Short abstract
Diminished IFN‐γ in the lungs of 21‐ day‐young (toddler) mice throughout infection corresponds to intrinsic rather than extrinsic CD4 T cell limitations in IFN‐γ transcription, indicating a limited CD4 T cell response in toddler age.
Keywords: pediatric immunology, cytokine responses, influenza virus
Abstract
Respiratory viral infections, such as influenza, can lead to delayed viral clearance in toddlers, possibly exacerbating disease morbidity. We hypothesized that defective CD4 T cells in toddlers may contribute to a failure to clear virus at a similar rate to adults. Thus, we developed a young mouse model to examine potential divergent responses between toddlers and adults. We determined that young mice (toddler mice, 21 d old) were actively generating and recruiting effector/memory T cells, whereas memory populations were firmly established in older, adult mice (8–10 wk old). We infected toddler and adult mice with influenza A/PR8/34 (H1N1) and found young mice had elevated morbidity, as measured by enhanced weight loss and lower partial pressure of oxygen levels, throughout the infection, thus, modeling the higher morbidity observed in children (<2 y old) during infection. Early viral loads were comparable to adult mice, but toddler mice failed to clear virus by 10 d postinfection. This delayed clearance corresponded to poor lung recruitment of CD4 T cells, lower antiviral T cell responses, and lower B cell/antibodies in the lungs. Mechanistically, diminished interferon‐γ was detected in the lungs of toddler mice throughout the infection and corresponded to intrinsic, rather than extrinsic, CD4 T cell limitations in interferon‐γ transcription. Moreover, defects in interferon‐γ production appeared downstream from signal transducer and activator of transcription 4 in the interleukin‐12 signaling pathway, suggesting maturational delays different from neonates. Importantly, recombinant interferon‐γ supplementation rescued CD4 T cell numbers in the lungs and influenza‐specific antibody formation. This study highlights the intrinsic limitations in CD4 T cell effector functions that may arise in toddlers and contribute to disease pathology.
Abbreviations
- ΔCt
comparative cycle threshold
- AM
adult mouse
- DC
dendritic cell
- fDC
follicular dendritic cell
- MFI
mean fluorescence intensity
- pO2
partial pressure of oxygen
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- rIFN‐γ
recombinant IFN‐γ
- TCID50
tissue culture‐infective dose 50
- TM
toddler mouse
Introduction
Influenza causes severe morbidity and mortality, with up to 15% of the global population infected yearly and one‐half a million people dying from complications from infection [1]. Young children (<4 y old) are especially susceptible to influenza infection, with toddlers (>6 mo old to <2 y old), frequently requiring hospitalization because of its severity [2, 3]. Moreover, 20,000 young children <5 y old [4] or 4 y old per 1000 toddlers [5] require yearly hospitalization because of influenza in the United States alone. Despite the availability of influenza vaccines, limited vaccination compliance (∼40% in the United States, CDC [6]), especially in the developing world; emergent or persistent strains, such as H1N1 2009 or H3N2v2; and suboptimal immunity in toddlers receiving their primary vaccinations [7, 8] continue to drive yearly infections (>15% mortality in children <5 y old in 2010) [9]. Although secondary bacterial infections are commonly associated with hospitalization, viral pneumonia alone accounts for approximately one third of all complications and deaths associated with influenza ([10]). While neonates (birth to 30 d old) may have Th2 T cell biases that relates to dysfunctional CD4 T cells and APCs [11, 12], the mechanisms responsible for increased influenza associated–morbidity in young children (hereafter, defined as those 6–24 mo), as compared to adults (<55 y old), are not fully defined. Moreover, although high morbidity in older adults is a function of immune senesce because of limited naïve T cell pools, the mechanisms associated with high morbidity in toddlers, with large naïve T cell pools, are not fully defined. Young children generate lower CD4 T cell responses to vaccines and pathogens than do older children or adults [13, 14, 15, 16, 17–18], but the mechanistic differences driving these lower responses are incompletely understood. In fact, unvaccinated young children can have diminished lung T cells responses [19, 20] and no detectable systemic CD4 T cell responses [21] to influenza during infection. By better understanding the mechanisms driving influenza pathogenesis and how host responses diverge in toddlers and adults, therapeutic targets may be identified that could diminish the morbidity in young children common during the many frequent respiratory viral infections in this population.
A limited cross‐protective pool of influenza‐specific memory, generated from seasonal exposure to influenza, may also explain why older children and adults do not exhibit higher morbidity during secondary infections [22] than younger children do, who are characterized by T cell pools dominated by influenza‐naïve cells. However, there are key immunologic maturational shifts in T cell types and numbers from neonates to toddlers to older children that could influence the antiviral responses [23]. T cells are associated with some protection from influenza [24], but the role of T cells in influenza protection is not well defined in young children outside of responses to flu vaccines, although we know they generate T cell responses that are quantitatively lower than vaccinated adults [25]. In addition, the immunologic maturity of the immune system, particularly APCs, may impair the capacity of the young immune system to respond to influenza infection allowing for greater inflammation and lung tissue damage, which, in turn, drives higher morbidity and mortality and initiates conditions favorable to secondary bacterial infection of the lungs in young children. Prior influenza studies were performed primarily on human neonates and neonatal mice [26], which may not translate to toddlers who have more‐mature immune responses. Therefore, the mechanisms driving higher morbidity in this population of children with respect to T cells function and as compared with adults remains to be determined.
To further define the differential host responses between adults and toddlers to influenza infection, we investigated the convergent and divergent molecular and cellular responses throughout infection using a murine model and concentrating on CD4 T cell function. Toddlers commonly have delayed viral clearance to many respiratory viral infections [27], and thus, we sought to determine what the effect of lower IFN‐γ generation by CD4 T cells has on antiviral responses in the lungs and what intrinsic mechanisms might be impairing IFN‐γ secretion. Although neonatal murine influenza infection demonstrated delayed T cell recruitment to the lungs [26], these mice started with limited T cells in the mucosa and had delayed T cell recruitment to the lungs (15 d postinfection), which would not be similar to human toddlers. Moreover, mice do not generate functional germinal centers until 14 d of age [14, 15], which diverges from human neonates (exhibit primitive germinal centers [28, 29]) and toddlers (early germinal centers are present in the prior infant stage [30]). Thus, we sought to use a toddler model to further explore mechanisms that could be driving delayed viral clearance in human toddlers. Our selection of the 21‐d‐old mouse (referred to as a TM) as a model for infection stems from multiple reasons: 1) TMs represent the point at which they are no longer breast‐fed, similar to children >6 mo old; 2) our study focuses on CD4 T cells that are generally impaired without competent B cells [31, 32], which occurs in mice <21 d old [13]; 3) neonatal mice exhibit similar APC and T cell dysfunctions as neonatal humans do [11, 12, 33] but are potentially unlike toddlers; and 4) neonatal mice have few T cells in the lungs [26] before infection, unlike human toddlers. Although we don't see much difference histologically between 21‐d and 28‐d‐old mice (with respect to germinal centers in spleens), the rapid aging of the mice during the course of the study would make starting infections in 28‐d‐old mice difficult because 38‐d‐old mice (age at 10 d postinfection) are much more immunologically mature than either 21‐ or 28‐d‐old mice (unpublished data). Thus, starting at 21‐d‐old and finishing examining the responses at or before 31‐d‐old would keep our TMs closer to human toddlers. However, we understand the rapid aging of TMs remains a limitation of this model because young children undergo immunologic maturation much more slowly, with clear differences in immune cell absolute counts and function with age. Nonetheless, we believe TMs model the physiological aspects and responses of toddlers better than do neonatal mice.
MATERIALS AND METHODS
Mice
The 8–10‐wk‐old, male and female, BALB/c or DO11.1 mice were purchased from the National Cancer Institute's Biologic Testing Branch (Frederick, MD, USA) or DO11.1 and BALB/c were bred and maintained under specific‐pathogen–free conditions and used at 21 d old. The Rochester General Hospital Institutional Animal Care and Use Committee approved all animal studies. Pulse oximetry (Star Life Sciences, Holliston MA, USA) was performed as previously described [34]. Mice were labeled by intraperitoneal injection (100 μl) with 10 mg/ml BrdU (Sigma‐Aldrich, St Louis, MO, USA) at day −3 and day 0 postinfection.
Influenza virus infection
Influenza virus (A/PR/8/34: PR8) was grown in the allantoic fluid of 10‐d‐old, embryonated chicken eggs (Charles River Laboratories, Kingston, NY, USA) as previously described [35]. Determination of influenza viral titers in 100mg lung homogenates was accomplished by the TCID50 assay as described previously [36], with titers expressed as the reciprocal of the dilution of lung extract that corresponds to 50% virus growth in Madin‐Darby canine kidney cells or calculated by the Reed Muench method. For in vivo infection, mice were anesthetized with isoflurane and 20–40 μl of PR8 influenza virus containing 250 TCID50 (TMs) or 500 TCID50 (adults) was administered intranasally. Influenza OVA [37] was also expanded and used for infection of mice, after autologous transfers of DO11.1 CD4 T cells, similar to PR8. All infected mice were housed in the biocontainment suite at the Rochester General Hospital animal facility or Iowa State University, where tissue harvest was also performed.
Histology
Lung tissue sections were harvested from formalin‐perfused lungs after vascular PBS perfusion, stored in formalin at 4°C, and H&E stain was performed by AML Laboratories (Baltimore, MD, USA). Histopathologic scores were calculated similar to prior studies by blinded reading. Pathologic scores were assigned similar to Belser et al. [38] by blinded review.
Abs and Reagents
Fluorochrome‐conjugated anti‐CD4, CD8, CD3, CD62L, CD27, CD44, CD45, CD95, B220, IFN‐γ, IL‐2, IL‐17A, IL‐5, CD19, pan‐DC, and TNF‐α, were obtained from BioLegend (San Diego, CA, USA). Anti‐BrdU was obtained from BD Biosciences (San Jose, CA, USA). Cells were surface stained with or without intracellularly staining with the fluorochrome‐conjugated Abs as previously described [39].
Cytokine assays
For intracellular cytokine staining analysis, CD4 T cells (106/well) were stimulated by PMA/ionomycin (Sigma‐Aldrich) for 6 h with brefeldin A (Sigma‐Aldrich) added in the last 5 h. Additional intracellular staining was done with culturing CD4 T cells in the presence of APCs (3:1 ratio), with anti‐CD28 and 10,000 TCID50 of influenza (heat‐inactivated) for 18 h, followed by brefeldin A for the last 5 h. The 23‐plex murine cytokine assays were performed with Luminex (Bio‐Rad Laboratories, Hercules, CA, USA) on lung homogenates from 50‐mg sections in cold PBS with 1% Igepals (Sigma‐Aldrich), generated by repeated grinding with a pestle. Lungs were clarified by centrifugation at 10,000 rpm for 30 s. Supernatants were transferred to new, cold eppendorf tubes and snap‐frozen in liquid nitrogen.
Cell Isolations
The 5 × 106 CD4 T cells from either young or adult DO11.1 mice were obtained from the spleens by negative selection (antibodies to CD8, MHCII, and GR‐1) and transferred by tail vein transfers into age‐matched BALB/c mice or reciprocally from toddler into adult, toddler into toddler, adult into toddler, or adult into AMs. Mice were infected with PR8 with OVA epitope [35]. DO11.1 T cells (KJ1‐26, biotin antibody; BioLegend) or APCs (MHCII, Bio X Cell, West Lebanon, NH, USA) were isolated by magnetic sorting (Polysciences, Warrington, PA, USA) at 6 d postinfection and transferred into RNAlater (Qiagen, Gaithersburg, MD, USA).
In vivo cytokine administration
Recombinant IFN‐γ was purchased from BioLegend, and 650 pg of cytokine was diluted into 100 μl of PBS and injected i.p. at 5 d postinfection. Mice received an additional i.p. injection of cytokine at 6 d postinfection in conjunction with 650 pg of cytokine by intranasal injection in 20–40 μl of PBS. Control mice received only the PBS vehicle.
Immunohistochemistry
Lungs were inflated with 25% OCT compound in PBS, dissected, and placed in OCT compound before freezing in cold isopentane. Then, 5‐µm sections were cut and fixed by cold acetone for 10 min. Sections were blocked with PBS/10% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 30 min. Endogenous peroxide was blocked by PBS containing 3% H2O2 for 30 min. CD3 staining was performed with purified anti‐CD3 obtained from Serotec (Raleigh, NC, USA), and sections were stained overnight at 1:50 dilution in PBS containing 1% donkey serum. Slides were washed with PBS containing 0.5% Triton X‐100 and anti‐goat biotin (Jackson ImmunoResearch) was added at 1:200 dilution followed by streptavidin HRP (Jackson ImmunoResearch) at 0.5 μg/ml. Slides were developed with 3,3′‐diaminobenzidine (KPL, Gaithersburg, MD, USA) for 10 min and counterstained with Contrast Green (KPL). Slides were photographed from young and AMs from the upper and lower lungs containing similar architecture, and the numbers of CD3+ cells were counted in 1‐mm2 grids using Photoshop (Adobe Systems, San Jose, CA, USA) from a minimum of 10 photographs for each lung. Influenza antigen staining was performed with influenza HA1–biotin antibodies obtained from Serotec. Lungs were blocked with PBS with 3% donkey serum. Slides from 3 and 7 d postinfected mice were prepared and stained with hemagglutinin antibodies overnight at 4°C in a 1:50 dilution of PBS containing 1% donkey serum. Slides were washed as previously described, and streptavidin‐Cy3 (Jackson ImmunoResearch) was used at 0.5 μg/ml in PBS with 1% donkey serum. Slides were counterstained with DAPI gold (Invitrogen, Carlsbad, CA, USA). Influenza receptor staining was performed with biotinylated Sambucus nigra agglutinin that was obtained from Vector Laboratories (Burlingame, CA, USA). Slides prepared from lungs of uninfected mice were blocked for endogenous biotin per manufacturer's instructions followed by the addition of 3% donkey serum. Agglutinin was diluted in PBS with 1% donkey serum at 1:50 and incubated overnight. Streptavidin conjugated to Cy3 was used, as previously described.
Flow cytometry
Cells were acquired using an LSR II flow cytometer (BD Biosciences) with a minimum acquisition of 300,000 events. BrdU was determined after surface staining followed by BrdU detected according to manufacturer's directions (BD Biosciences). Annexin V staining was determined according to manufacturer's directions (BD Biosciences). Additional cells were isolated and cultured at 37°C with FAM FLICA poly caspase according to manufacturer's directions (ImmunoChemistry Technologies, Bloomington, MN, USA). Analysis of acquisition events was accomplished using FlowJo software (Tree Star, Ashland, OR, USA) with gating using live amine dye (Invitrogen) and double discrimination.
Western blotting
Isolated CD4+ T cells were harvested from spleens at 6 d postinfection by magnetic isolation (Miltenyi Biotec, San Diego, CA, USA). Cells were snap‐frozen in liquid nitrogen and extracted in RIPA buffer containing protease inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). Cell extracts were run by electrophoresis, followed by transfer onto polyvinylidene difluoride membranes, blocked by 2% FBS in PBS, and probed with anti‐G3PDH and either p‐Zap70 or p‐STAT4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies (1:1000) overnight. Membranes were further stained with anti‐goat HRP (BioLegend), followed by color development using 3,3′‐diaminobenzidine substrate (KPL).
Real‐time RT‐PCR
RNA was extracted from tissues using RNeasy Plus (Qiagen). For detecting changes in gene expression in the influenza‐infected young and AMs, the RNA levels for each were compared with the levels in uninfected, young or AMs (calibrators), and data are presented as the change in expression of each gene. The ΔCt value for the tissue sample from the calibrator was then subtracted from the ΔCt value of the corresponding lung tissue of infected mice (ΔΔCt). The increase in cytokine mRNA levels in lung‐tissue samples of the infected animals compared with tissue samples of the baseline (calibrator) animals was then calculated as follows: increase = 2ΔΔCt. Lungs and spleens were isolated from uninfected mice and from 6‐d‐postinfected adult and young mice and stored in RNAlater. Superarray (Qiagen) qRT‐PCR was performed per the manufacturer's instructions using 260 ng of RNA extracted from lungs (inflammatory cytokine and receptors panel) or spleens (T cell/B cell activation panel). Data were analyzed with the RT2 Profiler PCR array analysis program (Qiagen) to calculate calibrated fold changes. Cytokine transcripts for GAPDH, ERM (ETV5), Jnk1, cJun, p38, MKK6, Tyk, IL‐12rb, STAT5, IL‐18r, IFN‐γ, CCR5, and IFN‐γR were performed on frozen CD4 T cells from 6 d postinfected mice using iScript One‐Step RT PCR with Sybergreen (Bio‐Rad) with 30 ng of RNA for each reaction. Data were calculated as the fold change of young mice above or below adult controls, with a 2‐fold cutoff representing a significant expression value. The target gene and the reference gene (GAPDH) were amplified with the same efficiency (data not shown). T bet or IFN‐γ transcripts were detected similar to that in mice, but calibration was performed using CD3 levels because young children generally have higher peripheral CD4 T cell counts than adults. Primers for each cytokine were designed with PrimerBank software [40].
Serum antibody titers
Serum was obtained from mice at 7 or 9 d postinfection from young mice with/without rIFN‐γ supplementation, with adult controls with no supplementation. End‐point titers were determined by ELISA using whole PR8 influenza virus plated in PBS on Immulon II plates (Thermo Fisher) overnight followed by blocking with 3% nonfat milk. Secondary anti‐mouse IgG AP (Jackson ImmunoResearch) was used at 1:10,000 in nonfat milk and incubated for 1 h at 37°C. Cutoff points were determined by 3 times the sd of the mean titer of the negative controls.
Statistical analysis
The 2‐tailed Student's t tests, the Mann‐Whitney U test, and 1‐way ANOVA were performed, with P < 0.05 considered significant.
RESULTS
Young mice are acquiring mucosal memory mirroring human toddlers
To assess differences in the systemic and mucosal immune system of TMs vs. AMs before infection, immunophenotyping of CD4 and CD8 T cells was performed on splenic and lung T cells. We found similar absolute counts for CD4 and CD8 T cells in the lungs and spleens of TMs and AMs ( Fig. 1A and B ). In contrast to AMs, we found higher levels of CD95 and B220 expression on CD4 T cells in the lungs of TMs, indicating recruitment of activated T cells to the lung mucosa (Supplemental Fig. 1A and B), and similar numbers (statistically insignificant) of B cells (CD3−CD45+B220+) in the spleens and lungs of AMs, as compared with TMs (Supplemental Fig. 1C).
Figure 1.
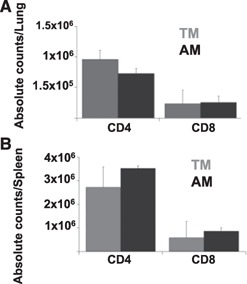
TMs rapidly acquire mucosal memory. We assessed the absolute numbers of CD4 and CD8 T cells in TMs and AMs residing in the lungs (A) and the spleens (B) of uninfected mice. Data shown represent means ± se. n = 3 for each group, 3 replicates each. Representative dot plots are shown.
Convergent viral kinetics but divergent viral distribution in TMs and AMs
Differences in influenza receptor expression in lungs of TMs and adults could dramatically affect influenza hemagglutinin binding/entry into lung epithelial cells and replication kinetics; therefore, we determined the level of expression in the lungs of α‐2,3–linked sialic acid (flu receptor in mice) in both groups of mice. We found that expression of this receptor was not significantly different in the lungs of TMs and AMs, as assessed by staining lung tissue with S. nigra agglutinin by immunohistochemistry (Supplemental Fig. 2). Specifically, heaviest staining patterns appeared around the bronchioles with more‐diffuse staining patterns spreading to the lungs.
Next, we infected TMs and AMs with 50, 125, 250, and 500 TCID50 to establish the sublethal dose in TMs. Dosages <100 TCID50 gave no productive infection in either TMs or AMs, as evidenced by no weight loss or signs of infection (labored breathing, changes in activity, etc). Here, we used 500 TCID50 to reproducibly ensure sublethal infections in AMs, similar to that used in other studies [34, 41, 42]. Although 250 TCID50 led to a LD50 of 0.1, the total body weight and lung weights of uninfected TMs was approximately 2‐fold less than that of adults, and thus, we used this dose for TMs to potentially infect similar numbers of lung epithelial cells.
To further examine differences in viral replication kinetics that could drive the observed higher morbidity in TMs, we assessed lung viral loads by TCID50 assay ( Fig. 2 ). We found similar peak lung viral burdens in TMs and AMs at 4 and 7 d postinfection. In contrast to adult mice, which cleared viral infection by 10–12 d postinfection, TMs still had virus in their lungs at 12 d postinfection and took longer to clear the virus.
Figure 2.
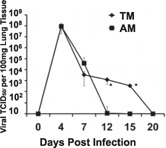
Delayed viral clearance in TMs mirrors human respiratory viral infection. We determined the viral lung burdens by TCID50 assay throughout infection. Data represent means ± se (n = 3 for each group, 3 replicates each; TMs, n = 6 at >10 d postinfection because of death). *P < 0.05
Enhanced morbidity during influenza infection is associated with higher histopathology in TMs
To determine the level of morbidity in TMs and AMs after infection, we assessed the level of weight loss and the pO2 levels with a murine pulse oximeter and compared those results with lung histopathology. TMs exhibited greater weight loss than AMs did by 5 d postinfection, which continued throughout the infection ( Fig. 3A ). In addition, TMs had significantly lower levels of pO2 at 7 d postinfection (Fig. 3B). In contrast to adults, we found evidence of thickening of airway epithelium as early as 3 d postinfection in TMs (Fig. 3C). By 7 d postinfection, TMs had higher levels of mononuclear recruitment to the lungs, particularly around the bronchioles that extended out into the surrounding alveolar spaces, whereas adults had moderate lung consolidation. By 10 d postinfection, the lungs of AMs mirrored the histology of uninfected adults, whereas TMs continued to exhibit mononuclear cell lung infiltration into the alveolar spaces, albeit at lower levels than found at 7 d postinfection. Importantly, TMs consistently had worse clinical histopathologic scores than adults did throughout the infection (Fig. 3D).
Figure 3.
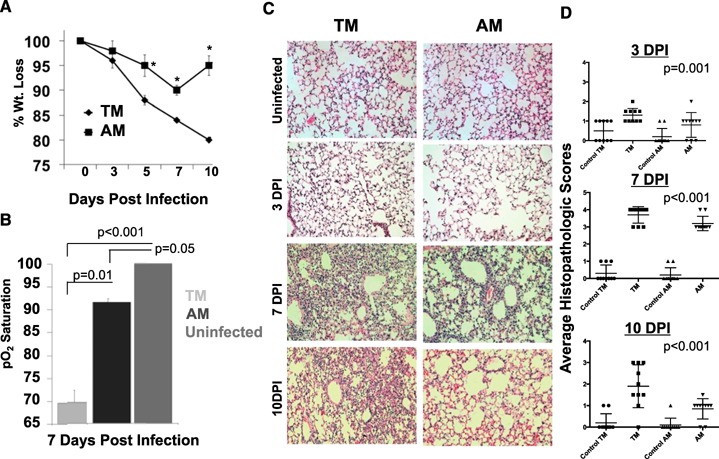
TMs recapitulate the enhanced influenza‐induced morbidity of toddler children. We assessed morbidity throughout infection by weight (Wt) loss (A) and pulse oximetry (B). *P < 0.05 between weight time points of TMs and AMs. Uninfected mice were a mix of both TMs and AMs, which both gave approximately 100% pO2. (C) We assessed lung pathology by H&E staining of tissue sections throughout infection and by quantifying the histopathologic scores by blinded review (D). Data shown represents means ± se. n = 3 for each group, 3 replicates each (TM, n = 7 at >10 days postinfection [DPI] because of death). Representative photos are shown. ×20 magnification, n = 3 for each group, 3 replicates each.
CD4 T cell recruitment differs in TMs and AMs during infection
Because viral clearance in mice is a function of the generation of influenza‐specific antibodies late in the infection [43] and viral suppression can occur through the combined effects of CD4 and CD8 T cells or innate immune cells [44, 45], we chose to focus further on limitations in CD4 T cells because of their importance for immunologic helper functions for B cells and direct antiviral activity. To examine the level of CD4 T cell in the lungs throughout the infection, we determined the absolute counts of CD4 T cells in the spleens and lungs of TMs and AMs at 7 and 10 d postinfection ( Fig. 4A and B ). Unsurprisingly, we found significant increases in the number of T cells in the lungs of adults at 7 d postinfection, which contrasted with a limited increase in T cells in the lungs of TMs. By 10 d postinfection, the recruitment to the lungs of AMs appeared to drop, which coincided with viral resolution. In contrast, limited accumulation of T cells occurred throughout infection in TMs. The failure to drive CD4 T cell recruitment to the lungs did not appear to be a function of lower proliferation because we found high BrdU staining in CD4 T cells from the lungs, mesenteric lymph nodes, and spleens of TMs at 7 d postinfection (Supplemental Fig. 3). We next examined the level of apoptosis on 6 d postinfected CD4 T cells in the lungs by Annexin V staining and found significantly greater staining (MFI) on TMs than on adults during infection (Fig. 4C). We further confirmed the levels of apoptosis using FLICA reagent that binds to poly caspases and found similar trends to Annexin V staining (Fig. 4D).
Figure 4.
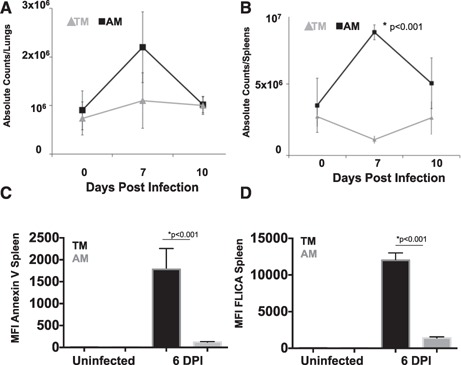
Diminished T cell infiltration into the lungs of infected TMs. Absolute numbers of CD4 T cells were determined in the lungs (A) and the spleens (B) during infection. Data represent means ± se, n = 3 for each group, 3 replicates each. *P < 0.05 for similar cell type comparisons. (C) Mean fluorescence intensity (MFI) of Annexin V staining on CD4 T cells isolated from spleens at 0 and 6 d postinfection. n = 6 each group. (D) MFI of FLICA‐incubated (poly caspases) with isolated CD4 T cells from the lungs at 0 and 6 d postinfection. Necrotic cells were negative‐gated using propidium iodide. n = 6 each group.
Diminished CD4 T cell responses characterize the TM response to infection
To determine the contribution of the adaptive immune system in driving viral clearance, we assessed the activity of CD4 T cells throughout infection. We found that, at 7 d postinfection, TMs had lower levels of Th1, Th2, and Th17 CD4 T cells in the lungs than did adults ( Fig. 5A ). The cytokine concentrations in the lungs were next examined by Luminex analysis of lung homogenates (Fig. 5B). We found significantly greater IFN‐γ production in the lungs of AMs at 7 and 10 d postinfection than TMs had. We did not detect high levels of Th2 cytokines (IL‐4 or IL‐13) by intracellular cytokine staining or by qRT‐PCR in TMs as compared with AMs during infection, and we did not detect increases in FoxP3+ regulatory T cell populations throughout infection of TMs or AMs (data not shown).
Figure 5.
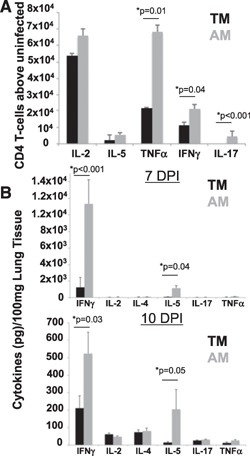
Diminished T cell functions in TMs correlate with delayed viral clearance. (A) We assessed cytokine‐positive CD4 T cells and in the lungs of TMs and AMs at 7 d postinfection (DPI). Cells were stimulated with PMA/ionomycin, and the percentage of uninfected controls was subtracted. Data represent means ± se, n = 6 each group. (B) We assessed lung cytokines by Luminex assay at 7 d and 10 DPI in TMs and AMs. Data represent means ± se with cytokines from uninfected, age‐matched mice subtracted, n = 8 each group.
Blocks to CD4 T cell IFN‐γ secretion appear downstream of IL‐12–induced STAT4
We next infected TMs and AMs, after transfer of equal numbers of autologous CD4 T cells from age‐matched DO11.1 mice (TMs into TMs vs. adults into adults), with influenza PR8 containing the OVA epitope in the neuraminidase stalk. We chose to use DO11.1 mice to ensure that our analysis would consist of very similar CD4 T cell effectors and possibly reduce confounding effects of other polarized T cell subsets while providing enough anti‐viral CD4 T cells for analysis. We determined the phosphorylation of p‐STAT4 and p‐Zap70 was not different between CD4 T cells by Western blotting (data not shown). We next preformed qRT‐PCR on the cDNA obtained from those cells, concentrating on the IL‐12/TCR signaling cascades. Importantly, we did not detect a difference in T bet, suggesting that TCR stimulation and Th1 polarization were similar between TMs and adults (shown as the fold increase/decrease of TM CD4 T cells over that of adults). In the IL‐12 signaling pathway, we found no difference in STAT4 expression but did detect a significant decrease (highlighted in red) in the expression of ERM and its downstream transcripts: IFN‐γ, CCR5, and IL‐18R ( Fig. 6A and B ). Importantly, we did not observe a significant difference in IL‐12 levels in the lungs (Luminex and qRT‐PCR) or spleens (qRT‐PCR from isolated APCs), suggesting the IL‐12 deficiency of neonates [39, 46] may be outgrown in TMs (Fig. 6C) and may not be contributing to lower CD4 T cell secretion of IFN‐γ detected in TMs. Similar limitations, albeit at greater magnitudes, in IFN‐γ transcripts were observed from isolated wild‐type TM CD4 T cells stimulated with CD3/CD28, as compared with adults, despite similar levels (<2‐fold difference) of T bet transcription (Fig. 6D).
Figure 6.
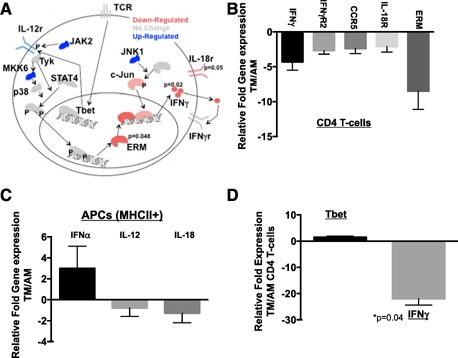
IFN‐γ pathway is down‐regulated in TMs at 6 d postinfection. We measured transcript expression in TMs that received DO11.1 CD4 T cells from TM donors and compared them with AMs that received similar transgenic cells from adult donors. (A) IL‐12 and TCR mediated signaling markers by qRT‐PCR from DO11.1 CD4 T cells isolated from 6 d postinfected autologously transferred BALB/c mice. n = 6 each group (2 replications of n = 3 each). Data represent the fold change in DO11.1 CD4 T cells from TMs over AMs, with adult values set to baseline. (B) Relative expression for significant cytokine transcripts is shown as TMs over AMs, with adult values set to baseline. P values are shown in (A). (C) Transcript expression from APCs isolated from the spleens of infected mice at 7 d postinfection shown as the relative fold increase of TMs over AMs, with adult values set to baseline. (D) Relative fold change in expression in wild‐type CD4 T cells from TMs over adults (aged 9 mo to 15 mo) after 72‐h stimulation with CD3/CD28 beads. n = 3 each. Negative values represent a down‐regulation in expression.
To further examine whether CD4 T cell limitations stemmed from intrinsic or extrinsic blocks in IFN‐γ secretion, we used adoptive transfers again but reciprocally (i.e., TM‐derived CD4 T cells placed into adult recipients). We isolated the adult‐ or TM‐donated CD4 T cells from the mediastinal lymph nodes 6 d postinfection and compared them with the values obtained from each host (shown as fold change above uninfected controls). Similar to the spleen shown in Fig. 6A, we found TM‐derived CD4 T cells transferred into young mice or adults exhibited similar lower expression of IFN‐γ (Supplemental Fig. 4A) and ERM transcripts (Supplemental Fig. 4B), which was significantly lower than they were with adult CD4 T cells transferred into AMs or adult CD4 T cells transferred into TMs.
Exogenous IFN‐γ administration rescues T cell activation and infiltration into the lungs of TMs
Although the tissue environment sets the stage for activation and recruitment of T cells to the site of infection, blocks to activation could account for diminished cytokine responses observed in TMs. The minimized levels of IFN‐γ production in the mucosa of TMs could not only have a dramatic effect on the capability of T cells to clear virus but also have an effect on T cell effector activation [35]. We determined that exogenous administration of rIFN‐γ before the early lung T cell infiltration stages (4 d postinfection) led to a significant increase in the absolute number of CD4 T cells entering the lungs ( Fig. 7A ) and in the spleens (Fig. 7B) at 7 d postinfection. We found that supplementation with IFN‐γ did not protect TMs from histopathology (Fig. 7C and E), and we are currently exploring the mechanism or mechanisms for this. However, addition of rIFN‐γ reduced the frequency of caspase3+ CD4 T cells in TMs to levels similar to that in adults (Fig. 7E). These data suggest that mechanisms diminishing the capacity for IFN‐γ production in TMs increase apoptosis in CD4 T cell in the lungs and survival in the spleen after activation.
Figure 7.
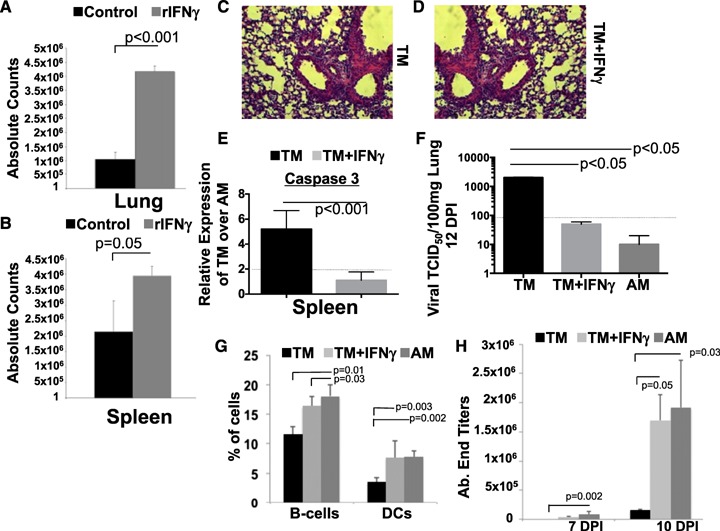
Diminished IFN‐γ impairs lung recruitment of T cells in TMs during infection. Absolute CD4 T cells in the lungs (A) and the spleens (B) were determined at 7 d postinfection in TMs treated with PBS or with rIFN‐γ (starting at 5 d postinfection). Data represent means ± se, n = 6 each group. Histology of TMs either treated with PBS (C) or rIFN‐γ (D). Representative photo is shown at ×20 magnification. (E) Caspase 3 transcript expression was measured in isolated CD4 T cells by qRT‐PCR and is expressed as fold change over adult infected controls. Line represents a significant 2‐fold expression. Data represent means ± se, n = 6 each group. (F) Viral loads were assessed in untreated or IFN‐γ treated TMs at 10 d postinfection. AMs are shown for comparison. The dashed line represents the limits of detection in our assays. (G) Percentages of B cells or DCs were determined in TMs with/without rIFN‐γ treatment with adult controls. (H) Serum endpoint titers specific for whole PR8 virus were determined for TMs with/without rIFN‐γ or for adult controls. n = 8 each group (2 replications of n = 4).
We next determined the effect of rIFN‐γ supplementation on the persistence of viral burdens. We found that by 10 d postinfection, both TMs with rIFN‐γ supplementation and adult controls were able to clear virus, whereas TMs without rIFN‐γ supplementation continued to have detectable viral loads (Fig. 7F). To further define the mechanism of clearance, we examined the percentages of CD19+ B cells and fDCs (CD19+pDC+) in the spleens at 7 d postinfection (Fig. 7G). We found that TMs with rIFN‐γ supplementation had higher B cells and had fDCs on par with adults, whereas nonsupplemented control TMs had lower percentages of these cells. We next examined the kinetics of antiviral IgG in the TMs and found that TMs with rIFN‐γ supplementation had higher antibodies to PR8 virus at 7 and 10 d postinfection than did nonsupplemented TMs that exhibited no generation of PR8‐specific IgG (Fig. 7H).
DISCUSSION
Influenza infection in toddlers leads to significant morbidity and frequently requires hospitalizations because of complications associated with viral and bacterial pneumonia [2]. The divergent immune responses to infection between toddlers and adults are not completely understood nor are the mechanisms driving increased lung pathology. However, young children have a delayed capacity to clear virus, which correlates with diminished systemic and mucosal T cell responses [36], which may relate to continued lung pathology during the later stages of infection. The immunologic maturity of neonates is well described as defects in APC function (IL‐12 secretion), T cells (IL‐4/IL‐13R), and epigenetic regulation of cytokines [12]. However, the level of immunologic maturity of toddlers is not well described, although T cells are known to secrete more IFN‐γ as compared to neonatal children, whereas older children appear to have a similar immune response capacity as adults [47]. We sought to use a mouse model of infection to better simulate infection of toddlers and found our model not only mirrored the enhanced influenza‐induced morbidity in toddlers but also accurately modeled the diminished T cell responses that correlated with a delay in viral clearance. We found the TMs were actively producing mucosal memory (effector markers), as would be expected of young children no longer protected by maternal antibodies and exposed to pathogens and environmental antigens. In addition, the similar absolute numbers of T cells in the spleens (higher counts based on tissue volume) are not surprising because young children generally have higher peripheral T cell counts than adults have. Although an exact model of infection in children is difficult to model in mice, given their rapid aging, mice <2 wk old do not exhibit germinal center formation (unlike human toddlers) [48], representing a similar point in weaning as that of children >6 mo old who are now reliant on their own immune systems for protection, and they exhibit functional DC populations like that of young children.
In 2009–2010, H1N1 2009 led to greater morbidity and mortality in young children than observed in adults [49, 50], similar to H3N2v2 in 2012–2013 [51]. A study of lower levels of respiratory influenza infections in toddlers demonstrated a higher cytokine response in the lungs but a nearly complete lack of CD4 T cell effectors because of the apoptosis of the responding cells as they entered the lungs [20], and strongly demonstrating that toddler and adult responses are different during influenza infection. In agreement with those studies, we found the toddler murine T cell response was diminished with respect to Th1 responses (but still dominant) as compared with that of AMs throughout infection. Although faulty DC functions have been implicated in having a role in lower CD4 T cell responses in neonates [11], our data demonstrate that TMs outgrow the neonatal phenotype with respect to T cell activation–induced cytokine secretion. Moreover, unlike neonates, the TM's APC stimulatory capacity appears to be mature, as evidenced by secretion of equivalent levels of IL‐12 between TMs and adults.
Previous studies of toddlers with lethal lower respiratory viral infections demonstrated poor control of viral replication coupled with T cell apoptosis in the lungs, but the exact mechanisms were unknown [19, 20]. In addition, multiple studies have demonstrated lower IFN‐γ secretion by young children's T cells, which increase with age and which may also extend to other chronic and acute viral infections [47, 52, 53]. However, the mechanisms for these diminished cytokines are unknown as is whether epigenetic blocks to cytokine production in neonates extend into toddlerhood. Here, we also found lower IFN‐γ production in the lungs by T cells in TMs, which may also extend to the innate immune cells. Interestingly, we found similar viral titers between TMs and adults at or before 7 d postinfection, suggesting that the innate immune response may be comparable between the 2. Lower IFN‐γ production by NK cells could also have a dramatic effect on APC activation and survival [54, 55–56], which, in turn, could also affect T cell activation/survival. Although we did not detect differences in expression of IFN‐γR1 and IFN‐γR2, we cannot rule out a poor association between the 2 receptors as having a role in limited IFN‐γ autocrine signaling. Moreover, we detected higher levels of type I IFN in TMs. and it is known that subthreshold levels of these cytokines are required for interactions between the 2 IFN‐γ receptors [57]. Thus, too‐high levels of type I IFN could have a role in lower IFN‐γ receptor sensitivity, which could be exacerbated by low IFN‐γ secretion levels. The lower levels of IFN‐γ in TMs clearly have an effect on T cell numbers in the lungs, potentially by influencing the level of apoptosis. We do not yet know whether there is an epigenetic block of ERM (ETV‐5) transcription after p‐STAT4 binding or whether some other defect in STAT4 signaling is involved (e.g., microRNA). However, clearly toddlers have an immunologic increase in maturation with the development of APC capacity to stimulate T cells and functional activity of STAT4 (both limitations in neonates).
The effects of IFN‐γ on T cell survival and polarization [45] are well known, and supplementation with this cytokine rescues CD4 T cells during infection. Future studies will determine the potential differential requirements for optimal activation of T cells in toddlers. However, the effects of rIFN‐γ on rescuing CD4 T cells may affect the activation/survival of fDC and CD19+ B cells in the spleens, which, in turn, may lead to increased antiviral antibody secretion and viral clearance kinetics on par with adults. The direct effect of rIFN‐γ on CD4 T cell subsets, such as follicular Th cells are a subject for future studies because we did detect lower CXCR5+CD4 T cells in draining lymph nodes of toddlers.
In summary, we investigated divergent immune responses to infection in toddler and adult models of influenza infection and found increased morbidity in TMs, thus accurately modeling human toddler infection. Prolonged viral clearance appeared to be associated with lower CD4 T cell antiviral and helper activity through blocks in IFN‐γ signaling. Importantly, CD4 T cells are not the only immune cell that appears to have limitations in IFN‐γ secretion; thus, future studies could also focus on the role of NK cells or macrophages and monocytes. However, given that we found APCs are functionally equivalent between TMs and adults, NK function with respect to APC priming may not be limited during infection of toddlers. The lack of a robust, adaptive response and continuation of viral replication in toddlers may significantly contribute to the higher morbidity observed in influenza infections in children between the ages of 6 mo and 2 y.
AUTHORSHIP
D.V. designed the study, performed assays, analyzed data, and wrote the manuscript. S.P. and K.P. performed assays and some data analysis.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Supplementary data
Supplementary data
Supplementary data
Supplementary data
ACKNOWLEDGMENTS
This work was supported by an American Lung Association Grant in Aid/NY Lung Association award to D.V. D.V. had additional support from U.S. National Institutes of Health (NIH) Grant A1089975 and an NIH National Institute of Allergy and Infectious Disease Loan Repayment Program award. We thank Dr. Donna Farber for early discussions about, and help with, the project.
REFERENCES
- 1.World Health Organization (2003) Influenza: WHO fact sheet. No. 211. Available at: http://www.who.int/mediacentre/factsheets/2003/fs211/en/.
- 2.Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. (2006) Disease. Available at: http://www.cdc.gov.
- 3. Cox, C. M. , D'Mello, T. , Perez, A. , Reingold, A. , Gershman, K. , Yousey‐Hindes, K. , Arnold, K. E. , Farley, M. M. , Ryan, P. , Lynfield, R. , Morin, C. , Baumbach, J. , Hancock, E. B. , Zansky, S. , Bennett, N. M. , Thomas, A. , Schaffner, W. , Finelli, L. (2012) Increase in rates of hospitalization due to laboratory‐confirmed influenza among children and adults during the 2009–10 influenza pandemic. J. Infect. Dis. 206, 1350–1358. [DOI] [PubMed] [Google Scholar]
- 4. Thompson, W. W. , Shay, D. K. , Weintraub, E. , Brammer, L. , Bridges, C. B. , Cox, N. J. , Fukuda, K. (2004) Influenza‐associated hospitalizations in the United States. JAMA 292, 1333–1340. [DOI] [PubMed] [Google Scholar]
- 5. Neuzil, K. M. , Zhu, Y. , Griffin, M. R. , Edwards, K. M. , Thompson, J. M. , Tollefson, S. J. , Wright, P. F. (2002) Burden of interpandemic influenza in children younger than 5 years: a 25‐year prospective study. J. Infect. Dis. 185, 147–152. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (2010) Interim results: state‐specific seasonal influenza vaccination coverage ‐ United States, August 2009–January 2010. Morb. Mortal. Wkly Rpt. 59, 477–484. [PubMed] [Google Scholar]
- 7. Fiore, A. E. , Bridges, C. B. , Cox, N. J. (2009) Seasonal influenza vaccines. Curr. Top. Microbiol. Immunol. 333, 43–82. [DOI] [PubMed] [Google Scholar]
- 8. Englund, J. A. , Walter, E. B. , Gbadebo, A. , Monto, A. S. , Zhu, Y. , Neuzil, K. M. (2006) Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics 118, e579–e585. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Infectious Disease, Centers for Disease Control and Prevention. (2010) Title of article. Available at: http://www.cdc.gov.
- 10. Boyd, M. , Clezy, K. , Lindley, R. , Pearce, R. (2006) Pandemic influenza: clinical issues. Med. J. Aust. 185, S44–S47. [DOI] [PubMed] [Google Scholar]
- 11. Lee, H. H. , Hoeman, C. M. , Hardaway, J. C. , Guloglu, F. B. , Ellis, J. S. , Jain, R. , Divekar, R. , Tartar, D. M. , Haymaker, C. L. , Zaghouani, H. (2008) Delayed maturation of an IL‐12‐producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J. Exp. Med. 205, 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaghouani, H. , Hoeman, C. M. , Adkins, B. (2009) Neonatal immunity: faulty T helpers and the shortcomings of dendritic cells. Trends Immunol. 30, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrios, C. , Brawand, P. , Berney, M. , Brandt, C. , Lambert, P. H. , Siegrist, C. A. (1996) Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2‐biased pattern which persists after adult boosting. Eur. J. Immunol. 26, 1489–1496. [DOI] [PubMed] [Google Scholar]
- 14. Siegrist, C. A. (2001) Neonatal and early life vaccinology. Vaccine 19, 3331–3346. [DOI] [PubMed] [Google Scholar]
- 15. Siegrist, C. A. (2007) The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 137 (Suppl 1), S4–S9. [DOI] [PubMed] [Google Scholar]
- 16. Siegrist, C. A. , Aspinall, R. (2009) B‐cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9, 185–194. [DOI] [PubMed] [Google Scholar]
- 17. Lewinsohn, D. A. , Gennaro, M. L. , Scholvinck, L. , Lewinsohn, D. M. (2004) Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int. J. Tuberc. Lung Dis. 8, 658–674. [PubMed] [Google Scholar]
- 18. Cusi, M. G. , Martorelli, B. , Di Genova, G. , Terrosi, C. , Campoccia, G. , Correale, P. (2010) Age related changes in T cell mediated immune response and effector memory to Respiratory Syncytial Virus (RSV) in healthy subjects. Immun. Ageing 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welliver, T. P. , Garofalo, R. P. , Hosakote, Y. , Hintz, K. H. , Avendano, L. , Sanchez, K. , Velozo, L. , Jafri, H. , Chavez‐Bueno, S. , Ogra, P. L. , McKinney, L. , Reed, J. L. , Welliver, R. C., Sr. (2007) Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welliver, T. P. , Reed, J. L. , Welliver, R. C., Sr. (2008) Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr. Infect. Dis. J. 27 (10:, Suppl)S92–S96. [DOI] [PubMed] [Google Scholar]
- 21. Clerici, M. , DePalma, L. , Roilides, E. , Baker, R. , Shearer, G. M. (1993) Analysis of T helper and antigen‐presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J. Clin. Invest. 91, 2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Combadière, B. , Sibéril, S. , Duffy, D. (2010) Keeping the memory of influenza viruses. Pathol. Biol. (Paris) 58, e79–e86. [DOI] [PubMed] [Google Scholar]
- 23. Schatorjé, E. J. , Gemen, E. F. , Driessen, G. J. , Leuvenink, J. , van Hout, R. W. , de Vries, E. (2012) Paediatric reference values for the peripheral T cell compartment. Scand. J. Immunol. 75, 436–444. [DOI] [PubMed] [Google Scholar]
- 24. Biddison, W. E. , Sharrow, S. O. , Shearer, G. M. (1981) T cell subpopulations required for the human cytotoxic T lymphocyte response to influenza virus: evidence for T cell help. J. Immunol. 127, 487–491. [PubMed] [Google Scholar]
- 25. Kumagai, T. , Nagai, K. , Okui, T. , Tsutsumi, H. , Nagata, N. , Yano, S. , Nakayama, T. , Okuno, Y. , Kamiya, H. (2004) Poor immune responses to influenza vaccination in infants. Vaccine 22, 3404–3410. [DOI] [PubMed] [Google Scholar]
- 26. Lines, J. L. , Hoskins, S. , Hollifield, M. , Cauley, L. S. , Garvy, B. A. (2010) The migration of T cells in response to influenza virus is altered in neonatal mice. J. Immunol. 185, 2980–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruest, A. , Michaud, S. , Deslandes, S. , Frost, E. H. (2003) Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription‐PCR for rapid diagnosis of influenza virus infection. J. Clin. Microbiol. 41, 3487–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Westerga, J. , Timens, W. (1989) Immunohistological analysis of human fetal lymph nodes. Scand. J. Immunol. 29, 103–112. [DOI] [PubMed] [Google Scholar]
- 29. Timens, W. , Rozeboom, T. , Poppema, S. (1987) Fetal and neonatal development of human spleen: an immunohistological study. Immunology 60, 603–609. [PMC free article] [PubMed] [Google Scholar]
- 30. Al Barzanji, A. J. , Penny, S. R. , Emery, J. L. (1976) Development of germinal centres in the spleen in infants related to birth and unexpected death. J. Clin. Pathol. 29, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kleindienst, P. , Brocker, T. (2005) Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T cell immunity in vivo. Immunology 115, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodríguez‐Pinto, D. , Moreno, J. (2005) B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen‐presenting cells in a CD154‐CD40‐dependent manner. Eur. J. Immunol. 35, 1097–1105. [DOI] [PubMed] [Google Scholar]
- 33. Velilla, P. A. , Rugeles, M. T. , Chougnet, C. A. (2006) Defective antigen‐presenting cell function in human neonates. Clin. Immunol. 121, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verhoeven, D. , Teijaro, J. R. , Farber, D. L. (2009) Pulse‐oximetry accurately predicts lung pathology and the immune response during influenza infection. Virology 390, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miner, K. T. , Croft, M. (1998) Generation, persistence, and modulation of Th0 effector cells: role of autocrine IL‐4 and IFN‐γ. J. Immunol. 160, 5280–5287. [PubMed] [Google Scholar]
- 36. Tregoning, J. S. , Schwarze, J. (2010) Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 23, 74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chapman, T. J. , Castrucci, M. R. , Padrick, R. C. , Bradley, L. M. , Topham, D. J. (2005) Antigen‐specific and non‐specific CD4+ T cell recruitment and proliferation during influenza infection. Virology 340, 296–306. [DOI] [PubMed] [Google Scholar]
- 38. Belser, J. A. , Wadford, D. A. , Pappas, C. , Gustin, K. M. , Maines, T. R. , Pearce, M. B. , Zeng, H. , Swayne, D. E. , Pantin‐Jackwood, M. , Katz, J. M. , Tumpey, T. M. (2010) Pathogenesis of pandemic influenza A (H1N1) and triple‐reassortant swine influenza A (H1) viruses in mice. J. Virol. 84, 4194–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goriely, S. , Van Lint, C. , Dadkhah, R. , Libin, M. , De Wit, D. , Demonté, D. , Willems, F. , Goldman, M. (2004) A defect in nucleosome remodeling prevents IL‐12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med. 199, 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang, X. , Spandidos, A. , Wang, H. , Seed, B. (2012) PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update., [Database issue] Nucleic Acids Res. 40, D1144–D1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chandran, S. S. , Verhoeven, D. , Teijaro, J. R. , Fenton, M. J. , Farber, D. L. (2009) TLR2 engagement on dendritic cells promotes high frequency effector and memory CD4 T cell responses. J. Immunol. 183, 7832–7841. [DOI] [PubMed] [Google Scholar]
- 42. Teijaro, J. R. , Jr, Njau, M. N. , Verhoeven, D. , Chandran, S. , Nadler, S. G. , Hasday, J. , Farber, D. L. (2009) Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J. Immunol. 182, 6834–6843. [DOI] [PubMed] [Google Scholar]
- 43. Epstein, S. L. , Lo, C. Y. , Misplon, J. A. , Lawson, C. M. , Hendrickson, B. A. , Max, E. E. , Subbarao, K. (1997) Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell‐depleted, β2‐microglobulin‐deficient, and J chain‐deficient mice. J. Immunol. 158, 1222–1230. [PubMed] [Google Scholar]
- 44. Epstein, S. L. , Lo, C. Y. , Misplon, J. A. , Bennink, J. R. (1998) Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 160, 322–327. [PubMed] [Google Scholar]
- 45. Benton, K. A. , Misplon, J. A. , Lo, C. Y. , Brutkiewicz, R. R. , Prasad, S. A. , Epstein, S. L. (2001) Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or γδ T cells. J. Immunol. 166, 7437–7445. [DOI] [PubMed] [Google Scholar]
- 46. Renneson, J. , Dutta, B. , Goriely, S. , Danis, B. , Lecomte, S. , Laes, J. F. , Tabi, Z. , Goldman, M. , Marchant, A. (2009) IL‐12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur. J. Immunol. 39, 2789–2799. [DOI] [PubMed] [Google Scholar]
- 47. Hanna‐Wakim, R. , Yasukawa, L. L. , Sung, P. , Fang, M. , Sullivan, B. , Rinki, M. , DeHovitz, R. , Arvin, A. M. , Gans, H. A. (2009) Age‐related increase in the frequency of CD4+ T cells that produce interferon‐gamma in response to staphylococcal enterotoxin B during childhood. J. Infect. Dis. 200, 1921–1927. [DOI] [PubMed] [Google Scholar]
- 48. Pihlgren, M. , Tougne, C. , Bozzotti, P. , Fulurija, A. , Duchosal, M. A. , Lambert, P. H. , Siegrist, C. A. (2003) Unresponsiveness to lymphoid‐mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T dependent antigens. J. Immunol. 170, 2824–2832. [DOI] [PubMed] [Google Scholar]
- 49. Lister, P. , Reynolds, F. , Parslow, R. , Chan, A. , Cooper, M. , Plunkett, A. , Riphagen, S. , Peters, M. (2009) Swine‐origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet 374, 605–607. [DOI] [PubMed] [Google Scholar]
- 50. Bozzola, E. , Krzysztofiak, A. , Lancella, L. , Tozzi, A. (2010) Risk factors of complicated H1N1 influenza in hospitalized Italian children. Vaccine 28, 5387–5388. [DOI] [PubMed] [Google Scholar]
- 51. Jhung, M. A. , Epperson, S. , Biggerstaff, M. , Allen, D. , Balish, A. , Barnes, N. , Beaudoin, A. , Berman, L. , Bidol, S. , Blanton, L. , Blythe, D. , Brammer, L. , D'Mello, T. , Danila, R. , Davis, W. , de Fijter, S. , Diorio, M. , Durand, L. O. , Emery, S. , Fowler, B. , Garten, R. , Grant, Y. , Greenbaum, A. , Gubareva, L. , Havers, F. , Haupt, T. , House, J. , Ibrahim, S. , Jiang, V. , Jain, S. , Jernigan, D. , Kazmierczak, J. , Klimov, A. , Lindstrom, S. , Longenberger, A. , Lucas, P. , Lynfield, R. , McMorrow, M. , Moll, M. , Morin, C. , Ostroff, S. , Page, S. L. , Park, S. Y. , Peters, S. , Quinn, C. , Reed, C. , Richards, S. , Scheftel, J. , Simwale, O. , Shu, B. , Soyemi, K. , Stauffer, J. , Steffens, C. , Su, S. , Torso, L. , Uyeki, T. M. , Vetter, S. , Villanueva, J. , Wong, K. K. , Shaw, M. , Bresee, J. S. , Cox, N. , Finelli, L. (2013) Outbreak of variant influenza A(H3N2) virus in the United States. Clin. Infect. Dis. 57, 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chung, H. L. , Park, H. J. , Kim, S. Y. , Kim, S. G. (2007) Age‐related difference in immune responses to respiratory syncytial virus infection in young children. Pediatr. Allergy Immunol. 18, 94‐99. [DOI] [PubMed] [Google Scholar]
- 53. Lidehäll, A. K. , Engman, M. L. , Sund, F. , Malm, G. , Lewensohn‐Fuchs, I. , Ewald, U. , Tötterman, T. H. , Karltorp, E. , Korsgren, O. , Eriksson, B. M. (2013) Cytomegalovirus‐specific CD4 and CD8 T cell responses in infants and children. Scand. J. Immunol. 77, 135–143. [DOI] [PubMed] [Google Scholar]
- 54. Kamath, A. T. , Sheasby, C. E. , Tough, D. F. (2005) Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN‐αβ and IFN‐γ. J. Immunol. 174, 767–776. [DOI] [PubMed] [Google Scholar]
- 55. Riise, R. E. , Bernson, E. , Aurelius, J. , Martner, A. , Pesce, S. , Della Chiesa, M. , Marcenaro, E. , Bylund, J. , Hellstrand, K. , Moretta, L. , Moretta, A. , Thorén, F. B. (2015) TLR‐stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses. J. Immunol. 195, 1121–1128. [DOI] [PubMed] [Google Scholar]
- 56. Walzer, T. , Dalod, M. , Robbins, S. H. , Zitvogel, L. , Vivier, E. (2005) Natural‐killer cells and dendritic cells: “l'union fait la force.” Blood 106, 2252–2258. [DOI] [PubMed] [Google Scholar]
- 57. Takaoka, A. , Mitani, Y. , Suemori, H. , Sato, M. , Yokochi, T. , Noguchi, S. , Tanaka, N. , Taniguchi, T. (2000) Cross talk between interferon‐γ and ‐α/β signaling components in caveolar membrane domains. Science 288, 2357–2360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data


