Short abstract
TGF‐ β differentially impairs IL‐7‐induced proliferation in memory and naiüve CD4+ T cells.
Keywords: cell cycle, cell proliferation, T lymphocytes, cytokines, homeostasis
Abstract
TGF‐β is a potent suppressor of T cell activation and expansion. Although the antiproliferative effects of TGF‐β are well characterized in TCR‐activated cells, the effects of TGF‐β on T cell proliferation driven by homeostatic cytokines, such as IL‐7, are poorly defined. In the current study, we found that TGF‐β inhibits IL‐7‐induced proliferation in memory, but not in naive human CD4+ T cells. TGF‐β impaired c‐myc induction in all CD4+ T cell maturation subsets, although the impairment was less sustained in naive CD4+ T cells. TGF‐β had no discernible effect on IL‐7R signaling (p‐STAT‐5, p‐Akt, or p‐S6) in memory T cells but selectively enhanced p‐S6 signaling in naive T cells. The inhibitory effects of TGF‐β on memory T cell proliferation were partially overcome by chemical inhibition of GSK‐3, which also led to enhanced c‐myc expression. These data suggest that TGF‐β could play an important role in limiting homeostatic proliferation of memory T cells. Our observations also point toward a novel strategy to subvert TGF‐β‐mediated inhibition of memory T cells by targeting GSK‐3 for inhibition.
Abbreviations
- %CFSElow
percentage of cells with diluted CFSE staining
- γc
γ chain
- Akt
protein kinase B
- CH
CHIR‐99021 (GSK‐3 inhibitor)
- CM
central memory
- EM
extended memory
- GSK
glycogen synthase kinase
- PB
pacific blue (dye)
- PeCy
phycoerythrin‐cyanine
- PerCP
peridinin chlorophyll protein
Introduction
TGF‐β1 is a cytokine that affects various cellular activities, including proliferation, differentiation, and apoptosis [1]. TGF‐β has well‐defined immunosuppressive effects on the immune system [2, 3, 4, 5, 6–7] and is therefore important in protection from immunopathology. Deficiencies in TGF‐β receptor expression can lead to T cell hyperactivation [8, 9], whereas inhibition of TGF‐β during parasitic infections may lead to poorly controlled immunopathology [10]. Overexpression of TGF‐β can also be detrimental in certain circumstances, as TGF‐β expression has been linked to susceptibility to certain parasites [11] and to impaired immune responses in tumor microenvironments [12]. Thus, an appropriate balance in T cell exposure to TGF‐β is important for efficient immune responses and homeostasis.
To mediate complex biologic effects, TGF‐β signals through its receptor to activate Smad‐2 and ‐3. Activated Smad‐2/3 binds to Smad‐4, and this complex translocates to the nucleus to regulate gene transcription [13, 14]. Cellular responses to TGF‐β are complex and may be determined by expression of cell‐type–specific cofactors [15, 16–17], TGF‐β receptors, signaling pathway regulators, and epigenetic mechanisms [18]. Although there are multiple levels of complexity by which TGF‐β affects cell responses, various studies have defined TGF‐β signaling through Smad‐3 as a potent suppressor of proliferation in epithelial cells, keratinocytes, and TCR‐activated T cells [19, 20–21]. In epithelial cells and keratinocytes, TGF‐β arrests the cell cycle at the G1–S phase transition by down‐regulating c‐myc expression [22], which is a key determinant in cell cycle progression [23, 24]. In TCR‐activated T cells, TGF‐β suppresses the expression of metabolic genes and diminished mTORC1‐mediated S6 kinase activity [19]. Early studies also suggested that TGF‐β could impair JAK/STAT signaling in T cells stimulated with IL‐2 [25]; however, this observation was later disputed [26]. The collective results of these studies suggest that TGF‐β inhibits the induction of c‐myc expression and the cellular metabolic program to impair cellular proliferation.
Despite our understanding of the activity of TGF‐β in TCR‐activated cells, the potential importance of this cytokine in T cells, responding to homoeostatic cytokines such as IL‐7, is poorly defined. Recent studies in mice with deficiencies in TGF‐β receptor demonstrated that these mice had a loss of naive T cells and a corresponding decrease in expression of IL‐7Rα [27]. This observation raises the possibility that TGF‐β has differential effects on naive and memory T cells, particularly in the context of IL‐7 stimulation. Because IL‐7 is a critical mediator of T cell restoration in lymphopenic conditions and has a nonredundant role in sustaining T cell numbers in the periphery [28], the potential interactions of TGF‐β with IL‐7 represent an important consideration in T cell homeostasis.
IL‐7 signals through the IL‐7 receptor and comprises IL‐7 receptor α (IL‐7Rα or CD127) and the common γ chain (γc or CD132) [29]. IL‐7 receptor engagement activates JAK‐1 and ‐3, leading to STAT‐5 phosphorylation and subsequent expression of genes promoting cell survival [30]. Additional signaling through PI3K is activated by IL‐7, resulting in delayed phosphorylation and activation of Akt [31, 32] and S6 [33], the 40s ribosomal subunit. Phosphorylation of S6 is associated with increased protein translation [34], whereas p‐Akt inactivates the negative regulator of proliferation, glycogen synthase kinase (GSK)‐3 [35]. In resting cells, GSK‐3 is a constitutively active kinase that interacts with many kinases [36] and promotes the degradation of c‐myc [37]. Thus, IL‐7 promotes c‐myc expression and global protein translation to drive cellular proliferation.
In this study, we examined the effects of TGF‐β on human naive and memory T cell proliferation that is induced by IL‐7. We found that TGF‐β inhibits IL‐7‐induced memory, but not naive T cell proliferation. We also uncovered differential effects of TGF‐β on S6 signaling and the duration of c‐myc inhibition in IL‐7‐stimulated memory and naive T cells. Furthermore, we find that chemical inhibition of GSK‐3 at least partially overcomes TGF‐β‐mediated inhibition of c‐myc expression and proliferation in IL‐7‐stimulated memory T cells. Overall, our studies highlight striking differences in the effects of TGF‐β on IL‐7 responses in naive and memory T cells, suggesting that TGF‐β plays a differential role in the homeostasis of these cellular subsets.
MATERIALS AND METHODS
PBMCs and CD4+ T cell subset isolation
Whole blood was collected from healthy adult volunteers who provided signed informed consent. The protocol was approved by the University Hospitals of Cleveland Institutional Review Board. PBMCs were isolated from whole blood by centrifugation over a Ficoll‐paque cushion (GE Life Sciences, Pittsburgh, PA, USA). Naive CD4+ T cells were isolated with a naive human CD4+ T cell isolation kit II (Miltenyi Biotec, San Diego, CA, USA). Memory CD4+ T cells were isolated with Miltenyi Biotec reagents by negative selection to remove CD8+ T cells, non‐T cells, and CD45RA+ naive T cells. The purity of the sorted naive and memory CD4+ T cells was assessed by flow cytometry.
Stimulation conditions
To assess proliferation, PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE; 0.25 mmol/L at 37°C for 10 min; Thermo Fisher Scientific, Waltham, MA, USA). Staining was quenched with FBS for 5 min on ice, and the cells were washed with RPMI1640. PBMCs were resuspended in an X‐Vivo 15 serum‐free medium supplemented with 1% penicillin–streptomycin and incubated at a concentration of 1 million cells/ml with or without rIL‐7 (5 ng/ml; Cytheris, SA, Issy le Molineaux, France) with or without rTGF‐β1 (5 ng/ml; R&D Systems, Minneapolis, MN, USA). In experiments testing inactivation of GSK‐3, small molecule inhibitor, CH 99021 (Axon Medchem, Gronigen, The Netherlands) were stimulated with rIL‐7±rTGF‐β1 at described concentrations in the Results section.
Flow cytometry
Freshly isolated PBMCs were sorted for naive and memory CD4+ T cells, which were incubated with the following fluorochrome‐labeled monoclonal antibodies for 30 min: anti‐CD3 peridinin chlorophyll protein (PerCP), anti‐CD45RA phycoerythrin‐cyanine 7 (PE‐Cy7), anti‐CD27 Alexa Fluor 700 (AF‐700) (all from BD Biosciences, San Jose, CA, USA), and anti‐CD4+ pacific blue (PB) (BioLegend, San Diego, CA, USA). Some studies included anti‐CD127 antibody (BD Biosciences) for IL‐7 receptor detection.
To measure proliferation and c‐myc expression, CFSE‐labeled PBMCs were incubated with fluorochrome‐labeled mAbs anti‐CD3 brilliant violet 711, CD45RA PE‐Cy7, CD27 PerCP (all from BD Biosciences), and CD4+ PB (BioLegend). Cells were washed, fixed, and permeabilized with 2× perm/wash buffer (BD Biosciences) and incubated with anti‐hc‐Myc AF‐700 (from R&D Systems, Inc.) overnight. After intracellular staining, cells were washed with 2× perm/wash buffer and assessed by flow cytometry. To assess p‐S6, p‐Akt, and p‐STAT‐5, cells were fixed with fixation buffer at 37°C for 10 min and permeabilized with Perm III buffer at 4°C for 30 min before labeled with anti‐CD3 PerCP, anti‐CD45RA PE‐Cy7, anti‐CD27 FITC, anti‐Akt (pS473), anti‐STAT‐5 (pY694) (all from BD Biosciences), anti‐CD4+ PB (BioLegend),and anti‐S6 pS240‐APC (Miltenyi Biotec), for 30 min in 25°C.
Statistical analyses
Prism 5 software (GraphPad, La Jolla, CA, USA) was used to generate the figures and perform statistical analyses. The nonparametric paired test, Wilcoxon matched‐pairs signed rank test was used to compare responses to IL‐7 in the presence or absence of TGF‐β. To compare the percentage of TGF‐β‐mediated inhibition of c‐myc expression and proliferation in response to IL‐7 among the CD4+ T cell maturation subsets, Kruskal‐Wallis t test was used and followed by Dunn's multiple comparison post hoc test. All tests were 2‐sided with a significance cutoff of P < 0.05.
RESULTS
TGF‐β inhibits memory but not naive CD4+ T cell proliferation in response to IL‐7
To characterize the effects of TGF‐β on naive and memory CD4+ T cell proliferation in response to IL‐7, we labeled PBMCs with CFSE tracking dye and stimulated the cells with rIL‐7, in the presence or absence of TGF‐β. We used flow cytometric analysis to gate on cells with naive (CD45RA+CD27+), central memory (CM; CD45RA−CD27+), and effector memory (EM; CD45RA−CD27−) phenotypes 7 d after IL‐7 stimulation and assessed the percentage of each population that had diluted CFSE tracking dye (%CFSElow) as a measure of cellular proliferation ( Fig. 1 ). The median percentage of TGF‐β‐mediated inhibition of proliferation was 50 and 62% in CM and EM CD4+ T cells, respectively. In contrast, TGF‐β did not inhibit naive T cell proliferation. Expression of CD45RA and CD27 on CD4+ T cell subsets did not appear to change during these cultures, given that stimulation with IL‐7 in the presence or absence of TGF‐β did not markedly alter the proportions of naive, CM, or EM cells compared with unstimulated cells (data not shown). To further characterize the inhibitory effect of TGF‐β on memory CD4+ T cell proliferation, we used FlowJo analytical software (FlowJo, LLC, Ashland, OR, USA) to calculate the proportion of precursor cells that divided at least once (percentage divided) and the average number of cell divisions among the divided cells (proliferation index). Compared with cells stimulated with IL‐7 alone, cells stimulated with IL‐7+TGF‐β displayed significantly reduced percentage divided indices (CM and EM cells) and significantly reduced proliferation indices (EM cells only; Supplemental Fig. 1). These data suggest that TGF‐β limits both the efficiency of initial cell cycle progression and the capacity of proliferating cells to undergo multiple rounds of division in memory CD4+ T cells.
Figure 1.
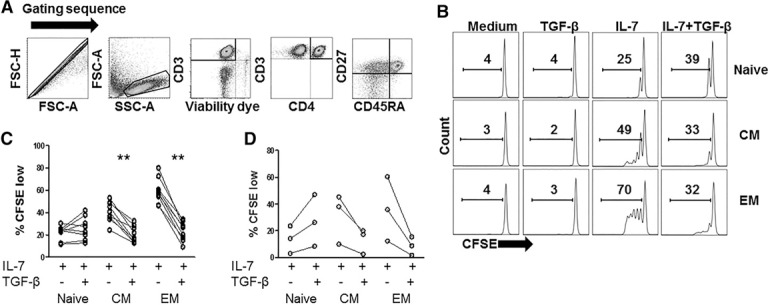
TGF‐β differentially affects naive and memory CD4+ T cell proliferation that is induced by IL‐7. (A–C) PBMCs were labeled with CFSE and incubated with rIL‐7 (5 ng/ml) in the presence or absence of rTGF‐β1 (5 ng/ml). After 7 d, cells were analyzed by flow cytometry for proliferation or CFSE dilution as measured by %CFSElow. (A) The gating sequence is provided. Doublets were excluded from analysis by the forward scatter area (FSC‐A) and forward scatter height (FSC‐H) gate, lymphocytes were identified by forward and side scatter, CD3+ cells that were viability dye low were selected, CD3+CD4+ cells were then selected and further divided into naive (CD45RA+CD27+), CM (CD45RA−CD27+), and EM (CD45RA−CD27−) subset. (B) The representative data show %CFSElow in CD4 T cell maturation subsets (naive, CM, EM). (C) Summary data show %CFSElow in CD4 T cell maturation subsets (n = 8). (D) Purified naive or memory CD4+ T cells were labeled with CFSE and stimulated with rIL‐7 (10 ng/ml) in the presence or absence of rTGF‐β1 (5 ng/ml). After 9 d, cells were analyzed by flow cytometry for %CFSElow. Data show summary data from 3 different donors. Proliferation of T cells incubated in medium alone or TGF‐β alone was consistently low (<2%; not shown). Significant differences were determined by Wilcoxon matched‐pairs signed rank test.
To determine whether the inhibitory effect of TGF‐β on IL‐7‐driven memory CD4+ T cell proliferation was related to a direct effect on T cells, we assessed the effects of TGF‐β on IL‐7‐induced proliferation using negatively selected, purified naive, or memory CD4+ T cells. Cell purity reached a minimum of 98.5 and 99.1% for naive (CD4+CD45RA+CD27+) and memory (CD4+CD45RA−) T cells, respectively. We found that TGF‐β inhibited proliferation in purified CD45RA− memory T cells that were further defined by expression of CD27+ (CM cells) and CD27− (EM cells) (Fig. 1D). In contrast, TGF‐β did not inhibit proliferation of purified naive T cells stimulated with rIL‐7.
TGF‐β suppresses IL‐7‐mediated induction of c‐myc expression in naive and memory CD4+ T cells
In keratinocytes and epithelial cells, TGF‐β has been found to inhibit cell proliferation in an Smad‐3‐dependent manner by suppressing c‐myc transcription [21]. To test whether TGF‐β inhibits IL‐7‐induced c‐myc expression in T cells, we stimulated PBMCs with IL‐7 ± TGF‐β and measured intracellular c‐myc protein by flow cytometry. In preliminary studies, we found evidence that IL‐7 mediated up‐regulation of c‐myc expression in CD4+ T cells within 3–5 d of stimulation (data not shown). At poststimulation day 5, IL‐7 treatment caused some cells to increase in size (defined by increased forward scatter by flow cytometry) in the CD4+ T cell maturation subsets, corresponding to increased expression of c‐myc. Both IL‐7‐mediated c‐myc up‐regulation and increased cell size were diminished by TGF‐β treatment in naive and memory CD4+ T cell subsets ( Fig. 2A and B ). The magnitude of c‐myc inhibition was compared among the T cell subsets at days 3 and 5 after stimulation with IL‐7+TGF‐β . Whereas similar levels of inhibition were noted at 3 d post stimulation among the T cell subsets, naive T cells tended to display a lesser degree of TGF‐β‐mediated inhibition at poststimulation 5 d, compared to memory cells and particularly compared with EM cells. This finding suggests that the inhibitory effect of TGF‐β on c‐myc expression diminished more substantially over time in the naive T cell subset compared with the memory subsets (Fig. 2C). Nonetheless, TGF‐β inhibited induction of IL‐7‐induced c‐myc expression in all T cell subsets.
Figure 2.
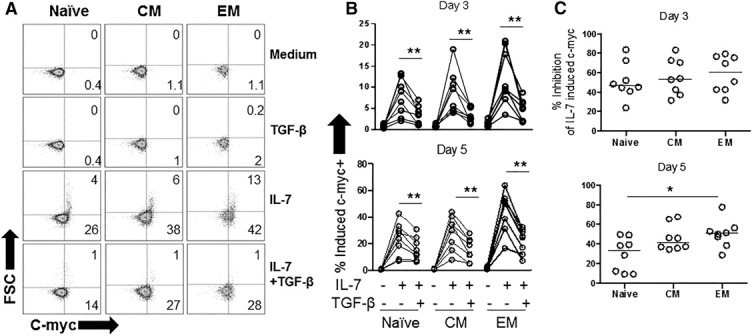
TGF‐β inhibits IL‐7‐mediated induction of c‐myc expression in naive and memory CD4+ T cells. PBMCs were incubated with rIL‐7 (5 ng/ml) in the presence or absence of rTGF‐β1 (5 ng/ml). After 3 or 5 d, cells were analyzed by flow cytometry for intracellular c‐myc expression. (A) Representative graph showing relative cell size as measured by forward scatter (FSC) vs. c‐myc expression in CD4 maturation subsets at day 5. (B) Summary data show c‐myc expression of CD4 T cell maturation subsets after 3 and 5 d of stimulation. (C) Summary data of TGF‐β‐mediated inhibition of IL‐7‐induced c‐myc expression in CD4 T cell maturation subsets after 3 and 5 d after stimulation. Percentage inhibition of IL‐7‐induced c‐myc was calculated as the difference between c‐myc expression in IL‐7‐stimulated cells and IL‐7+TGF‐β‐stimulated cells, divided by c‐myc expression in IL‐7‐stimulated cells × 100. Significant difference between percentage c‐myc+ in rIL‐7 in the presence or absence of rTGF‐β1 was assessed by Wilcoxon matched‐pairs signed rank test. Significant differences in percentage of TGF‐β‐mediated inhibition of IL‐7‐induced c‐myc expression between CD4+ T cells subsets were assessed by Dunn's multiple‐comparison test.
TGF‐β does not inhibit IL‐7 receptor signaling in memory CD4+ T cells and enhances S6 kinase signaling in naive CD4+ T cells
We and others have shown that p‐Akt signaling in response to IL‐7 is delayed and important to proliferation [31, 32]. Phosphorylation of S6 is downstream of p‐Akt and promotes proliferation by increasing protein translation [33]. TGF‐β has been shown to inhibit S6 kinase phosphorylation and activity in TCR‐activated CD4+ T cells [19]. To further examine the mechanism for the differential effects of TGF‐β on proliferation in response to IL‐7 between the CD4+ T cell maturation subsets, we assessed the effects of TGF‐β on the induction of p‐STAT‐5 and p‐S6 in response to IL‐7 at poststimulation 3 d. This time point was chosen based on our previous experience with IL‐7‐induced p‐Akt signaling that becomes detectable by flow cytometry 2–3 d after IL‐7 stimulation [32] ( Fig. 3A ). TGF‐β did not have a consistent effect on p‐STAT‐5, p‐Akt, or p‐S6 in memory CD4+ T cells, but unexpectedly caused a significant enhancement of p‐S6 expression in naive T cells stimulated by IL‐7. The capacity of TGF‐β to enhance S6 signaling in naive T cells may have been independent of Akt, given that we did not see a consistent effect of TGF‐β on Akt signaling in any T cell subset (Fig. 3B). Overall, TGF‐β had no discernible effects in memory T cell subsets but enhanced p‐S6 signaling in naive T cells.
Figure 3.
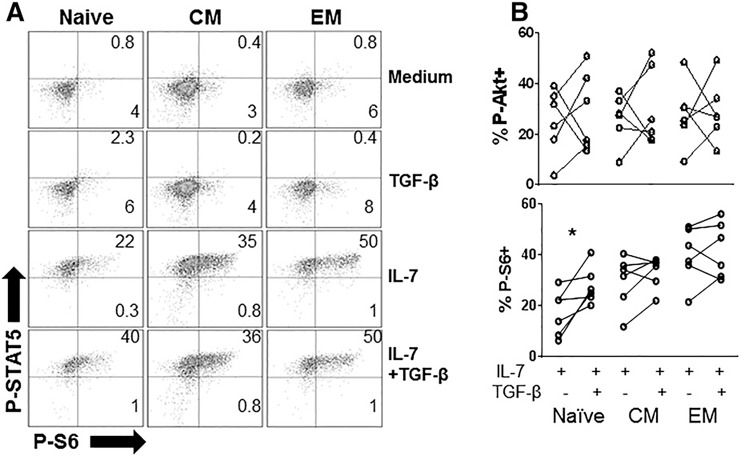
TGF‐β does not inhibit IL‐7 receptor signaling in memory CD4+ T cells and enhances S6 phosphorylation in naive CD4+ T cells. PBMCs were incubated with rIL‐7 (5 ng/ml) in the presence or absence of rTGF‐β1 (5 ng/ml), rTGF‐β1 (5 ng/ml) alone, or medium alone. After 3 d, cells were analyzed by flow cytometry for p‐S6. (A) Cells incubated in medium alone were used to set quadrants. Expression of p‐STAT‐5 and p‐S6 in naive, CM, and EM CD4+ T cells is shown in each indicated condition 3 d after stimulation. (B) Summary data comparing p‐Akt and p‐S6 induction in T cell subsets 3 d after stimulation with rIL‐7 or with rIL‐7+rTGF‐β1. Significant difference between rIL‐7 in the presence or absence of rTGF‐β1 was assessed by Wilcoxon matched‐pairs signed rank test.
We considered the possibility that TGF‐β may enhance CD127 expression in naive T cells as a means to increase S6 signaling in these cells. Using PBMCs from 3 donors, we evaluated CD127 receptor expression in CD4+ T cell subsets that had been incubated for 3 d in the presence of IL‐7±TGF‐β. Compared to cells incubated in medium alone, CD127 cell surface density was not affected by incubating cells in TGF‐β but was downregulated in cells incubated with IL‐7. The addition of TGF‐β to IL‐7‐stimulated cells tended to cause further loss of CD127 expression (Supplemental Fig. 2). Notably, we found that IL‐7 receptor occupancy, resulting from treating freshly isolated PBMCs with high concentrations of rIL‐7 (75 ng/ml) for 1 h on ice, had little effect on CD127 detection, suggesting that the loss of CD127 observed in these studies was not simply due to receptor occupancy that interfered with antibody–receptor interaction (data not shown). Thus, TGF‐β did not appear to enhance CD127 cell surface expression, either alone or in combination with IL‐7 in any CD4+ T cell subset.
GSK‐3 inactivation partially overcomes TGF‐β‐mediated inhibition of IL‐7‐induced proliferation and c‐myc induction in memory CD4+ T cells
P‐Akt phosphorylates GSK‐3, resulting in its inactivation in response to IL‐7 stimulation. Since GSK‐3 constitutively promotes c‐myc degradation, inactivated GSK‐3 results in increased c‐myc expression [35]. Therefore, we asked if a chemical inhibitor of GSK‐3 might overcome TGF‐β‐mediated inhibition of T cell proliferation and c‐myc suppression in IL‐7‐stimulated memory T cells. CHIR‐99021 (CH) is a highly specific small molecular inhibitor of GSK‐3α and ‐3β isoforms, which inactivates GSK‐3 by competitive ATP binding [38]. We assessed T cell proliferation percentage of CFSElow cells) in response to IL‐7 ± TGF‐β, in the presence or absence of CH. The addition of CH to the PBMCs resulted in modest increases in memory T cell proliferation compared to cells incubated in medium alone, and CH also increased proliferation in cells stimulated with IL‐7 ( Fig. 4A ). The addition of CH to cells treated with IL‐7+TGF‐β resulted in proliferation responses that were greater than those observed in cells treated with IL‐7+TGF‐β and in the case of CM cells, similar to the responses observed in T cells stimulated with IL‐7 alone (Fig. 4A and B). Thus, CH largely overcame the TGF‐β‐mediated inhibition of IL‐7‐induced proliferation in the CM CD4+ T cell subset and partially overcame the inhibition of proliferation in the EM CD4 T cell subset (Fig. 4A and B). Similarly, CH reversed the TGF‐β‐mediated inhibition of c‐myc expression in CM CD4+ T cells and partially reversed inhibition EM CD4+ T cells at days 3 and 5 following IL‐7 stimulation (Fig. 4C). In comparison to cells stimulated with rIL‐7 and TGF‐β, cells stimulated with IL‐7+TGF‐β+CH displayed significantly improved proliferation responses measured by percentage of divided cells within the CM subset (Fig. 4D). A tendency toward improvement in proliferation indices also was noted in memory subsets (comparing IL‐7+TGF‐β to IL‐7+TGF‐β+CH) although this effect did not reach statistical significance (Supplemental Fig. 3). Notably, proliferation of T cells stimulated with CH GF‐β+IL‐7 was consistently lower than proliferation of cells stimulated with CH+IL‐7 (Fig. 4A and data not shown), suggesting that some antiproliferative effects of TGF‐β remained in the presence of CH.
Figure 4.
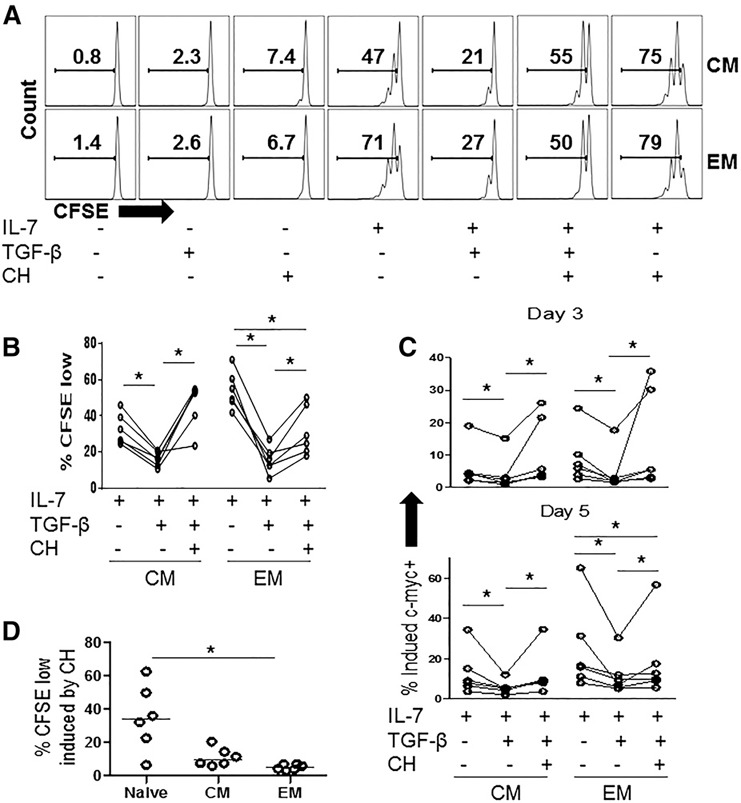
TGF‐β‐mediated inhibition of IL‐7‐induced proliferation and c‐myc expression in memory CD4+ T cells is reversed by the inactivation of GSK‐3. PBMCs were labeled with CFSE and were incubated in medium alone or with rIL‐7(5 ng/ml), rTGF‐β1 (5 ng/ml), CH (1 µM) or the indicated combinations of these treatments. After 7 d, cells were analyzed by flow cytometry. (A) Representative graphs from a single donor showing CFSE dye dilution of CM and EM cells identified by flow cytometry and (B) summary data showing %CFSElow in CD4+ T cell maturation subsets following stimulation with rIL‐7 ± TGF‐β ± CH. (C) Additional PBMCs were stimulated in the same conditions and intracellular c‐myc expression was assessed by flow cytometry. Graphs show expression of c‐myc after days 3 and 5 after stimulation. Significant differences were assessed by Wilcoxon matched‐pairs signed rank test. (D) %CFSElow cells are shown for PBMCs that were incubated in medium+CH inhibitor. Each symbol represents cells from a different donor.
Although the purpose of the above experiments was to determine the effect of the GSK‐3 inhibitor, CH, on memory T cells, we also found striking effects of the inhibitor in naive T cells. For example, at the concentration of CH (1 μM) used to overcome TGF‐β inhibition in memory T cells, we found that CH alone markedly induced naive T cell proliferation (median percentage of CFSElow cells = 34% in CH‐treated PBMCs after 7 d; n = 6; Fig. 4D). Moreover, the addition of CH to IL‐7‐stimulated PBMCs induced marked proliferation in naive T cells that far exceeded the proliferation observed in cells stimulated with IL‐7 alone (medium percentage of CFSElow = 18% and 86%, for naive T cells stimulated with IL‐7 or IL‐7+CH, respectively, n = 6; P = 0.0313). These observations suggest that GSK‐3 plays a more critical role in regulating proliferation in naive T cells than memory T cells.
DISCUSSION
Differential effects of TGF‐β on naive and memory CD4 T cell proliferation has been described previously in T cells activated by anti‐CD3 plus IL‐2. In these studies, TGF‐β inhibited memory, but enhanced naive TCR‐driven proliferation, in the presence of IL‐2 but not in the presence of IL‐7. The TGF‐β‐mediated enhancement of proliferation in naive CD4+ T cells was associated with increased IL‐2Rα (CD25) expression [39]. Our data also point to differences in the effects of TGF‐β on proliferation in naive and memory T cells. Our studies did not include TCR stimulation and centered instead on the direct effects of TGF‐β on T cell proliferation in response to the homeostatic cytokine IL‐7. We find that TGF‐β impairs memory but not naive CD4+ T cell responses to IL‐7 stimulation.
One limitation of our study was that we did not include analyses of Treg cells, which may have either naive or memory phenotype and may respond differently to either TGF‐β or IL‐7 stimulation compared to conventional T cells. TGF‐β induces FOXP3 expression and mediates Treg cell differentiation T cell cultures [40, 41]. Studies in TGF‐β and ‐βRII knockout mice suggest that TGF‐β has an important role for Treg cell homeostasis and maintenance [42, 43]. Thus, it is possible that TGF‐β could affect Treg in a manner that is distinct from conventional T cells. Moreover, although human Tregs have limited CD127 expression, these cells are still able to mount responses to rIL‐7 stimulation in vitro [44] and recent data suggest that IL‐7 may play a role in thymus‐independent maintenance of naive Tregs in persons experiencing thymectomy [45]. Thus, further studies that explore the possible interactions of TGF‐β and IL‐7 in naive and memory Treg cell homeostasis may uncover additional complexities.
Because TGF‐β inhibits c‐myc expression in epithelial cells [21], we speculated that a similar effect may occur in T cells. We further hypothesized that inhibition of T cell proliferation would correspond to inhibition of c‐myc expression and therefore may be more pronounced in memory CD4+ T cells than in naive CD4+ T cells. Our data demonstrate that the TGF‐β mediates inhibition of c‐myc expression in both memory and naive T cells; however, it only impairs proliferation of memory CD4+ T cells. The reason behind this observation is unclear but may be related to the TGF‐β‐mediated increase in p‐S6 induction in response to IL‐7 in naive but not memory CD4+ T cells (Fig. 3). Enhancement of IL‐7‐induced p‐S6 by TGF‐β in naive T cells may enhance global protein translation, perhaps compensating in part for lower c‐myc expression. Overall, these observations suggest that TGF‐β can exert stimulatory and inhibitory effects on naive CD4+ T cells, while primarily delivering inhibitory signals to memory CD4+ T cells.
TGF‐β did not have consistent effects on IL‐7 receptor signaling measured by p‐STAT‐5, p‐Akt, or p‐S6 in memory T cell subsets and no discernible effects on p‐Akt or p‐STAT‐5 in naive T cells. These findings are consistent with reports that TGF‐β does not impair JAK/STAT signaling [26]. We found unexpected evidence that TGF‐β could enhance IL‐7‐induced p‐S6 signaling in naive T cells. The mechanism underlining this effect of TGF‐β is uncertain but may be related to the reported role of TGF‐β in enhancing CD127 expression in naive T cells [27]. Nonetheless, in preliminary experiments, we did not find consistent evidence of higher CD127 expression in naive T cells incubated with TGF‐β compared to unstimulated cells (data not shown), and we found no evidence of higher CD127 expression in cells incubated with TGF‐β+IL‐7 compared to cells incubated with IL‐7 alone (Supplemental Fig. 2). Also, we did not find a consistent enhancement of p‐Akt signaling or p‐STAT‐5 signaling in naive T cells exposed to IL‐7+TGF‐β compared with IL‐7‐stimulated cells alone. These observations suggest that the effects of TGF‐β on p‐S6 signaling in naive T cells may occur independently of effects on CD127 expression. Further studies with more detailed kinetic and dose–response analyses of CD127 expression and signaling pathway activation may ultimately help to discern the mechanism behind this effect.
The importance of IL‐7 and TGF‐β in maintaining T cell homeostasis have been highlighted in mouse models, where IL‐7 deficiency results in T cell lymphopenia [28], whereas loss of TGF‐βR results in T cell hyperactivation [8, 9]. Moreover, in mice, TGF‐β plays an important role in enhancing naive T cell survival by sustaining CD127 expression. This effect involved suppression of Gfi‐1, a regulatory factor that reduces CD127 transcription [27]. Thus, although recognized for its immunosuppressive activities, there is a growing appreciation that TGF‐β plays an important role in sustaining the number of naive T cell in vivo.
The interplay between IL‐7 and TGF‐β may also be important in inflamed lymph node microenvironments, such as those observed in HIV disease. Memory and naive CD4+ T cells are exposed to IL‐7 expressed by stromal cells in reticular networks within the lymph node [46, 47–48], and TGF‐β may be secreted from activated or regulatory T cells during HIV infection [2, 49] [46]. Thus, memory CD4+ T cells that rely on IL‐7 to proliferate and maintain homeostasis could be inhibited by TGF‐β exposure in T cell zones within lymph nodes. Similar interactions may occur in mucosal microenvironments where IL‐7 is produced by epithelial cells [50] and TGF‐β may be produced by various cells [51]. It is likely that TGF‐β and IL‐7 interactions influence homeostasis of other cell types that rely on IL‐7, such as γ/δ T cells [52] in the mucosa and that these interactions could also be modified in the context of inflammatory conditions.
We show here that chemical inhibition of GSK‐3 can at least partially overcome TGF‐β‐mediated inhibition of IL‐7‐induced memory T cell proliferation. TGF‐β causes downregulation of c‐myc expression via Smad‐dependent transcriptional suppression, whereas GSK‐3 mediates degradation of c‐myc protein [21, 53, 54] Therefore, we speculate that GSK‐3 inhibition likely overcomes TGF‐β inhibition by prolonging the half‐life of c‐myc protein, thereby, compensating for diminished transcriptional production. An alternative, and not mutually exclusive hypothesis, is that GSK‐3 inhibition may affect CD98/SLCA5 expression, leading to increased amino acid uptake, which in turn, provides a mechanism to boost protein synthesis and c‐myc translation [55]. The latter has been implicated as a mechanism to enhance c‐myc expression in mouse effector CTLs maintained in cell culture with rIL‐2, although notably, IL‐7 appeared to be far less effective at sustaining c‐myc expression in these experimental conditions [56].
It is also striking that chemical inhibition of GSK‐3 was sufficient to induce T cell proliferation and that the effects were most pronounced among naive T cells. These data will need further study to fully understand the underlying mechanisms, and it will be interesting to explore the possibility that inhibition or disruption of GSK‐3 expression in vivo may have similar effects. Taken together, our observations suggest that the role of GSK‐3 in regulating IL‐7 proliferation responses in T cells is likely to change as cells mature, with the most substantial effects being observed in less mature cells (naive > CM > EM). These data are consistent with previous studies indicating that CD28‐mediated control of GSK‐3 is more important in naive than in memory T cells that proliferate in response to TCR triggering. Our studies also raise the intriguing possibility that targeting GSK‐3 for inhibition in vivo is a strategy to at least partially circumvent TGF‐β‐mediated inhibition of memory T cells. This strategy may be desirable, for example, to enhance T cell responses to tumors or pathogens. It may be limited, however, by the potential for TGF‐β to enhance production of other immunosuppressive cytokines such as IL‐10 in memory T cells [57].
Overall, our studies uncover novel interactions of TGF‐β and IL‐7 that have distinct implications for naive and memory T cell proliferation and homeostasis. Our studies with human T cells are consistent with studies in mice, suggesting that TGF‐β plays an important role in maintaining naive T cell homeostasis while limiting memory T cell expansion under steady state conditions. Understanding the molecular mechanisms that account for these differential outcomes may ultimately be important for designing intervention strategies that support T cell recovery from lymphopenia while inhibiting T cell hyperactivation in vivo.
AUTHORSHIP
T.N. performed experiments and analyzed data; S.S. and T.N. designed studies and wrote the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grant AI‐36219 (the CRWU Center for Aids Research).
REFERENCES
- 1. Letterio, J. J. , Roberts, A. B. (1998) Regulation of immune responses by TGF‐beta. Annu. Rev. Immunol. 16, 137–161. [DOI] [PubMed] [Google Scholar]
- 2. Kehrl, J. H. , Wakefield, L. M. , Roberts, A. B. , Jakowlew, S. , Alvarez‐Mon, M. , Derynck, R. , Sporn, M. B. , Fauci, A. S. (1986) Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brabletz, T. , Pfeuffer, I. , Schorr, E. , Siebelt, F. , Wirth, T. , Serfling, E. (1993) Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin‐2 gene in T cells through a noncanonical octamer‐binding site. Mol. Cell. Biol. 13, 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshimura, A. , Muto, G. (2011) TGF‐β function in immune suppression. Curr. Top. Microbiol. Immunol. 350, 127–147. [DOI] [PubMed] [Google Scholar]
- 5. Kuruvilla, A. P. , Shah, R. , Hochwald, G. M. , Liggitt, H. D. , Palladino, M. A. , Thorbecke, G. J. (1991) Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc. Natl. Acad. Sci. USA 88, 2918–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Racke, M. K. , Cannella, B. , Albert, P. , Sporn, M. , Raine, C. S. , McFarlin, D. E. (1992) Evidence of endogenous regulatory function of transforming growth factor‐beta 1 in experimental allergic encephalomyelitis. Int. Immunol. 4, 615–620. [DOI] [PubMed] [Google Scholar]
- 7. Miller, A. , Lider, O. , Roberts, A. B. , Sporn, M. B. , Weiner, H. L. (1992) Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen‐specific triggering. Proc. Natl. Acad. Sci. USA 89, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kulkarni, A. B. , Huh, C. G. , Becker, D. , Geiser, A. , Lyght, M. , Flanders, K. C. , Roberts, A. B. , Sporn, M. B. , Ward, J. M. , Karlsson, S. (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90, 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shull, M. M. , Ormsby, I. , Kier, A. B. , Pawlowski, S. , Diebold, R. J. , Yin, M. , Allen, R. , Sidman, C. , Proetzel, G. , Calvin, D. , et al. (1992) Targeted disruption of the mouse transforming growth factor‐beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin, D. L. , Postan, M. , Lucas, P. , Gress, R. , Tarleton, R. L. (2007) TGF‐beta regulates pathology but not tissue CD8+ T cell dysfunction during experimental Trypanosoma cruzi infection. Eur. J. Immunol. 37, 2764–2771. [DOI] [PubMed] [Google Scholar]
- 11. Barral, A. , Teixeira, M. , Reis, P. , Vinhas, V. , Costa, J. , Lessa, H. , Bittencourt, A. L. , Reed, S. , Carvalho, E. M. , Barral‐Netto, M. (1995) Transforming growth factor‐beta in human cutaneous leishmaniasis. Am. J. Pathol. 147, 947–954. [PMC free article] [PubMed] [Google Scholar]
- 12. Yang, L. , Pang, Y. , Moses, H. L. (2010) TGF‐beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmierer, B. , Hill, C. S. (2007) TGFbeta‐SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 8, 970–982. [DOI] [PubMed] [Google Scholar]
- 14. Feng, X. H. , Derynck, R. (2005) Specificity and versatility in tgf‐beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693. [DOI] [PubMed] [Google Scholar]
- 15. Massagué, J. , Gomis, R. R. (2006) The logic of TGFbeta signaling. FEBS Lett. 580, 2811–2820. [DOI] [PubMed] [Google Scholar]
- 16. Mizutani, A. , Koinuma, D. , Tsutsumi, S. , Kamimura, N. , Morikawa, M. , Suzuki, H. I. , Imamura, T. , Miyazono, K. , Aburatani, H. (2011) Cell type‐specific target selection by combinatorial binding of Smad2/3 proteins and hepatocyte nuclear factor 4alpha in HepG2 cells. J. Biol. Chem. 286, 29848–29860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mullen, A. C. , Orlando, D. A. , Newman, J. J. , Lovén, J. , Kumar, R. M. , Bilodeau, S. , Reddy, J. , Guenther, M. G. , DeKoter, R. P. , Young, R. A. (2011) Master transcription factors determine cell‐type‐specific responses to TGF‐β signaling. Cell 147, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Massagué, J. (2012) TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delisle, J. S. , Giroux, M. , Boucher, G. , Landry, J. R. , Hardy, M. P. , Lemieux, S. , Jones, R. G. , Wilhelm, B. T. , Perreault, C. (2013) The TGF‐β‐Smad3 pathway inhibits CD28‐dependent cell growth and proliferation of CD4+ T cells. Genes Immun. 14, 115–126. [DOI] [PubMed] [Google Scholar]
- 20. Siegel, P. M. , Massagué, J. (2003) Cytostatic and apoptotic actions of TGF‐beta in homeostasis and cancer. Nat. Rev. Cancer 3, 807–821. [DOI] [PubMed] [Google Scholar]
- 21. Frederick, J. P. , Liberati, N. T. , Waddell, D. S. , Shi, Y. , Wang, X. F. (2004) Transforming growth factor beta‐mediated transcriptional repression of c‐myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol. Cell. Biol. 24, 2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen, C. R. , Kang, Y. , Siegel, P. M. , Massagué, J. (2002) E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c‐myc repression. Cell 110, 19–32. [DOI] [PubMed] [Google Scholar]
- 23. Heikkila, R. , Schwab, G. , Wickstrom, E. , Loke, S. L. , Pluznik, D. H. , Watt, R. , Neckers, L. M. (1987) A c‐myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature 328, 445–449. [DOI] [PubMed] [Google Scholar]
- 24. Harel‐Bellan, A. , Ferris, D. K. , Vinocour, M. , Holt, J. T. , Farrar, W. L. (1988) Specific inhibition of c‐myc protein biosynthesis using an antisense synthetic deoxy‐oligonucleotide in human T lymphocytes. J. Immunol. 140, 2431–2435. [PubMed] [Google Scholar]
- 25. Bright, J. J. , Sriram, S. (1998) TGF‐beta inhibits IL‐12‐induced activation of Jak‐STAT pathway in T lymphocytes. J. Immunol. 161, 1772–1777. [PubMed] [Google Scholar]
- 26. Sudarshan, C. , Galon, J. , Zhou, Y. , O'Shea, J. J. (1999) TGF‐beta does not inhibit IL‐12‐ and IL‐2‐induced activation of Janus kinases and STATs. J. Immunol. 162, 2974–2981. [PubMed] [Google Scholar]
- 27. Ouyang, W. , Oh, S. A. , Ma, Q. , Bivona, M. R. , Zhu, J. , Li, M. O. (2013) TGF‐β cytokine signaling promotes CD8+ T cell development and low‐affinity CD4+ T cell homeostasis by regulation of interleukin‐7 receptor a expression. Immunity 39, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schluns, K. S. , Kieper, W. C. , Jameson, S. C. , Lefrançois, L. (2000) Interleukin‐7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432. [DOI] [PubMed] [Google Scholar]
- 29. Lai, S. Y. , Molden, J. , Goldsmith, M. A. (1997) Shared gamma(c) subunit within the human interleukin‐7 receptor complex: a molecular basis for the pathogenesis of X‐linked severe combined immunodeficiency. J. Clin. Invest. 99, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofmeister, R. , Khaled, A. R. , Benbernou, N. , Rajnavolgyi, E. , Muegge, K. , Durum, S. K. (1999) Interleukin‐7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 10, 41–60. [DOI] [PubMed] [Google Scholar]
- 31. Dadi, H. , Ke, S. , Roifman, C. M. (1994) Activation of phosphatidylinositol‐3 kinase by ligation of the interleukin‐7 receptor is dependent on protein tyrosine kinase activity. Blood 84, 1579–1586. [PubMed] [Google Scholar]
- 32. Nguyen, T. P. , Bazdar, D. A. , Mudd, J. C. , Lederman, M. M. , Harding, C. V. , Hardy, G. A. , Sieg, S. F. (2015) Interferon‐α inhibits CD4+ T cell responses to interleukin‐7 and interleukin‐2 and selectively interferes with Akt signaling. J. Leukoc. Biol. 97, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takada, K. , Jameson, S. C. (2009) Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9, 823–832. [DOI] [PubMed] [Google Scholar]
- 34. Ruvinsky, I. , Sharon, N. , Lerer, T. , Cohen, H. , Stolovich‐Rain, M. , Nir, T. , Dor, Y. , Zisman, P. , Meyuhas, O. (2005) Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 19, 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barata, J. T. , Silva, A. , Brandao, J. G. , Nadler, L. M. , Cardoso, A. A. , Boussiotis, V. A. (2004) Activation of PI3K is indispensable for interleukin 7‐mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 200, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thorne, C. A. , Wichaidit, C. , Coster, A. D. , Posner, B. A. , Wu, L. F. , Altschuler, S. J. (2015) GSK‐3 modulates cellular responses to a broad spectrum of kinase inhibitors. Nat. Chem. Biol. 11, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gregory, M. A. , Qi, Y. , Hann, S. R. (2003) Phosphorylation by glycogen synthase kinase‐3 controls c‐myc proteolysis and subnuclear localization. J. Biol. Chem. 278, 51606–51612. [DOI] [PubMed] [Google Scholar]
- 38. Meijer, L. , Flajolet, M. , Greengard, P. (2004) Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 25, 471–480. [DOI] [PubMed] [Google Scholar]
- 39. De Jong, R. , van Lier, R. A. , Ruscetti, F. W. , Schmitt, C. , Debré, P. , Mossalayi, M. D. (1994) Differential effect of transforming growth factor‐beta 1 on the activation of human naive and memory CD4+ T lymphocytes. Int. Immunol. 6, 631–638. [DOI] [PubMed] [Google Scholar]
- 40. Chen, W. , Jin, W. , Hardegen, N. , Lei, K. J. , Li, L. , Marinos, N. , McGrady, G. , Wahl, S. M. (2003) Conversion of peripheral CD4+CD25‐ naive T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fantini, M. C. , Becker, C. , Monteleone, G. , Pallone, F. , Galle, P. R. , Neurath, M. F. (2004) Cutting edge: TGF‐beta induces a regulatory phenotype in CD4+CD2– T cells through Foxp3 induction and down‐regulation of Smad7. J. Immunol. 172, 5149–5153. [DOI] [PubMed] [Google Scholar]
- 42. Marie, J. C. , Letterio, J. J. , Gavin, M. , Rudensky, A. Y. (2005) TGF‐beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li, M. O. , Sanjabi, S. , Flavell, R. A. (2006) Transforming growth factor‐beta controls development, homeostasis, and tolerance of T cells by regulatory T cell‐dependent and ‐independent mechanisms. Immunity 25, 455–471. [DOI] [PubMed] [Google Scholar]
- 44. Dupont, G. , Demaret, J. , Venet, F. , Malergue, F. , Malcus, C. , Poitevin‐Later, F. , Morel, J. , Monneret, G. (2014) Comparative dose‐responses of recombinant human IL‐2 and IL‐7 on STAT5 phosphorylation in CD4+FOXP3– cells versus regulatory T cells: a whole blood perspective. Cytokine 69, 146–149. [DOI] [PubMed] [Google Scholar]
- 45. Silva, S. L. , Albuquerque, A. S. , Serra‐Caetano, A. , Foxall, R. B. , Pires, A. R. , Matoso, P. , Fernandes, S. M. , Ferreira, J. , Cheynier, R. , Victorino, R. M. , Caramalho, I. , Barata, J. T. , Sousa, A. E. (2016) Human naive regulatory T‐cells feature high steady‐state turnover and are maintained by IL‐7. Oncotarget 7, 12163–12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng, M. , Smith, A. J. , Wietgrefe, S. W. , Southern, P. J. , Schacker, T. W. , Reilly, C. S. , Estes, J. D. , Burton, G. F. , Silvestri, G. , Lifson, J. D. , Carlis, J. V. , Haase, A. T. (2011) Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV‐1 and SIV infections. J. Clin. Invest. 121, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deeks, S. G. (2012) HIV infection, lymphoid fibrosis, and disease. Blood 120, 1753–1754. [DOI] [PubMed] [Google Scholar]
- 48. MacLeod, M. K. , Kappler, J. W. , Marrack, P. (2010) Memory CD4+ T cells: generation, reactivation and re‐assignment. Immunology 130, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kekow, J. , Wachsman, W. , McCutchan, J. A. , Cronin, M. , Carson, D. A. , Lotz, M. (1990) Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA 87, 8321–8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watanabe, M. , Ueno, Y. , Yajima, T. , Iwao, Y. , Tsuchiya, M. , Ishikawa, H. , Aiso, S. , Hibi, T. , Ishii, H. (1995) Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95, 2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Konkel, J. E. , Chen, W. (2011) Balancing acts: the role of TGF‐b in the mucosal immune system. Trends Mol. Med. 17, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baccala, R. , Witherden, D. , Gonzalez‐Quintial, R. , Dummer, W. , Surh, C. D. , Havran, W. L. , Theofilopoulos, A. N. (2005) Gamma delta T cell homeostasis is controlled by IL‐7 and IL‐15 together with subset‐specific factors. J. Immunol. 174, 4606–4612. [DOI] [PubMed] [Google Scholar]
- 53. Yagi, K. , Furuhashi, M. , Aoki, H. , Goto, D. , Kuwano, H. , Sugamura, K. , Miyazono, K. , Kato, M. (2002) c‐myc is a downstream target of the Smad pathway. J. Biol. Chem. 277, 854–861. [DOI] [PubMed] [Google Scholar]
- 54. Stephen, T. L. , Rutkowski, M. R. , Allegrezza, M. J. , Perales‐Puchalt, A. , Tesone, A. J. , Svoronos, N. , Nguyen, J. M. , Sarmin, F. , Borowsky, M. E. , Tchou, J. , Conejo‐Garcia, J. R. (2014) Transforming growth factor β‐mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity 41, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sinclair, L. V. , Rolf, J. , Emslie, E. , Shi, Y. B. , Taylor, P. M. , Cantrell, D. A. (2013) Control of amino‐acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Preston, G. C. , Sinclair, L. V. , Kaskar, A. , Hukelmann, J. L. , Navarro, M. N. , Ferrero, I. , MacDonald, H. R. , Cowling, V. H. , Cantrell, D. A. (2015) Single cell tuning of Myc expression by antigen receptor signal strength and interleukin‐2 in T lymphocytes. EMBO J. 34, 2008–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garcia, C. A. , Benakanakere, M. R. , Alard, P. , Kosiewicz, M. M. , Kinane, D. F. , Martin, M. (2008) Antigenic experience dictates functional role of glycogen synthase kinase‐3 in human CD4+ T cell responses. J. Immunol. 181, 8363–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files


