Short abstract
TLT2 expression is conserved between mouse and human, and increased on leukocytes, in response to microbial products.
Keywords: TLT2, leukocyte, exocytosis
Abstract
The triggering receptor expressed on myeloid cell locus encodes a family of receptors that is emerging as an important class of molecules involved in modulating the innate immune response and inflammation. Of the 4 conserved members, including triggering receptor expressed on myeloid cells 1 and 2 and triggering receptor expressed on myeloid cell‐like transcripts 1 and 2, relatively little is known about triggering receptor expressed on myeloid cell‐like transcript 2 expression and function, particularly in humans. In this study, experiments were performed to determine if triggering receptor expressed on myeloid cell‐like transcript 2 expression is conserved between mouse and human, demonstrating that human triggering receptor expressed on myeloid cell‐like transcript 2 is expressed on cells of the lymphoid, as well as myeloid/granuloid lineages, similar to murine triggering receptor expressed on myeloid cell‐like transcript 2. Consistent with studies in the mouse, triggering receptor expressed on myeloid cell‐like transcript 2 expression is up‐regulated in response to inflammatory mediators on human neutrophils. Importantly, it was shown that triggering receptor expressed on myeloid cell‐like transcript 2, in resting human neutrophils, is predominantly localized to intracellular vesicles, including secretory vesicles and primary granules; with the majority of triggering receptor expressed on myeloid cell‐like transcript 2 stored in primary granules. In contrast to other primary granule proteins, triggering receptor expressed on myeloid cell‐like transcript 2 is not expelled on neutrophil extracellular traps but is retained in the plasma membrane following primary granule exocytosis. In summary, these findings establish that triggering receptor expressed on myeloid cell‐like transcript 2 expression is conserved between species and is likely to be important in regulating neutrophil antimicrobial function following primary granule exocytosis.
Abbreviations
- Cyto B
cytochalasin B
- GPCR
G‐protein‐coupled receptor
- MFI
mean fluorescence intensity
- MPO
myeloperoxidase
- NET
neutrophil extracellular trap
- PBL
peripheral blood leukocyte
- PFA
paraformaldehyde
- PTK
protein tyrosine kinase
- RT
room temperature
- TLT1/2
triggering receptor expressed on myeloid cell‐like transcript 1/2
- TREM
triggering receptor expressed on myeloid cells
Introduction
Cell‐to‐cell communication and modulation of effector function are both important for regulating the inflammatory, as well as the innate immune response, and are controlled via receptors belonging to the Ig superfamily [1, 2–3]. Members of the TREM family have been shown to modulate cellular responses to inflammatory and pathogenic stimuli and play a role in mediating cellular crosstalk [4, 5]. There are 4 conserved members of the TREM locus expressed in mice and humans: TREM‐1, TREM‐2, TLT1, and TLT2 [6]. In the past decade, studies have focused on characterizing the functional role played by TREM locus receptors during the innate immune response. Of the conserved receptors, TLT2 is the least well characterized with respect to its expression and function in the immune system.
Previous studies in mice have demonstrated that TLT2 is expressed on B cells, neutrophils, and macrophages, and this expression is up‐regulated in response to inflammatory stimuli in vivo on innate immune cells but not B cells [7]. Studies have demonstrated that ligation of TLT2 synergistically enhances neutrophil migration, degranulation, and the respiratory burst in response to agonists that signal via GPCRs [8]. Administration of TLT2 mAb in vivo has been shown to enhance neutrophil recruitment to local sites of inflammation [8], as a result of the direct action of TLT2 on neutrophils, resulting in their enhanced responsiveness to chemoattractants produced in association with inflammation [8, 9]. Additional studies have demonstrated that TLT2 appears to play a role in mediating efferocytosis of apoptotic cells by macrophages [10]. Thus, it appears that TLT2 is indeed involved in modulating innate immune cell function, as is the case for other TREM locus receptors [11, 12, 13, 14, 15–16].
To date, little is known about TLT2 expression and function in humans. It has been reported that TLT2 is expressed on T and B lymphocytes, as well as monocytes in humans [17], although a complete analysis of PBL populations has yet to be conducted. In this study, it was determined that TLT2 is expressed on monocytic and granulocytic cells in the human, as well as B cells, similar to what is observed in the mouse, although in contrast to mice, TLT2 is highly expressed on human monocytes. Analysis of TLT2 expression on human neutrophils revealed that TLT2 is up‐regulated in response to a range of agonists, albeit maximal expression is not observed unless cells are incubated with Cyto B, leading to release of primary granules. Studies further demonstrated that TLT2 is contained in secretory vesicles, as well as primary granules, but that the majority of intracellular TLT2 is stored in primary granules and is presumably released in conjunction with terminal activation of the neutrophil. In summary, these findings support the conclusion that TLT2 plays a role in modulating effector function in human neutrophils in agreement with previous findings in the mouse [7, 8].
MATERIALS AND METHODS
Patient samples and cell isolation
Venous blood was isolated from healthy volunteers in accordance with a protocol approved by the University of Alabama Institutional Review Board. PBLs were isolated using sodium dextran sedimentation. Neutrophils were isolated using Polymorphprep (Axis‐Shield, Oslo, Norway).
Phenotypic analysis
Human PBLs were resuspended (1 × 106 cells/ml) in FACS buffer (PBS + 2% FCS), blocked with TruStain fcX (BioLegend, San Diego, CA, USA), and stained with lineage‐specific antibodies (from BioLegend or BD Biosciences, San Jose, CA, USA; see Supplemental Materials for clone information) or TLT2 antibody (monoclonal 1C5 conjugated to A647 or A488, generated as described previously [7]). Stained cells were acquired using a BD LSR II (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Neutrophil activation
Neutrophils were placed in 96‐well polypropylene round‐bottom plates (1 × 106 cells/ml) and rested (15 min, 37°C, 5% CO2). Activating factors from R&D Systems (Minneapolis, MN, USA; C5a, 50 ng/ml; GM‐CSF, 50 ng/ml; IFN‐γ, 1000 μ/ml), Sigma (St. Louis, MO, USA; fMLP, 100 nM; LPS, 100 ng/ml), or PeproTech (Rocky Hill, NJ, USA; IL‐8, 100 ng/ml) were added to cells for times indicated (30, 60, 90, or 120 min; 37°C; 5% CO2). Cells were placed on ice to stop the reaction, blocked with TruStain fcX, stained with CD11b and TLT2, and analyzed by flow cytometry.
Intracellular granule colocalization studies
Isolated neutrophils (5 × 105 cells/ml) were fixed in 4% PFA (10 min, RT) and permeabilized (0.5% saponin, 10 min, RT). Cells blocked with TruStain fcX and stained with antibodies against granule types, with all washes containing 0.2% saponin. Stained cells were cytospun (350 rpm, 3 min, RT, medium acceleration) onto slides and stained with DAPI and coverslips mounted with ProLong Gold Antifade (#P36930; Life Technologies, Thermo Fisher Scientific, Grand Island, NY, USA). Slides were imaged with a Nikon Eclipse Ti microscope. Colocalization studies were performed using ImageJ software (Coloc_2 plugin), and Pearson's correlation coefficients were reported for 5 healthy donors (10 replicates for each donor).
Degranulation assays
Neutrophils (1 × 106 cells/ml in RPMI) were pretreated with DMSO or Cyto B (5 μg/ml; Sigma) for 10 min (37°C, 5% CO2) before addition of fMLP for 30 or 60 min (1 μM; Sigma). For inhibition of degranulation, cells were incubated for 15 min with DMSO, genistein (100 μM; Sigma), or Rac inhibitor NSC23766 (10, 25, 50 μM; R&D Systems) before addition of fMLP and Cyto B. Cells were placed on ice to stop the reaction, blocked with TruStain fcX, stained with antibodies, and analyzed by flow cytometry.
NET induction
Neutrophils (1 × 106 cells/ml in RPMI) were added to wells of a chamber slide (Millipore, Billerica, MA, USA) and rested (30 min, 37°C, 5% CO2) to allow for adherence. Supernatants were removed carefully and replaced with RPMI containing DMSO or PMA (100 nM; Sigma) and incubated (4 h, 37°C, 5% CO2). After stimulation, cells were washed gently, so as not to disturb NET formation, and fixed (4% PFA, 10 min, RT). Slides were blocked (PBS + 2.5% FCS + TruStain fcX in PBS, 25 min, RT), stained with MPO antibody (1:250, 30 min; AbD Serotec, Kidlington, United Kingdom), and then stained with TLT2‐A488 or isotype control antibody (rat IgG1 A488; Life Technologies, Thermo Fisher Scientific) and a goat anti‐mouse IgG2b‐A555 (Life Technologies, Thermo Fisher Scientific) for 30 min. Slides were washed and stained with DAPI and coverslips mounted and imaged as mentioned above.
Statistical analysis
Statistical significance was determined by paired Studentˈs t test using Prism software (GraphPad, La Jolla, CA, USA), unless noted otherwise. These statistical values are denoted in the figure legends.
RESULTS AND DISCUSSION
TLT2 expression is conserved on mouse and human leukocytes
Previous studies analyzed murine TLT2 expression on hematopoietic lineage cells, demonstrating that TLT2 is unique among the TREM locus receptors in that it is expressed by cells of the myeloid/granuloid and lymphoid lineages. To ascertain whether TLT2 expression is conserved between mice and humans, resting PBLs were isolated from venous blood and surface TLT2 expression assessed by flow cytometry. Analysis of side scatter vs. TLT2 revealed a distinct pattern of TLT2 expression ( Fig. 1 ). When each subpopulation is gated and stained with lineage markers, TLT2 is most highly expressed on B cells (CD19+), followed by monocytes (CD14+) and granulocytes (high side‐scatter, CD14low) in the blood (Fig. 1B and C).
Figure 1.
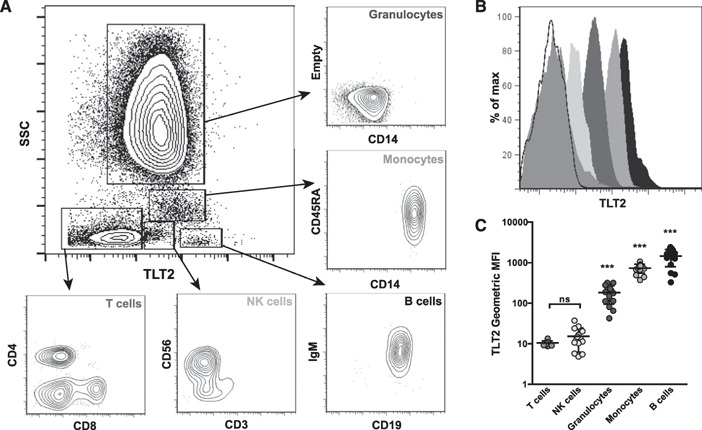
TLT2 is expressed on human lymphoid and myeloid/granuloid lineages. PBLs were isolated and stained with mAb directed against CD19, IgM, CD14, CD45RA, CD3, CD56, CD4, CD8, and TLT2 and then analyzed by a BD LSR II flow cytometer and FlowJo software. (A) Representative scatter plot of distinct PBL subpopulations distinguished by side‐scatter (SSC) and TLT2 expression. (B) Representative histogram depicting relative expression of TLT2 on specific PBL subsets. Histogram shading reflects the subpopulations denoted in C. The open histogram is the staining control depicting autofluorescence of ungated PBL. (C) MFI ± sd for TLT2 expression on PBL subsets (unstained control MFI = 10.16). Data shown are from healthy donors, n = 10 (individual MFIs are depicted as circles). ***P < 0.001; ns, not significant.
The relative level of TLT2 expression observed on distinct PBL subpopulations (B cell > monocyte > neutrophil) is similar to that observed upon analysis of TLT2 expression in the mouse. Whereas TLT2 is highly expressed on human monocytes, it is not detected on monocytes isolated from mice, representing the 1 significant difference in the expression pattern of TLT2 between species [7]. TLT2 expression is unaffected when human monocytes are differentiated into macrophages in vitro (data not shown). Analysis of TLT2 expression on human NK cells in the blood consistently revealed very low levels on the surface (2–3 logs lower than that on monocytes or B cells) but was not statistically significant in terms of MFI compared with staining controls (Fig. 1B and C).
In contrast to a previous report [17], analysis of T cells failed to demonstrate significant expression of TLT2, regardless of CD4+ or CD8+ specificity. Moreover, activation of human T cells with CD3/CD28 mAb or PMA/ionomycin failed to increase TLT2 expression, as has been reported in the mouse and human (data not shown) [17, 18]. Our laboratory has generated a total of 14 TLT2 mAb, and none has detected significant TLT2 expression on T cells, regardless of their activation state (data not shown). Thus, it is not likely that TLT2 serves a functional role on T cells.
TLT2 expression on neutrophils is up‐regulated in response to inflammatory mediators
Previous studies have shown that expression of TREM locus receptors is modulated in response to inflammatory stimuli [19, 20], and TLT2 expression is up‐regulated on mouse neutrophils in response to in vivo administration of LPS [7]. Neutrophils respond to several different classes of molecules, including growth factors (e.g., GM‐CSF), chemotactic factors (e.g., C5a and IL‐8), bacterial products (e.g., LPS and fMLP), and cytokines (e.g., IFN−γ), resulting in activation and enhanced microbicidal activity. As shown, multiple classes of stimuli, with the exception of IFN‐γ, caused immediate and sustained neutrophil activation based on up‐regulation of CD11b expression ( Fig. 2A ). Although TLT2 expression was increased in response to GPCR (C5a and IL‐8) and growth factor receptor (GM‐CSF) agonists, fMLP and LPS resulted in the most significant increases in TLT2 expression (Fig. 2B). These data demonstrate that TLT2 expression on neutrophils is expressed constitutively at moderate levels on the cell surface and that expression is up‐regulated 2‐fold in response to LPS‐mediated activation, similar to that observed in murine neutrophils in vivo. This suggests that neutrophils up‐regulate TLT2 surface expression, primarily in response to bacterial products as opposed to other inflammatory mediators, and could be indicative of a proinflammatory function for TLT2.
Figure 2.
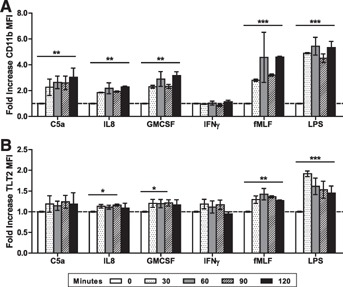
Up‐regulation of TLT2 expression in response to inflammatory mediators. Isolated neutrophils were stimulated with C5a (50 ng/ml), IL‐8 (100 ng/ml), GM‐CSF (50 ng/ml), IFN‐γ (1000 μ/ml), fMLP (fMLF; 100 nM), or LPS (100 ng/ml) for 30, 60, 90, or 120 min at 37°C. Cells were stained with CD11b‐PE and TLT2‐A647. The MFI for CD11b (A) or TLT2 (B) was determined using FlowJo software. The means ± sd of the fold increase in MFI are depicted. Data shown are from healthy donors, n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
TLT2 is stored in primary (azurophilic) granules in neutrophils
Other reports have established that members of the TREM family are stored in vesicles and that surface expression can be modulated through movement of these stores upon cellular activation [20, 21]. As a result of the rapid increase in TLT2 surface expression in neutrophils stimulated with bacterial products (Fig. 2), we hypothesized that TLT2 may be stored in granules for immediate transport to the cell surface in response to inflammation or infection. On permeabilized, resting neutrophils, TLT2 exhibits a punctate staining pattern, suggesting that it is likely to be stored in intracellular vesicles or granules ( Fig. 3A–C ). To determine in which vesicles or granules TLT2 was stored, neutrophils were costained with markers specific for different types of granules. TLT2 did not colocalize completely with any 1 type of granule, as is seen with other markers [22, 23–24]. However, an overlap of TLT2 was seen with the secretory vesicle marker CD35 (complement receptor 1; Fig. 3A) and CD63 (tetraspanin lysosome‐associated membrane protein 3), a membrane component of primary granules (Fig. 3C). Conversely, TLT2 and CD66b, a marker of secondary and tertiary granules, constitute distinct subcellular compartments, as shown by lack of overlap (Fig. 3B). Analysis of TLT2 colocalization, based on plotting the pixel intensity of red (TLT2) vs. green (CD35, CD66b, or CD63), reveals that TLT2 staining exhibits a direct, proportional distribution with respect to that of CD63 (Fig. 3F), whereas TLT2 staining vs. CD35 exhibits a modest degree of proportionality with increasing red and green pixel intensity exhibiting a divergent pattern (Fig. 3D). In contrast, TLT2 staining with respect to CD66b exhibits little to no colocalization (Fig. 3E). Analysis by Pearson's correlation coefficient further supports the conclusion that TLT2 expression correlates most highly with that of CD63, as opposed to CD35 or CD66b (Fig. 3G). In summary, intracellular TLT2 colocalizes primarily with CD63, a marker of primary granules. Although TLT2 exhibits modest colocalization with CD35, it is important to note that this may be a result of the fact that TLT2, expressed in the plasma membrane, is trapped in secretory vesicles as a result of normal recycling, whereas the bulk of intracellular TLT2 may be synthesized and stored in primary granules during neutrophil maturation in the bone marrow [25, 26].
Figure 3.
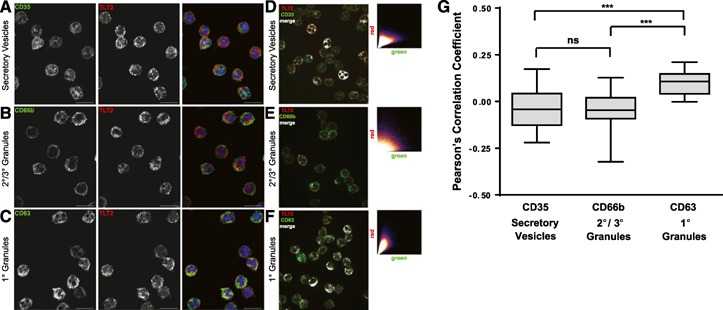
TLT2 colocalizes with secretory vesicles and primary granules in neutrophils. Resting neutrophils were fixed, permeabilized, and stained with DAPI, TLT2‐A647, and granule‐specific markers: (A) CD35‐A488 (secretory vesicles), (B) CD66b‐A488 (secondary/tertiary granules), or (C) CD63‐A488 (primary/azurophilic granules). Images shown are representative of 5 different donors. Original scale bars, 10 μm. Colocalization studies were performed on images of neutrophils stained with DAPI, TLT2‐A647, and granule‐specific markers: (D) CD35‐A488 (E) CD66b‐A488, and (F) CD63‐A488. Scatter plots depict the relative intensity of staining for TLT2 vs. vesicle/granule markers; with the relative pixel intensity for green (granule protein) on the x‐axis and red (TLT2) on the y‐axis. (G) Box and whisker plot of the Pearson's correlation coefficient for colocalization of TLT2 with each granule protein marker, n = 5 [5 healthy donors with 10 cells (replicates)/donor]. One‐way ANOVA analysis was performed. ***P < 0.001.
Neutrophils contain 4 granule subtypes: primary (azurophilic), secondary (specific), and tertiary (gelatinase) granules and secretory vesicles. These intracellular granules differ in terms of the signaling pathways that are involved in mediating their exocytosis and with respect to their functional role in the response to microbial challenge. Importantly, they are released in a hierarchical manner, with secretory vesicles mobilized first, followed by secondary/tertiary granules, and finally primary granules. From a functional perspective, secretory vesicles are highly specialized for rapid transport to the cell surface to deliver proteins important for priming neutrophils to respond to microbially derived stimuli, as well as to promote cell adhesion and migration, thereby facilitating neutrophil recruitment to sites of inflammation/infection [27, 28]. Previous studies have demonstrated that TLT2 ligation enhances murine neutrophil chemotaxis toward various chemotactic factors, including IL‐8 and C5a [8]. Thus, storage of TLT2 in secretory vesicles may facilitate a feed‐forward process, in which TLT2 expression can be up‐regulated rapidly to promote neutrophil activation and migration. To determine the relative proportion of TLT2 that is stored in primary granules vs. secretory vesicles, neutrophils were activated with LPS ± Cyto B ( Fig. 4A ) or fMLP ± Cyto B (Fig. 4B). Cyto B is a known actin‐depolymerizing agent, which induces complete degranulation, leading to mobilization of primary granules in the presence of fMLP but not LPS [29]. Activation of human neutrophils with LPS induces Cyto B‐independent secretory vesicle mobilization, as denoted by increases in surface CD11b and TLT2 expression (3‐ to 6‐fold and ≤2 fold, respectively; Figs. 2 and 4A). Importantly, LPS in the presence or absence of Cyto B induces only modest exocytosis of secondary/tertiary granules (∼2‐fold increase in CD66b expression) and no exocytosis of primary granules (no increase in CD66b expression; Fig. 4A). These data suggest that relatively low levels of TLT2 are contained in secretory vesicles and are readily mobilized in response to LPS. Treatment of human neutrophils with fMLP alone results in a 5‐ to 7‐fold increase in CD11b and CD66b, no increase in CD63, and ≤2‐fold change in TLT2 (Fig. 4B). Thus, even though fMLP induces a significant increase in secretory vesicle and secondary/tertiary granule exocytosis, this is not accompanied by a proportional increase in TLT2 expression on the surface. Importantly, addition of fMLP in the presence of Cyto B does not result in a further increase in CD11b expression, suggesting that fMLP alone induces complete exocytosis of secretory vesicles. In contrast, treatment of cells with fMLP + Cyto B results in a large increase in CD66b and CD63 expression, which is accompanied by a proportional increase in TLT2 expression. Thus, only under conditions in which exocytosis of primary granules is induced is there a significant increase in TLT2 expression (Fig. 4B). The relative change in MFI for TLT2 under conditions in which secretory vesicle exocytosis is maximal is ≤2‐fold, whereas upon primary granule release, this maximally increases to ∼25‐fold. Together, with the immunofluorescence data depicted in Fig. 3, these results support the conclusion that low levels of TLT2 are stored in secretory vesicles, whereas the largest stores of TLT2 are contained in primary granules. This observation is striking, as primary granules contain relatively few membrane proteins, whereas they store soluble, potent antimicrobial proteases, cytolytic enzymes, and defensins. Primary granules do not typically exocytose to the plasma membrane but instead, fuse with phagolysosomes, where they empty their microbicidal contents [30]. This raises the possibility that TLT2 is stored in a specific subset of primary granules that are preferentially targeted to the plasma membrane, as opposed to phagolysosomes. Previous studies support the conclusion that primary granules can be segregated into subsets according to their composition of defensins [31].
Figure 4.
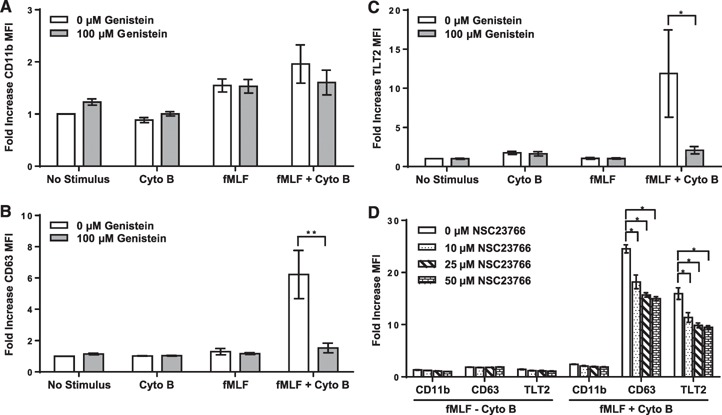
Up‐regulation of TLT2 expression correlates with primary granule exocytosis. Neutrophils were treated with (A) LPS (1 μM) ± Cyto B (5 μM) or (B) fMLP (1 μM) ± Cyto B (5 μM) to induce full degranulation for 0, 30, or 60 min at 37°C. Cells were fixed, then stained with mAb against TLT2, CD11b (activation and secretory vesicles), CD66b (secondary/tertiary granules), and CD63 (primary granules). The means ± sd of the fold increase in MFI for each marker are depicted. Data are from healthy donors, n = 3. *P < 0.05.
Studies were subsequently performed to assess the biochemical pathways that are required for exocytosis of TLT2 in response to fMLP + Cyto B. Previous work has demonstrated that primary and secondary granule exocytosis is dependent on activation of Src family PTKs [32, 33–34]. To confirm that increased expression of TLT2 is indeed dependent on PTK activation, experiments were performed in which human neutrophils were treated with fMLP + Cyto B in the presence or absence of genistein (PTK inhibitor). Treatment of neutrophils with genistein was observed to block up‐regulation of TLT2 in response to fMLP + Cyto B ( Fig. 5C ). As demonstrated previously, inhibition of Src family PTKs does not affect secretory vesicle release based on analysis of CD11b expression (Fig. 5A). However, exocytosis of primary granules (based on CD63 expression) is blocked completely by genistein, as is expression of TLT2 (Fig. 5B), confirming that TLT2 is stored in primary granules, and its mobilization is dependent on Src family PTK activation. In addition to Src family PTK activation, it has been shown that mobilization of primary granules is dependent on Rac‐mediated F‐actin formation, as this promotes primary granule movement to the cell membrane, in conjunction with actin depolarization at the cell cortex (mediated by Cyto B), which induces optimal exocytosis [35, 36]. In agreement with these findings, treatment of human neutrophils with NSC23766 (Rac inhibitor) significantly attenuates CD63 and TLT2 expression in response to fMLP + Cyto B (Fig. 5D). These data support the conclusion that mobilization of TLT2 to the cell surface is indeed dependent on activation of PTK‐dependent signaling pathways and Rac‐mediated F‐actin remodeling in association with cortical actin depolarization.
Figure 5.
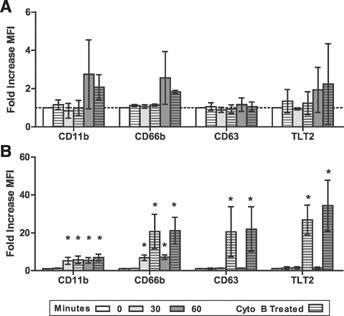
Inhibition of PTK and Rac GTPase activation abrogates TLT2 translocation to the cell surface. Neutrophils were pretreated with genistein (100 μM) and then activated with fMLP (fMLF; 1 μM) or fMLP + Cyto B (5 μM) for 30 min at 37°C to induce degranulation. Flow cytometric analysis was used to monitor the fold increase in expression for (A) CD11b, (B) CD63, and (C) TLT2. The data are depicted as means ± sd of the fold increase in MFI. Data are from healthy donors, n = 5. *P < 0.05, **P < 0.01. (D) Neutrophils were pretreated with various doses of the Rac inhibitor NSC23766 and activated with fMLP (1 μM) or fMLP + Cyto B (5 μM) for 30 min at 37°C to induce degranulation. Surface expression of CD11b, CD63, and TLT2 was measured by flow cytometry. The means ± sd of the fold increase in MFI are depicted. Data are from healthy donors, n = 3. *P < 0.05.
TLT2 is not a component of NETs
Upon activation in response to microbial pathogens, neutrophils expel their DNA as an antimicrobial weapon, on which microbicidal contents contained within the primary granules (e.g., MPO, defensins) are displayed across the DNA, creating NETs [37, 38]. As TLT2 expression on neutrophils is modulated in response to bacterial products (Fig. 2), and the major intracellular pool of TLT2 is stored in primary granules (Figs. 3 and 4), it was of interest to determine if soluble forms of TLT2 are generated before or in conjunction with primary granule exocytosis as a result of the action of proteases. Others have demonstrated that members of the TREM family, including TREM‐1 and TLT1, are produced in a soluble form [12, 39, 40]. Thus, soluble TLT2 may be generated and displayed on NETs, where it plays a role in the antimicrobial response. To this end, neutrophils were activated with the classic NET inducer PMA and stained for TLT2 and MPO, an antimicrobial enzyme stored in primary granules that coats the surface of NETs. Unlike MPO (red), which exhibits a clear association with extruded NETs (DAPI, blue; Fig. 6A ), TLT2 (green) is not associated with NETs and exhibits a staining pattern suggestive of plasma‐membrane retention and to some extent, within the cytosolic remnants of the cell (Fig. 6B). These data clearly demonstrate that a soluble form of TLT2 is not associated with NETs and remains localized to the plasma membrane following netosis.
Figure 6.
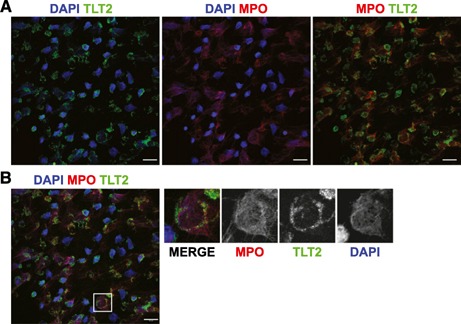
Primary granule exocytosis leads to association of TLT2 with the plasma membrane but not NETs. Neutrophils were treated with PMA (100 nM) for 4 h at 37°C to induce NET formation. (A) Cells were fixed and stained with antibody against MPO (red), DAPI (blue), and TLT2 (green). (B) Three–color overlay with colors representing the same markers as in A. The region denoted by the white box has been magnified and depicted as 3‐color and single‐color images. The images are representative of 3 different healthy donors. Original scale bars, 20 μm.
The results from these studies support the conclusion that TLT2 expression is conserved between mouse and human. Additionally, TLT2 expression on mouse and human neutrophils can be up‐regulated in response to a range of agonists, most likely as a result of secretory vesicle mobilization. However, it was striking to find that the largest pool of intracellular TLT2 is stored in primary granules, and upon stimulation, primary granules containing TLT2 are exocytosed, resulting in deposition of TLT2 onto the plasma membrane, where it presumably interacts with its ligand to regulate terminal neutrophil effector function.
AUTHORSHIP
K.A.T. designed and performed experiments, analyzed data, and prepared the manuscript. R.G.K. contributed to experimental design, data analysis, and manuscript preparation. C.M.S. helped perform experiments. L.B.J. directed and financed the research, as well as contributed to experimental design, data analysis, and manuscript preparation.
DISCLOSURES
The authors have no conflicts of interest to declare.
Supporting information
Supplementary data
Supplementary data
ACKNOWLEDGMENTS
This study was supported, in part, by the U.S. National Institutes of Health (NIH) Grant AI107232‐01 (to L.B.J.). The authors thank the laboratories of Chad Steele, Amit Gaggar, Phillip Smith, and Zdenek Hel at University of Alabama at Birmingham (UAB) for the use of equipment and sharing of reagents. The authors also thank Christine Sestero and Preeyam Patel for sample isolation and Mary Ann Accavatti‐Loper of the UAB Hybridoma Core for mAb generation and Shawn Williams of the UAB High Resolution Imaging Core (both supported by NIH Grant P30 AR048311).
REFERENCES
- 1. Barclay, A. N. (2003) Membrane proteins with immunoglobulin‐like domains—a master superfamily of interaction molecules. Semin. Immunol. 15, 215–223. [DOI] [PubMed] [Google Scholar]
- 2. Chavakis, T. (2012) Leucocyte recruitment in inflammation and novel endogenous negative regulators thereof. Eur. J. Clin. Invest. 42, 686–691. [DOI] [PubMed] [Google Scholar]
- 3. Golias, C. , Batistatou, A. , Bablekos, G. , Charalabopoulos, A. , Peschos, D. , Mitsopoulos, P. , Charalabopoulos, K. (2011) Physiology and pathophysiology of selectins, integrins, and IgSF cell adhesion molecules focusing on inflammation. A paradigm model on infectious endocarditis. Cell Commun. Adhes. 18, 19–32. [DOI] [PubMed] [Google Scholar]
- 4. Ford, J. W. , McVicar, D. W. (2009) TREM and TREM‐like receptors in inflammation and disease. Curr. Opin. Immunol. 21, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klesney‐Tait, J. , Turnbull, I. R. , Colonna, M. (2006) The TREM receptor family and signal integration. Nat. Immunol. 7, 1266–1273. [DOI] [PubMed] [Google Scholar]
- 6. Allcock, R. J. , Barrow, A. D. , Forbes, S. , Beck, S. , Trowsdale, J. (2003) The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur. J. Immunol. 33, 567–577. [DOI] [PubMed] [Google Scholar]
- 7. King, R. G. , Herrin, B. R. , Justement, L. B. (2006) TREM‐like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up‐regulated in response to inflammation. J. Immunol. 176, 6012–6021. [DOI] [PubMed] [Google Scholar]
- 8. Halpert, M. M. , Thomas, K. A. , King, R. G. , Justement, L. B. (2011) TLT2 potentiates neutrophil antibacterial activity and chemotaxis in response to G protein‐coupled receptor‐mediated signaling. J. Immunol. 187, 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossi, D. , Zlotnik, A. (2000) The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242. [DOI] [PubMed] [Google Scholar]
- 10. De Freitas, A. , Banerjee, S. , Xie, N. , Cui, H. , Davis, K. I. , Friggeri, A. , Fu, M. , Abraham, E. , Liu, G. (2012) Identification of TLT2 as an engulfment receptor for apoptotic cells. J. Immunol. 188, 6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barrow, A. D. , Astoul, E. , Floto, A. , Brooke, G. , Relou, I. A. , Jennings, N. S. , Smith, K. G. , Ouwehand, W. , Farndale, R. W. , Alexander, D. R. , Trowsdale, J. (2004) Cutting edge: TREM‐like transcript‐1, a platelet immunoreceptor tyrosine‐based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP‐2. J. Immunol. 172, 5838–5842. [DOI] [PubMed] [Google Scholar]
- 12. Morales, J. , Villa, K. , Gattis, J. , Castro, W. , Colon, K. , Lubkowski, J. , Sanabria, P. , Hunter, R. , Washington, A. V. (2010) Soluble TLT‐1 modulates platelet‐endothelial cell interactions and actin polymerization. Blood Coag. Fibrinolysis 21, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamerman, J. A. , Jarjoura, J. R. , Humphrey, M. B. , Nakamura, M. C. , Seaman, W. E. , Lanier, L. L. (2006) Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)‐2 and DAP12. J. Immunol. 177, 2051–2055. [DOI] [PubMed] [Google Scholar]
- 14. Piccio, L. , Buonsanti, C. , Mariani, M. , Cella, M. , Gilfillan, S. , Cross, A. H. , Colonna, M. , Panina‐Bordignon, P. (2007) Blockade of TREM‐2 exacerbates experimental autoimmune encephalomyelitis. Eur. J. Immunol. 37, 1290–1301. [DOI] [PubMed] [Google Scholar]
- 15. Bleharski, J. R. , Kiessler, V. , Buonsanti, C. , Sieling, P. A. , Stenger, S. , Colonna, M. , Modlin, R. L. (2003) A role for triggering receptor expressed on myeloid cells‐1 in host defense during the early‐induced and adaptive phases of the immune response. J. Immunol. 170, 3812–3818. [DOI] [PubMed] [Google Scholar]
- 16. Bouchon, A. , Facchetti, F. , Weigand, M. A. , Colonna, M. (2001) TREM‐1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107. [DOI] [PubMed] [Google Scholar]
- 17. Xu, J. C. , Gao, F. , Fu, F. Q. , Chen, Y. J. , Xu, P. , Zhou, B. , Zhang, X. G. (2013) Generation and characterization of two novel monoclonal antibodies produced against human TLT‐2 molecule. Monoclon. Antib. Immunodiagn. Immunother. 32, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashiguchi, M. , Kobori, H. , Ritprajak, P. , Kamimura, Y. , Kozono, H. , Azuma, M. (2008) Triggering receptor expressed on myeloid cell‐like transcript 2 (TLT‐2) is a counter‐receptor for B7‐H3 and enhances T cell responses. Proc. Natl. Acad. Sci. USA 105, 10495–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouchon, A. , Dietrich, J. , Colonna, M. (2000) Cutting edge: inflammatory responses can be triggered by TREM‐1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995. [DOI] [PubMed] [Google Scholar]
- 20. Washington, A. V. , Schubert, R. L. , Quigley, L. , Disipio, T. , Feltz, R. , Cho, E. H. , McVicar, D. W. (2004) A TREM family member, TLT‐1, is found exclusively in the alpha‐granules of megakaryocytes and platelets. Blood 104, 1042–1047. [DOI] [PubMed] [Google Scholar]
- 21. Prada, I. , Ongania, G. N. , Buonsanti, C. , Panina‐Bordignon, P. , Meldolesi, J. (2006) Triggering receptor expressed in myeloid cells 2 (TREM2) trafficking in microglial cells: continuous shuttling to and from the plasma membrane regulated by cell stimulation. Neuroscience 140, 1139–1148. [DOI] [PubMed] [Google Scholar]
- 22. R⊘rvig, S. , Honore, C. , Larsson, L. I. , Ohlsson, S. , Pedersen, C. C. , Jacobsen, L. C. , Cowland, J. B. , Garred, P. , Borregaard, N. (2009) Ficolin‐1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J. Leukoc. Biol. 86, 1439–1449. [DOI] [PubMed] [Google Scholar]
- 23. Luerman, G. C. , Powell, D. W. , Uriarte, S. M. , Cummins, T. D. , Merchant, M. L. , Ward, R. A. , McLeish, K. R. (2011) Identification of phosphoproteins associated with human neutrophil granules following chemotactic peptide stimulation. Mol. Cell. Proteomics 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerecedo, D. , Cisneros, B. , Gomez, P. , Galvan, I. J. (2010) Distribution of dystrophin‐ and utrophin‐associated protein complexes during activation of human neutrophils. Exp. Hematol. 38, 618–628. [DOI] [PubMed] [Google Scholar]
- 25. Mollinedo, F. (2003) Human neutrophil granules and exocytosis molecular control. Inmunologia 22, 340–358. [Google Scholar]
- 26. Borregaard, N. , Cowland, J. B. (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521. [PubMed] [Google Scholar]
- 27. Borregaard, N. , Kjeldsen, L. , Lollike, K. , Sengelov, H. (1995) Granules and secretory vesicles of the human neutrophil. Clin. Exp. Immunol. 101 (Suppl 1), 6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sengel⊘v, H. , Follin, P. , Kjeldsen, L. , Lollike, K. , Dahlgren, C. , Borregaard, N. (1995) Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 154, 4157–4165. [PubMed] [Google Scholar]
- 29. Nogare, A. R. , Yarbrough, W. C., Jr. (1990) A comparison of the effects of intact and deacylated lipopolysaccharide on human polymorphonuclear leukocytes. J. Immunol. 144, 1404–1410. [PubMed] [Google Scholar]
- 30. Hirsch, J. G. , Cohn, Z. A. (1960) Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J. Exp. Med. 112, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rice, W. G. , Ganz, T. , Kinkade, J. M., Jr. , Selsted, M. E. , Lehrer, R. I. , Parmley, R. T. (1987) Defensin‐rich dense granules of human neutrophils. Blood 70, 757–765. [PubMed] [Google Scholar]
- 32. Meshki, J. , Tuluc, F. , Bredetean, O. , Ding, Z. , Kunapuli, S. P. (2004) Molecular mechanism of nucleotide‐induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am. J. Physiol. Cell Physiol. 286, C264–C271. [DOI] [PubMed] [Google Scholar]
- 33. Tuluc, F. , Garcia, A. , Bredetean, O. , Meshki, J. , Kunapuli, S. P. (2004) Primary granule release from human neutrophils is potentiated by soluble fibrinogen through a mechanism depending on multiple intracellular signaling pathways. Am. J. Physiol. Cell Physiol. 287, C1264–C1272. [DOI] [PubMed] [Google Scholar]
- 34. Lacy, P. , Eitzen, G. (2008) Control of granule exocytosis in neutrophils. Front. Biosci. 13, 5559–5570. [DOI] [PubMed] [Google Scholar]
- 35. Abdel‐Latif, D. , Steward, M. , Lacy, P. (2005) Neutrophil primary granule release and maximal superoxide generation depend on Rac2 in a common signalling pathway. Can. J. Physiol. Pharmacol. 83, 69–75. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell, T. , Lo, A. , Logan, M. R. , Lacy, P. , Eitzen, G. (2008) Primary granule exocytosis in human neutrophils is regulated by Rac‐dependent actin remodeling. Am. J. Physiol. Cell Physiol. 295, C1354–C1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Branzk, N. , Papayannopoulos, V. (2013) Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 35, 513–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brinkmann, V. , Reichard, U. , Goosmann, C. , Fauler, B. , Uhlemann, Y. , Weiss, D. S. , Weinrauch, Y. , Zychlinsky, A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 39. Gibot, S. , Kolopp‐Sarda, M. N. , Béné, M. C. , Bollaert, P. E. , Lozniewski, A. , Mory, F. , Levy, B. , Faure, G. C. (2004) A soluble form of the triggering receptor expressed on myeloid cells‐1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Derive, M. , Bouazza, Y. , Sennoun, N. , Marchionni, S. , Quigley, L. , Washington, V. , Massin, F. , Max, J. P. , Ford, J. , Alauzet, C. , Levy, B. , McVicar, D. W. , Gibot, S. (2012) Soluble TREM‐like transcript‐1 regulates leukocyte activation and controls microbial sepsis. J. Immunol. 188, 5585–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data


