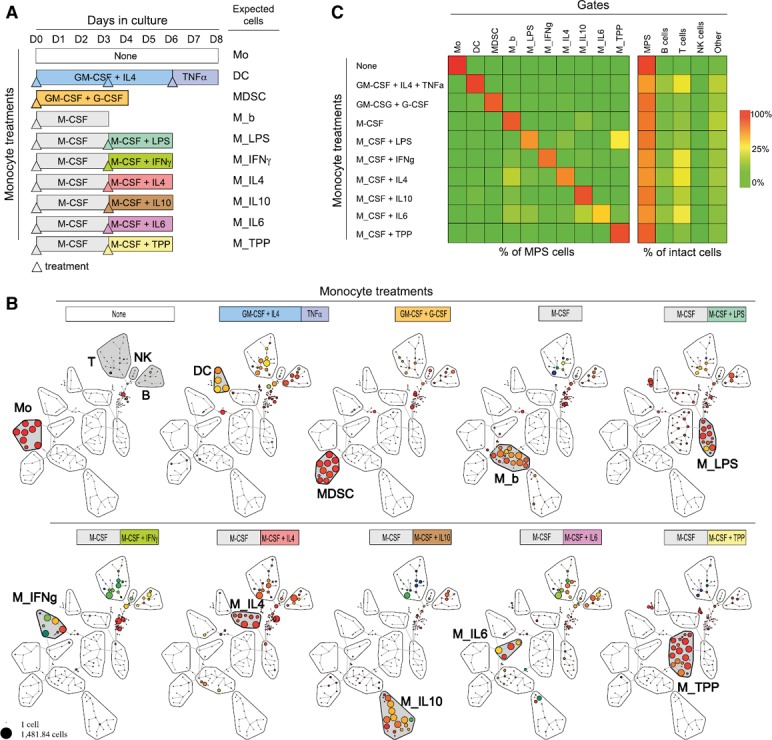
CyTOF profiles of DCs, MDSCs, and macrophages, derived in vitro from monocytes. (A) Experimental procedure to derive DCs, MDSCs, and macrophages at baseline (M_b) or polarized under various stimuli (M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP [a cocktail including TNF‐α, PGE2, and Pam3]). Peripheral blood monocytes were obtained from blood donors and purified by elutriation. Expected cells from the stimuli conditions are indicated on the right. Days of treatment (colored, up‐pointing triangles) or of collection (end of bar) were specific to the culture condition. (B) After CyTOF analysis, cells were defined as Ir+CD45+. Then, a SPADE analysis with 200 nodes and down‐sampling at 10% was performed. Adjacent nodes with an increase in cell abundance and phenotypic similarity were labeled in red with the name of the expected cells from the culture condition. Monocyte, DC, MDSC, M_b, M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP gates were positive for myeloid markers, whereas T, NK, and B gates expressed CD3, CD16/CD45RA, CD19, respectively. Nodes outside gates were considered unclassified (C, left) Cell abundance in gate (monocyte, DC, MDSC, M_b, M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP) reported to MPS gate and related to each condition of stimulation. (C, right) Cell abundance in monocyte, DC, MDSC, M_b, M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP gates (sum in MPS) and B, T, and NK gate or unclassified, reported to intact cells (Ir+CD45+) and related to each condition of stimulation. Average percentage of 2 independent experiments.
MDSCs and polarized macrophages have specific phenotypes
Next, the phenotype of cell types obtained after differentiation of monocytes and polarization of macrophages was examined. To broadly assess the modulation of protein expression, median expression was assessed for each population ( Fig. 3A ). Average, transformed median expression was then calculated from nodes included in each gate identity (Fig. 3B). Monocytes were distinguished by high expression of CD33, CD36, and CCR2 and low CD163 and CD274 expression. DCs were CD11chigh and HLA‐DRhigh. M_b expressed CD14, CD206, and HLA‐DR. Statistical differences among all conditions are summarized in Supplemental Fig. 2. In particular, various polarized macrophages were compared with M_b (Fig. 3C). M_LPS was distinguished by high levels of CD13 and CD86 and low levels of CD163 and CD206 (P < 0.01). M_IL‐4 was CD274high and CD64low (P < 0.01). M_TPP expressed CD14high and HL‐DRlow (P < 0.001). M_IFN‐g was CD64high and CD86high (P < 0.001). M_IL‐10 was CD14high, CCR2high, and CD163high (P < 0.01); of note, significantly more CD163 was expressed in M_IL‐10 than in M_b (P < 0.01) (Supplemental Fig. 2). Finally, M_IL‐6 was CD11chigh and CD33high (P < 0.05). Then, MDSCs were compared with monocytes, DCs, and M_b (Fig. 3C). MDSCs showed greater expression of CD32, CD206, and CD13 (P < 0.05) and less expression of CD36, CD163, S100A9, CD33, and HLA‐DR (P < 0.05), when compared with monocytes. Compared with DCs, MDSCs expressed greater amounts of CD32, CD206, CD64, CCR2, CD14 (P < 0.05) and lesser amounts of CD13, CD274, CD33, and HLA‐DR (P < 0.05). Finally, compared to M_b, MDSCs expressed greater CD64 and CCR2 (P < 0.05), and less CD14, CD13, CD11c, CD36, CD163, S100A9, CD33, and HLA‐DR (P < 0.05). Peripheral blood–derived MDSCs were distinguished by the expected low expression of HLA‐DR and by an unexpectedly low expression of S100A9, in contrast to other peripheral blood mononuclear myeloid cell populations, with the exception of DCs.
Figure 3.
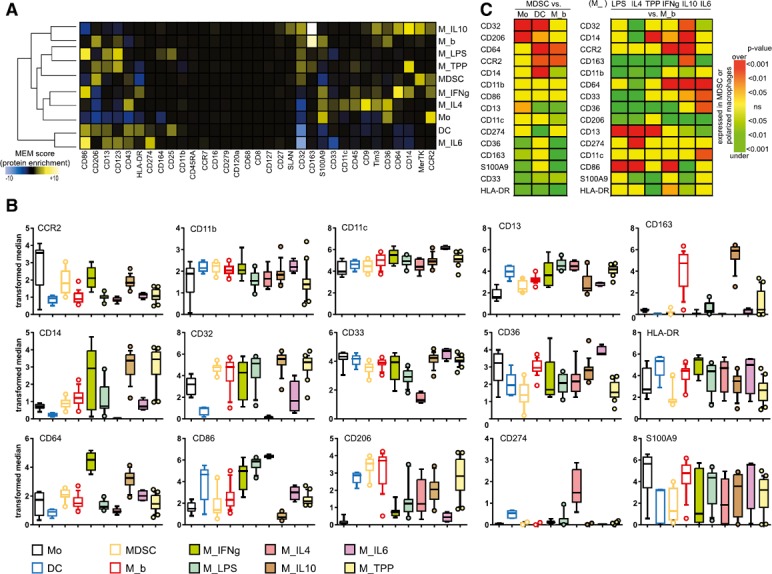
MDSC and polarized macrophages derived in vitro have specific phenotypes. (A) For monocytes, DC, MDSC, M_b, M_LPS, M_IFN‐γ, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP gates, transformed median expression for each marker was averaged from all nodes included in the gate. After normalization, results are shown on heat map after hierarchical clustering. (B) Comparison of markers for each node (each dot represents a node). Box and whisker plots show the 10–90 percentiles and the outliers. Nodes from 2 or 3 different experiments are shown. One‐way ANOVA tests (parametric or nonparametric as appropriate after normality test) with posttest comparing all pairs of columns are summarized in Supplemental Fig. 2. (C, left) Comparison of P values among MDSCs and monocytes, DCs, and M_b. (C, right) Comparison of various polarized macrophages (M_IFN‐g, M_LPS, M_IL‐4, M_IL‐10, M_IL‐6, and M_TPP) to M_b. Rows and columns were arranged after hierarchical clustering (not shown). Only markers that were, at least once, statistically different are shown. Unpaired t tests (parametric or nonparametric as appropriate after normality test) were performed. Yellow, nonsignificant (ns); light to dark green, significantly underexpressed in MDSCs or polarized macrophages; orange to red, significantly overexpressed in MDSCs or polarized macrophages.
MDSCs derived from bone marrow were S100A9+
Published protocols have established methods to derive MDSCs, including combining cytokines or culturing peripheral blood or bone marrow. We derived MDSCs from bone marrow to investigate their phenotypes, following the protocol published by Marigo et al. [41]. As published, we cultured human bone marrow for 4 d with GM‐CSF + G‐CSF or GM‐CSF + IL‐6 before CyTOF analysis ( Fig. 4A ). Median protein expression is shown on hierarchically clustered heat maps (Fig. 4B). A first group of nodes (in green) was mainly CD11c+, CD11b+, CD36+, CD14+ CD13+, CD64+, and HLA‐DR+ but also CD274+ and CD86+. Those cells displayed heterogeneous expression of S100A9; in particular, node 7 (S100A9low) was increased only with GM‐CSF + G‐CSF. One group of cells (in purple) displayed the expected MDSC phenotype (i.e., S100A9high, CD33+, CD14+, and HLA‐DRlow); in addition, those cells were also CD64+, CD11b+, CCR2+, CD36+, CD13+, and CD32+. Of note, node 24 was only increased under GM‐CSF and G‐CSF and was characterized by very high expression of CD32. Finally, a third group of nodes was found (in orange) in which cells were CD123+ and HLA‐DR+, whereas CD14, CD11b, CD36, CD64, and S100A9 were not expressed; thus, those cells were labeled DCs (Fig. 4B). The increase in abundance for those cells was assessed in 3 different human bone marrow samples. All three phenotypes (i.e., monocytes that were CD86+ and CD274+, MDSCs, and DCs) were significantly increased after GM‐CSF + G‐CSF or GM‐CSF + IL‐6 culture when compared with the vehicle (Fig. 4C). No difference in cell frequency was found between either condition (Fig. 4C). Finally, because of the phenotypic differences observed between MDSCs derived from PBMCs and those from bone marrow, and to demonstrate their suppressive capabilities, an allogeneic 3‐way MLR assay was performed ( Fig. 5 ). MDSCs obtained were suppressive at a ratio of 1:8, 1:4, and 1:2 when derived from bone marrow and 1:4 and 1:2 when derived from PBMCs (P < 0.05).
Figure 4.
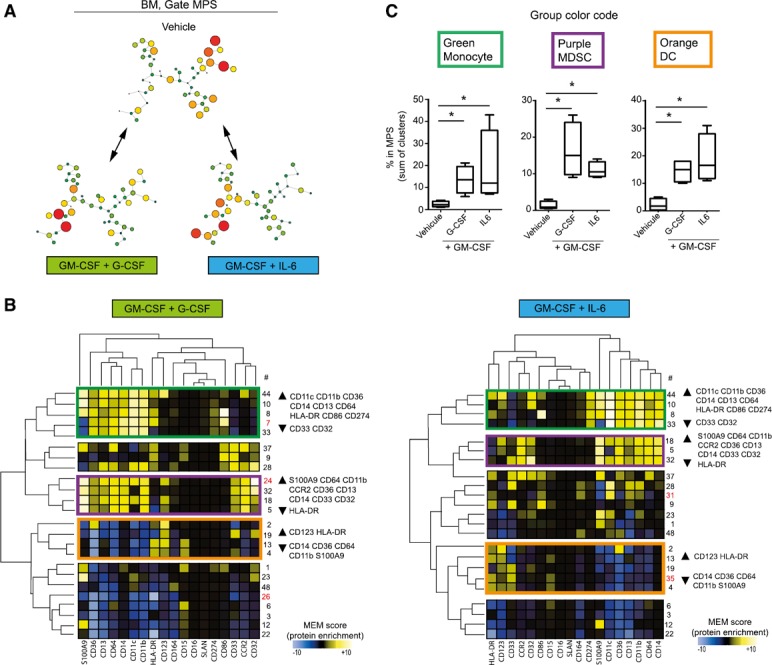
MDSCs obtained from bone marrow are S100A9+. (A) Human bone marrow was cultured for 4 d with GM‐CSF + IL‐6 or GM‐CSF + G‐CSF or with the vehicle. By mass cytometry analysis >100,000 Ir+CD45+ cells were defined on a biaxial plot, before classification with a viSNE algorithm. MPS (>20,000 cells) was gated as the remaining cells after the exclusion of B (CD19+), T (CD3+), and NK (CD3−CD16+CD45RA+) lymphocytes and doublets. Events in the MPS gate were then parsed with SPADE, arbitrarily restricted to 50 nodes, using all clustering markers, except CD19 and CD3. Then, comparisons were made among each culture conditions, and cells were treated with vehicle. Nodes with a 2‐fold increase in cell abundance (percentage FC > 1) between GM‐CSF + G‐CSF and vehicle or between GM‐CSF + IL‐6 and vehicle were retained for further analysis (B) Transformed median expression for each markers was averaged from each nodes (percentage FC > 1). After normalization, results are shown on heat map after hierarchical clustering. (B, left) Nodes with an increase in cell abundance after GM‐CSF + G‐CSF culture. (B, right) Nodes with an increase in cell abundance after GM‐CSF + IL‐6 culture. No. of nodes: red, nodes increased in only one condition; rectangles in green, purple, or orange indicate various phenotypes of interest. A representative experiment is shown. (C) Abundance of cells in the MPS gate for each phenotype of interest, with or without GM‐CSF + G‐CSF or GM‐CSF + IL‐6 (n = 4). ∗P < 0.05.
Figure 5.
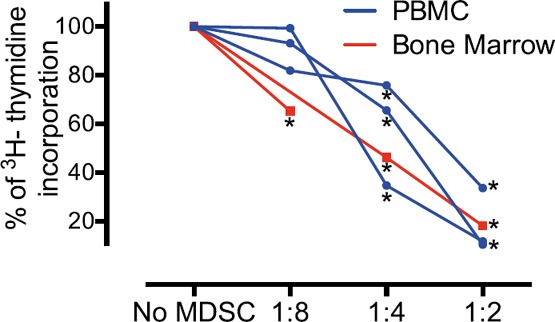
MDSCs derived from PBMC or bone marrow are both suppressive. An allogeneic 3‐way MLR was performed on MDSCs derived from PBMCs or bone marrow. APCs and T cells were cultured with no MDSCs and various ratios of MDSCs to T cells (1:8, 1:4, and 1:2). The inhibition of [3H]thymidine incorporation was evaluated. Results are represented as percentages of inhibition, where 100% is the condition without MDSCs. Replicate (3–5) wells were performed for each condition. ∗P < 0.05 indicates significant difference when compared with the condition without MDSCs.
Mass cytometry identifies phenotypic MDSCs in the peripheral blood of patients with melanoma
The mass cytometry panel, unsupervised analysis approach, and myeloid cell definitions were finally evaluated in clinical samples. MDSCs have previously been reported to be increased in the peripheral blood from patients with solid tumor, irrespective of the disease stage, including patients with melanoma [49, 50, 51, 52–53]. Here, an abundance of cells with an MDSC phenotype, including high S100A9 protein expression, were observed in the peripheral blood of patients with melanoma ( Fig. 6A ). This cell type was significantly increased in 8 samples from 4 patients compared with HDs, with an abundance at 3% and 15.5% from the MPS gate, respectively (P = 0.019) (Fig. 6B).
Figure 6.
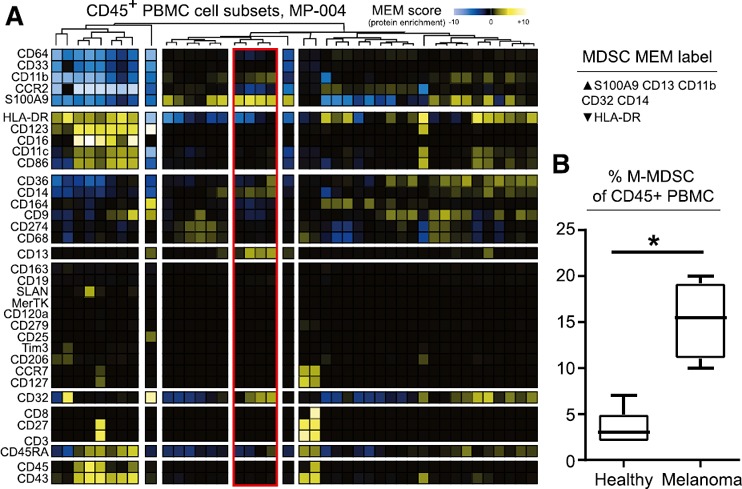
MDSCs accumulated in the peripheral blood of patients with melanoma are revealed by mass cytometry. (A) By mass cytometry analysis, >100,000 Ir+CD45+ cells were defined on a biaxial plot before viSNE analysis. MPS cells (>20,000 cells) were gated as remaining cells after the exclusion of B (CD19+), T (CD3+), and NK (CD3−CD16+CD45RA+) lymphocytes and doublets. Events in the MPS gate were then parsed with SPADE, arbitrarily restricted to 50 nodes, using all markers for clustering except CD19 and CD3. After normalization, transformed median expression for each markers and each node are shown on a heat map after hierarchical clustering; red, nodes increased with an increase of CD14+ S100A9+ cells. (B) Abundance of CD14+S100A9+ cells in the MPS gate in PBMCs from HDs (n = 4) and patients with melanoma (n = 5). ∗P < 0.05.
DISCUSSION
The MPS compartment includes monocytes, DCs, and macrophages, cells that are extremely heterogeneous in their phenotypes and functions. Recently, their nomenclature has been extensively revised and clarified [1, 8, 10, 11]. Because there are no unique identity markers and an overlap in their phenotypes, their definition at the protein level is still being debated. Here, we hypothesized that mass cytometry data, parsed by high‐dimensional approaches, such as SPADE, viSNE, and hierarchical clustering, would clarify, at the protein level, the human spectrum of the MPS compartment. To that aim, various in vitro culture conditions and peripheral blood from patients with cancer were compared to build a reference data framework including 1) monocyte subsets and MDSCs, 2) DCs, and 3) macrophages under basal conditions or treated with various canonical, polarization stimuli.
To date, mass cytometry analyses have been performed on only a few myeloid populations. In humans, peripheral blood, bone marrow, or tissues from HDs [21], inflammatory or septic patients [28, 32, 54, 55], and patients with AML [43, 56, 57–58] have been analyzed for myeloid cells. Of note, except in AML, panels employed were not dedicated specifically for deep analysis of the myeloid compartment. Markers used in those studies included mostly CD13, CD33, CD36, CD14, CD16, HLA‐DR, CD11b, CD11c, and CD123. In a recent comprehensive panel dedicated to the monitoring of immunomodulatory therapies on PBMCs, CD14, CD15, HLA‐DR, CD11c, CD36, CD16, CD169, CD123, CD303, Siglec‐8, and CD1c were proposed to delineate neutrophils, monocytes, basophils, and eosinophils, as well as DC subsets [59]. In mice, more complete myeloid‐targeted panels have been published, in particular, with the use of the specific myeloid markers F4/80, Ly6C, and Ly6G [29, 60]. The panel was built by including 1) canonical markers from prior studies of the human MPS [40], 2) markers known to be modulated in specific monocyte subsets or macrophage polarization stages (viz, CCR2, CD163, CD206, CD32, and CD64), and 3) markers differentially expressed during monocyte/DC activation (viz, CD86, CD274, CD45RA). The panel was validated with PBMCs in recognizing, in HDs, the 4 already described monocytes subsets (CD14+CD16−, CD14+CD16+, CD14dimCD16+Slanlow, and CD14dimCD16+Slanhigh) [10, 12].
Then, to explore the full spectrum of the MPS compartment, we took advantage of recent nomenclature articles [11], resource work refining the macrophage transcriptomic landscape [16], and studies on MDSCs [42] or on DCs [61, 62]. In particular, Xue et al. [16] described 9 different clusters of transcription networks. We decided to align, as much as possible, with those conditions and thus derived from monocytes, M_b, M_IL‐4, M_IL‐10, M_LPS, M_IFN‐γ, M_IL‐6, and M_TPP, but also from DCs and MDSCs, given that their phenotypes are overlapping. Regarding macrophages, each stimulation condition gave rise to a specific phenotype of polarized macrophage (Fig. 2B and C). There was no or little overlap between M_IFN‐g and M_LPS (both previously known as M1) and M_IL‐4 and M_IL‐10 (both previously known as M2). M_TPP also represented a separate cluster of nodes. This was in agreement with previous findings at the transcriptomic level, where macrophages polarized by IL‐4, IL‐10, IFN‐γ, and LPS clustered separately based on RNA expression profiles [16]. Novel patterns of phenotypes within MPS were discovered and remarkable. CD32, CD14, CCR2, CD163, CD64, and CD33 were highly expressed in M_IL‐10. CD274 and CD86 were highly expressed, whereas CD14, CD32, and CD33 were expressed at low level, in M_IL‐4 (Fig. 2B and C and Supplemental Fig. 2). Surprisingly, phenotypic patterns of M_LPS and M_IL‐4 were separated only by CD32 and CD33, with more expressed in M_LPS, whereas CD274 was less expressed, and CD163 was not differently expressed. CD163 is considered a key marker of tumor‐associated macrophages and sometimes, by extension, for the historical M2 macrophages; however, greater expression in M_IL‐10 than in M_IL‐4 has been shown [63]. M_TPP expressed high levels of CD14 and CD13, whereas HLA‐DR was expressed at low level, and M_TPPs were shown to be immunosuppressive [16]. MDSCs were also clearly separated from M_b, DCs, and monocytes (Fig. 2B and C) by especially high levels of CD32, CD206, CD64, CCR2, and CD14 and by low levels of CD33 and HLA‐DR. MDSCs were also phenotypically different from M_IL‐4, M_IL‐10, and M_TPP, 3 polarized macrophages with anti‐inflammatory functions, because of greater expression of CCR2 and CD206 and lower expression of CD13 (Supplemental Fig. 2). Because HLA‐DR expression is continuous across myeloid cells, it has been challenging to distinguish monocytic MDSCs from monocytes in peripheral blood. Based on observations here, we propose using CD32, CD206, and S100A9, in addition to CD14 and HLA‐DR (Fig. 3C).
Surprisingly, S100A9, a highly expressed protein marker of MDSCs [8, 64, 65–66], was expressed at low levels in MDSCs generated from peripheral blood (Fig. 3B and C). Despite lower S100A9 than other MDSCs, peripheral blood–derived MDSCs were functional and effective at suppressing T cell proliferation (Fig. 5). In previous works, human MDSCs were derived either from peripheral blood or from bone marrow [41, 42]. Thus, we hypothesized that MDSCs derived from bone marrow would have a different phenotype. Monocytes, DCs, and MDSCs were increased in abundance when bone marrow was cultured with GM‐CSF + G‐CSF or with GM‐CSF + IL‐6 (Fig. 4C). This observation has not, to our knowledge, been reported in published protocols for deriving MDSCs, and it would have been difficult to identify without the single‐cell, high‐dimensional, mass cytometry approach used in our study. In agreement, GM‐CSF–cultured murine bone marrow has been shown to generate both macrophages and DCs [62]. We also found that MDSCs derived from human marrow expressed a more‐consistent phenotype, highly expressing S100A9, CD14, CD64, CD11b, CCR2, and CD32, while remaining HLA‐DRlow, making bone marrow MDSCs an ideal, if more difficult to obtain, reference point. Finally, this approach was employed to characterize clinical samples from patients with melanoma because, in that cancer, high levels of circulating MDSCs have been described across grades [49, 53]. MDSCs with the same phenotype as those derived from bone marrow were enriched in the blood of patients with melanoma.
In summary, a broad phenotypic analysis of the human MPS compartment characterizes know cell populations and brings increased clarity to the definitions of cell types, including MDSCs and polarized mononuclear phagocytes. In particular, the multidimensional approach at the protein level might constitute the first step of efforts in unifying transcriptomic to proteomic and functional approaches in a multiomics era [67]. It would be interesting to expand the panel to have a clear view of signaling pathways involved. Finally, this study also highlights the potential value of mass cytometry in systems immune monitoring of the myeloid compartment for patients in clinical trials.
AUTHORSHIP
M.R. and J.M.I. conceived the study. M.R., P.B.F., A.R.G., F.L., and S.L.G. performed the experiments. D.B.J. provided samples. M.R., P.B.F., A.R.G., F.L., S.L.G., K.E.D., and J.M.I. analyzed data and revised figures. M.R. and J.M.I. wrote the paper, and all authors revised the manuscript.
DISCLOSURES
J.M.I. is cofounder and a board member of Cytobank Inc. and received research support from Incyte Corp and Janssen. The other authors declare no conflicts of interest.
Supporting information
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
ACKNOWLEDGMENTS
M.R. is the recipient of a fellowship from the Nuovo‐Soldati Foundation (Switzerland). This study was supported by Grants F31 CA199993 (A.R.G.), K12 CA090625 (P.B.F.), R25 CA136440‐04 (K.E.D.), R00 CA143231‐03 (J.M.I.), and the Vanderbilt‐Ingram Cancer Center (VICC, Grant P30 CA68485), VICC Ambassadors, a VICC Hematology Helping Hands award (J.M.I., P.B.F., and K.E.D.), and the Tinsley R. Harrison Society (P.B.F.). The authors thank Emmanuel Gautherot from Beckman Coulter Immunotech (Marseille, France) for the gift of purified CD206.
Contributor Information
Mikael Roussel, Email: mikael.roussel@chu-rennes.fr.
Jonathan M. Irish, Email: jonathan.irish@vanderbilt.edu
REFERENCES
- 1. Guilliams, M. , Ginhoux, F. , Jakubzick, C. , Naik, S. H. , Onai, N. , Schraml, B. U. , Segura, E. , Tussiwand, R. , Yona, S. (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engblom, C. , Pfirschke, C. , Pittet, M. J. (2016) The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462. [DOI] [PubMed] [Google Scholar]
- 3. Lavin, Y. , Mortha, A. , Rahman, A. , Merad, M. (2015) Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 15, 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merad, M. , Sathe, P. , Helft, J. , Miller, J. , Mortha, A. (2013) The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor, P. R. , Martinez‐Pomares, L. , Stacey, M. , Lin, H.‐H. , Brown, G. D. , Gordon, S. (2005) Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944. [DOI] [PubMed] [Google Scholar]
- 6. Hamann, J. , Koning, N. , Pouwels, W. , Ulfman, L. H. , van Eijk, M. , Stacey, M. , Lin, H.‐H. , Gordon, S. , Kwakkenbos, M. J. (2007) EMR1, the human homolog of F4/80, is an eosinophil‐specific receptor. Eur. J. Immunol. 37, 2797–2802. [DOI] [PubMed] [Google Scholar]
- 7. Rabinovich, G. A. , Gabrilovich, D. , Sotomayor, E. M. (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bronte, V. , Brandau, S. , Chen, S.‐H. , Colombo, M. P. , Frey, A. B. , Greten, T. F. , Mandruzzato, S. , Murray, P. J. , Ochoa, A. , Ostrand‐Rosenberg, S. , Rodriguez, P. C. , Sica, A. , Umansky, V. , Vonderheide, R. H. , Gabrilovich, D. I. (2016) Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ancuta, P. (2015) A Slan‐based nomenclature for monocytes? Blood 126, 2536–2538. [DOI] [PubMed] [Google Scholar]
- 10. Ziegler‐Heitbrock, L. , Ancuta, P. , Crowe, S. , Dalod, M. , Grau, V. , Hart, D. N. , Leenen, P. J. M. , Liu, Y.‐J. , MacPherson, G. , Randolph, G. J. , Scherberich, J. , Schmitz, J. , Shortman, K. , Sozzani, S. , Strobl, H. , Zembala, M. , Austyn, J. M. , Lutz, M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80. [DOI] [PubMed] [Google Scholar]
- 11. Murray, P. J. , Allen, J. E. , Biswas, S. K. , Fisher, E. A. , Gilroy, D. W. , Goerdt, S. , Gordon, S. , Hamilton, J. A. , Ivashkiv, L. B. , Lawrence, T. , Locati, M. , Mantovani, A. , Martinez, F. O. , Mege, J.‐L. , Mosser, D. M. , Natoli, G. , Saeij, J. P. , Schultze, J. L. , Shirey, K. A. , Sica, A. , Suttles, J. , Udalova, I. , van Ginderachter, J. A. , Vogel, S. N. , Wynn, T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cros, J. , Cagnard, N. , Woollard, K. , Patey, N. , Zhang, S.‐Y. , Senechal, B. , Puel, A. , Biswas, S. K. , Moshous, D. , Picard, C. , Jais, J.‐P. , D'Cruz, D. , Casanova, J.‐L. , Trouillet, C. , Geissmann, F. (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Palma, M. , Venneri, M. A. , Galli, R. , Sergi Sergi, L. , Politi, L. S. , Sampaolesi, M. , Naldini, L. (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226. [DOI] [PubMed] [Google Scholar]
- 14. Martinez, F. O. , Gordon, S. , Locati, M. , Mantovani, A. (2006) Transcriptional profiling of the human monocyte‐to‐macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311. [DOI] [PubMed] [Google Scholar]
- 15. Ginhoux, F. , Schultze, J. L. , Murray, P. J. , Ochando, J. , Biswas, S. K. (2016) New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17, 34–40. [DOI] [PubMed] [Google Scholar]
- 16. Xue, J. , Schmidt, S. V. , Sander, J. , Draffehn, A. , Krebs, W. , Quester, I. , De Nardo, D. , Gohel, T. D. , Emde, M. , Schmidleithner, L. , Ganesan, H. , Nino‐Castro, A. , Mallmann, M. R. , Labzin, L. , Theis, H. , Kraut, M. , Beyer, M. , Latz, E. , Freeman, T. C. , Ulas, T. , Schultze, J. L. (2014) Transcriptome‐based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mosser, D. M. , Edwards, J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards, J. P. , Zhang, X. , Frauwirth, K. A. , Mosser, D. M. (2006) Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biswas, S. K. , Allavena, P. , Mantovani, A. (2013) Tumor‐associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 35, 585–600. [DOI] [PubMed] [Google Scholar]
- 20. Greenplate, A. R. , Johnson, D. B. , Roussel, M. , Savona, M. R. , Sosman, J. A. , Puzanov, I. , Ferrell, P. B., Jr. , Irish, J. M. (2016) Myelodysplastic syndrome revealed by systems immunology in a melanoma patient undergoing anti‐PD‐1 therapy. Cancer Immunol. Res. 4, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bendall, S. C. , Simonds, E. F. , Qiu, P. , Amir, A. D. , Krutzik, P. O. , Finck, R. , Bruggner, R. V. , Melamed, R. , Trejo, A. , Ornatsky, O. I. , Balderas, R. S. , Plevritis, S. K. , Sachs, K. , Pe'er, D. , Tanner, S. D. , Nolan, G. P. (2011) Single‐cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spitzer, M. H. , Nolan, G. P. (2016) Mass cytometry: single cells, many features. Cell 165, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saeys, Y. , Gassen, S. V. , Lambrecht, B. N. (2016) Computational flow cytometry: helping to make sense of high‐dimensional immunology data. Nat. Rev. Immunol. 16, 449–462. [DOI] [PubMed] [Google Scholar]
- 24. Diggins, K. E. , Greenplate, A. R. , Leelatian, N. , Wogsland, C. E. , Irish, J. M. (2017) Characterizing cell subsets using marker enrichment modeling. Nat. Methods 14, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amir, A. D. , Davis, K. L. , Tadmor, M. D. , Simonds, E. F. , Levine, J. H. , Bendall, S. C. , Shenfeld, D. K. , Krishnaswamy, S. , Nolan, G. P. , Pe'er, D. (2013) viSNE enables visualization of high dimensional single‐cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu, P. , Simonds, E. F. , Bendall, S. C. , Gibbs, K. D., Jr. , Bruggner, R. V. , Linderman, M. D. , Sachs, K. , Nolan, G. P. , Plevritis, S. K. (2011) Extracting a cellular hierarchy from high‐dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong, M. T. , Chen, J. , Narayanan, S. , Lin, W. , Anicete, R. , Kiaang, H. T. K. , De Lafaille, M. A. C. , Poidinger, M. , Newell, E. W. (2015) Mapping the diversity of follicular helper t cells in human blood and tonsils using high‐dimensional mass cytometry analysis. Cell Reports 11, 1822–1833. [DOI] [PubMed] [Google Scholar]
- 28. Van Unen, V. , Li, N. , Molendijk, I. , Temurhan, M. , Höllt, T. , van der Meulen‐de Jong, A. E. , Verspaget, H. W. , Mearin, M. L. , Mulder, C. J. , van Bergen, J. , Lelieveldt, B. P. F. , Koning, F. (2016) Mass cytometry of the human mucosal immune system identifies tissue‐ and disease‐associated immune subsets. Immunity 44, 1227–1239. [DOI] [PubMed] [Google Scholar]
- 29. Becher, B. , Schlitzer, A. , Chen, J. , Mair, F. , Sumatoh, H. R. , Teng, K. W. W. , Low, D. , Ruedl, C. , Riccardi‐Castagnoli, P. , Poidinger, M. , Greter, M. , Ginhoux, F. , Newell, E. W. (2014) High‐dimensional analysis of the murine myeloid cell system. Nat. Immunol. 15, 1181–1189. [DOI] [PubMed] [Google Scholar]
- 30. Sen, N. , Mukherjee, G. , Sen, A. , Bendall, S. C. , Sung, P. , Nolan, G. P. , Arvin, A. M. (2014) Single‐cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Reports 8, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horowitz, A. , Strauss‐Albee, D. M. , Leipold, M. , Kubo, J. , Nemat‐Gorgani, N. , Dogan, O. C. , Dekker, C. L. , Mackey, S. , Maecker, H. , Swan, G. E. , Davis, M. M. , Norman, P. J. , Guethlein, L. A. , Desai, M. , Parham, P. , Blish, C. A. (2013) Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 5, 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaudillière, B. , Fragiadakis, G. K. , Bruggner, R. V. , Nicolau, M. , Finck, R. , Tingle, M. , Silva, J. , Ganio, E. A. , Yeh, C. G. , Maloney, W. J. , Huddleston, J. I. , Goodman, S. B. , Davis, M. M. , Bendall, S. C. , Fantl, W. J. , Angst, M. S. , Nolan, G. P. (2014) Clinical recovery from surgery correlates with single‐cell immune signatures. Sci. Transl. Med. 6, 255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mason, G. M. , Lowe, K. , Melchiotti, R. , Ellis, R. , de Rinaldis, E. , Peakman, M. , Heck, S. , Lombardi, G. , Tree, T. I. M. (2015) Phenotypic complexity of the human regulatory T cell compartment revealed by mass cytometry. J. Immunol. 195, 2030–2037. [DOI] [PubMed] [Google Scholar]
- 34. Hansmann, L. , Blum, L. , Ju, C.‐H. , Liedtke, M. , Robinson, W. H. , Davis, M. M. (2015) Mass cytometry analysis shows that a novel memory phenotype B cell is expanded in multiple myeloma. Cancer Immunol. Res. 3, 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strauss‐Albee, D. M. , Horowitz, A. , Parham, P. , Blish, C. A. (2014) Coordinated regulation of NK receptor expression in the maturing human immune system. J. Immunol. 193, 4871–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bendall, S. C. , Davis, K. L. , Amir, A. D. , Tadmor, M. D. , Simonds, E. F. , Chen, T. J. , Shenfeld, D. K. , Nolan, G. P. , Pe'er, D. (2014) Single‐cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell 157, 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nicholas, K. J. , Greenplate, A. R. , Flaherty, D. K. , Matlock, B. K. , Juan, J. S. , Smith, R. M. , Irish, J. M. , Kalams, S. A. (2016) Multiparameter analysis of stimulated human peripheral blood mononuclear cells: A comparison of mass and fluorescence cytometry. Cytometry A 89, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guilliams, M. , Dutertre, C.‐A. , Scott, C. L. , McGovern, N. , Sichien, D. , Chakarov, S. , VanGassen, S. , Chen, J. , Poidinger, M. , De Prijck, S. , Tavernier, S. J. , Low, I. , Irac, S. E. , Mattar, C. N. , Sumatoh, H. R. , Low, G. H. L. , Chung, T. J. K. , Chan, D. K. H. , Tan, K. K. , Hon, T. L. K. , Fossum, E. , Bogen, B. , Choolani, M. , Chan, J. K. Y. , Larbi, A. , Luche, H. , Henri, S. , Saeys, Y. , Newell, E. W. , Lambrecht, B. N. , Malissen, B. , Ginhoux, F. (2016) Unsupervised high‐dimensional analysis aligns dendritic cells across tissues and species. Immunity 45, 669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irish, J. M. (2014) Beyond the age of cellular discovery. Nat. Immunol. 15, 1095–1097. [DOI] [PubMed] [Google Scholar]
- 40. Roussel, M. , Greenplate, A. R. , Irish, J. M. (2016) Dissecting complex cellular systems with high dimensional single cell mass cytometry. In Experimental Approaches for the Investigation of Innate Immunity: The Human Innate Immunity Handbook (Montgomery R. R. and Bucala R., eds.), World Scientific, Hackensack, NJ, 15–26. [Google Scholar]
- 41. Marigo, I. , Bosio, E. , Solito, S. , Mesa, C. , Fernández, A. , Dolcetti, L. , Ugel, S. , Sonda, N. , Bicciato, S. , Falisi, E. , Calabrese, F. , Basso, G. , Zanovello, P. , Cozzi, E. , Mandruzzato, S. , Bronte, V. (2010) Tumor‐induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 32, 790–802. [DOI] [PubMed] [Google Scholar]
- 42. Lechner, M. G. , Liebertz, D. J. , Epstein, A. L. (2010) Characterization of cytokine‐induced myeloid‐derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferrell, P. B., Jr. , Diggins, K. E. , Polikowsky, H. G. , Mohan, S. R. , Seegmiller, A. C. , Irish, J. M. (2016) High‐dimensional analysis of acute myeloid leukemia reveals phenotypic changes in persistent cells during induction therapy. PLoS One 11, e0153207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fienberg, H. G. , Simonds, E. F. , Fantl, W. J. , Nolan, G. P. , Bodenmiller, B. (2012) A platinum‐based covalent viability reagent for single‐cell mass cytometry. Cytometry A 81, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finck, R. , Simonds, E. F. , Jager, A. , Krishnaswamy, S. , Sachs, K. , Fantl, W. , Pe'er, D. , Nolan, G. P. , Bendall, S. C. (2013) Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diggins, K. E. , Ferrell, P. B., Jr. , Irish, J. M. (2015) Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods 82, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kotecha, N. , Krutzik, P. O. , Irish, J. M. (2010) Web‐based analysis and publication of flow cytometry experiments. In Curr. Protoc. Cytom., John Wiley & Sons, New York, 10.17.1–10.17.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hofer, T. P. , Zawada, A. M. , Frankenberger, M. , Skokann, K. , Satzl, A. A. , Gesierich, W. , Schuberth, M. , Levin, J. , Danek, A. , Rotter, B. , Heine, G. H. , Ziegler‐Heitbrock, L. (2015) Slan‐defined subsets of CD16‐positive monocytes: impact of granulomatous inflammation and M‐CSF receptor mutation. Blood 126, 2601–2610. [DOI] [PubMed] [Google Scholar]
- 49. Sade‐Feldman, M. , Kanterman, J. , Klieger, Y. , Ish‐Shalom, E. , Olga, M. , Saragovi, A. , Shtainberg, H. , Lotem, M. , Baniyash, M. (2016) Clinical significance of circulating CD33+CD11b+HLA‐DR‐ myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin. Cancer Res. 22, 5661–5672. [DOI] [PubMed] [Google Scholar]
- 50. Rudolph, B. M. , Loquai, C. , Gerwe, A. , Bacher, N. , Steinbrink, K. , Grabbe, S. , Tuettenberg, A. (2014) Increased frequencies of CD11b+ CD33+ CD14+ HLA‐DRlow myeloid‐derived suppressor cells are an early event in melanoma patients. Exp. Dermatol. 23, 202–204. [DOI] [PubMed] [Google Scholar]
- 51. Chevolet, I. , Speeckaert, R. , Schreuer, M. , Neyns, B. , Krysko, O. , Bachert, C. , Van Gele, M. , van Geel, N. , Brochez, L. (2015) Clinical significance of plasmacytoid dendritic cells and myeloid‐derived suppressor cells in melanoma. J. Transl. Med. 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weide, B. , Martens, A. , Zelba, H. , Stutz, C. , Derhovanessian, E. , Di Giacomo, A. M. , Maio, M. , Sucker, A. , Schilling, B. , Schadendorf, D. , Büttner, P. , Garbe, C. , Pawelec, G. (2014) Myeloid‐derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY‐ESO‐1‐ or Melan‐A‐specific T cells. Clin. Cancer Res. 20, 1601–1609. [DOI] [PubMed] [Google Scholar]
- 53. Mao, Y. , Poschke, I. , Wennerberg, E. , Pico de Coaña, Y. , Egyhazi Brage, S. , Schultz, I. , Hansson, J. , Masucci, G. , Lundqvist, A. , Kiessling, R. (2013) Melanoma‐educated CD14+ cells acquire a myeloid‐derived suppressor cell phenotype through COX‐2‐dependent mechanisms. Cancer Res. 73, 3877–3887. [DOI] [PubMed] [Google Scholar]
- 54. Mingueneau, M. , Boudaoud, S. , Haskett, S. , Reynolds, T. L. , Nocturne, G. , Norton, E. , Zhang, X. , Constant, M. , Park, D. , Wang, W. , Lazure, T. , Le Pajolec, C. , Ergun, A. , Mariette, X. (2016) Cytometry by time‐of‐flight immunophenotyping identifies a blood Sjögren's signature correlating with disease activity and glandular inflammation. J. Allergy Clin. Immunol. 137, 1809–1821.e12. [DOI] [PubMed] [Google Scholar]
- 55. Gaudillière, B. , Ganio, E. A , Tingle, M. , Lancero, H. L. , Fragiadakis, G. K. , Baca, Q. J. , Aghaeepour, N. , Wong, R. J. , Quaintance, C. , El‐Sayed, Y. Y. , Shaw, G. M. , Lewis, D. B. , Stevenson, D. K. , Nolan, G. P. , Angst, M. S. (2015) Implementing mass cytometry at the bedside to study the immunological basis of human diseases: distinctive immune features in patients with a history of term or preterm birth. Cytometry A 87, 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Behbehani, G. K. , Samusik, N. , Bjornson, Z. B. , Fantl, W. J. , Medeiros, B. C. , Nolan, G. P. (2015) Mass cytometric functional profiling of acute myeloid leukemia defines cell‐cycle and immunophenotypic properties that correlate with known responses to therapy. Cancer Discov. 5, 988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levine, J. H. , Simonds, E. F. , Bendall, S. C. , Davis, K. L. , Amir, A. D. , Tadmor, M. D. , Litvin, O. , Fienberg, H. G. , Jager, A. , Zunder, E. R. , Finck, R. , Gedman, A. L. , Radtke, I. , Downing, J. R. , Pe'er, D. , Nolan, G. P. (2015) Data‐driven phenotypic dissection of AML reveals progenitor‐like cells that correlate with prognosis. Cell 162, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han, L. , Qiu, P. , Zeng, Z. , Jorgensen, J. L. , Mak, D. H. , Burks, J. K. , Schober, W. , McQueen, T. J. , Cortes, J. , Tanner, S. D. , Roboz, G. J. , Kantarjian, H. M. , Kornblau, S. M. , Guzman, M. L. , Andreeff, M. , Konopleva, M. (2015) Single‐cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3‐ITD‐mutated AML stem/progenitor cells. Cytometry A 87, 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baumgart, S. , Peddinghaus, A. , Schulte‐Wrede, U. , Mei, H. E. , Grützkau, A. (2016) OMIP‐034: comprehensive immune phenotyping of human peripheral leukocytes by mass cytometry for monitoring immunomodulatory therapies. Cytometry A. 91, 34–38. [DOI] [PubMed] [Google Scholar]
- 60. Spitzer, M. H. , Gherardini, P. F. , Fragiadakis, G. K. , Bhattacharya, N. , Yuan, R. T. , Hotson, A. N. , Finck, R. , Carmi, Y. , Zunder, E. R. , Fantl, W. J. , Bendall, S. C. , Engleman, E. G. , Nolan, G. P. (2015) IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science 349, 1259425–1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Son, Y.‐I. , Egawa, S. , Tatsumi, T. , Redlinger, R. E., Jr. , Kalinski, P. , Kanto, T. (2002) A novel bulk‐culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 262, 145–157. [DOI] [PubMed] [Google Scholar]
- 62. Helft, J. , Böttcher, J. , Chakravarty, P. , Zelenay, S. , Huotari, J. , Schraml, B. U. , Goubau, D. , Reis e Sousa, C. (2015) GM‐CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity 42, 1197–1211. [DOI] [PubMed] [Google Scholar]
- 63. Van de Garde, M. D. B. , Martinez, F. O. , Melgert, B. N. , Hylkema, M. N. , Jonkers, R. E. , Hamann, J. (2014) Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J. Immunol. 192, 1196–1208. [DOI] [PubMed] [Google Scholar]
- 64. Feng, P.‐H. , Lee, K.‐Y. , Chang, Y.‐L. , Chan, Y.‐F. , Kuo, L.‐W. , Lin, T.‐Y. , Chung, F.‐T. , Kuo, C.‐S. , Yu, C.‐T. , Lin, S.‐M. , Wang, C.‐H. , Chou, C.‐L. , Huang, C.‐D. , Kuo, H.‐P. (2012) CD14+S100A9+ monocytic myeloid‐derived suppressor cells and their clinical relevance in non‐small cell lung cancer. Am. J. Respir. Crit. Care Med. 186, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao, F. , Hoechst, B. , Duffy, A. , Gamrekelashvili, J. , Fioravanti, S. , Manns, M. P. , Greten, T. F. , Korangy, F. (2012) S100A9 a new marker for monocytic human myeloid‐derived suppressor cells. Immunology 136, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen, X. , Eksioglu, E. A. , Zhou, J. , Zhang, L. , Djeu, J. , Fortenbery, N. , Epling‐Burnette, P. , Van Bijnen, S. , Dolstra, H. , Cannon, J. , Youn, J.‐I. , Donatelli, S. S. , Qin, D. , De Witte, T. , Tao, J. , Wang, H. , Cheng, P. , Gabrilovich, D. I. , List, A. , Wei, S. (2013) Induction of myelodysplasia by myeloid‐derived suppressor cells. J. Clin. Invest. 123, 4595–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gordon, S. , Plüddemann, A. , Martinez Estrada, F. (2014) Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 262, 36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files


