Short abstract
Review on autophagy as a regulation factor in the function of the endothelium in health and disease.
Keywords: atherosclerosis, cardiovascular, ATG, HMGB1
Abstract
Current studies of vascular health, aging, and autophagy emphasize how the endothelium adapts to stress and contributes to disease. The endothelium is far from an inert barrier to blood‐borne cells, pathogens, and chemical signals; rather, it actively translates circulating mediators into tissue responses, changing rapidly in response to physiologic stressors. Macroautophagy—the cellular ingestion of effete organelles and protein aggregates to provide anabolic substrates to fuel bioenergetics in times of stress—plays an important role in endothelial cell homeostasis, vascular remodeling, and disease. These roles include regulating vascular tone, sustaining or limiting cell survival, and contributing to the development of atherosclerosis secondary to infection, inflammation, and angiogenesis. Autophagy modulates these critical functions of the endothelium in a dynamic and perpetual response to tissue and intravascular cues.
Abbreviations
- 3MA
3‐methyladenine
- ABO
6‐amino‐3‐dihydro‐3‐hydroxymethyl‐1,4‐benzoxazine
- ATG
autophagy‐related genes
- BBB
blood‐brain barrier
- CQ
chloroquine
- EC
endothelial cell
- GBS
group B Streptococcus
- HMGB1
high‐mobility group box‐1
- KCNK3
potassium channel subfamily K member 3
- LC3
protein microtubule‐associated protein 1 light chain‐3
- MLEC
mouse lung endothelial cell
- mTOR
mammalian target of rapamycin
- NLR
NOD1‐like receptor
- PA
palmitic acid
- PH
pulmonary hypertension
- PINK1
phosphatase and tensin homolog‐induced kinase 1
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- RSV
reservatrol
- siRNA
small interfering RNA
- SMAD2
mothers against decapentaplegic homolog 2
- SQSTM1
sequestesome 1 (p62)
- Ucp2
uncoupling protein 2
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
There is nothing permanent except change.
Heraclitus
Introduction
The vascular endothelium serves as a barrier and entry point for nutrient, oxygen, carbon dioxide, and catabolic byproduct transport. It is a platform for inflammatory cell recruitment and coagulation and a regulator of vascular tone. To carry out these functions, the endothelium uses various mechanisms to rapidly respond to injury or tissue stress. Autophagy is a highly conserved process that is critical to cell stress response and is present in all plants and all animals dating back to the last common eukaryotic ancestor. In ECs, stressors include hypoxia, starvation, infection, inflammation, vascular disruption, genomic stress, and oxidant damage. Autophagy, literally translated as “self‐eating,” was recognized with the Nobel Prize awarded to yeast cellular biologist, Yoshinori Ohsumi, in 2016 for work carried out over the last four decades. Recently, autophagy has also been studied in the context of cardiac and other vascular diseases [1, 2, 3, 4, 5, 6–7]. In this review, we examine how autophagy regulates the known functions and disease processes that are associated with the endothelium including and beyond those that contribute to cardiovascular pathology. Whereas autophagy has been reviewed in a variety of cell types, including circulating leukocytes, the literature on autophagy is more limited with regard to the endothelium [8]; therefore, we draw from primary reports and extensive new data that have been produced by our own laboratories, as well as a number of others delineating the role of autophagy in ECs and other cell types. In the endothelium, autophagy emerges as a primary protective mechanism [9]; however, autophagy may also enable EC death, thereby contributing to inflammation and thrombosis.
As the primary interface between blood and tissue, the endothelium has the dual role of both limiting and promoting leukocyte and platelet adhesion. These interactions are central to systemic, tissue, and vessel stress and injury. Thus, a meaningful understanding of autophagic processes within the endothelium will help elucidate its interaction with circulating cells.
REVIEW OF MACROAUTOPHAGY
Autophagy describes the lysosomal‐mediated consumption of cytoplasmic organelles and proteins in times of cell stress ( Fig. 1 ). This process involves engulfment and degradation of cytosolic constituents, which contributes to organelle quality control. Autophagy‐based unconventional secretory pathways have also been described that contribute to both thrombosis and inflammation [10, 11, 12, 13, 14, 15–16]. There are three recognized forms of autophagy, namely macroautophagy, microautophagy, and chaperone‐mediated autophagy [17]. The term, immune cell–mediated autophagy, describes the inflammatory cell–mediated induction of autophagy in other cells. This has been observed in the interaction of tumors with NK and T cells [18]. Macroautophagy (autophagy subsequently) begins with vesicle nucleation in which double‐membrane vacuoles develop from several potential sources, including the endoplasmic reticulum, mitochondria (with starvation), or the Golgi apparatus [17]. Vesicles then elongate into double‐membrane autophagosomes that sequester protein aggregates or organelles to be consumed, transporting them along tubulin tracks to lysosomal compartments. Complete autophagosomes then join with lysosomes to form autophagolysosomes within which the contents are degraded and recycled into the cytoplasm [17]. This process is initiated by starvation as well as by compounds or signals that inhibit mTOR, which is a constitutive inhibitor of ATGs, including Unc‐51–like kinase 1. Unc‐51–like kinase 1/ATG1 is critical to the early stages of autophagy induction [19]. Rapamycin inhibits mTOR, which, in turn, augments autophagy. Conversely, the availability of amino acids inhibits autophagy by activating mTOR, possibly via interactions at the level of the lysosome [20, 21].
Figure 1.
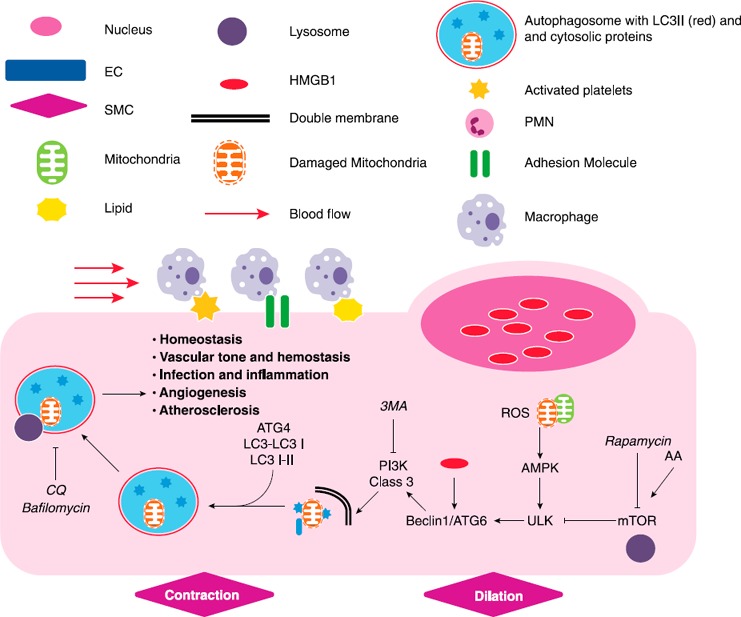
Macroautophagy in ECs. Schematic that describes pathways that mediate the induction of autophagy in ECs. Exogenous mediators known to either enhance or inhibit designated pathways are shown in italics. The common legend is utilized for all subsequent figures. Arrows designate an activating relationship. “T” lines indicate an inhibitory effect. AA, amino acids, PMN, polymorphonuclear cell, SMC, smooth muscle cell, ULK, Unc‐51–like kinase 1.
Autophagy is a dynamic process that is best measured as flux; thus, its recognition requires the identification of autophagosome formation and protein/organelle/organism degradation from lysosomal consumption. Initiators and inhibitors of autophagy, coupled with timed experiments, can help assess which points within the autophagic cycle are important for an individual cellular function or phenotype [17]. The class III PI3K inhibitor, 3MA, disrupts autophagy early in the autophagic process [22]. In contrast, CQ and bafilomycin result in late autophagic inhibition by disrupting lysosomal function and autophagosome degradation. CQ and bafilomycin promote autophagosome accumulation and lack of internalized cytosolic protein degradation [22, 23–24]. Vinca alkaloids and taxanes interrupt autophagy at later stages, similar to bafilomycin and CQ, which halts autophagic flux and increases autophagosome accumulation [25]. Quantifying the number of autophagosomes in cells is not sufficient to confirm that autophagy is active, as their increase within the cytoplasm can occur as a result of diminished degradation or increased production [17].
Autophagosome formation is typically confirmed by LC3B (herein LC3) [26]. During autophagosome formation, LC3 is cleaved by ATG4 to generate LC3I. LC3I is converted to a lipidated form by conjugation to phosphotidylethanolamine via mediating ATG12‐ATG5 complex [27]. The phosphotidylethanolamine‐conjugated form of LC3 (LC3‐II) is incorporated into autophagosomes that appear as punctae on immunohistochemistry and migrates distally from LC3‐I during electrophoresis. Western blotting of the LC3 protein thus reveals two bands that represent the unmodified and conjugated forms. By using immunohistochemistry, LC3‐II on autophagosomes appear as puncta rather than diffuse particles within the cytoplasm. Evaluating organelle/substrate degradation over time, such as mitochondria or the SQSTM1/p62 protein, along with LC3 expression, can help determine whether autophagy is increased or decreased [17].
VASCULAR TONE, HOMEOSTASIS, AND THE RESPONSE TO METABOLIC STRESS
Autophagy and vascular tone
The endothelium is critical in maintaining normal vascular tone by producing the signals that modulate minute‐to‐minute vasodilation or vasoconstriction. Loss of vasomotor function is typically manifested by dysregulation of NO signaling, which is affected by autophagic processes ( Fig. 2 ) [28]. Starvation of HUVECs results in an increase in acidic vesicular organelle formation, LC3‐I to LC3‐II conversion, and SQSTM‐1/p62 degradation associated with increased autophagy [24]. Serum starvation also results in a significant decrease in NO production, which is reversed with CQ treatment [24]. CQ attenuates phenylephrine‐induced vasoconstriction in aortic rings in a manner that is reversible by application of the selective NO synthase inhibitor, N (ω)‐nitro‐l‐arginine methylester. Aortic rings stripped of endothelium do not reverse their vasoconstrictive phenotype with CQ. Thus, autophagic signaling seems to directly modulate endothelial NO release, with prominent effects on smooth muscle cell contraction [24].
Figure 2.
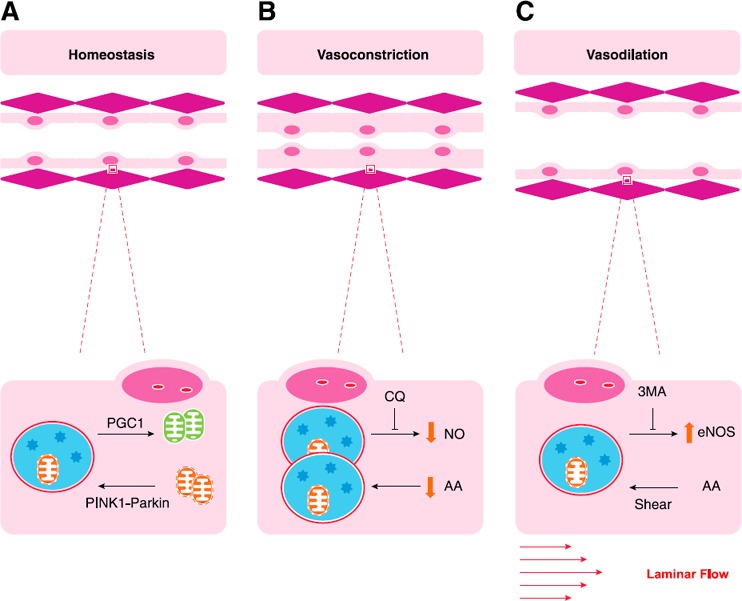
Autophagy and vascular tone. (A) Autophagic clearance of damaged mitochondria (mitophagy) is critical to maintaining homeostasis. Mitophagy is induced by PINK1‐Parkin signaling as described in the section on vascular tone, homeostasis, and the response to metabolic stress, and is balanced by mitochondrial biogenesis, induced by peroxisome proliferator‐activated receptor γ co‐activator (PGC) 1α. Effects of autophagy on ECs include modulation of NO signaling, where it has both positive and negative effects. (B and C) Whereas potassium channel ion flow is also critically important to vascular tone, these images depict autophagic regulation of NO induced vasoconstriction (B) and vasodilation (C). AA, amino acids.
Autophagy also mediates eNOS expression in response to shear stress [29]. In some circumstances, shear stress increases adhesion molecule expression on endothelium, which promotes leukocyte recruitment, inflammation, arteriogenesis, and atherogenesis [30, 31, 32, 33, 34, 35, 36, 37–38]. Shear is also present during normal arterial flow and may be more or less relevant depending on the vascular bed [39]. In an ex vivo model that uses both HUVECs and rabbit carotid artery segments, changes in shear flow promote concomitant LC3‐I to LC3‐II conversion and Beclin‐1/ATG6 expression and SQSTM1/p62 degradation. In this setting, shear stress also increases eNOS expression. Pretreatment of arterial segments and cells with 3MA abolishes eNOS up‐regulation, whereas rapamycin enhances it [29]. In addition, ECs that are treated with nontargeting siRNA increase eNOS expression and phosphorylation in response to shear. Those treated with siRNA to limit ATG3 reduce eNOS expression, which enhances ROS generation and increases IL‐8 production [40]. Here, elevated eNOS expression correlates with a protective, dilatory effect of autophagy in ECs that may promote tissue perfusion [29].
Both arterial endothelial and smooth muscle cells also maintain vascular tone through the regulation of potassium ion channels, which are modified, in part, by enzymes that interact directly with SQSTM1, a chaperone and autophagy substrate [41, 42]. Potassium channels are activated by voltage and/or calcium and consist of α‐ and β‐subunits. β‐Subunits undergo conformational changes with phosphorylation (and other modifications), which results in changes in channel function [43]. SQSTM1, a protein with a ubiquitin binding site and a target for autophagic degradation, acts as a scaffold for PKCζ in arterial smooth muscle cells, which affects the phosphorylation state of the β‐subunit—reviewed extensively by Ishii [41]. The SQSTM1 protein itself also interacts with potassium channels, which promotes K+ channel β‐subunit phosphorylation by the tyrosine kinase, p56lck [41]. In vascular cells, the clearance of SQSTM1 by autophagic processes may therefore indirectly influence baseline vascular tone via potassium channel activation.
Potassium channel–induced vasomotor tone contributes to disease states, including PH. Inhibition of potassium ion channel flow contributes to calcium‐mediated vasoconstriction and is a possible etiologic factor in idiopathic PH [42, 44]. KCNK3 is diminished in pulmonary artery ECs in patients with PH and in rat models. In isolated arterial segments, inhibition of KCNK3 potassium ion flow vasoconstricts, whereas in vivo administration of KCNK3 inhibitors results in arterial EC proliferation and muscularization as well as tissue inflammation [42].
Autophagy, homeostasis, and response to metabolic stress
Endothelial function depends on sources of energy that are provided by a healthy mitochondrial pool and basal glycolysis. Mitochondrial quality control is regulated by mitophagy, in which malfunctioning mitochondria are engulfed by autophagosomes and digested within the lysosome after fusion (Fig. 2A) [8]. To restore mitochondrial numbers, mitophagy is balanced by mitochondrial biogenesis via up‐regulation of the transcription factor, peroxisome proliferator‐activated receptor γ co‐activator 1α, and enhanced by peroxisome proliferator‐activated receptor‐γ agonists [45, 46]. The importance of mitochondrial function in EC is reviewed extensively by Kluge et al. [9]. Mitochondria are an important source of ROS during EC stress and can induce autophagy via AMPK‐mediated pathways. Ubiquinated proteins that are otherwise destined for proteosomal degradation can result in mitochondrial ROS generation and AMPK activation if allowed to accumulate [47]. In turn, AMPK‐mediated autophagic pathways remove damaged and dysfunctional mitochondria, which restores homeostatic balance [27]. Although mitochondrial stores are generally low in ECs compared with cardiac or skeletal myocytes, their dysfunction can lead to oxidative damage and systemic vascular disease in disordered states. Radiation, oxidative, and chemical damage to ECs can promote mitochondrial depolarization and injury, which, in turn, promotes mitophagy [9]. Reduced mitophagy is thought to be associated with aging and metabolic syndrome [27, 48].
Autophagy plays a critical role in modulating the endothelium's response to metabolic changes that occur with normal and deranged cellular respiration. The ability of ECs to maintain a nonreactive surface is affected both positively and negatively by active autophagy induced by metabolic stress (described in detail in the ECs and circulating cells section below). Of note, whereas autophagy may rescue a metabolically challenged cell, it can also induce inflammatory pathways that result in immune‐modulating cell death [49].
Examples of both the protective and damaging effects of autophagy and mitophagy have been described. PINK1 and Parkin‐mediated pathways actively promote EC mitophagy in response to PA, which induces oxidative damage [48]. In the absence of PINK1, PA exposure causes accumulation of damaged mitochondria within ECs as well as apoptosis. Obese mice have a higher expression of PINK1 and Parkin within arterial endothelium. These data highlight a protective role for mitophagy that helps attenuate oxidative stress induced by fatty acids [48].
In hemolytic anemia, autophagy is also active, responding to a heme‐induced oxidative state that drives iron‐dependent lipid peroxidation, NO scavenging, and ischemic tissue damage with inflammatory cell recruitment [50]. Heme‐induced cell death caused by disrupted bioenergetics and mitochondrial dysfunction is potentiated by the autophagy inhibitor, 3MA. These findings suggest that autophagy is initiated during hemolytic anemia to help assuage damage but still actively promotes bioenergetic substrate production until and during cell death [50]. Others have also linked EC autophagy to an adaptive response to oxidative stress. Caveolin‐1 diminishes the accumulation of mitochondrial ROS in bovine arterial ECs [51]. Knockdown of caveolin‐1 with siRNA increases H2O2 and ROS production and diminishes catalase activity. These findings correlate with an increase in LC3‐I to LC3‐II conversion as well as an accumulation of intracellular dipeptides, which is consistent with the induction of autophagy in response to a redox challenge [51].
Oxidant‐induced models of PH have emphasized the role of autophagy in the etiology of the disease rather than as a protective mechanism. Mice that are exposed to intermittent hypoxia in a murine model of PH demonstrate a requirement for EC‐specific Ucp2, an anion transporter that dissipates the protein gradient that is formed by the electron transport chain. MLECs that have been harvested from mice that lack endothelial‐specific Ucp2 have severe metabolic derangements, excessive mitophagy, and increased autophagic flux [52]. Those mice also develop PH and right heart ventricular dysfunction. MLECs taken from knockout mice and those treated with siRNA to Ucp2 could be partially rescued by inhibiting a mitochondrial membrane protein that is responsible for initiating mitophagy [52]. These findings highlight that excessive mitophagy can result in destabilization and cellular damage even in the setting of an underlying mitochondrial disturbance.
Excessive mitophagy also exacerbates injury that results from growth factor deficiency. In addition to promoting capillary leak and EC proliferation in response to angiogenic cues, VEGF is a homeostatic growth factor that maintains mitochondrial integrity. VEGF deficiency results in mitochondrial disruption and conspicuous mitophagy, which leads to cellular instability and death [53]. In an inducible model of murine, endothelial‐specific VEGF depletion (VEGF‐iECKO), hypoxic exposure results in sudden death associated with intestinal damage and perforation and brain hemorrhage. To understand this phenomenon on a cellular level, knockdown of VEGF in cultured ECs promotes aberrant mitochondrial function, a concomitant increase in autophagic vacuoles, and increased expression of autophagy‐inducing forkhead transcription factors. Administration of forkhead transcription factor inhibitors to VEGF‐depleted ECs reverses the metabolic derangements that arise from heightened autophagy [53].
ECs AND CIRCULATING CELLS—THROMBOSIS, INFECTION, AND INFLAMMATION
Autophagy and hemostasis
Critical functions of the endothelium are to aid in hemostasis and help prevent hemorrhage. Autophagic regulation of secretory functions in ECs has defined a role for ATGs in thrombus formation ( Fig. 3 ). Key findings that support this assertion suggest that ATG7 regulates vWF secretion by EC Wiebel‐Palade bodies. vWF multimers bind to the extracellular matrix and subendothelial layers upon vessel disruption, which allows for platelet recruitment to the site of injury. In cultured ECs, autophagosomes cluster around Wiebel‐Palade bodies. Knockdown of ATG7 in human cultured ECs reduces vWF secretion in response to histamine and growth factors. CQ has a similar effect. In vivo, EC‐specific ATG7KO mice do not increase serum vWF in response to an epinephrine challenge, whereas wild‐type mice do. Furthermore, EC‐specific ATG7KO mice have prolonged bleeding times compared with WT mice. Similar effects on bleeding time were observed with CQ administration. These data highlight a prohemostatic function for autophagic pathways in ECs that results in the secretion of thrombotic molecules [16].
Figure 3.
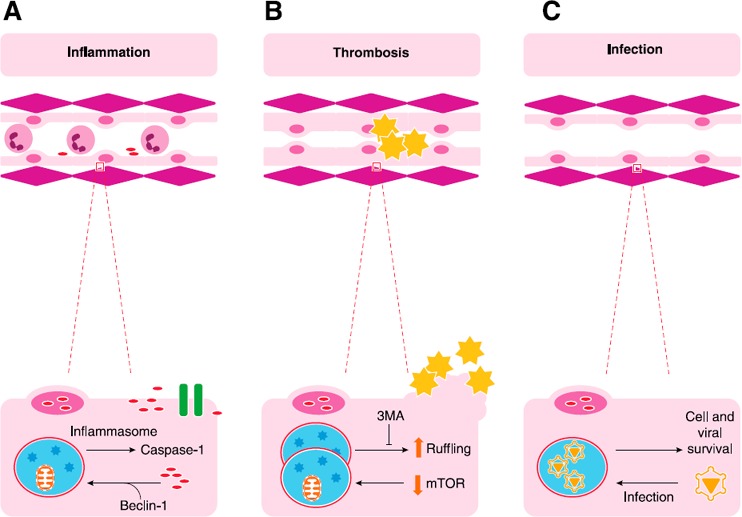
EC autophagy and cell‐cell interaction. A critical role of the endothelium is modulation of cell‐cell interactions, particularly those involving leukocytes and platelets. (A) Translocation of HMGB1 from nuclei displaces beclin‐1 from BCL2, which induces autophagy. Autophagic pathways can interact with the inflammasome to release of cytokines, such as IL‐1b and IL‐18, through activation of caspase‐1. HMGB1, a nuclear protein and damage‐associated molecular pattern (DAMP) molecule, can also be released by caspase‐1–mediated pathways in Mϕs and can recruit inflammatory cells to areas of damage. (B) Typically, quiescent endothelium should not attract platelets; however, EC autophagy has been implicated in platelet recruitment as a result of cytoskeletal changes that lead to membrane ruffling. These mechanisms are thought to lead to thrombosis induced by rapamycin and sirolimus‐coated coronary stents. (C) Of interest, organisms, such as EBV, can usurp autophagic pathways to maintain a viral reservoir by promoting cell survival. Alternatively, GBS infection of cells induces autophagy, which clears bacteria, perhaps via TLR2‐mediated pathways (not shown).
Regulation of autophagy in ECs also contributes to the maintenance of an inert antithrombotic surface within the vasculature. Drug‐eluting stents that inhibit intimal hyperplasia after endovascular interventions have provided insight into how endothelial autophagy can promote or inhibit thrombosis. Both sirolimus and paclitaxel were directly studied for their ability to induce autophagy in ECs, thus diminishing their proliferation. Sluggish re‐endothelialization enables and sustains the exposure of subendothelial components, such as collagen, which are themselves thrombogenic [54]. Sirolimus has a profound proautophagic effect in aortic ECs and is associated with an antiproliferative, antiapoptotic phenotype that is consistent with negative vascular remodeling [54]. Whereas apoptosis is the primary form of programmed cell death, it also balances autophagy and has positive effects in tissue restoration and regeneration [55].
Rapamycin, an inducer of autophagy and a commonly used coating for coronary stents, causes EC cytoskeletal ruffling that promotes platelet recruitment and thrombosis [56]. Unlike studies that have highlighted the role of re‐endothelialization in thrombosis, this study highlights a direct effect of autophagy on EC thrombogenicity. Platelet adhesion to cultured rapamycin‐treated ECs is higher than it is in cells that are pretreated with DMSO. Platelet adhesion to EC is deterred by cytochalasin D treatment, which implicates EC cytoskeletal changes in platelet recruitment. 3MA also diminishes cytoskeletal remodeling and, presumably, would have an antiadhesion phenotype in this model [56]. Because maintenance of an antithrombotic surface is a critical role of endothelium under homeostatic conditions, autophagy can be considered to promote thrombosis.
Autophagy and EC adhesion molecule expression
In addition to playing hemostatic and prothrombotic roles, autophagy in ECs contributes to inflammation, recruitment, and regulation of innate and adaptive immune cells, as well as infection by invading pathogens [57]. These relationships are less well characterized in ECs compared with Mϕs and other cell types, as extensively reviewed by Deretic [8]. What is clear is that inflammatory cytokines can influence adhesion molecule expression via autophagic processes in both ECs and epithelial cells. Enhancing endothelial autophagy can directly promote leukocyte recruitment. For example, HUVECs that are treated with TNF‐α express ICAM‐1 in a manner that is attenuated by the SIRT1 and tRNA synthetase binder RSV [58, 59]. RSV concomitantly increases the LC3‐I to LC3‐II conversion and promotes SQSTM1/p62 turnover. These anti‐inflammatory protective effects of RSV can be reversed when HUVECS are also exposed to 3MA, CQ, bafilomycin, or siRNA‐targeting beclin‐1/ATG6 [59]. Activation of autophagy in this setting reduces the inflammatory phenotype that is typically induced by TNF‐α and, presumably, is a potential mechanism that mediates the protective effects of RSV.
In mice, pretreatment with rapamycin before administration of intratracheal LPS attenuates neutrophil recruitment in bronchoalveolar lavage samples, which implies diminished adhesion molecule expression. Neutrophil myeloperoxidase staining within lung alveolar spaces is also diminished by rapamycin pretreatment, although this was not necessarily associated with diminishing mortality [60].
Others have found the opposite effect. Enhancing autophagic pathways can facilitate TNF‐α–induced ICAM expression in ECs and lung epithelial cells. 3MA inhibits this expression in human aortic ECs and decreases monocyte adhesion to lung epithelial cells in vitro [61]. These conflicting results may be a result of differences in the timing of autophagy induction and the cell types examined. Rapamycin that is administered as a pretreatment before LPS exposure may create a metabolic environment that hinders leukocyte recruitment in injured lung, contributing to mortality rather than protection.
Autophagy, infection, and TLRs
PRRs, including the TLR, detect both pathogen‐ and damage‐associated molecular pattern molecules, which initiates an early immune response [62]. Pathogen‐associated molecular pattern molecules include bacterial LPS (a constitutive component of the outer cell membrane of gram‐negative bacteria), bacterial flagellin (a component of bacterial flagellum), peptidoglycans, and bacterial nucleic acid components, including hypomethylated DNA (CpG) [63, 64, 65–66]. Endogenous damage‐associated molecular pattern molecules include HMGB1, heat shock protein, S100 proteins, uric acid, histones, and degraded matrix components associated with tissue damage [67].
TLR activation allows the innate immune system to rapidly recruit Mϕs and leukocytes during infection and injury, and their expression on the endothelium has been well documented [68, 69–70]. TLR crosstalk with autophagic pathways has been described in Mϕs, circulating leukocytes, and, to a lesser extent, ECs [26, 71, 72]. Autophagic clearance of microbes is analogous to processes that clear cytosolic proteins and organelles [26]. In Mϕs, a connection between autophagy and TLR7 activation has been well established, which implicates autophagy in bacterial clearance. Autophagy is induced by administering single stranded RNA, a TLR7 agonist that induces the formation of prominent cytoplasmic LC3 puncta. Administration of single stranded RNA to Mϕs also promotes intracellular engulfment of bacillus Calmette‐Guerin, presumably via autophagic pathways within the cell [73]. This consumption of pathogens through an autophagic process is referred to as xenophagy [74].
Similarly, in BBB ECs, autophagy promotes the clearance of bacterial GBS. In this case, however, TLR involvement is implied as GBS is recognized by TLR2, and TLR2 deficiency exacerbates GBS infection [75, 76–77]. Autophagy in BBB ECs increases after GBS infection [78]. Administration of live, but not heat‐inactivated GBS to cultured BBB ECs increases autophagic flux characterized by LC3‐I to LC3‐II conversion and enhanced SQSTM1/p62 turnover. Treatment of BBB ECs with rapamycin results in less bacterial accrual within the cell. Treatment of cells with bafilomycin predictably results in autophagosome accumulation and reduced bacterial clearance. Similar effects were not observed with inactivation of more proximal signaling molecules in the autophagic pathway, which suggests that the machinery required to deliver autophagosome contents to lysosomes are critical to bacterial clearance [78].
Autophagic pathways can also be usurped by invading pathogens to facilitate infectivity and vascular spread, which is reflected in the infectious etiology of γ herpes virus 68 (an EBV homolog and γ herpes virus) [79]. In a murine model of EBV infection, the survival of viral particles depends on autophagy to promote persistence within the cell. Specifically, ECs that are infected with this virus undergo significant increases in autophagy, which promotes the survival of the cell and perpetuation of the virus. Knockdown of autophagy genes abrogates EC survival but also limits the viral reservoir [79].
Autophagy, inflammation, and HMGB1
TLRs promote the initiation of inflammation from both exogenous and endogenous damage‐associated proteins, including HMGB1, which has been a focus of many of our own studies. HMGB1—a ubiquitously expressed nuclear protein—has prominent proinflammatory effects when translocated, secreted, or passively released into the systemic circulation. Locally, HMGB1 can also be proregenerative and proangiogenic, recruiting tissue repair cells, including ECs, muscle stem cells, and mesangioblasts to areas of damage [80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90–91]. We have shown that ECs release HMGB1 when exposed to hypoxia and can also proliferate in response to HMGB1 [92]. In certain cell types, HMGB1 can sustain autophagy by displacing BCL2 from beclin‐1 and can be released from epithelial cells via pathways that are modified by autophagic signaling [93]. We determined that disulfide bonds in the vicinal cysteines at C23 and C45 promote the translocation of HMGB1 out of the nucleus, which increases its binding to cytoplasmic beclin‐1 and the concomitant dissociation of beclin‐1 from BCL2 [93, 94].
In Mϕs, autophagy can promote HMGB1 release via its interaction with intracellular inflammatory pathways. Autophagy intersects with the inflammasome, an oligomer of cytosolic PRR that induces the secretion of IL‐1β and IL‐18 after activation of caspase‐1. Specifically, NLRs, such as NLRP‐3, are cytosolic PRRs that assemble to form the inflammasome complex. Multimeric NLRs bind procaspase‐1 with or without the adaptor signaling caspase recruitment domain. This complex then initiates cleavage of the zymogen procaspase‐1 to activated caspase‐1 subunits, which, in turn, cleaves pro–IL‐1β to create an active form suitable for secretion [95, 96]. In mice, caspase‐1 and caspase‐11 are proinflammatory caspases, differing from caspase‐3 and caspase‐9, which promote apoptosis [97]. IL‐18 and HMGB1 can be secreted by inflammasome activation in Mϕs in pathways that intersect with autophagy [98, 99].
In MLECs, the NLRP3 inflammasome contributes to damage from hyperoxia, a condition that can often exacerbate critical illness in intubated patients. NLRP3KO mice are protected from lethal injury that is induced by hyperoxia by up‐regulating PINK1, autophagy, and mitophagy at the expense of caspase‐3 and EC apoptosis. Loss of NLRP3 signaling also results in less IL‐1β in bronchoalveolar lavage samples after hyperoxic challenge; however, additional knockout of PINK1 or intranasal delivery of PINK1 silencing RNA reversed this protective effect [100]. Thus, inflammasome signaling and mitophagy in lung ECs are linked to and contribute to the inflammatory effects of oxidative stress.
Modulating autophagy can attenuate systemic inflammation in sepsis. Mice that receive LPS to induce sepsis are protected when treated with CQ [101]. CQ systematically reduces serum HMGB1 and TNF‐α and improves survival in that model. In vitro, CQ also attenuates the expression of TNF‐α and IL‐6 and limits NF‐κB translocation in HUVECs in response to LPS treatment. As the primary receptor for LPS, TLR4 may also be implicated in this pathway [101].
The inflammatory function of extracellular HMGB1 may be critically important in cancer by activating innate and adaptive immunity pathways [102, 103–104]. Modulating cell death in a manner that induces autophagy and HMGB1 release could acutely increase tumor immunogenicity and improve outcomes [105, 106, 107, 108–109]. Chronic release of HMGB1 results in largely immunomodulatory functions that promote the recruitment of regulatory T cells and myeloid‐derived suppressor cells [110, 111–112]. For example, in glioblastoma, epidermal growth factor receptor–targeted diphtheria toxoid results in cell death associated with enhanced autophagy in addition to the release of immune‐modulating HMGB1. If autophagy is inhibited with siRNA to ATG5, HMGB1 is not released. A potential benefit of HMGB1 release from tumors is increased immunogenicity that may improve organism survival by destroying cancer cells [105, 106]; however, HMGB1 can also promote tumor EC proliferation, angiogenesis, and autophagy‐mediated survival. As a result, the role of inflammatory HMGB1 in cancer likely includes beneficial as well as disadvantageous effects [113, 114].
ANGIOGENESIS
Angiogenesis is the formation of new blood vessels from preexisting capillaries [30, 31, 115, 116] and it plays a critical role in both tumor and vascular biology ( Fig. 4 ). Within the emergent tumor, autophagy is suppressed, apoptosis is promoted, and reparative proliferation ensues. As the tumor becomes established, autophagy predominates within the microenvironment, associated with hypoxia and nutrient depletion, which enables tumor cell survival [18, 117, 118]. Antiautophagic agents, such as hydroxychloroquine, are included in newer chemotherapeutic protocols to enhance tumor cell killing by decreasing survival signals [119, 120].
Figure 4.
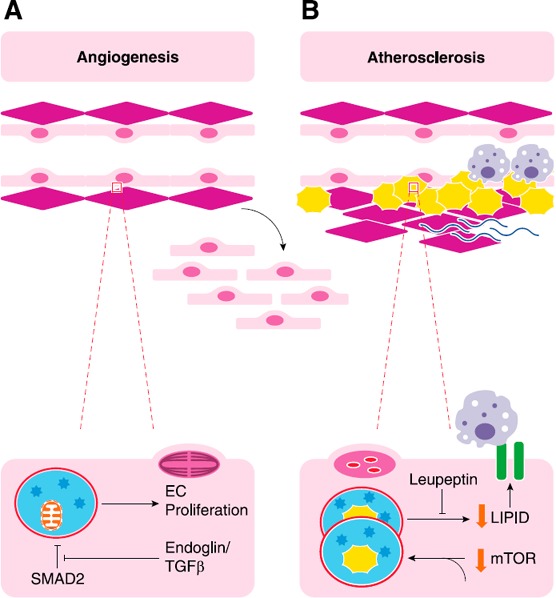
Autophagy, neovascularization, and atherosclerosis. (A) EC autophagy has been implicated in angiogenesis through many mechanisms. TGF‐β and coreceptor endoglin can promote EC proliferation, a critical component of angiogenesis in which new vessels are formed from old ones, by inhibiting the inhibition of SMAD2 on autophagy. Loss of endoglin reduces autophagic markers and capillary sprouting. (B) Development of atheroma require both lipid accumulation and inflammation. Autophagosomes can eliminate lipid droplets and is thought to be promoted by the active ingredient in green tea. Leupeptin inhibits lysosomal function and prevents degradation of lipid. The accumulation of lipids within cells can promote adhesion molecule expression and Mϕ recruitment, both of which are required for atheroma formation.
One way in which autophagy promotes tumor cell survival is by promoting reparative and restorative angiogenesis, which involves multiple mediators, receptors, and cell types [31, 115, 121, 122]. Expression of EC receptors that may play a role in angiogenesis both regulate and are regulated by autophagy [123]. One such receptor is neuropilin‐1, which mediates axonal growth and EC migration and tube formation [124]. Metabolic stressors, such as hypoxia, induce degradation of neuropilin‐1, which promotes EC migration and tube formation in response to VEGF. The pattern of neuropilin‐1 degradation could be disrupted by using either 3MA or bafilomycin, which suggests a role for autophagy in modulating receptor density and expression [124].
Bone morphogenic protein and bone active receptor membrane‐bound inhibitor is a receptor that is found on ECs that inhibits TGF‐β signaling, as a pseudoreceptor, that lacks an intracellular serine/threonine kinase domain. It inhibits EC tube formation when overexpressed in HUVECs and can be regulated by autophagic degradation [125, 126]. In vivo, it is localized to the renal endothelium and glomeruli, but it is also found in cultured ECs. In HUVECs, serum starvation results in the disappearance of the receptor from cells in a manner that could be mimicked by rapamycin and inhibited by bafilomycin. Inhibition of proteosomal function does not phenocopy these changes, which suggests that autophagic degradation regulates the expression of bone active receptor membrane‐bound inhibitors on HUVECs [125].
The TGF‐β signaling pathway employs SMAD2 to regulate EC beclin‐1/ATG6 expression. Endoglin—a coreceptor for TGF‐β—suppresses SMAD2 activation, which normally inhibits beclin‐1/ATG6 and the autophagic cascade that ensues. Thus, knockdown of endoglin allows SMAD2 to inhibit autophagic signaling, which diminishes beclin‐1/ATG6 expression, ATG12 expression, and LC3‐I to LC3‐II conversion. Subsequently, EC proliferation and capillary sprouting are inhibited [127].
Because autophagy is a dynamic process, interrupting autophagic flux at individual steps in the process may have differential effects on angiogenesis. Late inhibition of autophagy with CQ results in tumor vessel normalization and, thus, improvements in chemotherapeutic delivery. These results were not observed with the inhibition of autophagy at the early steps of autophagosome formation [122]. In ATG5‐silenced tumors, the vasculature is notably tortuous, incomplete, and inefficient at perfusion [122]. Furthermore, in ECs, CQ treatment disrupts endothelial sprouting and migration, whereas knockdown of ATG5 does not [122]. Although these findings may initially seem to be counterintuitive, the overgrowth of ECs can lead to disorganized vasculature, which is not effective for tissue perfusion [128, 129].
Muscle ischemia promotes angiogenesis, which is protective in myocardial infarction, peripheral arterial disease, and diabetic wound healing [80, 130, 131, 132, 133, 134–135]. Autophagy is also important for the angiogenic behavior of aortic and otherwise arterial ECs [136]. In our studies, HMGB1 and autophagy are both necessary for endothelial tube formation in culture. Human dermal microvascular ECs that were cultured on Matrigel formed complex networks of tubules in response to hypoxia but not in the presence of 3MA or the Ab to HMGB1 [92]. Conversely, administration of rapamycin increased endothelial tube formation in culture. We also demonstrated a relationship between HMGB1 and autophagic signaling. Human dermal microvascular endothelial cells that were treated with HMGB1 demonstrated LC3‐I to LC3‐II conversion in a manner that could be inhibited by the application of an HMGB1 neutralizing Ab [92]. We have shown that HMGB1 is critical for muscle regeneration and angiogenesis in the setting of chronic muscle ischemia in a manner that likely involves TLR2 and ‐4 as well as their downstream adaptors [70, 88, 137]. Given the direct effects of HMGB1 on EC autophagy, it is likely that autophagic signaling also plays a role in the recovery from muscle ischemia, which may have important implications for peripheral arterial disease.
ATHEROSCLEROSIS
Certain compounds that attenuate atherosclerosis also seem to have autophagy‐promoting effects (Fig. 4) [7, 37, 138, 139, 140, 141–142]. Atherosclerosis is an inflammatory process that responds to lipid‐induced EC injury. Adhesion molecule expression on damaged endothelium recruits Mϕs to the sites of injury, which, in and of themselves, contribute to atherogenesis and progression [143]. Indeed, the benefit of statin therapy is likely related to its role in attenuating inflammation, particularly as other lipid‐reducing modalities do not provide similar benefits. To highlight the role of reducing inflammation in the clinical control of atherosclerosis, reducing elevated C‐reactive protein is recognized as a potential goal of lipid management to reduce cardiovascular risk [144, 145].
Autophagy can result in increased lysosomal degradation of lipid droplets within ECs that are treated with palmitate and epigallocatechin gallate, the active ingredient in green tea [146]. Epigallocatechin gallate induces autophagy, which increases lipid accumulation in autophagic vacuoles. Ammonium chloride/leupeptin prevents lysosomal degradation, which results in the accumulation of LC3‐II–positive puncta that colocalize with lipids. These results suggest that autophagy enhances lipid processing and elimination, which reduces its accumulation within cells [146].
A number of studies have demonstrated increased autophagy in ECs that are exposed to oxidized LDL, a major contributor to atherosclerosis [147, 148–149]. Knockdown of ATG7 deters LC3‐II formation in response to oxidized LDL. GFP‐conjugated LC3 delivered by electroporation to ECs coalesce into puncta that surround oxidized LDL within cells. This imaging seemed to suggest trafficking of oxidized LDL to autophagosomes [147]. In endothelial‐specific ATG7KO mice, lipid is retained with ECs of the retina after injection of oxidized LDL conjugated to a fluorescent tag. In wild‐type mice, retained lipid in ECs was not observed [147]. These data suggest that autophagic processing of lipids may help prevent accumulation within the endothelium and the resultant atherosclerotic damage that may ensue.
Reducing intracellular lipid accumulation accompanied by a reduction of inflammation has promised to offer the greatest clinical benefit in atherosclerosis [144, 145, 150, 151]; thus, to provide a protective role, autophagic clearance of lipids ideally should be associated with a reduction in inflammation. This protective effect has been shown to be pharmacologically inducible. A small molecule, ABO, limits apoptosis, which promotes autophagy in ECs that are exposed to oxidized LDL. ECs that are treated with oxidized LDL without ABO have diminished autophagy. ABO increases LC3‐I to LC3‐II conversion and degradation of SQSTM1/p62. As a result, ABO decreases lipid accumulation within experimental plaques in ApoE knockout mice. Furthermore, inflammatory‐type Mϕs (CD68/CD11c) are also decreased, whereas Mϕs that express inflammation‐resolving (CD68/CD206) receptors are increased [152].
As in many other processes, whereas autophagy seems to have a promising role in protecting against, or at least attenuating, atherosclerotic disease, it also is associated with damage. Enhanced autophagic phenotypes in ECs are found after treatment with the aforementioned PA [153]. In this scenario, autophagy is prominent before cell death from palmitate exposure. Palmitate increases autophagy and decreases EC viability. In contrast, wortmannin and 3MA diminish LC3‐I to LC3‐II conversion and promotes greater cell viability [154]. These inhibitor experiments suggest that the ECs are dying with autophagy.
CONCLUDING REMARKS
EC autophagy is complex and affects normal vascular homeostasis as well as the modulation of disease. The potential role of autophagy in the beat to beat variation in the endothelium invites additional studies to assess how EC‐specific deletions of autophagic machinery affect function. Modulation of autophagy has already been demonstrated as a potent therapeutic target for patients with cancer [18, 117, 118]. Additional research that highlights genetic deficiencies and pharmacologic manipulation of autophagy may similarly determine its clinical potential in modulating vascular disease.
AUTHORSHIP
U.S. and M.T.L. conceived of and designed the study, performed data analysis, and wrote the manuscript.
DISCLOSURES
The authors declare no conflicts of interest. M.T.L. is currently employed as Chief Scientific Officer at Lion Biopharmaceuticals on entrepreneurial leave. None of the work reported here is derived from that role.
ACKNOWLEDGMENTS
Supported in part by the U.S. National Institutes of Health [NIH; Grants K08HL103899 (to U.S.) and CA181450 (to M.T.L.)], charitable contributions to the Center for DAMP Biology at the University of Pittsburgh, the University of Pittsburgh Cancer Institute [Grant P30CA047904 (to M.T.L.)], and the Doris Duke Foundation for the Academy for Clinical Research (to M.T.L.).
Contributor Information
Ulka Sachdev, Email: sachdevu2@upmc.edu.
Michael T. Lotze, Email: lotzemt@upmc.edu
REFERENCES
- 1. Larroque‐Cardoso, P. , Swiader, A. , Ingueneau, C. , Nègre‐Salvayre, A. , Elbaz, M. , Reyland, M. E. , Salvayre, R. , Vindis, C. (2013) Role of protein kinase C δ in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell Death Dis. 4, e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li, W. , Sultana, N. , Siraj, N. , Ward, L. J. , Pawlik, M. , Levy, E. , Jovinge, S. , Bengtsson, E. , Yuan, X.‐M. (2016) Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J. Cell. Mol. Med. 20, 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuan, X.‐M. , Sultana, N. , Siraj, N. , Ward, L. J. , Ghafouri, B. , Li, W. (2016) Autophagy induction protects against 7‐oxysterol‐induced cell death via lysosomal pathway and oxidative stress. J. Cell Death 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kassiotis, C. , Ballal, K. , Wellnitz, K. , Vela, D. , Gong, M. , Salazar, R. , Frazier, O. H. , Taegtmeyer, H. (2009) Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 120 (Suppl 11), S191–S197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirakabe, A. , Zhai, P. , Ikeda, Y. , Saito, T. , Maejima, Y. , Hsu, C.‐P. , Nomura, M. , Egashira, K. , Levine, B. , Sadoshima, J. (2016) Drp1‐dependent mitochondrial autophagy plays a protective role against pressure overload‐induced mitochondrial dysfunction and heart failure. Circulation 133, 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakayama, H. , Nishida, K. , Otsu, K. (2016) Macromolecular degradation systems and cardiovascular aging. Circ. Res. 118, 1577–1592. [DOI] [PubMed] [Google Scholar]
- 7. Nussenzweig, S. C. , Verma, S. , Finkel, T. (2015) The role of autophagy in vascular biology. Circ. Res. 116, 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deretic, V. (2016) Autophagy in leukocytes and other cells: mechanisms, subsystem organization, selectivity, and links to innate immunity. J. Leukoc. Biol. 100, 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kluge, M. A. , Fetterman, J. L. , Vita, J. A. (2013) Mitochondria and endothelial function. Circ. Res. 112, 1171–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keulers, T. G. , Schaaf, M. B. E. , Rouschop, K. M. A. (2016) Autophagy‐dependent secretion: contribution to tumor progression. Front. Oncol. 6, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pallet, N. , Sirois, I. , Bell, C. , Hanafi, L.‐A. , Hamelin, K. , Dieudé, M. , Rondeau, C. , Thibault, P. , Desjardins, M. , Hebert, M.‐J. (2013) A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13, 1108–1120. [DOI] [PubMed] [Google Scholar]
- 12. Zeng, J. , Feng, S. , Wu, B. , Guo, W. (2017) Polarized exocytosis. Cold Spring Harb. Perspect. Biol. a027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis, L. C. , Platt, F. M. , Galione, A. (2015) Preferential coupling of the NAADP pathway to exocytosis in T‐cells. Messenger (Los Angel.) 4, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Najafinobar, N. , Mellander, L. J. , Kurczy, M. E. , Dunevall, J. , Angerer, T. B. , Fletcher, J. S. , Cans, A. S. (2016) Cholesterol alters the dynamics of release in protein independent cell models for exocytosis. Sci. Rep. 6, 33702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang, B. L. (2017) Sec16 in conventional and unconventional exocytosis: working at the interface of membrane traffic and secretory autophagy? J. Cell. Physiol. doi: 10.1002/jcp.25842 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Torisu, T. , Torisu, K. , Lee, I. H. , Liu, J. , Malide, D. , Combs, C. A. , Wu, X. S. , Rovira, I. I. , Fergusson, M. M. , Weigert, R. , Connelly, P. S. , Daniels, M. P. , Komatsu, M. , Cao, L. , Finkel, T. (2013) Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med. 19, 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klionsky, D. J. , Abdelmohsen, K. , Abe, A. , Abedin, M. J. , Abeliovich, H. , Acevedo Arozena, A. , Adachi, H. , Adams, C. M. , Adams, P. D. , Adeli, K. , Adhihetty, P. J. , Adler, S. G. , Agam, G. , Agarwal, R. , Aghi, M. K. , Agnello, M. , Agostinis, P. , Aguilar, P. V. , Aguirre‐Ghiso, J. , Airoldi, E. M. , Ait‐Si‐Ali, S. , Akematsu, T. , Akporiaye, E. T. , Al‐Rubeai, M. , Albaiceta, G. M. , Albanese, C. , Albani, D. , Albert, M. L. , Aldudo, J. , Algül, H. , Alirezaei, M. , Alloza, I. , Almasan, A. , Almonte‐Beceril, M. , Alnemri, E. S. , Alonso, C. , Altan‐Bonnet, N. , Altieri, D. C. , Alvarez, S. , Alvarez‐Erviti, L. , Alves, S. , Amadoro, G. , Amano, A. , Amantini, C. , Ambrosio, S. , Amelio, I. , Amer, A. O. , Amessou, M. , Amon, A. , An, Z. , Anania, F. A. , Andersen, S. U. , Andley, U. P. , Andreadi, C. K. , Andrieu‐Abadie, N. , Anel, A. , Ann, D. K. , Anoopkumar‐Dukie, S. , Antonioli, M. , Aoki, H. , Apostolova, N. , Aquila, S. , Aquilano, K. , Araki, K. , Arama, E. , Aranda, A. , Araya, J. , Arcaro, A. , Arias, E. , Arimoto, H. , Ariosa, A. R. , Armstrong, J. L. , Arnould, T. , Arsov, I. , Asanuma, K. , Askanas, V. , Asselin, E. , Atarashi, R. , Atherton, S. S. , Atkin, J. D. , Attardi, L. D. , Auberger, P. , Auburger, G. , Aurelian, L. , Autelli, R. , Avagliano, L. , Avantaggiati, M. L. , Avrahami, L. , Awale, S. , Azad, N. , Bachetti, T. , Backer, J. M. , Bae, D. H. , Bae, J. S. , Bae, O. N. , Bae, S. H. , Baehrecke, E. H. , Baek, S. H. , Baghdiguian, S. , Bagniewska‐Zadworna, A. , Bai, H. , Bai, J. , Bai, X. Y. , Bailly, Y. , Balaji, K. N. , Balduini, W. , Ballabio, A. , Balzan, R. , Banerjee, R. , Bánhegyi, G. , Bao, H. , Barbeau, B. , Barrachina, M. D. , Barreiro, E. , Bartel, B. , Bartolomé, A. , Bassham, D. C. , Bassi, M. T. , Bast, R. C., Jr. , Basu, A. , Batista, M. T. , Batoko, H. , Battino, M. , Bauckman, K. , Baumgarner, B. L. , Bayer, K. U. , Beale, R. , Beaulieu, J. F. , Beck, G. R., Jr. , Becker, C. , Beckham, J. D. , Bédard, P. A. , Bednarski, P. J. , Begley, T. J. , Behl, C. , Behrends, C. , Behrens, G. M. , Behrns, K. E. , Bejarano, E. , Belaid, A. , Belleudi, F. , Bénard, G. , Berchem, G. , Bergamaschi, D. , Bergami, M. , Berkhout, B. , Berliocchi, L. , Bernard, A. , Bernard, M. , Bernassola, F. , Bertolotti, A. , Bess, A. S. , Besteiro, S. , Bettuzzi, S. , Bhalla, S. , Bhattacharyya, S. , Bhutia, S. K. , Biagosch, C. , Bianchi, M. W. , Biard‐Piechaczyk, M. , Billes, V. , Bincoletto, C. , Bingol, B. , Bird, S. W. , Bitoun, M. , Bjedov, I. , Blackstone, C. , Blanc, L. , Blanco, G. A. , Blomhoff, H. K. , Boada‐Romero, E. , Böckler, S. , Boes, M. , Boesze‐Battaglia, K. , Boise, L. H. , Bolino, A. , Boman, A. , Bonaldo, P. , Bordi, M. , Bosch, J. , Botana, L. M. , Botti, J. , Bou, G. , Bouché, M. , Bouchecareilh, M. , Boucher, M. J. , Boulton, M. E. , Bouret, S. G. , Boya, P. , Boyer‐Guittaut, M. , Bozhkov, P. V. , Brady, N. , Braga, V. M. , Brancolini, C. , Braus, G. H. , Bravo‐San Pedro, J. M. , Brennan, L. A. , Bresnick, E. H. , Brest, P. , Bridges, D. , Bringer, M. A. , Brini, M. , Brito, G. C. , Brodin, B. , Brookes, P. S. , Brown, E. J. , Brown, K. , Broxmeyer, H. E. , Bruhat, A. , Brum, P. C. , Brumell, J. H. , Brunetti‐Pierri, N. , Bryson‐Richardson, R. J. , Buch, S. , Buchan, A. M. , Budak, H. , Bulavin, D. V. , Bultman, S. J. , Bultynck, G. , Bumbasirevic, V. , Burelle, Y. , Burke, R. E. , Burmeister, M. , Bütikofer, P. , Caberlotto, L. , Cadwell, K. , Cahova, M. , Cai, D. , Cai, J. , Cai, Q. , Calatayud, S. , Camougrand, N. , Campanella, M. , Campbell, G. R. , Campbell, M. , Campello, S. , Candau, R. , Caniggia, I. , Cantoni, L. , Cao, L. , Caplan, A. B. , Caraglia, M. , Cardinali, C. , Cardoso, S. M. , Carew, J. S. , Carleton, L. A. , Carlin, C. R. , Carloni, S. , Carlsson, S. R. , Carmona‐Gutierrez, D. , Carneiro, L. A. , Carnevali, O. , Carra, S. , Carrier, A. , Carroll, B. , Casas, C. , Casas, J. , Cassinelli, G. , Castets, P. , Castro‐Obregon, S. , Cavallini, G. , Ceccherini, I. , Cecconi, F. , Cederbaum, A. I. , Ceña, V. , Cenci, S. , Cerella, C. , Cervia, D. , Cetrullo, S. , Chaachouay, H. , Chae, H. J. , Chagin, A. S. , Chai, C. Y. , Chakrabarti, G. , Chamilos, G. , Chan, E. Y. , Chan, M. T. , Chandra, D. , Chandra, P. , Chang, C. P. , Chang, R. C. , Chang, T. Y. , Chatham, J. C. , Chatterjee, S. , Chauhan, S. , Che, Y. , Cheetham, M. E. , Cheluvappa, R. , Chen, C. J. , Chen, G. , Chen, G. C. , Chen, G. , Chen, H. , Chen, J. W. , Chen, J. K. , Chen, M. , Chen, M. , Chen, P. , Chen, Q. , Chen, Q. , Chen, S. D. , Chen, S. , Chen, S. S. , Chen, W. , Chen, W. J. , Chen, W. Q. , Chen, W. , Chen, X. , Chen, Y. H. , Chen, Y. G. , Chen, Y. , Chen, Y. , Chen, Y. , Chen, Y. J. , Chen, Y. Q. , Chen, Y. , Chen, Z. , Chen, Z. , Cheng, A. , Cheng, C. H. , Cheng, H. , Cheong, H. , Cherry, S. , Chesney, J. , Cheung, C. H. , Chevet, E. , Chi, H. C. , Chi, S. G. , Chiacchiera, F. , Chiang, H. L. , Chiarelli, R. , Chiariello, M. , Chieppa, M. , Chin, L. S. , Chiong, M. , Chiu, G. N. , Cho, D. H. , Cho, S. G. , Cho, W. C. , Cho, Y. Y. , Cho, Y. S. , Choi, A. M. , Choi, E. J. , Choi, E. K. , Choi, J. , Choi, M. E. , Choi, S. I. , Chou, T. F. , Chouaib, S. , Choubey, D. , Choubey, V. , Chow, K. C. , Chowdhury, K. , Chu, C. T. , Chuang, T. H. , Chun, T. , Chung, H. , Chung, T. , Chung, Y. L. , Chwae, Y. J. , Cianfanelli, V. , Ciarcia, R. , Ciechomska, I. A. , Ciriolo, M. R. , Cirone, M. , Claerhout, S. , Clague, M. J. , Clària, J. , Clarke, P. G. , Clarke, R. , Clementi, E. , Cleyrat, C. , Cnop, M. , Coccia, E. M. , Cocco, T. , Codogno, P. , Coers, J. , Cohen, E. E. , Colecchia, D. , Coletto, L. , Coll, N. S. , Colucci‐Guyon, E. , Comincini, S. , Condello, M. , Cook, K. L. , Coombs, G. H. , Cooper, C. D. , Cooper, J. M. , Coppens, I. , Corasaniti, M. T. , Corazzari, M. , Corbalan, R. , Corcelle‐Termeau, E. , Cordero, M. D. , Corral‐Ramos, C. , Corti, O. , Cossarizza, A. , Costelli, P. , Costes, S. , Cotman, S. L. , Coto‐Montes, A. , Cottet, S. , Couve, E. , Covey, L. R. , Cowart, L. A. , Cox, J. S. , Coxon, F. P. , Coyne, C. B. , Cragg, M. S. , Craven, R. J. , Crepaldi, T. , Crespo, J. L. , Criollo, A. , Crippa, V. , Cruz, M. T. , Cuervo, A. M. , Cuezva, J. M. , Cui, T. , Cutillas, P. R. , Czaja, M. J. , Czyzyk‐Krzeska, M. F. , Dagda, R. K. , Dahmen, U. , Dai, C. , Dai, W. , Dai, Y. , Dalby, K. N. , Dalla Valle, L. , Dalmasso, G. , D'Amelio, M. , Damme, M. , Darfeuille‐Michaud, A. , Dargemont, C. , Darley‐Usmar, V. M. , Dasarathy, S. , Dasgupta, B. , Dash, S. , Dass, C. R. , Davey, H. M. , Davids, L. M. , Dávila, D. , Davis, R. J. , Dawson, T. M. , Dawson, V. L. , Daza, P. , de Belleroche, J. , de Figueiredo, P. , de Figueiredo, R. C. , de la Fuente, J. , De Martino, L. , De Matteis, A. , De Meyer, G. R. , De Milito, A. , De Santi, M. , de Souza, W. , De Tata, V. , De Zio, D. , Debnath, J. , Dechant, R. , Decuypere, J. P. , Deegan, S. , Dehay, B. , Del Bello, B. , Del Re, D. P. , Delage‐Mourroux, R. , Delbridge, L. M. , Deldicque, L. , Delorme‐Axford, E. , Deng, Y. , Dengjel, J. , Denizot, M. , Dent, P. , Der, C. J. , Deretic, V. , Derrien, B. , Deutsch, E. , Devarenne, T. P. , Devenish, R. J. , Di Bartolomeo, S. , Di Daniele, N. , Di Domenico, F. , Di Nardo, A. , Di Paola, S. , Di Pietro, A. , Di Renzo, L. , DiAntonio, A. , Díaz‐Araya, G. , Díaz‐Laviada, I. , Diaz‐Meco, M. T. , Diaz‐Nido, J. , Dickey, C. A. , Dickson, R. C. , Diederich, M. , Digard, P. , Dikic, I. , Dinesh‐Kumar, S. P. , Ding, C. , Ding, W. X. , Ding, Z. , Dini, L. , Distler, J. H. , Diwan, A. , Djavaheri‐Mergny, M. , Dmytruk, K. , Dobson, R. C. , Doetsch, V. , Dokladny, K. , Dokudovskaya, S. , Donadelli, M. , Dong, X. C. , Dong, X. , Dong, Z. , Donohue, T. M., Jr. , Doran, K. S. , D'Orazi, G. , Dorn, G. W. , 2nd., Dosenko, V. , Dridi, S. , Drucker, L. , Du, J. , Du, L. L. , Du, L. , du Toit, A. , Dua, P. , Duan, L. , Duann, P. , Dubey, V. K. , Duchen, M. R. , Duchosal, M. A. , Duez, H. , Dugail, I. , Dumit, V. I. , Duncan, M. C. , Dunlop, E. A. , Dunn, W. A., Jr. , Dupont, N. , Dupuis, L. , Durán, R. V. , Durcan, T. M. , Duvezin‐Caubet, S. , Duvvuri, U. Eapen, V. , Ebrahimi‐Fakhari, D. , Echard, A. , Eckhart, L. , Edelstein, C. L. , Edinger, A. L. , Eichinger, L. , Eisenberg, T. , Eisenberg‐Lerner, A. , Eissa, N. T. , El‐Deiry, W. S. , El‐Khoury, V. , Elazar, Z. , Eldar‐Finkelman, H. , Elliott, C. J. , Emanuele, E. , Emmenegger, U. , Engedal, N. , Engelbrecht, A. M. , Engelender, S. , Enserink, J. M. , Erdmann, R. , Erenpreisa, J. , Eri, R. , Eriksen, J. L. , Erman, A. , Escalante, R. , Eskelinen, E. L. , Espert, L. , Esteban‐Martínez, L. , Evans, T. J. , Fabri, M. , Fabrias, G. , Fabrizi, C. , Facchiano, A. , Færgeman, N. J. , Faggioni, A. , Fairlie, W. D. , Fan, C. , Fan, D. , Fan, J. , Fang, S. , Fanto, M. , Fanzani, A. , Farkas, T. , Faure, M. , Favier, F. B. , Fearnhead, H. , Federici, M. , Fei, E. , Felizardo, T. C. , Feng, H. , Feng, Y. , Feng, Y. , Ferguson, T. A. , Fernández, Á. F. , Fernandez‐Barrena, M. G. , Fernandez‐Checa, J. C. , Fernández‐López, A. , Fernandez‐Zapico, M. E. , Feron, O. , Ferraro, E. , Ferreira‐Halder, C. V. , Fesus, L. , Feuer, R. , Fiesel, F. C. , Filippi‐Chiela, E. C. , Filomeni, G. , Fimia, G. M. , Fingert, J. H. , Finkbeiner, S. , Finkel, T. , Fiorito, F. , Fisher, P. B. , Flajolet, M. , Flamigni, F. , Florey, O. , Florio, S. , Floto, R. A. , Folini, M. , Follo, C. , Fon, E. A. , Fornai, F. , Fortunato, F. , Fraldi, A. , Franco, R. , Francois, A. , François, A. , Frankel, L. B. , Fraser, I. D. , Frey, N. , Freyssenet, D. G. , Frezza, C. , Friedman, S. L. , Frigo, D. E. , Fu, D. , Fuentes, J. M. , Fueyo, J. , Fujitani, Y. , Fujiwara, Y. , Fujiya, M. , Fukuda, M. , Fulda, S. , Fusco, C. , Gabryel, B. , Gaestel, M. , Gailly, P. , Gajewska, M. , Galadari, S. , Galili, G. , Galindo, I. , Galindo, M. F. , Galliciotti, G. , Galluzzi, L. , Galluzzi, L. , Galy, V. , Gammoh, N. , Gandy, S. , Ganesan, A. K. , Ganesan, S. , Ganley, I. G. , Gannagé, M. , Gao, F. B. , Gao, F. , Gao, J. X. , García Nannig, L. , García Véscovi, E. , Garcia‐Macía, M. , Garcia‐Ruiz, C. , Garg, A. D. , Garg, P. K. , Gargini, R. , Gassen, N. C. , Gatica, D. , Gatti, E. , Gavard, J. , Gavathiotis, E. , Ge, L. , Ge, P. , Ge, S. , Gean, P. W. , Gelmetti, V. , Genazzani, A. A. , Geng, J. , Genschik, P. , Gerner, L. , Gestwicki, J. E. , Gewirtz, D. A. , Ghavami, S. , Ghigo, E. , Ghosh, D. , Giammarioli, A. M. , Giampieri, F. , Giampietri, C. , Giatromanolaki, A. , Gibbings, D. J. , Gibellini, L. , Gibson, S. B. , Ginet, V. , Giordano, A. , Giorgini, F. , Giovannetti, E. , Girardin, S. E. , Gispert, S. , Giuliano, S. , Gladson, C. L. , Glavic, A. , Gleave, M. , Godefroy, N. , Gogal, R. M., Jr. , Gokulan, K. , Goldman, G. H. , Goletti, D. , Goligorsky, M. S. , Gomes, A. V. , Gomes, L. C. , Gomez, H. , Gomez‐Manzano, C. , Gómez‐Sánchez, R. , Gonçalves, D. A. , Goncu, E. , Gong, Q. , Gongora, C. , Gonzalez, C. B. , Gonzalez‐Alegre, P. , Gonzalez‐Cabo, P. , González‐Polo, R. A. , Goping, I. S. , Gorbea, C. , Gorbunov, N. V. , Goring, D. R. , Gorman, A. M. , Gorski, S. M. , Goruppi, S. , Goto‐Yamada, S. , Gotor, C. , Gottlieb, R. A. , Gozes, I. , Gozuacik, D. , Graba, Y. , Graef, M. , Granato, G. E. , Grant, G. D. , Grant, S. , Gravina, G. L. , Green, D. R. , Greenhough, A. , Greenwood, M. T. , Grimaldi, B. , Gros, F. , Grose, C. , Groulx, J. F. , Gruber, F. , Grumati, P. , Grune, T. , Guan, J. L. , Guan, K. L. , Guerra, B. , Guillen, C. , Gulshan, K. , Gunst, J. , Guo, C. , Guo, L. , Guo, M. , Guo, W. , Guo, X. G. , Gust, A. A. , Gustafsson, Å. B. , Gutierrez, E. , Gutierrez, M. G. , Gwak, H. S. , Haas, A. , Haber, J. E. , Hadano, S. , Hagedorn, M. , Hahn, D. R. , Halayko, A. J. , Hamacher‐Brady, A. , Hamada, K. , Hamai, A. , Hamann, A. , Hamasaki, M. , Hamer, I. , Hamid, Q. , Hammond, E. M. , Han, F. , Han, W. , Handa, J. T. , Hanover, J. A. , Hansen, M. , Harada, M. , Harhaji‐Trajkovic, L. , Harper, J. W. , Harrath, A. H. , Harris, A. L. , Harris, J. , Hasler, U. , Hasselblatt, P. , Hasui, K. , Hawley, R. G. , Hawley, T. S. , He, C. , He, C. Y. , He, F. , He, G. , He, R. R. , He, X. H. , He, Y. W. , He, Y. Y. , Heath, J. K. , Hébert, M. J. , Heinzen, R. A. , Helgason, G. V. , Hensel, M. , Henske, E. P. , Her, C. , Herman, P. K. , Hernández, A. , Hernandez, C. , Hernández‐Tiedra, S. , Hetz, C. , Hiesinger, P. R. , Higaki, K. , Hilfiker, S. , Hill, B. G. , Hill, J. A. , Hill, W. D. , Hino, K. , Hofius, D. , Hofman, P. , Höglinger, G. U. , Höhfeld, J. , Holz, M. K. , Hong, Y. , Hood, D. A. , Hoozemans, J. J. , Hoppe, T. , Hsu, C. , Hsu, C. Y. , Hsu, L. C. , Hu, D. , Hu, G. , Hu, H. M. , Hu, H. , Hu, M. C. , Hu, Y. C. , Hu, Z. W. , Hua, F. , Hua, Y. , Huang, C. , Huang, H. L. , Huang, K. H. , Huang, K. Y. , Huang, S. , Huang, S. , Huang, W. P. , Huang, Y. R. , Huang, Y. , Huang, Y. , Huber, T. B. , Huebbe, P. , Huh, W. K. , Hulmi, J. J. , Hur, G. M. , Hurley, J. H. , Husak, Z. , Hussain, S. N. , Hussain, S. , Hwang, J. J. , Hwang, S. , Hwang, T. I. , Ichihara, A. , Imai, Y. , Imbriano, C. , Inomata, M. , Into, T. , Iovane, V. , Iovanna, J. L. , Iozzo, R. V. , Ip, N. Y. , Irazoqui, J. E. , Iribarren, P. , Isaka, Y. , Isakovic, A. J. , Ischiropoulos, H. , Isenberg, J. S. , Ishaq, M. , Ishida, H. , Ishii, I. , Ishmael, J. E. , Isidoro, C. , Isobe, K. , Isono, E. , Issazadeh‐Navikas, S. , Itahana, K. , Itakura, E. , Ivanov, A. I. , Iyer, A. K. , Izquierdo, J. M. , Izumi, Y. , Izzo, V. , Jäättelä, M. , Jaber, N. , Jackson, D. J. , Jackson, W. T. , Jacob, T. G. , Jacques, T. S. , Jagannath, C. , Jain, A. , Jana, N. R. , Jang, B. K. , Jani, A. , Janji, B. , Jannig, P. R. , Jansson, P. J. , Jean, S. , Jendrach, M. , Jeon, J. H. , Jessen, N. , Jeung, E. B. , Jia, K. , Jia, L. , Jiang, H. , Jiang, H. , Jiang, L. , Jiang, T. , Jiang, X. , Jiang, X. , Jiang, X. , Jiang, Y. , Jiang, Y. , Jiménez, A. , Jin, C. , Jin, H. , Jin, L. , Jin, M. , Jin, S. , Jinwal, U. K. , Jo, E. K. , Johansen, T. , Johnson, D. E. , Johnson, G. V. , Johnson, J. D. , Jonasch, E. , Jones, C. , Joosten, L. A. , Jordan, J. , Joseph, A. M. , Joseph, B. , Joubert, A. M. , Ju, D. , Ju, J. , Juan, H. F. , Juenemann, K. , Juhász, G. , Jung, H. S. , Jung, J. U. , Jung, Y. K. , Jungbluth, H. , Justice, M. J. , Jutten, B. , Kaakoush, N. O. , Kaarniranta, K. , Kaasik, A. , Kabuta, T. , Kaeffer, B. , Kågedal, K. , Kahana, A. , Kajimura, S. , Kakhlon, O. , Kalia, M. , Kalvakolanu, D. V. , Kamada, Y. , Kambas, K. , Kaminskyy, V. O. , Kampinga, H. H. , Kandouz, M. , Kang, C. , Kang, R. , Kang, T. C. , Kanki, T. , Kanneganti, T. D. , Kanno, H. , Kanthasamy, A. G. , Kantorow, M. , Kaparakis‐Liaskos, M. , Kapuy, O. , Karantza, V. , Karim, M. R. , Karmakar, P. , Kaser, A. , Kaushik, S. , Kawula, T. , Kaynar, A. M. , Ke, P. Y. , Ke, Z. J. , Kehrl, J. H. , Keller, K. E. , Kemper, J. K. , Kenworthy, A. K. , Kepp, O. , Kern, A. , Kesari, S. , Kessel, D. , Ketteler, R. , Kettelhut Ido, C. , Khambu, B. , Khan, M. M. , Khandelwal, V. K. , Khare, S. , Kiang, J. G. , Kiger, A. A. , Kihara, A. , Kim, A. L. , Kim, C. H. , Kim, D. R. , Kim, D. H. , Kim, E. K. , Kim, H. Y. , Kim, H. R. , Kim, J. S. , Kim, J. H. , Kim, J. C. , Kim, J. H. , Kim, K. W. , Kim, M. D. , Kim, M. M. , Kim, P. K. , Kim, S. W. , Kim, S. Y. , Kim, Y. S. , Kim, Y. , Kimchi, A. , Kimmelman, A. C. , Kimura, T. , King, J. S. , Kirkegaard, K. , Kirkin, V. , Kirshenbaum, L. A. , Kishi, S. , Kitajima, Y. , Kitamoto, K. , Kitaoka, Y. , Kitazato, K. , Kley, R. A. , Klimecki, W. T. , Klinkenberg, M. , Klucken, J. , Knævelsrud, H. , Knecht, E. , Knuppertz, L. , Ko, J. L. , Kobayashi, S. , Koch, J. C. , Koechlin‐Ramonatxo, C. , Koenig, U. , Koh, Y. H. , Köhler, K. , Kohlwein, S. D. , Koike, M. , Komatsu, M. , Kominami, E. , Kong, D. , Kong, H. J. , Konstantakou, E. G. , Kopp, B. T. , Korcsmaros, T. , Korhonen, L. , Korolchuk, V. I. , Koshkina, N. V. , Kou, Y. , Koukourakis, M. I. , Koumenis, C. , Kovács, A. L. , Kovács, T. , Kovacs, W. J. , Koya, D. , Kraft, C. , Krainc, D. , Kramer, H. , Kravic‐Stevovic, T. , Krek, W. , Kretz‐Remy, C. , Krick, R. , Krishnamurthy, M. , Kriston‐Vizi, J. , Kroemer, G. , Kruer, M. C. , Kruger, R. , Ktistakis, N. T. , Kuchitsu, K. , Kuhn, C. , Kumar, A. P. , Kumar, A. , Kumar, A. , Kumar, D. , Kumar, D. , Kumar, R. , Kumar, S. , Kundu, M. , Kung, H. J. , Kuno, A. , Kuo,. S. H. , Kuret, J. , Kurz, T. , Kwok, T. , Kwon, T. K. , Kwon, Y. T. , Kyrmizi, I. La Spada, A. R. , Lafont, F. , Lahm, T. , Lakkaraju, A. , Lam, T. , Lamark, T. , Lancel, S. , Landowski, T. H. , Lane, D. J. , Lane, J. D. , Lanzi, C. , Lapaquette, P. , Lapierre, L. R. , Laporte, J. , Laukkarinen, J. , Laurie, G. W. , Lavandero, S. , Lavie, L. , LaVoie, M. J. , Law, B. Y. , Law, H. K. , Law, K. B. , Layfield, R. , Lazo, P. A. , Le Cam, L. , Le Roch, K. G. , Le Stunff, H. , Leardkamolkarn, V. , Lecuit, M. , Lee, B. H. , Lee, C. H. , Lee, E. F. , Lee, G. M. , Lee, H. J. , Lee, H. , Lee, J. K. , Lee, J. , Lee, J. H. , Lee, J. H. , Lee, M. , Lee, M. S. , Lee, P. J. , Lee, S. W. , Lee, S. J. , Lee, S. J. , Lee, S. Y. , Lee, S. H. , Lee, S. S. , Lee, S. J. , Lee, S. , Lee, Y. R. , Lee, Y. J. , Lee, Y. H. , Leeuwenburgh, C. , Lefort, S. , Legouis, R. , Lei, J. , Lei, Q. Y. , Leib, D. A. , Leibowitz, G. , Lekli, I. , Lemaire, S. D. , Lemasters, J. J. , Lemberg, M. K. , Lemoine, A. , Leng, S. , Lenz, G. , Lenzi, P. , Lerman, L. O. , Lettieri Barbato, D. , Leu, J. I. , Leung, H. Y. , Levine, B. , Lewis, P. A. , Lezoualc'h, F. , Li, C. , Li, F. , Li, F. J. , Li, J. , Li, K. , Li, L. , Li, M. , Li, M. , Li, Q. , Li, R. , Li, S. , Li, W. , Li, W. , Li, X. , Li, Y. , Lian, J. , Liang, C. , Liang, Q. , Liao, Y. , Liberal, J. , Liberski, P. P. , Lie, P. , Lieberman, A. P. , Lim, H. J. , Lim, K. L. , Lim, K. , Lima, R. T. , Lin, C. S. , Lin, C. F. , Lin, F. , Lin, F. , Lin, F. C. , Lin, K. , Lin, K. H. , Lin, P. H. , Lin, T. , Lin, W. W. , Lin, Y. S. , Lin, Y. , Linden, R. , Lindholm, D. , Lindqvist, L. M. , Lingor, P. , Linkermann, A. , Liotta, L. A. , Lipinski, M. M. , Lira, V. A. , Lisanti, M. P. , Liton, P. B. , Liu, B. , Liu, C. , Liu, C. F. , Liu, F. , Liu, H. J. , Liu, J. , Liu, J. J. , Liu, J. L. , Liu, K. , Liu, L. , Liu, L. , Liu, Q. , Liu, R. Y. , Liu, S. , Liu, S. , Liu, W. , Liu, X. D. , Liu, X. , Liu, X. H. , Liu, X. , Liu, X. , Liu, X. , Liu, Y. , Liu, Y. , Liu, Z. , Liu, Z. , Liuzzi, J. P. , Lizard, G. , Ljujic, M. , Lodhi, I. J. , Logue, S. E. , Lokeshwar, B. L. , Long, Y. C. , Lonial, S. , Loos, B. , López‐Otín, C. , López‐Vicario, C. , Lorente, M. , Lorenzi, P. L. , Lõrincz, P. , Los, M. , Lotze, M. T. , Lovat, P. E. , Lu, B. , Lu, B. , Lu, J. , Lu, Q. , Lu, S. M. , Lu, S. , Lu, Y. , Luciano, F. , Luckhart, S. , Lucocq, J. M. , Ludovico, P. , Lugea, A. , Lukacs, N. W. , Lum, J. J. , Lund, A. H. , Luo, H. , Luo, J. , Luo, S. , Luparello, C. , Lyons, T. , Ma, J. , Ma, Y. , Ma, Y. , Ma, Z. , Machado, J. , Machado‐Santelli, G. M. , Macian, F. , MacIntosh, G. C. , MacKeigan, J. P. , Macleod, K. F. , MacMicking, J. D. , MacMillan‐Crow, L. A. , Madeo, F. , Madesh, M. , Madrigal‐Matute, J. , Maeda, A. , Maeda, T. , Maegawa, G. , Maellaro, E. , Maes, H. , Magariños, M. , Maiese, K. , Maiti, T. K. , Maiuri, L. , Maiuri, M. C. , Maki, C. G. , Malli, R. , Malorni, W. , Maloyan, A. , Mami‐Chouaib, F. , Man, N. , Mancias, J. D. , Mandelkow, E. M. , Mandell, M. A. , Manfredi, A. A. , Manié, S. N. , Manzoni, C. , Mao, K. , Mao, Z. , Mao, Z. W. , Marambaud, P. , Marconi, A. M. , Marelja, Z. , Marfe, G. , Margeta, M. , Margittai, E. , Mari, M. , Mariani, F. V. , Marin, C. , Marinelli, S. , Mariño, G. , Markovic, I. , Marquez, R. , Martelli, A. M. , Martens, S. , Martin, K. R. , Martin, S. J. , Martin, S. , Martin‐Acebes, M. A. , Martín‐Sanz, P. , Martinand‐Mari, C. , Martinet, W. , Martinez, J. , Martinez‐Lopez, N. , Martinez‐Outschoorn, U. , Martínez‐Velázquez, M. , Martinez‐Vicente, M. , Martins, W. K. , Mashima, H. , Mastrianni, J. A. , Matarese, G. , Matarrese, P. , Mateo, R. , Matoba, S. , Matsumoto, N. , Matsushita, T. , Matsuura, A. , Matsuzawa, T. , Mattson, M. P. , Matus, S. , Maugeri, N. , Mauvezin, C. , Mayer, A. , Maysinger, D. , Mazzolini, G. D. , McBrayer, M. K. , McCall, K. , McCormick, C. , McInerney, G. M. , McIver, S. C. , McKenna, S. , McMahon, J. J. , McNeish, I. A. , Mechta‐Grigoriou, F. , Medema, J. P. , Medina, D. L. , Megyeri, K. , Mehrpour, M. , Mehta, J. L. , Mei, Y. , Meier, U. C. , Meijer, A. J. , Meléndez, A. , Melino, G. , Melino, S. , de Melo, E. J. , Mena, M. A. , Meneghini, M. D. , Menendez, J. A. , Menezes, R. , Meng, L. , Meng, L. H. , Meng, S. , Menghini, R. , Menko, A. S. , Menna‐Barreto, R. F. , Menon, M. B. , Meraz‐Ríos, M. A. , Merla, G. , Merlini, L. , Merlot, A. M. , Meryk, A. , Meschini, S. , Meyer, J. N. , Mi, M. T. , Miao, C. Y. , Micale, L. , Michaeli, S. , Michiels, C. , Migliaccio, A. R. , Mihailidou, A. S. , Mijaljica, D. , Mikoshiba, K. , Milan, E. , Miller‐Fleming, L. , Mills, G. B. , Mills, I. G. , Minakaki, G. , Minassian, B. A. , Ming, X. F. , Minibayeva, F. , Minina, E. A. , Mintern, J. D. , Minucci, S. , Miranda‐Vizuete, A. , Mitchell, C. H. , Miyamoto, S. , Miyazawa, K. , Mizushima, N. , Mnich, K. , Mograbi, B. , Mohseni, S. , Moita, L. F. , Molinari, M. , Molinari, M. , Møller, A. B. , Mollereau, B. , Mollinedo, F. , Mongillo, M. , Monick, M. M. , Montagnaro, S. , Montell, C. , Moore, D. J. , Moore, M. N. , Mora‐Rodriguez, R. , Moreira, P. I. , Morel, E. , Morelli, M. B. , Moreno, S. , Morgan, M. J. , Moris, A. , Moriyasu, Y. , Morrison, J. L. , Morrison, L. A. , Morselli, E. , Moscat, J. , Moseley, P. L. , Mostowy, S. , Motori, E. , Mottet, D. , Mottram, J. C. , Moussa, C. E. , Mpakou, V. E. , Mukhtar, H. , Mulcahy Levy, J. M. , Muller, S. , Muñoz‐Moreno, R. , Muñoz‐Pinedo, C. , Münz, C. , Murphy, M. E. , Murray, J. T. , Murthy, A. , Mysorekar, I. U. , Nabi, I. R. , Nabissi, M. , Nader, G. A. , Nagahara, Y. , Nagai, Y. , Nagata, K. , Nagelkerke, A. , Nagy, P. , Naidu, S. R. , Nair, S. , Nakano, H. , Nakatogawa, H. , Nanjundan, M. , Napolitano, G. , Naqvi, N. I. , Nardacci, R. , Narendra, D. P. , Narita, M. , Nascimbeni, A. C. , Natarajan, R. , Navegantes, L. C. , Nawrocki, S. T. , Nazarko, T. Y. , Nazarko, V. Y. , Neill, T. , Neri, L. M. , Netea, M. G. , Netea‐Maier, R. T. , Neves, B. M. , Ney, P. A. , Nezis, I. P. , Nguyen, H. T. , Nguyen, H. P. , Nicot, A. S. , Nilsen, H. , Nilsson, P. , Nishimura, M. , Nishino, I. , Niso‐Santano, M. , Niu, H. , Nixon, R. A. , Njar, V. C. , Noda, T. , Noegel, A. A. , Nolte, E. M. , Norberg, E. , Norga, K. K. , Noureini, S. K. , Notomi, S. , Notterpek, L. , Nowikovsky, K. , Nukina, N. , Nürnberger, T. , O'Donnell, V. B. , O'Donovan, T. , O'Dwyer, P. J. , Oehme, I. , Oeste, C. L. , Ogawa, M. , Ogretmen, B. , Ogura, Y. , Oh, Y. J. , Ohmuraya, M. , Ohshima, T. , Ojha, R. , Okamoto, K. , Okazaki, T. , Oliver, F. J. , Ollinger, K. , Olsson, S. , Orban, D. P. , Ordonez, P. , Orhon, I. , Orosz, L. , O'Rourke, E. J. , Orozco, H. , Ortega, A. L. , Ortona, E. , Osellame, L. D. , Oshima, J. , Oshima, S. , Osiewacz, H. D. , Otomo, T. , Otsu, K. , Ou, J. H. , Outeiro, T. F. , Ouyang, D. Y. , Ouyang, H. , Overholtzer, M. , Ozbun, M. A. , Ozdinler, P. H. , Ozpolat, B. , Pacelli, C. , Paganetti, P. , Page, G. , Pages, G. , Pagnini, U. , Pajak, B. , Pak, S. C. , Pakos‐Zebrucka, K. , Pakpour, N. , Palková, Z. , Palladino, F. , Pallauf, K. , Pallet, N. , Palmieri, M. , Paludan, S. R. , Palumbo, C. , Palumbo, S. , Pampliega, O. , Pan, H. , Pan, W. , Panaretakis, T. , Pandey, A. , Pantazopoulou, A. , Papackova, Z. , Papademetrio, D. L. , Papassideri, I. , Papini, A. , Parajuli, N. , Pardo, J. , Parekh, V. V. , Parenti, G. , Park, J. I. , Park, J. , Park, O. K. , Parker, R. , Parlato, R. , Parys, J. B. , Parzych, K. R. , Pasquet, J. M. , Pasquier, B. , Pasumarthi, K. B. , Patschan, D. , Patterson, C. , Pattingre, S. , Pattison, S. , Pause, A. , Pavenstädt, H. , Pavone, F. , Pedrozo, Z. , Peña, F. J. , Peñalva, M. A. , Pende, M. , Peng, J. , Penna, F. , Penninger, J. M. , Pensalfini, A. , Pepe, S. , Pereira, G. J. , Pereira, P. C. , Pérez‐de la Cruz, V. , Pérez‐Pérez, M. E. , Pérez‐Rodríguez, D. , Pérez‐Sala, D. , Perier, C. , Perl, A. , Perlmutter, D. H. , Perrotta, I. , Pervaiz, S. , Pesonen, M. , Pessin, J. E. , Peters, G. J. , Petersen, M. , Petrache, I. , Petrof, B. J. , Petrovski, G. , Phang, J. M. , Piacentini, M. , Pierdominici, M. , Pierre, P. , Pierrefite‐Carle, V. , Pietrocola, F. , Pimentel‐Muiños, F. X. , Pinar, M. , Pineda, B. , Pinkas‐Kramarski, R. , Pinti, M. , Pinton, P. , Piperdi, B. , Piret, J. M. , Platanias, L. C. , Platta, H. W. , Plowey, E. D. , Pöggeler, S. , Poirot, M. , Polčic, P. , Poletti, A. , Poon, A. H. , Popelka, H. , Popova, B. , Poprawa, I. , Poulose, S. M. , Poulton, J. , Powers, S. K. , Powers, T. , Pozuelo‐Rubio, M. , Prak, K. , Prange, R. , Prescott, M. , Priault, M. , Prince, S. , Proia, R. L. , Proikas‐Cezanne, T. , Prokisch, H. , Promponas, V. J. , Przyklenk, K. , Puertollano, R. , Pugazhenthi, S. , Puglielli, L. , Pujol, A. , Puyal, J. , Pyeon, D. Qi, X. , Qian, W. B. , Qin, Z. H. , Qiu, Y. , Qu, Z. , Quadrilatero, J. , Quinn, F. , Raben, N. , Rabinowich, H. , Radogna, F. , Ragusa, M. J. , Rahmani, M. , Raina, K. , Ramanadham, S. , Ramesh, R. , Rami, A. , Randall‐Demllo, S. , Randow, F. , Rao, H. , Rao, V. A. , Rasmussen, B. B. , Rasse, T. M. , Ratovitski, E. A. , Rautou, P. E. , Ray, S. K. , Razani, B. , Reed, B. H. , Reggiori, F. , Rehm, M. , Reichert, A. S. , Rein, T. , Reiner, D. J. , Reits, E. , Ren, J. , Ren, X. , Renna, M. , Reusch, J. E. , Revuelta, J. L. , Reyes, L. , Rezaie, A. R. , Richards, R. I. , Richardson, D. R. , Richetta, C. , Riehle, M. A. , Rihn, B. H. , Rikihisa, Y. , Riley, B. E. , Rimbach, G. , Rippo, M. R. , Ritis, K. , Rizzi, F. , Rizzo, E. , Roach, P. J. , Robbins, J. , Roberge, M. , Roca, G. , Roccheri, M. C. , Rocha, S. , Rodrigues, C. M. , Rodríguez, C. I. , de Cordoba, S. R. , Rodriguez‐Muela, N. , Roelofs, J. , Rogov, V. V. , Rohn, T. T. , Rohrer, B. , Romanelli, D. , Romani, L. , Romano, P. S. , Roncero, M. I. , Rosa, J. L. , Rosello, A. , Rosen, K. V. , Rosenstiel, P. , Rost‐Roszkowska, M. , Roth, K. A. , Roué, G. , Rouis, M. , Rouschop, K. M. , Ruan, D. T. , Ruano, D. , Rubinsztein, D. C. , Rucker, E. B. , 3rd., Rudich, A. , Rudolf, E. , Rudolf, R. , Ruegg, M. A. , Ruiz‐Roldan, C. , Ruparelia, A. A. , Rusmini, P. , Russ, D. W. , Russo, G. L. , Russo, G. , Russo, R. , Rusten, T. E. , Ryabovol, V. , Ryan, K. M. , Ryter, S. W. , Sabatini, D. M. , Sacher, M. , Sachse, C. , Sack, M. N. , Sadoshima, J. , Saftig, P. , Sagi‐Eisenberg, R. , Sahni, S. , Saikumar, P. , Saito, T. , Saitoh, T. , Sakakura, K. , Sakoh‐Nakatogawa, M. , Sakuraba, Y. , Salazar‐Roa, M. , Salomoni, P. , Saluja, A. K. , Salvaterra, P. M. , Salvioli, R. , Samali, A. , Sanchez, A. M. , Sánchez‐Alcázar, J. A. , Sanchez‐Prieto, R. , Sandri, M. , Sanjuan, M. A. , Santaguida, S. , Santambrogio, L. , Santoni, G. , Dos Santos, C. N. , Saran, S. , Sardiello, M. , Sargent, G. , Sarkar, P. , Sarkar, S. , Sarrias, M. R. , Sarwal, M. M. , Sasakawa, C. , Sasaki, M. , Sass, M. , Sato, K. , Sato, M. , Satriano, J. , Savaraj, N. , Saveljeva, S. , Schaefer, L. , Schaible, U. E. , Scharl, M. , Schatzl, H. M. , Schekman, R. , Scheper, W. , Schiavi, A. , Schipper, H. M. , Schmeisser, H. , Schmidt, J. , Schmitz, I. , Schneider, B. E. , Schneider, E. M. , Schneider, J. L. , Schon, E. A. , Schönenberger, M. J. , Schönthal, A. H. , Schorderet, D. F. , Schröder, B. , Schuck, S. , Schulze, R. J. , Schwarten, M. , Schwarz, T. L. , Sciarretta, S. , Scotto, K. , Scovassi, A. I. , Screaton, R. A. , Screen, M. , Seca, H. , Sedej, S. , Segatori, L. , Segev, N. , Seglen, P. O. , Seguí‐Simarro, J. M. , Segura‐Aguilar, J. , Seki, E. , Sell, C. , Seiliez, I. , Semenkovich, C. F. , Semenza, G. L. , Sen, U. , Serra, A. L. , Serrano‐Puebla, A. , Sesaki, H. , Setoguchi, T. , Settembre, C. , Shacka, J. J. , Shajahan‐Haq, A. N. , Shapiro, I. M. , Sharma, S. , She, H. , Shen, C. K. , Shen, C. C. , Shen, H. M. , Shen, S. , Shen, W. , Sheng, R. , Sheng, X. , Sheng, Z. H. , Shepherd, T. G. , Shi, J. , Shi, Q. , Shi, Q. , Shi, Y. , Shibutani, S. , Shibuya, K. , Shidoji, Y. , Shieh, J. J. , Shih, C. M. , Shimada, Y. , Shimizu, S. , Shin, D. W. , Shinohara, M. L. , Shintani, M. , Shintani, T. , Shioi, T. , Shirabe, K. , Shiri‐Sverdlov, R. , Shirihai, O. , Shore, G. C. , Shu, C. W. , Shukla, D. , Sibirny, A. A. , Sica, V. , Sigurdson, C. J. , Sigurdsson, E. M. , Sijwali, P. S. , Sikorska, B. , Silveira, W. A. , Silvente‐Poirot, S. , Silverman, G. A. , Simak, J. , Simmet, T. , Simon, A. K. , Simon, H. U. , Simone, C. , Simons, M. , Simonsen, A. , Singh, R. , Singh, S. V. , Singh, S. K. , Sinha, D. , Sinha, S. , Sinicrope, F. A. , Sirko, A. , Sirohi, K. , Sishi, B. J. , Sittler, A. , Siu, P. M. , Sivridis, E. , Skwarska, A. , Slack, R. , Slaninová, I. , Slavov, N. , Smaili, S. S. , Smalley, K. S. , Smith, D. R. , Soenen, S. J. , Soleimanpour, S. A. , Solhaug, A. , Somasundaram, K. , Son, J. H. , Sonawane, A. , Song, C. , Song, F. , Song, H. K. , Song, J. X. , Song, W. , Soo, K. Y. , Sood, A. K. , Soong, T. W. , Soontornniyomkij, V. , Sorice, M. , Sotgia, F. , Soto‐Pantoja, D. R. , Sotthibundhu, A. , Sousa, M. J. , Spaink, H. P. , Span, P. N. , Spang, A. , Sparks, J. D. , Speck, P. G. , Spector, S. A. , Spies, C. D. , Springer, W. , Clair, D. S. , Stacchiotti, A. , Staels, B. , Stang, M. T. , Starczynowski, D. T. , Starokadomskyy, P. , Steegborn, C. , Steele, J. W. , Stefanis, L. , Steffan, J. , Stellrecht, C. M. , Stenmark, H. , Stepkowski, T. M. , Stern, S. T. , Stevens, C. , Stockwell, B. R. , Stoka, V. , Storchova, Z. , Stork, B. , Stratoulias, V. , Stravopodis, D. J. , Strnad, P. , Strohecker, A. M. , Ström, A. L. , Stromhaug, P. , Stulik, J. , Su, Y. X. , Su, Z. , Subauste, C. S. , Subramaniam, S. , Sue, C. M. , Suh, S. W. , Sui, X. , Sukseree, S. , Sulzer, D. , Sun, F. L. , Sun, J. , Sun, J. , Sun, S. Y. , Sun, Y. , Sun, Y. , Sun, Y. , Sundaramoorthy, V. , Sung, J. , Suzuki, H. , Suzuki, K. , Suzuki, N. , Suzuki, T. , Suzuki, Y. J. , Swanson, M. S. , Swanton, C. , Swärd, K. , Swarup, G. , Sweeney, S. T. , Sylvester, P. W. , Szatmari, Z. , Szegezdi, E. , Szlosarek, P. W. , Taegtmeyer, H. , Tafani, M. , Taillebourg, E. , Tait, S. W. , Takacs‐Vellai, K. , Takahashi, Y. , Takáts, S. , Takemura, G. , Takigawa, N. , Talbot, N. J. , Tamagno, E. , Tamburini, J. , Tan, C. P. , Tan, L. , Tan, M. L. , Tan, M. , Tan, Y. J. , Tanaka, K. , Tanaka, M. , Tang, D. , Tang, D. , Tang, G. , Tanida, I. , Tanji, K. , Tannous, B. A. , Tapia, J. A. , Tasset‐Cuevas, I. , Tatar, M. , Tavassoly, I. , Tavernarakis, N. , Taylor, A. , Taylor, G. S. , Taylor, G. A. , Taylor, J. P. , Taylor, M. J. , Tchetina, E. V. , Tee, A. R. , Teixeira‐Clerc, F. , Telang, S. , Tencomnao, T. , Teng, B. B. , Teng, R. J. , Terro, F. , Tettamanti, G. , Theiss, A. L. , Theron, A. E. , Thomas, K. J. , Thomé, M. P. , Thomes, P. G. , Thorburn, A. , Thorner, J. , Thum, T. , Thumm, M. , Thurston, T. L. , Tian, L. , Till, A. , Ting, J. P. , Titorenko, V. I. , Toker, L. , Toldo, S. , Tooze, S. A. , Topisirovic, I. , Torgersen, M. L. , Torosantucci, L. , Torriglia, A. , Torrisi, M. R. , Tournier, C. , Towns, R. , Trajkovic, V. , Travassos, L. H. , Triola, G. , Tripathi, D. N. , Trisciuoglio, D. , Troncoso, R. , Trougakos, I. P. , Truttmann, A. C. , Tsai, K. J. , Tschan, M. P. , Tseng, Y. H. , Tsukuba, T. , Tsung, A. , Tsvetkov, A. S. , Tu, S. , Tuan, H. Y. , Tucci, M. , Tumbarello, D. A. , Turk, B. , Turk, V. , Turner, R. F. , Tveita, A. A. , Tyagi, S. C. , Ubukata, M. , Uchiyama, Y. , Udelnow, A. , Ueno, T. , Umekawa, M. , Umemiya‐Shirafuji, R. , Underwood, B. R. , Ungermann, C. , Ureshino, R. P. , Ushioda, R. , Uversky, V. N. , Uzcátegui, N. L. , Vaccari, T. , Vaccaro, M. I. , Váchová, L. , Vakifahmetoglu‐Norberg, H. , Valdor, R. , Valente, E. M. , Vallette, F. , Valverde, A. M. , Van den Berghe, G. , Van Den Bosch, L. , van den Brink, G. R. , van der Goot, F. G. , van der Klei, I. J. , van der Laan, L. J. , van Doorn, W. G. , van Egmond, M. , van Golen, K. L. , Van Kaer, L. , van Lookeren Campagne, M. , Vandenabeele, P. , Vandenberghe, W. , Vanhorebeek, I. , Varela‐Nieto, I. , Vasconcelos, M. H. , Vasko, R. , Vavvas, D. G. , Vega‐Naredo, I. , Velasco, G. , Velentzas, A. D. , Velentzas, P. D. , Vellai, T. , Vellenga, E. , Vendelbo, M. H. , Venkatachalam, K. , Ventura, N. , Ventura, S. , Veras, P. S. , Verdier, M. , Vertessy, B. G. , Viale, A. , Vidal, M. , Vieira, H. L. , Vierstra, R. D. , Vigneswaran, N. , Vij, N. , Vila, M. , Villar, M. , Villar, V. H. , Villarroya, J. , Vindis, C. , Viola, G. , Viscomi, M. T. , Vitale, G. , Vogl, D. T. , Voitsekhovskaja, O. V. , von Haefen, C. , von Schwarzenberg, K. , Voth, D. E. , Vouret‐Craviari, V. , Vuori, K. , Vyas, J. M. , Waeber, C. , Walker, C. L. , Walker, M. J. , Walter, J. , Wan, L. , Wan, X. , Wang, B. , Wang, C. , Wang, C. Y. , Wang, C. , Wang, C. , Wang, C. , Wang, D. , Wang, F. , Wang, F. , Wang, G. , Wang, H. J. , Wang, H. , Wang, H. G. , Wang, H. , Wang, H. D. , Wang, J. , Wang, J. , Wang, M. , Wang, M. Q. , Wang, P. Y. , Wang, P. , Wang, R. C. , Wang, S. , Wang, T. F. , Wang, X. , Wang, X. J. , Wang, X. W. , Wang, X. , Wang, X. , Wang, Y. , Wang, Y. , Wang, Y. , Wang, Y. J. , Wang, Y. , Wang, Y. , Wang, Y. T. , Wang, Y. , Wang, Z. N. , Wappner, P. , Ward, C. , Ward, D. M. , Warnes, G. , Watada, H. , Watanabe, Y. , Watase, K. , Weaver, T. E. , Weekes, C. D. , Wei, J. , Weide, T. , Weihl, C. C. , Weindl, G. , Weis, S. N. , Wen, L. , Wen, X. , Wen, Y. , Westermann, B. , Weyand, C. M. , White, A. R. , White, E. , Whitton, J. L. , Whitworth, A. J. , Wiels, J. , Wild, F. , Wildenberg, M. E. , Wileman, T. , Wilkinson, D. S. , Wilkinson, S. , Willbold, D. , Williams, C. , Williams, K. , Williamson, P. R. , Winklhofer, K. F. , Witkin, S. S. , Wohlgemuth, S. E. , Wollert, T. , Wolvetang, E. J. , Wong, E. , Wong, G. W. , Wong, R. W. , Wong, V. K. , Woodcock, E. A. , Wright, K. L. , Wu, C. , Wu, D. , Wu, G. S. , Wu, J. , Wu, J. , Wu, M. , Wu, M. , Wu, S. , Wu, W. K. , Wu, Y. , Wu, Z. , Xavier, C. P. , Xavier, R. J. , Xia, G. X. , Xia, T. , Xia, W. , Xia, Y. , Xiao, H. , Xiao, J. , Xiao, S. , Xiao, W. , Xie, C. M. , Xie, Z. , Xie, Z. , Xilouri, M. , Xiong, Y. , Xu, C. , Xu, C. , Xu, F. , Xu, H. , Xu, H. , Xu, J. , Xu, J. , Xu, J. , Xu, L. , Xu, X. , Xu, Y. , Xu, Y. , Xu, Z. X. , Xu, Z. , Xue, Y. , Yamada, T. , Yamamoto, A. , Yamanaka, K. , Yamashina, S. , Yamashiro, S. , Yan, B. , Yan, B. , Yan, X. , Yan, Z. , Yanagi, Y. , Yang, D. S. , Yang, J. M. , Yang, L. , Yang, M. , Yang, P. M. , Yang, P. , Yang, Q. , Yang, W. , Yang, W. Y. , Yang, X. , Yang, Y. , Yang, Y. , Yang, Z. , Yang, Z. , Yao, M. C. , Yao, P. J. , Yao, X. , Yao, Z. , Yao, Z. , Yasui, L. S. , Ye, M. , Yedvobnick, B. , Yeganeh, B. , Yeh, E. S. , Yeyati, P. L. , Yi, F. , Yi, L. , Yin, X. M. , Yip, C. K. , Yoo, Y. M. , Yoo, Y. H. , Yoon, S. Y. , Yoshida, K. , Yoshimori, T. , Young, K. H. , Yu, H. , Yu, J. J. , Yu, J. T. , Yu, J. , Yu, L. , Yu, W. H. , Yu, X. F. , Yu, Z. , Yuan, J. , Yuan, Z. M. , Yue, B. Y. , Yue, J. , Yue, Z. , Zacks, D. N. , Zacksenhaus, E. , Zaffaroni, N. , Zaglia, T. , Zakeri, Z. , Zecchini, V. , Zeng, J. , Zeng, M. , Zeng, Q. , Zervos, A. S. , Zhang, D. D. , Zhang, F. , Zhang, G. , Zhang, G. C. , Zhang, H. , Zhang, H. , Zhang, H. , Zhang, H. , Zhang, J. , Zhang, J. , Zhang, J. , Zhang, J. , Zhang, J. P. , Zhang, L. , Zhang, L. , Zhang, L. , Zhang, L. , Zhang, M. Y. , Zhang, X. , Zhang, X. D. , Zhang, Y. , Zhang, Y. , Zhang, Y. , Zhang, Y. , Zhang, Y. , Zhao, M. , Zhao, W. L. , Zhao, X. , Zhao, Y. G. , Zhao, Y. , Zhao, Y. , Zhao, Y. X. , Zhao, Z. , Zhao, Z. J. , Zheng, D. , Zheng, X. L. , Zheng, X. , Zhivotovsky, B. , Zhong, Q. , Zhou, G. Z. , Zhou, G. , Zhou, H. , Zhou, S. F. , Zhou, X. J. , Zhu, H. , Zhu, H. , Zhu, W. G. , Zhu, W. , Zhu, X. F. , Zhu, Y. , Zhuang, S. M. , Zhuang, X. , Ziparo, E. , Zois, C. E. , Zoladek, T. , Zong, W. X. , Zorzano, A. , Zughaier, S. M. , (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buchser, W. J. , Laskow, T. C. , Pavlik, P. J. , Lin, H.‐M. , Lotze, M. T. (2012) Cell‐mediated autophagy promotes cancer cell survival. Cancer Res. 72, 2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ganley, I. G. , Lam, H. , Wang, J. , Ding, X. , Chen, S. , Jiang, X. (2009) ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 284, 12297–12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine, B. , Kroemer, G. (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jewell, J. L. , Russell, R. C. , Guan, K.‐L. (2013) Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shigemitsu, K. , Tsujishita, Y. , Hara, K. , Nanahoshi, M. , Avruch, J. , Yonezawa, K. (1999) Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J. Biol. Chem. 274, 1058–1065. [DOI] [PubMed] [Google Scholar]
- 23. Tasdemir, E. , Maiuri, M. C. , Galluzzi, L. , Vitale, I. , Djavaheri‐Mergny, M. , D'Amelio, M. , Criollo, A. , Morselli, E. , Zhu, C. , Harper, F. , Nannmark, U. , Samara, C. , Pinton, P. , Vicencio, J. M. , Carnuccio, R. , Moll, U. M. , Madeo, F. , Paterlini‐Brechot, P. , Rizzuto, R. , Szabadkai, G. , Pierron, G. , Blomgren, K. , Tavernarakis, N. , Codogno, P. , Cecconi, F. , Kroemer, G. (2008) Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pestana, C. R. , Oishi, J. C. , Salistre‐Araújo, H. S. , Rodrigues, G. J. (2015) Inhibition of autophagy by chloroquine stimulates nitric oxide production and protects endothelial function during serum deprivation. Cell. Physiol. Biochem. 37, 1168–1177. [DOI] [PubMed] [Google Scholar]
- 25. Yang, Z. J. , Chee, C. E. , Huang, S. , Sinicrope, F. A. (2011) The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 10, 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delgado, M. , Singh, S. , De Haro, S. , Master, S. , Ponpuak, M. , Dinkins, C. , Ornatowski, W. , Vergne, I. , Deretic, V. (2009) Autophagy and pattern recognition receptors in innate immunity. Immunol. Rev. 227, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan, Y. , Finkel, T. (2016) Autophagy as a regulator of cardiovascular redox homeostasis. Free Radic. Biol. Med. S0891‐5849(16)31091‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villalba, N. , Sonkusare, S. K. , Longden, T. A. , Tran, T. L. , Sackheim, A. M. , Nelson, M. T. , Wellman, G. C. , Freeman, K. (2014) Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function. J. Am. Heart Assoc. 3, e001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo, F. , Li, X. , Peng, J. , Tang, Y. , Yang, Q. , Liu, L. , Wang, Z. , Jiang, Z. , Xiao, M. , Ni, C. , Chen, R. , Wei, D. , Wang, G. X. (2014) Autophagy regulates vascular endothelial cell eNOS and ET‐1 expression induced by laminar shear stress in an ex vivo perfused system. Ann. Biomed. Eng. 42, 1978–1988. [DOI] [PubMed] [Google Scholar]
- 30. Buschmann, I. , Schaper, W. (2000) The pathophysiology of the collateral circulation (arteriogenesis). J. Pathol. 190, 338–342. [DOI] [PubMed] [Google Scholar]
- 31. Carmeliet, P. (2000) Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395. [DOI] [PubMed] [Google Scholar]
- 32. Sweet, D. T. , Chen, Z. , Givens, C. S. , Owens III, A. P. , Rojas, M. , Tzima, E. (2013) Endothelial Shc regulates arteriogenesis through dual control of arterial specification and inflammation via the notch and nuclear factor‐k‐light‐chain‐enhancer of activated B‐cell pathways. Circ. Res. 113, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gawaz, M. , Langer, H. , May, A. E. (2005) Platelets in inflammation and atherogenesis. J. Clin. Invest. 115, 3378–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartvigsen, K. , Chou, M.‐Y. , Hansen, L. F. , Shaw, P. X. , Tsimikas, S. , Binder, C. J. , Witztum, J. L. (2009) The role of innate immunity in atherogenesis. J. Lipid Res. 50(Suppl), S388–S393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuwahata, S. , Fujita, S. , Orihara, K. , Hamasaki, S. , Oba, R. , Hirai, H. , Nagata, K. , Ishida, S. , Kataoka, T. , Oketani, N. , Ichiki, H. , Iriki, Y. , Saihara, K. , Okui, H. , Ninomiya, Y. , Tei, C. (2010) High expression level of Toll‐like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis 209, 248–254. [DOI] [PubMed] [Google Scholar]
- 36. Michelsen, K. S. , Doherty, T. M. , Shah, P. K. , Arditi, M. (2004) TLR signaling: an emerging bridge from innate immunity to atherogenesis. J. Immunol. 173, 5901–5907. [DOI] [PubMed] [Google Scholar]
- 37. Razani, B. , Feng, C. , Coleman, T. , Emanuel, R. , Wen, H. , Hwang, S. , Ting, J. P. , Virgin, H. W. , Kastan, M. B. , Semenkovich, C. F. (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 15, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoletov, K. , Fang, L. , Choi, S. H. , Hartvigsen, K. , Hansen, L. F. , Hall, C. , Pattison, J. , Juliano, J. , Miller, E. R. , Almazan, F. , Crosier, P. , Witztum, J. L. , Klemke, R. L. , Miller, Y. I. (2009) Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ. Res. 104, 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Firasat, S. , Hecker, M. , Binder, L. , Asif, A. R. (2014) Advances in endothelial shear stress proteomics. Expert Rev. Proteomics 11, 611–619. [DOI] [PubMed] [Google Scholar]
- 40. Bharath, L. P. , Mueller, R. , Li, Y. , Ruan, T. , Kunz, D. , Goodrich, R. , Mills, T. , Deeter, L. , Sargsyan, A. , Anandh Babu, P. V. , Graham, T. E. , Symons, J. D. (2014) Impairment of autophagy in endothelial cells prevents shear‐stress‐induced increases in nitric oxide bioavailability. Can. J. Physiol. Pharmacol. 92, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishii, T. , Warabi, E. , Siow, R. C. M. , Mann, G. E. (2013) Sequestosome1/p62: a regulator of redox‐sensitive voltage‐activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic. Biol. Med. 65, 102–116. [DOI] [PubMed] [Google Scholar]
- 42. Antigny, F. , Hautefort, A. , Meloche, J. , Belacel‐Ouari, M. , Manoury, B. , Rucker‐Martin, C. , Péchoux, C. , Potus, F. , Nadeau, V. , Tremblay, E. , Ruffenach, G. , Bourgeois, A. , Dorfmüller, P. , Breuils‐Bonnet, S. , Fadel, E. , Ranchoux, B. , Jourdon, P. , Girerd, B. , Montani, D. , Provencher, S. , Bonnet, S. , Simonneau, G. , Humbert, M. , Perros, F. (2016) Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation 133, 1371–1385. [DOI] [PubMed] [Google Scholar]
- 43. Moreno, L. , Frazziano, G. , Cogolludo, A. , Cobeño, L. , Tamargo, J. , Perez‐Vizcaino, F. (2007) Role of protein kinase Czeta and its adaptor protein p62 in voltage‐gated potassium channel modulation in pulmonary arteries. Mol. Pharmacol. 72, 1301–1309. [DOI] [PubMed] [Google Scholar]
- 44. Coppock, E. A. , Tamkun, M. M. (2001) Differential expression of KV channel α‐ and β‐subunits in the bovine pulmonary arterial circulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1350–L1360. [DOI] [PubMed] [Google Scholar]
- 45. Palikaras, K. , Lionaki, E. , Tavernarakis, N. (2015) Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 22, 1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. LeBleu, V. S. , O'Connell, J. T. , Gonzalez Herrera, K. N. , Wikman, H. , Pantel, K. , Haigis, M. C. , de Carvalho, F. M. , Damascena, A. , Domingos Chinen, L. T. , Rocha, R. M. , Asara, J. M. , Kalluri, R. (2014) PGC‐1a mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang, S. , Park, D. W. , Gao, Y. , Ravi, S. , Darley‐Usmar, V. , Abraham, E. , Zmijewski, J. W. (2015) Participation of proteasome‐ubiquitin protein degradation in autophagy and the activation of AMP‐activated protein kinase. Cell. Signal. 27, 1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu, W. , Xu, H. , Wang, Z. , Mao, Y. , Yuan, L. , Luo, W. , Cui, Z. , Cui, T. , Wang, X. L. , Shen, Y. H. (2015) PINK1‐parkin‐mediated mitophagy protects mitochondrial integrity and prevents metabolic stress‐induced endothelial injury. PLoS One 10, e0132499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belloni, D. , Veschini, L. , Foglieni, C. , Dell'Antonio, G. , Caligaris‐Cappio, F. , Ferrarini, M. , Ferrero, E. (2010) Bortezomib induces autophagic death in proliferating human endothelial cells. Exp. Cell Res. 316, 1010–1018. [DOI] [PubMed] [Google Scholar]
- 50. Higdon, A. N. , Benavides, G. A. , Chacko, B. K. , Ouyang, X. , Johnson, M. S. , Landar, A. , Zhang, J. , Darley‐Usmar, V. M. (2012) Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 302, H1394–H1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shiroto, T. , Romero, N. , Sugiyama, T. , Sartoretto, J. L. , Kalwa, H. , Yan, Z. , Shimokawa, H. , Michel, T. (2014) Caveolin‐1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One 9, e87871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haslip, M. , Dostanic, I. , Huang, Y. , Zhang, Y. , Russell, K. S. , Jurczak, M. J. , Mannam, P. , Giordano, F. , Erzurum, S. C. , Lee, P. J. (2015) Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 35, 1166–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Domigan, C. K. , Warren, C. M. , Antanesian, V. , Happel, K. , Ziyad, S. , Lee, S. , Krall, A. , Duan, L. , Torres‐Collado, A. X. , Castellani, L. W. , Elashoff, D. , Christofk, H. R. , van der Bliek, A. M. , Potente, M. , Iruela‐Arispe, M. L. (2015) Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J. Cell Sci. 128, 2236–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hayashi, S. , Yamamoto, A. , You, F. , Yamashita, K. , Ikegame, Y. , Tawada, M. , Yoshimori, T. , Shimizu, S. , Nakashima, S. (2009) The stent‐eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy. Am. J. Pathol. 175, 2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang, K. (2015) Autophagy and apoptosis in liver injury. Cell Cycle 14, 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiang, P. , Lan, Y. , Luo, J. , Ren, Y. L. , Liu, D. G. , Pang, J. X. , Liu, J. , Li, J. , Wang, C. , Cai, J. P. (2014) Rapamycin promoted thrombosis and platelet adhesion to endothelial cells by inducing membrane remodeling. BMC Cell Biol. 15, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duraes, F. V. , Niven, J. , Dubrot, J. , Hugues, S. , Gannagé, M. (2015) Macroautophagy in endogenous processing of self‐ and pathogen‐derived antigens for MHC class II presentation. Front. Immunol. 6, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sajish, M. , Schimmel, P. (2015) A human tRNA synthetase is a potent PARP1‐activating effector target for resveratrol. Nature 519, 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen, M. L. , Yi, L. , Jin, X. , Liang, X. Y. , Zhou, Y. , Zhang, T. , Xie, Q. , Zhou, X. , Chang, H. , Fu, Y. J. , Zhu, J. D. , Zhang, Q. Y. , Mi, M. T. (2013) Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 9, 2033–2045. [DOI] [PubMed] [Google Scholar]
- 60. Fielhaber, J. A. , Carroll, S. F. , Dydensborg, A. B. , Shourian, M. , Triantafillopoulos, A. , Harel, S. , Hussain, S. N. , Bouchard, M. , Qureshi, S. T. , Kristof, A. S. (2012) Inhibition of mammalian target of rapamycin augments lipopolysaccharide‐induced lung injury and apoptosis. J. Immunol. 188, 4535–4542. [DOI] [PubMed] [Google Scholar]
- 61. Wang, C. Y. , Chiang, T. H. , Chen, C. L. , Tseng, P. C. , Chien, S. Y. , Chuang, Y. J. , Yang, T. T. , Hsieh, C. Y. , Choi, P. C. , Lin, C. F. (2014) Autophagy facilitates cytokine‐induced ICAM‐1 expression. Innate Immun. 20, 200–213. [DOI] [PubMed] [Google Scholar]
- 62. Janeway, C. A., Jr. , Medzhitov, R. (2002) Innate immune recognition. Annu. Rev. Immunol. 20, 197–216. [DOI] [PubMed] [Google Scholar]
- 63. Fitzgerald, K. A. , Rowe, D. C. , Barnes, B. J. , Caffrey, D. R. , Visintin, A. , Latz, E. , Monks, B. , Pitha, P. M. , Golenbock, D. T. (2003) LPS‐TLR4 signaling to IRF‐3/7 and NF‐kappaB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayashi, F. , Smith, K. D. , Ozinsky, A. , Hawn, T. R. , Yi, E. C. , Goodlett, D. R. , Eng, J. K. , Akira, S. , Underhill, D. M. , Aderem, A. (2001) The innate immune response to bacterial flagellin is mediated by Toll‐like receptor 5. Nature 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- 65. Gust, A. A. , Biswas, R. , Lenz, H. D. , Rauhut, T. , Ranf, S. , Kemmerling, B. , Götz, F. , Glawischnig, E. , Lee, J. , Felix, G. , Nürnberger, T. (2007) Bacteria‐derived peptidoglycans constitute pathogen‐associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem. 282, 32338–32348. [DOI] [PubMed] [Google Scholar]
- 66. Muta, T. (2006) Molecular basis for invertebrate innate immune recognition of (1–>3)‐beta‐d‐glucan as a pathogen‐associated molecular pattern. Curr. Pharm. Des. 12, 4155–4161. [DOI] [PubMed] [Google Scholar]
- 67. Lotze, M. T. , Tracey, K. J. (2005) High‐mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342. [DOI] [PubMed] [Google Scholar]
- 68. Li, Y. , Xiang, M. , Yuan, Y. , Xiao, G. , Zhang, J. , Jiang, Y. , Vodovotz, Y. , Billiar, T. R. , Wilson, M. A. , Fan, J. (2009) Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1670–R1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fan, J. , Frey, R. S. , Malik, A. B. (2003) TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J. Clin. Invest. 112, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sachdev, U. , Cui, X. , McEnaney, R. , Wang, T. , Benabou, K. , Tzeng, E. (2012) TLR2 and TLR4 mediate differential responses to limb ischemia through MyD88‐dependent and independent pathways. PLoS One 7, e50654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meng, N. , Wu, L. , Gao, J. , Zhao, J. , Su, L. , Su, H. , Zhang, S. , Miao, J. (2010) Lipopolysaccharide induces autophagy through BIRC2 in human umbilical vein endothelial cells. J. Cell. Physiol. 225, 174–179. [DOI] [PubMed] [Google Scholar]
- 72. Deretic, V. (2009) Links between autophagy, innate immunity, inflammation and Crohn's disease. Dig. Dis. 27, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Delgado, M. A. , Elmaoued, R. A. , Davis, A. S. , Kyei, G. , Deretic, V. (2008) Toll‐like receptors control autophagy. EMBO J. 27, 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pilla‐Moffett, D. , Barber, M. F. , Taylor, G. A. , Coers, J. (2016) Interferon‐inducible GTPases in host resistance, inflammation and disease. J. Mol. Biol. 428, 3495–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Puliti, M. , Uematsu, S. , Akira, S. , Bistoni, F. , Tissi, L. (2009) Toll‐like receptor 2 deficiency is associated with enhanced severity of group B streptococcal disease. Infect. Immun. 77, 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Henneke, P. , Dramsi, S. , Mancuso, G. , Chraibi, K. , Pellegrini, E. , Theilacker, C. , Hübner, J. , Santos‐Sierra, S. , Teti, G. , Golenbock, D. T. , Poyart, C. , Trieu‐Cuot, P. (2008) Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 180, 6149–6158. [DOI] [PubMed] [Google Scholar]
- 77. Asplin, I. R. , Carl, D. J. , Way, S. S. , Jones, A. L. (2008) Role of Toll‐like receptor 2 in innate resistance to group B Streptococcus. Microb. Pathog. 44, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cutting, A. S. , Del Rosario, Y. , Mu, R. , Rodriguez, A. , Till, A. , Subramani, S. , Gottlieb, R. A. , Doran, K. S. (2014) The role of autophagy during group B Streptococcus infection of blood‐brain barrier endothelium. J. Biol. Chem. 289, 35711–35723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Suárez, A. L. , Kong, R. , George, T. , He, L. , Yue, Z. , van Dyk, L. F. (2011) Gammaherpesvirus 68 infection of endothelial cells requires both host autophagy genes and viral oncogenes for optimal survival and persistence. J. Virol. 85, 6293–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Biscetti, F. , Straface, G. , De Cristofaro, R. , Lancellotti, S. , Rizzo, P. , Arena, V. , Stigliano, E. , Pecorini, G. , Egashira, K. , De Angelis, G. , Ghirlanda, G. , Flex, A. (2010) High‐mobility group box‐1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF‐dependent mechanism. Diabetes 59, 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chavakis, E. , Hain, A. , Vinci, M. , Carmona, G. , Bianchi, M. E. , Vajkoczy, P. , Zeiher, A. M. , Chavakis, T. , Dimmeler, S. (2007) High‐mobility group box 1 activates integrin‐dependent homing of endothelial progenitor cells. Circ. Res. 100, 204–212. [DOI] [PubMed] [Google Scholar]
- 82. De Mori, R. , Straino, S. , Di Carlo, A. , Mangoni, A. , Pompilio, G. , Palumbo, R. , Bianchi, M. E. , Capogrossi, M. C. , Germani, A. (2007) Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler. Thromb. Vasc. Biol. 27, 2377–2383. [DOI] [PubMed] [Google Scholar]
- 83. Fages, C. , Nolo, R. , Huttunen, H. J. , Eskelinen, E. , Rauvala, H. (2000) Regulation of cell migration by amphoterin. J. Cell Sci. 113, 611–620. [DOI] [PubMed] [Google Scholar]
- 84. Mitola, S. , Belleri, M. , Urbinati, C. , Coltrini, D. , Sparatore, B. , Pedrazzi, M. , Melloni, E. , Presta, M. (2006) Cutting edge: extracellular high mobility group box‐1 protein is a proangiogenic cytokine. J. Immunol. 176, 12–15. [DOI] [PubMed] [Google Scholar]
- 85. Palumbo, R. , Sampaolesi, M. , De Marchis, F. , Tonlorenzi, R. , Colombetti, S. , Mondino, A. , Cossu, G. , Bianchi, M. E. (2004) Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J. Cell Biol. 164, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Riuzzi, F. , Sorci, G. , Donato, R. (2006) The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo. J. Biol. Chem. 281, 8242–8253. [DOI] [PubMed] [Google Scholar]
- 87. Riuzzi, F. , Sorci, G. , Sagheddu, R. , Donato, R. (2012) HMGB1‐RAGE regulates muscle satellite cell homeostasis through p38‐MAPK‐ and myogenin‐dependent repression of Pax7 transcription. J. Cell Sci. 125, 1440–1454. [DOI] [PubMed] [Google Scholar]
- 88. Sachdev, U. , Cui, X. , Tzeng, E. (2013) HMGB1 and TLR4 mediate skeletal muscle recovery in a murine model of hindlimb ischemia. J. Vasc. Surg. 58, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schlueter, C. , Weber, H. , Meyer, B. , Rogalla, P. , Röser, K. , Hauke, S. , Bullerdiek, J. (2005) Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am. J. Pathol. 166, 1259–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sorci, G. , Riuzzi, F. , Arcuri, C. , Giambanco, I. , Donato, R. (2004) Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol. Cell. Biol. 24, 4880–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang, H. , Wang, H. , Czura, C. J. , Tracey, K. J. (2005) The cytokine activity of HMGB1. J. Leukoc. Biol. 78, 1–8. [DOI] [PubMed] [Google Scholar]
- 92. Sachdev, U. , Cui, X. , Hong, G. , Namkoong, S. , Karlsson, J. M. , Baty, C. J. , Tzeng, E. (2012) High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. J. Vasc. Surg. 55, 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang, D. , Kang, R. , Livesey, K. M. , Cheh, C. W. , Farkas, A. , Loughran, P. , Hoppe, G. , Bianchi, M. E. , Tracey, K. J. , Zeh III, H. J. , Lotze, M. T. (2010) Endogenous HMGB1 regulates autophagy. J. Cell Biol. 190, 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tang, D. , Kang, R. , Coyne, C. B. , Zeh, H. J. , Lotze, M. T. (2012) PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 249, 158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rodgers, M. A. , Bowman, J. W. , Liang, Q. , Jung, J. U. (2014) Regulation where autophagy intersects the inflammasome. Antioxid. Redox Signal. 20, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schaefer, L. (2014) Complexity of danger: the diverse nature of damage‐associated molecular patterns. J. Biol. Chem. 289, 35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. McIlwain, D. R. , Berger, T. , Mak, T. W. (2015) Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 7, a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lu, B. , Nakamura, T. , Inouye, K. , Li, J. , Tang, Y. , Lundbäck, P. , Valdes‐Ferrer, S. I. , Olofsson, P. S. , Kalb, T. , Roth, J. , Zou, Y. , Erlandsson‐Harris, H. , Yang, H. , Ting, J. P. Y. , Wang, H. , Andersson, U. , Antoine, D. J. , Chavan, S. S. , Hotamisligil, G. S. , Tracey, K. J. (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dupont, N. , Jiang, S. , Pilli, M. , Ornatowski, W. , Bhattacharya, D. , Deretic, V. (2011) Autophagy‐based unconventional secretory pathway for extracellular delivery of IL‐1β. EMBO J. 30, 4701–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang, Y. , Sauler, M. , Shinn, A. S. , Gong, H. , Haslip, M. , Shan, P. , Mannam, P. , Lee, P. J. (2014) Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J Immunol. 192, 5296–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yang, M. , Cao, L. , Xie, M. , Yu, Y. , Kang, R. , Yang, L. , Zhao, M. , Tang, D. (2013) Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem. Pharmacol. 86, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tang, D. , Kang, R. , Cheh, C.‐W. , Livesey, K. M. , Liang, X. , Schapiro, N. E. , Benschop, R. , Sparvero, L. J. , Amoscato, A. A. , Tracey, K. J. , Zeh, H. J. , Lotze, M. T. (2010) HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 29, 5299–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dong, Xda E. , Ito, N. , Lotze, M. T. , Demarco, R. A. , Popovic, P. , Shand, S. H. , Watkins, S. , Winikoff, S. , Brown, C. K. , Bartlett, D. L. , Zeh III, H. J. I. (2007) High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J. Immunother. 30, 596–606. [DOI] [PubMed] [Google Scholar]
- 104. Tang, D. , Kang, R. , Livesey, K. M. , Zeh III, H. J. , Lotze, M. T. (2011) High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid. Redox Signal. 15, 2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thorburn, J. , Horita, H. , Redzic, J. , Hansen, K. , Frankel, A. E. , Thorburn, A. (2009) Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 16, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thorburn, J. , Frankel, A. E. , Thorburn, A. (2009) Regulation of HMGB1 release by autophagy. Autophagy 5, 247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Suzuki, Y. , Mimura, K. , Yoshimoto, Y. , Watanabe, M. , Ohkubo, Y. , Izawa, S. , Murata, K. , Fujii, H. , Nakano, T. , Kono, K. (2012) Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 72, 3967–3976. [DOI] [PubMed] [Google Scholar]
- 108. Kono, K. , Mimura, K. (2013) Immunogenic tumor cell death induced by chemoradiotherapy in a clinical setting. OncoImmunology 2, e22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kono, K. , Mimura, K. , Kiessling, R. (2013) Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis. 4, e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wild, C. A. , Brandau, S. , Lotfi, R. , Mattheis, S. , Gu, X. , Lang, S. , Bergmann, C. (2012) HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral Oncol. 48, 409–416. [DOI] [PubMed] [Google Scholar]
- 111. Wild, C. A. , Bergmann, C. , Fritz, G. , Schuler, P. , Hoffmann, T. K. , Lotfi, R. , Westendorf, A. , Brandau, S. , Lang, S. (2012) HMGB1 conveys immunosuppressive characteristics on regulatory and conventional T cells. Int. Immunol. 24, 485–494. [DOI] [PubMed] [Google Scholar]
- 112. Zhu, X.‐M. , Yao, Y.‐M. , Liang, H.‐P. , Xu, C.‐T. , Dong, N. , Yu, Y. , Sheng, Z.‐Y. (2011) High mobility group box‐1 protein regulate immunosuppression of regulatory T cells through Toll‐like receptor 4. Cytokine 54, 296–304. [DOI] [PubMed] [Google Scholar]
- 113. Pistoia, V. , Pezzolo, A. (2016) Involvement of HMGB1 in resistance to tumor vessel‐targeted, monoclonal antibody‐based immunotherapy. J. Immunol. Res. 2016, 3142365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kang, R. , Zhang, Q. , Zeh, H. J. , Lotze, M. T. , Tang, D. (2013) HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 19, 4046–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carmeliet, P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936. [DOI] [PubMed] [Google Scholar]
- 116. Carmeliet, P. (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69 (Suppl 3), 4–10. [DOI] [PubMed] [Google Scholar]
- 117. Amaravadi, R. K. , Thompson, C. B. (2007) The roles of therapy‐induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 13, 7271–7279. [DOI] [PubMed] [Google Scholar]
- 118. Boone, B. A. , Orlichenko, L. , Schapiro, N. E. , Loughran, P. , Gianfrate, G. C. , Ellis, J. T. , Singhi, A. D. , Kang, R. , Tang, D. , Lotze, M. T. , Zeh, H. J. (2015) The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 22, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liang, X. , De Vera, M. E. , Buchser, W. J. , Romo de Vivar Chavez, A., Loughran, P. , Beer Stolz, D. , Basse, P. , Wang, T. , Van Houten, B. , Zeh III, H. J. , Lotze, M. T. (2012) Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long‐term tumor regression. Cancer Res. 72, 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kang, R. , Tang, D. , Lotze, M. T. , Zeh Iii, H. J. (2013) Autophagy is required for IL‐2‐mediated fibroblast growth. Exp. Cell Res. 319, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Salabei, J. K. , Hill, B. G. (2015) Autophagic regulation of smooth muscle cell biology. Redox Biol. 4, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Maes, H. , Kuchnio, A. , Peric, A. , Moens, S. , Nys, K. , De Bock, K. , Quaegebeur, A. , Schoors, S. , Georgiadou, M. , Wouters, J. , Vinckier, S. , Vankelecom, H. , Garmyn, M. , Vion, A.‐C. , Radtke, F. , Boulanger, C. , Gerhardt, H. , Dejana, E. , Dewerchin, M. , Ghesquière, B. , Annaert, W. , Agostinis, P. , Carmeliet, P. (2014) Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26, 190–206. [DOI] [PubMed] [Google Scholar]
- 123. Buraschi, S. , Neill, T. , Goyal, A. , Poluzzi, C. , Smythies, J. , Owens, R. T. , Schaefer, L. , Torres, A. , Iozzo, R. V. (2013) Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. USA 110, E2582–E2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bae, D. , Lu, S. , Taglienti, C. A. , Mercurio, A. M. (2008) Metabolic stress induces the lysosomal degradation of neuropilin‐1 but not neuropilin‐2. J. Biol. Chem. 283, 28074–28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xavier, S. , Gilbert, V. , Rastaldi, M. P. , Krick, S. , Kollins, D. , Reddy, A. , Bottinger, E. , Cohen, C. D. , Schlondorff, D. (2010) BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One 5, e12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Guillot, N. , Kollins, D. , Gilbert, V. , Xavier, S. , Chen, J. , Gentle, M. , Reddy, A. , Bottinger, E. , Jiang, R. , Rastaldi, M. P. , Corbelli, A. , Schlondorff, D. (2012) BAMBI regulates angiogenesis and endothelial homeostasis through modulation of alternative TGFβ signaling. PLoS One 7, e39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pan, C. C. , Kumar, S. , Shah, N. , Bloodworth, J. C. , Hawinkels, L. J. A. C. , Mythreye, K. , Hoyt, D. G. , Lee, N. Y. (2015) Endoglin regulation of Smad2 function mediates beclin1 expression and endothelial autophagy. J. Biol. Chem. 290, 14884–14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fukumura, D. , Jain, R. K. (2007) Tumor microvasculature and microenvironment: targets for anti‐angiogenesis and normalization. Microvasc. Res. 74, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jain, R. K. (2003) Molecular regulation of vessel maturation. Nat. Med. 9, 685–693. [DOI] [PubMed] [Google Scholar]
- 130. Liu, H. , Yu, S. , Zhang, H. , Xu, J. (2012) Angiogenesis impairment in diabetes: role of methylglyoxal‐induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS One 7, e46720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Baumgartner, I. , Pieczek, A. , Manor, O. , Blair, R. , Kearney, M. , Walsh, K. , Isner, J. M. (1998) Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 97, 1114–1123. [DOI] [PubMed] [Google Scholar]
- 132. Wagatsuma, A. (2007) Endogenous expression of angiogenesis‐related factors in response to muscle injury. Mol. Cell. Biochem. 298, 151–159. [DOI] [PubMed] [Google Scholar]
- 133. Lederman, R. J. , Mendelsohn, F. O. , Anderson, R. D. , Saucedo, J. F. , Tenaglia, A. N. , Hermiller, J. B. , Hillegass, W. B. , Rocha‐Singh, K. , Moon, T. E. , Whitehouse, M. J. , Annex, B. H. ; TRAFFIC Investigators . (2002) Therapeutic angiogenesis with recombinant fibroblast growth factor‐2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 359, 2053–2058. [DOI] [PubMed] [Google Scholar]
- 134. Limbourg, A. , Korff, T. , Napp, L. C. , Schaper, W. , Drexler, H. , Limbourg, F. P. (2009) Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind‐limb ischemia. Nat. Protoc. 4, 1737–1746. [DOI] [PubMed] [Google Scholar]
- 135. Messina, L. M. , Brevetti, L. S. , Chang, D. S. , Paek, R. , Sarkar, R. (2002) Therapeutic angiogenesis for critical limb ischemia: invited commentary. J. Control. Release 78, 285–294. [DOI] [PubMed] [Google Scholar]
- 136. Du, J. , Teng, R.‐J. , Guan, T. , Eis, A. , Kaul, S. , Konduri, G. G. , Shi, Y. (2012) Role of autophagy in angiogenesis in aortic endothelial cells. Am. J. Physiol. Cell Physiol. 302, C383–C391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sachdev, U. , Cui, X. , Xu, J. , Xu, J. , Tzeng, E. (2014) MyD88 and TRIF mediate divergent inflammatory and regenerative responses to skeletal muscle ischemia. Physiol. Rep. 2, e12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shao, B. Z. , Han, B. Z. , Zeng, Y. X. , Su, D. F. , Liu, C. (2016) The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol. Sin. 37, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xiong, Y. , Yepuri, G. , Forbiteh, M. , Yu, Y. , Montani, J.‐P. , Yang, Z. , Ming, X.‐F. (2014) ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy 10, 2223–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vindis, C. (2015) Autophagy: an emerging therapeutic target in vascular diseases. Br. J. Pharmacol. 172, 2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Grootaert, M. O. J. , da Costa Martins, P. A. , Bitsch, N. , Pintelon, I. , De Meyer, G. R. Y. , Martinet, W. , Schrijvers, D. M. (2015) Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 11, 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Geng, Z. , Xu, F. , Zhang, Y. (2016) MiR‐129‐5p‐mediated beclin‐1 suppression inhibits endothelial cell autophagy in atherosclerosis. Am. J. Transl. Res. 8, 1886–1894. [PMC free article] [PubMed] [Google Scholar]
- 143. Santovito, D. , Weber, C. (2017) Atherosclerosis revisited from a clinical perspective: still an inflammatory disease? Thromb. Haemost. 117, 231–237. [DOI] [PubMed] [Google Scholar]
- 144. Glynn, R. J. , MacFadyen, J. G. , Ridker, P. M. (2009) Tracking of high‐sensitivity C‐reactive protein after an initially elevated concentration: the JUPITER Study. Clin. Chem. 55, 305–312. [DOI] [PubMed] [Google Scholar]
- 145. Ridker, P. M. , Cannon, C. P. , Morrow, D. , Rifai, N. , Rose, L. M. , McCabe, C. H. , Pfeffer, M. A. , Braunwald, E. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 (PROVE IT‐TIMI 22) Investigators . (2005) C‐reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28. [DOI] [PubMed] [Google Scholar]
- 146. Kim, H. S. , Montana, V. , Jang, H. J. , Parpura, V. , Kim, J. A. (2013) Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation. J. Biol. Chem. 288, 22693–22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Torisu, K. , Singh, K. K. , Torisu, T. , Lovren, F. , Liu, J. , Pan, Y. , Quan, A. , Ramadan, A. , Al‐Omran, M. , Pankova, N. , Boyd, S. R. , Verma, S. , Finkel, T. (2016) Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 15, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nowicki, M. , Zabirnyk, O. , Duerrschmidt, N. , Borlak, J. , Spanel‐Borowski, K. (2007) No upregulation of lectin‐like oxidized low‐density lipoprotein receptor‐1 in serum‐deprived EA.hy926 endothelial cells under oxLDL exposure, but increase in autophagy. Eur. J. Cell Biol. 86, 605–616. [DOI] [PubMed] [Google Scholar]
- 149. Menghini, R. , Casagrande, V. , Marino, A. , Marchetti, V. , Cardellini, M. , Stoehr, R. , Rizza, S. , Martelli, E. , Greco, S. , Mauriello, A. , Ippoliti, A. , Martelli, F. , Lauro, R. , Federici, M. (2014) MiR‐216a: a link between endothelial dysfunction and autophagy. Cell Death Dis. 5, e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mora, S. , Ridker, P. M. (2006) Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)–can C‐reactive protein be used to target statin therapy in primary prevention? Am. J. Cardiol. 97(2A), 33A–41A. [DOI] [PubMed] [Google Scholar]
- 151. Ray, K. K. , Cannon, C. P. , Cairns, R. , Morrow, D. A. , Rifai, N. , Kirtane, A. J. , McCabe, C. H. , Skene, A. M. , Gibson, C. M. , Ridker, P. M. , Braunwald, E. ; PROVE IT‐TIMI 22 Investigators . (2005) Relationship between uncontrolled risk factors and C‐reactive protein levels in patients receiving standard or intensive statin therapy for acute coronary syndromes in the PROVE IT‐TIMI 22 trial. J. Am. Coll. Cardiol. 46, 1417–1424. [DOI] [PubMed] [Google Scholar]
- 152. Li, H. , Huang, S. , Wang, S. , Zhao, J. , Su, L. , Zhao, B. , Zhang, Y. , Zhang, S. , Miao, J. (2013) Targeting annexin A7 by a small molecule suppressed the activity of phosphatidylcholine‐specific phospholipase C in vascular endothelial cells and inhibited atherosclerosis in apolipoprotein E–/– mice. Cell Death Dis. 4, e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhang, Y. , Cao, X. , Zhu, W. , Liu, Z. , Liu, H. , Zhou, Y. , Cao, Y. , Liu, C. , Xie, Y. (2016) Resveratrol enhances autophagic flux and promotes OxLDL degradation in HUVECs via upregulation of SIRT1. Oxid. Med. Cell. Longev. 2016, 7589813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Khan, M. J. , Rizwan Alam, M. , Waldeck‐Weiermair, M. , Karsten, F. , Groschner, L. , Riederer, M. , Hallström, S. , Rockenfeller, P. , Konya, V. , Heinemann, A. , Madeo, F. , Graier, W. F. , Malli, R. (2012) Inhibition of autophagy rescues palmitic acid‐induced necroptosis of endothelial cells. J. Biol. Chem. 287, 21110–21120. [DOI] [PMC free article] [PubMed] [Google Scholar]


