Short abstract
A microfluidic assay enables precise characterization of neutrophil migration in the native blood environment, independent of the donor species, and at single cell resolution.
Keywords: neutrophil, migration, inbred lines
Abstract
Animal models of human disease differ in innate immune responses to stress, pathogens, or injury. Precise neutrophil phenotype measurements could facilitate interspecies comparisons. However, such phenotype comparisons could not be performed accurately with the use of current assays, as they require the separation of neutrophils from blood using species‐specific protocols, and they introduce distinct artifacts. Here, we report a microfluidic technology that enables robust characterization of neutrophil migratory phenotypes in a manner independent of the donor species and performed directly in a droplet of whole blood. The assay relies on the particular ability of neutrophils to deform actively during chemotaxis through microscale channels that block the advance of other blood cells. Neutrophil migration is measured directly in blood, in the presence of other blood cells and serum factors. Our measurements reveal important differences among migration counts, velocity, and directionality among neutrophils from 2 common mouse strains, rats, and humans.
Abbreviations
- FCC
focal chemotaxis chamber
- LTB4
leukotriene B4
- PDMS
polydimethylsiloxane
- WBLC
whole‐blood loading chamber
Introduction
Murine models are used in a wide variety of research areas, including inflammation [1] and infectious disease [2, 3], and are currently the most used models to screen drug candidates before human trials [4]. The overall structure of the immune system in mice, rats, and humans is quite similar [5]; however, controversy exists about how closely murine models of inflammation match corresponding human diseases [6, 7–8]. The proportion of neutrophils and lymphocytes in the blood of these mammals is considerably different: human blood is neutrophil rich (∼70% neutrophils), whereas mouse and rat blood is lymphocyte rich (10–20% neutrophils) [8]. Significant differences in WBC counts have been identified, even among common mouse strains [9]. Moreover, mouse and human neutrophils are known to have distinct chemoattractant receptor density and affinity [10], interact differently with selectins [11], and use distinct intracellular signaling pathways [12]. Some of these differences have been associated with physiologic [13] and pathologic differences [14], such as strain‐specific susceptibility to bacterial infections. For instance, some mouse strains are resistant to Salmonella enterica and survive acute infections (e.g., Sv129S6), whereas others are susceptible and die quickly after infections (e.g., the common C57BL/6) [15]. Recognition and quantification of the immune variations among commonly used inbred mouse strains are essential for the accurate interpretation of immune responses and for the efficient translation of findings from murine models to humans. If the differences among strains could be easily quantified, then researchers would be able to take into account not only the genes of interest but also how the background strain contributes to the manifestation of disease and therapeutic responses in various species [16, 17].
So far, all systematic comparisons of neutrophil migration in human and animal models have been performed using isolated cells, removed from their blood environment and lacking species‐specific serum influences. The Transwell (Boyden chamber) assay only provides a bulk end‐point measurement, and therefore, directionality of neutrophil migration cannot be measured [18, 19]. Current neutrophil separation methods, developed originally for human donors, require large volumes of blood [20, 21–22] and are less suitable for mice as a result of their significantly lower circulatory volume. Therefore, many studies on mouse neutrophils are done with bone marrow cells [23], which are heterogeneous and functionally immature. Standard negative enrichment of neutrophils includes a lengthy (∼3 h) protocol, during which the neutrophil phenotype can change. Moreover, antibody cocktails for neutrophil isolation are less specific for mouse than human, and activation levels of neutrophils affect the purity and yield [24]. Positive‐selection enrichment strategies that rely on neutrophil capture from blood by selective adhesion require species‐matched capture molecules and inevitably activate the neutrophils before the migration starts [25, 26].
To circumvent the limitations of current neutrophil separation and migration techniques, we have previously developed microfluidic assays to measure the migration of human neutrophils from a single droplet of unprocessed blood. These platforms used mechanical filters with right‐angle turns that selectively block RBCs without impeding on active neutrophil migration patterns [27, 28]. However, these assays could not be applied to murine neutrophils, as RBCs are significantly smaller than in humans (75% diameter, 50% thickness), obstructing the migration channels and preventing neutrophil migration. Consequently, new designs are required to be compatible with human and murine whole blood for neutrophil chemotaxis assays.
Here, we report the development of a neutrophil migration assay, which can use as little as 2 µl mouse whole blood pipetted directly into the device. The assay measures the migration of neutrophils in the presence of all blood components and is independent of the donor species. The assay relies on physical properties shared by neutrophils from various species, which allow them to deform actively during chemotaxis through microscale channels. A combination of constrictions and turns in these channels blocks murine RBCs, while allowing neutrophil migration. This novel microfluidic platform enables precision measurements, at single‐cell resolution, of several chemotaxis phenotype parameters—fraction of cells migrating, velocity, and directionality—for C57BL/6 and Sv129S6 mice, Wistar rats, and human neutrophils.
METHODS
Microfluidic device design
The microfluidic device to study mouse, rat, and human neutrophil chemotaxis from 1 droplet of whole blood (see Fig. 1) is designed with 3 main components: FCCs (200 × 200 µm), a central WBLC, and migration channels. All reagents and whole blood are pipetted into the device. There is no flow or requirement of external syringe pump, and the device has no moving parts. The RBC filtering regions consist of 10 short channels (length ∼75 µm) with 3.5 µm constrictions (“pinch”), connected horizontally through a common channel (∼200 µm) to create 90° bending sections capable of blocking RBCs in the whole‐blood sample from dispersing into the rest of the migration channel (see Fig. 2). All migration channels were designed to be 12 µm wide and 3 µm high to establish only a single column of RBCs for efficient trapping while allowing neutrophils to migrate easily through. Neutrophil migration is guided by a gradient of chemoattractant, which is established along the migration channels by diffusion between the FCCs and WBLC. Robust quantification of neutrophil migration in confined channels is more accurate than on a planar surface, as individual neutrophil trajectory, speed, and persistence are consistent over time.
Figure 1.
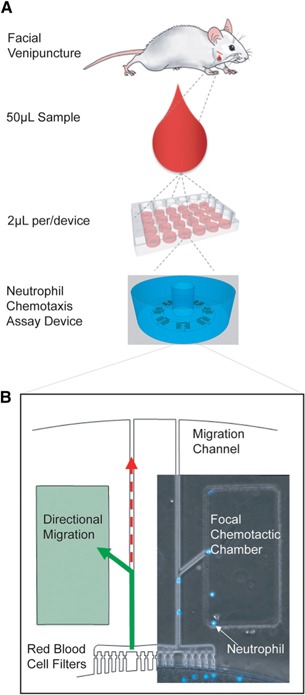
Microfluidic platform for nonlethal measurements of murine neutrophil migration patterns. (A) For mouse models, a 50 µl droplet of capillary whole blood is taken via facial venipuncture procedure without anesthesia. Heparin anticoagulant and Hoechst stain are added to sample, and 2 µl blood is added to each microfluidic neutrophil chemotaxis assay. Each neutrophil chemotaxis assay device includes 1 WBLC surrounded by 16 FCCs primed with chemoattractant. Each device is 5 mm in diameter, and 12 assays can be run in parallel in 12‐well, glass‐bottom plates. (B) Neutrophils migrate out of the WBLC along the chemoattractant gradient(green) in the migration channel. The RBC filter that includes a 3 µm pinch and right‐angle geometries traps RBCs but allows actively migrating neutrophils to pass. A bifurcation before each FCC facilitates quantification of directional neutrophils following the chemoattractant gradient from randomly migrating cells that exit the device. Neutrophil (blue) counts accumulating in the FCC are obtained with time‐lapse imaging.
Figure 2.
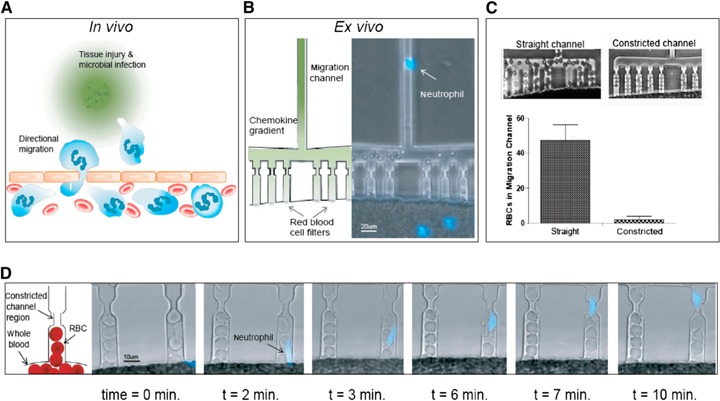
Microfluidic platform to measure mouse, rat, and human neutrophil migratory function from a droplet of whole blood. (A) After injury and microbial infection (green grains), damaged tissue and bacteria produce gradients of chemoattractants that act as a compass for neutrophil chemotaxis. (B) To prevent the granular flow of RBCs through the migration channels, we implemented mechanical filters that selectively block the advancement of human and murine RBCs. The RBC filter that includes a 3 µm constriction and right‐angle geometries traps RBCs but allows actively migrating neutrophils (blue) to pass. (C) The constriction just upstream of the neutrophil migration channel significantly reduces the number of mouse RBCs in the migration channel (P < 0.05). This prevents clogging of the migration channel and allows mouse neutrophils to migrate actively without obstruction. (D) RBC filtration comb. High‐resolution (40×), bright‐field images of the RBC filtration comb. A 3 µm pinch in the RBC filtration comb size excludes mouse RBCs from obstructing the upstream migration channel, whereas actively migrating neutrophils (blue‐stained nucleus—Hoechst dye) are deformable and can migrate through this bottleneck without slowing in velocity.
Microfluidic device fabrication
Microfluidic devices were produced by replica molding PDMS (Sylgard 184; Elsworth Adhesives, Wilmington, MA, USA) on a master wafer, fabricated using standard microfabrication techniques. First, a silicon wafer (Desert Silicon, Grandale, AZ, USA) was spun, coated with a 3 µm layer of photoresist (SU‐8 2; MicroChem, Newton, MA, USA), and patterned by photolithography using a Mylar photomask (FineLine Imaging, Colorado Springs, CO, USA) to define the migration channels. Afterward, a second layer of photoresist (SU‐8 50) was processed to define the 50 µm‐thick central WBLC and FCCs, aligned to the migration channels. PDMS and the curing agent were then mixed (10:1 ratio), poured on the master wafer, and left to de‐gas for 1 h in a vacuum chamber. After curing for at least 12 h in an oven set to 65°C, the PDMS layer was peeled off the master and punched with 1.5 and 5 mm punchers (Harris Uni‐Core; Ted Pella, Reading, CA, USA) to shape the blood loading well and the whole donut‐shaped devices, respectively. Finally, a 12‐well glass‐bottom plate (MatTek, Ashland, MA, USA) was plasma treated, along with the PDMS donut‐shaped devices, and the devices bonded to the plate on a hot plate (85°C for 10 min).
Whole‐blood sample collection
From mice, 50 µl capillary blood was collected by the facial vein method (in accordance with the guidelines of Massachusetts General Hospital, approved as Institutional Animal Care and Use Protocol #2007N000136). The protocol required no anesthesia. From rats, 50 µl venous blood was collected from the tail vein (in accordance with the guidelines of Massachusetts General Hospital, approved as Institutional Animal Care and Use Protocol #2012N000034) by use of 1–2% isoflurane inhalant. The blood from mice was collected directly into Eppendorf tubes containing a mixed solution of HBSS media, heparin anticoagulant (1.65 USP/50 µl blood), and Hoechst stain (10 µl, 32.4 µM). The blood from rats was first aspirated in a pipette tip and then transferred to Eppendorf tubes. The tubes were incubated for 10 min at 37°C and 5% CO2 to allow for proper staining of the nuclei. From humans, 50 µl blood samples were prepared from venous blood collected by phlebotomy in standard tubes with heparin anticoagulant (Vacutainer; Becton, Dickinson and Co., Franklin Lanes, NJ, USA). Previously, we have shown that the blood source for the assay (venous vs. finger prick) does not significantly alter neutrophil migration patterns [27]. Samples were obtained with written, informed consent and through procedures approved by and in accordance with the guidelines of the Institutional Review Board at Massachusetts General Hospital (2008‐P‐002123).
Device priming and cell loading
Immediately after bonding to the well plate and before cell loading, donut‐shaped devices were filled with the chemoattractant solution. Whenever possible, specific chemoattractants for each neutrophil species were used: peptide fMLP (MW 437.55; Sigma‐Aldrich, St. Louis, MO, USA), recombinant human complement component C5a protein (8.3 kDa; R&D Systems, Minneapolis, MN, USA), recombinant mouse complement component C5a (9.0 kDa; R&D Systems), and LTB4 (MW 336.5; Cayman Chemical, Ann Arbor, MI, USA). With the chemoattractant, 25 nM species‐specific fibronectin (Sigma‐Aldrich) was added to the microfluidic devices to coat the glass and PDMS surfaces (physical absorption). Fibronectin coating has been reported previously to facilitate neutrophil migration on flat surfaces [25] and in Transwell assays [29]. The well plate was then placed in a desiccator under vacuum to de‐gas for 15 min to ensure proper filling of the FCCs, while the PDMS surface was still hydrophilic. Afterward, the central WBLC and the outside region surrounding the donut were washed thoroughly in each well to establish the gradient along the migration channels. The wells of the plate were then filled with IMDM with 20% FBS and allowed to sit for a period of 15 min to generate stable chemoattractant gradients. Finally, with the use of a gel‐loading tip, 2 µl whole blood was slowly pipetted into the central WBLC (Supplemental Fig. 1).
Chemotaxis imaging and gradient measurements
Time‐lapse imaging was performed on a Nikon Eclipse Ti microscope inside of a biochamber heated to 37°C with 5% CO2 and 80% humidity. Separate experiments to characterize the formation of gradients along the migration channels in the absence of cells were performed under similar temperature and gas conditions by replacing the chemoattractant with fluorescein (Sigma‐Aldrich) of MW = 332, compared with that of fMLP (MW = 438) and LTB4 (MW = 336). For each experiment, at least 50 neutrophils were manually tracked. Directionality of neutrophils moving through the channels toward FCCs was quantified by calculating the fraction of cells that followed the chemotactic gradient and turned toward the FCC at the bifurcation. For experiments involving C5a chemoattractant, we tracked neutrophils moving on the flat bottom of the WBLC in the vicinity of the entrances to the migration channels. For all conditions, neutrophil migration velocities were calculated using ImageJ software (NIH, Bethesda, MD, USA).
Finite element model (COMSOL) of diffusion of chemoattractant gradient of device
The percentage of neutrophils migrating from the whole‐blood sample was estimated using a COMSOL simulation model (COMSOL, Burlington, MA, USA; Supplemental Fig. 2). For human whole‐blood samples, we calculated that ∼220 neutrophils in each well are exposed to on critical concentration of 1 nM and gradient concentration stronger than 1 pM/µm, considered to be compatible with cell migration (Supplemental Fig. 3). For some conditions, the percentage of neutrophils activated by specific chemokines was estimated based on the percentage of the neutrophils inside of the whole‐blood compartment, exposed to above‐threshold concentrations and gradients, that moved over distances larger than 1 cell length.
Transwell assay control experiments
Mouse neutrophil migration was evaluated by a Transwell assay, as described previously [30]. In brief, neutrophils were isolated from the bone marrow of C57BL/6 and Sv129S6 mice at 8–10 wk of age (Charles River Laboratories, Wilmington, MA, USA) by density gradient centrifugation by use of Histopaque 1.077 g/ml and 1.119 g/ml (Sigma‐Aldrich). Flow cytometry showed that >95% of the isolated cells were positive for anti‐LY6G antibody (BD Biosciences, Franklin Lakes, NJ, USA). Cells (105), resuspended in 100 μl RPMI 1640, supplemented with 10% FBS, were placed onto each upper chamber of a 96‐well chemotaxis plate with 3 μm pore diameter (EMD Millipore, Billerica, MA, USA). In the lower chambers, 150 μl of the RPMI medium, with or without LTB4 (100 nM) or fMLP (100 nM), was added. After incubated for 1 h at 37° under 5% CO2, the numbers of the cells in the lower chambers were counted.
Statistical analysis
All experiments were performed in triplicate. We calculated means and sd for each condition. We compared the conditions by use of 2‐tailed Studentˈs t test, performed using Prism (GraphPad Software, La Jolla, CA, USA). Differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Microfluidic platform design and validation
For studies in mice, blood was obtained using the facial vein technique [31], without euthanizing the animal and requiring no anesthesia. From rats, the blood was obtained from the tail vein, requiring anesthesia (isoflurane inhalation). From 1 droplet of blood (∼50 µl), we were able to run 24 microfluidic devices in a glass‐bottomed plate, with 1 device/well and 16 FCCs/microfluidic device ( Fig. 1 ).
The key design feature that enables the use of whole blood directly in the microfluidic device is the RBC filter. Murine RBCs (average diameter = 6 μm; thickness = 1 μm) are of smaller geometry than human (average diameter = 7–8 μm; thickness = 2 μm). RBCs, pushing on each other under the effect of gravity, are mechanically blocked at the entrance of the migration channels by the combined effects of the flat channel cross‐section, the 90° angle comb [27], and the 3.5 µm pinch and remain confined inside of the central loading chamber ( Fig. 2B ). The pinch is key to reducing the number of murine RBCs advancing inside of the migration channel, from 47.9 ± 9.8 to 2.3 ± 3.3 cells (P < 0.001; Fig. 2C). The trapped RBCs do not clog the channel at the pinch because of the difference in height between the channel (3 µm) and the thickness of murine RBCs that can only enter the channel on the side. Moreover, sufficient space remains between the RBCs and the channel walls to allow chemokine diffusion. The gradient that forms between the FCCs (source) and the WBLC (sink) guides neutrophil chemotaxis. Neutrophils are able to deform and migrate actively through the pinch. Once the neutrophils have passed the pinch, they continue to follow the chemoattractant gradient along the migration channel and enter the FCC (Fig. 2D and Supplemental Movie 1). A bifurcation in the channel creates a “decision point,” where neutrophils can migrate toward or away from the chemoattractant gradient, providing critical information about their directionality.
Device validation
We validated the assay using neutrophils collected from C57BL/6 and Sv129S6 mice, Wistar rats, and humans. We observed that neutrophils from Sv129S6 mice reach the FCC with LTB4 at 2 h and continue to accumulate for 3–4 h before their numbers inside of the FCCs reach a plateau. The size of the migration channels prevents the migration of lymphocytes and monocytes, which deform less and require larger channels for migration [21, 32]. We verified the selectivity of the assay for neutrophils by observing the characteristic polymorph (segmented) shape of the nucleus stained with fluorescent dye.
We compared the migration of neutrophils in blood samples obtained from the same mice once/wk for 3 wk (n = 3). The neutrophil accumulation curve varied <5% among the 3 samples ( Fig. 3A ). Therefore, perturbations from this baseline in subsequent samples could be representative of significant changes in the neutrophil migratory phenotype during physiologic or pathologic responses.
Figure 3.
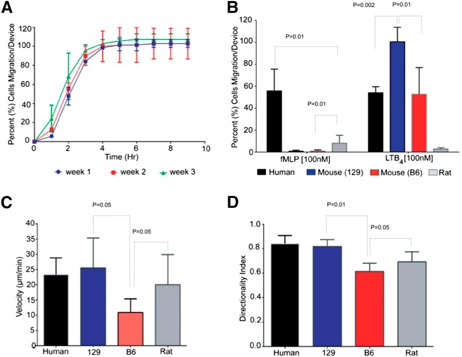
Characterization of mouse, rat, and human neutrophil migration from whole blood. (A) Neutrophil accumulation profiles in response to LTB4 were consistent over a 3 wk time period for the same mice (Sv129S6). This suggests that a neutrophil migration baseline is feasible and could provide a reliable reference in models of disease that evolve over time. (B) Neutrophil migration from a droplet of whole blood from human healthy donors (black bars), Sv129S6 mice (129; blue bars), C57BL/6 mice (B6; red bars), and Wistar rat (gray bars) toward 2 standard chemoattractants (fMLP and LTB4) was compared. Human cells that migrated to all chemoattractants and mouse cells (C57BL/6 and Sv129S6) only directionally migrated toward LTB4. Rat neutrophils migrated to fMLP in lower numbers (6.9‐fold less; P < 0.05) than human neutrophils. (C) Human, mouse, and rat neutrophil migration velocities toward LTB4 were compared. Human (22 µm/min), Sv129S6 (25 µm/min), and rat (20 µm/min) neutrophils migrated with comparable velocities, whereas C57BL/6 mouse neutrophil velocity was significantly lower (12 µm/min; P < 0.05). (D) Mouse and human neutrophil directional index toward LTB4 was compared. Human (0.85) and Sv129S6 (0.9) neutrophils migrated with comparable directionality, whereas C57BL/6 neutrophil directional index was significantly lower (0.6; P < 0.05). Experiments presented in B–D were repeated 3 times.
The measurements are performed in the presence of all blood components and could be highly multiplexed. Compared with traditional methods (i.e., Transwell assay), the device avoids lengthy neutrophil isolation steps, and it is performed in the same conditions and following the same sample preparation protocols regardless of the blood source. The assay requires microliter volumes of murine, rat, or human blood, which represent <0.1% of the total blood volume and can be collected from conscious animals. These capabilities allow for repeated measures without the potentially confounding effects of anesthetic drugs. The microfluidic device also has important advantages compared with recent techniques that rely on neutrophil capture from blood by selective adhesion, e.g., P‐selectin [25, 26]. By avoiding the cell‐washing steps, the new method preserves the integrity of the blood sample and with it, important cues that may modulate neutrophil activity from serum [33]. Interactions with other cells in the whole blood are also preserved. Moreover, by relying on physical (channel geometry) rather than biologic mechanisms (selectins or endothelial cells) to achieve selectivity for neutrophils, it eliminates the artificial activation of neutrophils via capture mechanisms and the need for species‐matched capture molecules.
Neutrophil migration characteristics: differences and similarities among mice, rats, and humans
To compare neutrophil migration phenotype among species, we measured chemotaxis to 2 standard chemoattractants (fMLP and LTB4) in humans, C57BL/6 and Sv129S6 mice, and Wistar rats. Human neutrophils in 2 µl whole‐blood samples migrated toward fMLP (55.8 ± 19.8%) and LTB4 (54.0 ± 5.6%; Fig. 3B). A lower percentage of Wistar rat neutrophils migrated to fMLP and LTB4 (10.9 ± 8.5 and 2.8 ± 1.1%, respectively). Surprisingly, LTB4 was the only chemoattractant able to induce significant neutrophil migration in all mouse neutrophils tested: Sv129S6 (99.5 ± 13%) and C57BL/6 (52.7 ± 24%). The velocity of C57BL/6 neutrophils toward LTB4 (13 ± 7.4 µm/min) was lower than that of Sv129S6 (19.7 ± 8.7 µm/min), rat (26.2 ± 5.9 µm/min), or human (23.4 ± 5.4 µm/min) neutrophils (Fig. 3C). The directional index of C57BL/6 neutrophils toward LTB4 (0.61 ± 0.07) was compared with that of rat neutrophils (0.62 ± 0.12) and lower than that of Sv129S6 (0.82 ± 0.06) or human (0.85 ± 0.06) neutrophils (Fig. 3D). A comparison between the whole‐blood assay and the traditional Transwell assay, in the presence of fMLP and LTB4 chemoattractants, showed essentially the same results in neutrophils in whole blood and neutrophils isolated from mouse bone marrow. In the Transwell assay, fMLP failed to increase significantly the number of migrated neutrophils compared with controls, whereas LTB4 effectively induced neutrophil migration in Sv129S6 (46.2 ± 3%) and C57BL/6 (37.8 ± 3%; Supplemental Fig. 4). More human neutrophils entered the FCC in response to C5a than mouse neutrophils (26.8 ± 5.9% vs. 1 ± 0.8%; Fig. 4A ). This difference correlates with more neutrophils being activated by C5a in human blood samples compared with the Sv129S6 mouse (63.3 ± 7.6% vs. 18.3 ± 3%; Fig. 4B). The directionality of Sv129S6 mouse neutrophils toward C5a was lower than in humans (Fig. 4C), whereas directionality toward LTB4 was comparable (Fig. 3D). These patterns of neutrophil migration in response to C5a are consistent with migration patterns reported previously from isolated human neutrophils [34].
Figure 4.
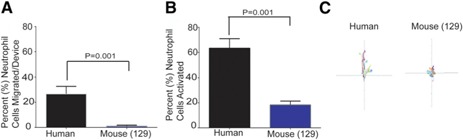
Directional versus nondirectional cell migration toward C5a in human and Sv129S6 mouse neutrophils. (A) The percentage of neutrophils migrating toward C5a (1 µM), from a droplet of whole blood is different for humans and Sv129S6 mice (n = 3; P < 0.05). (B) The percentage of neutrophils activated, based on migration phenotype inside of the whole‐blood compartment over distances larger than 1 cell length, reveals differences between human and Sv129S6 mouse neutrophils (n = 3, P < 0.05). (C) C5a causes less‐persistent directional migration in humans and nondirectional cell activation in mouse (Sv129S6) neutrophils compared with migration toward LTB4 (total length of axis is 1 mm distance inside of the device).
Several factors could explain the lack of mouse neutrophil migration toward fMLP in our whole‐blood devices and the apparent contradiction with previous reports [10, 35]. First, during the sample ‐preparation steps for the whole‐blood assay and for the Transwell assay, we were cautious not to activate the neutrophils. This approach is distinct from common protocols that use preactivated neutrophils and show sizeable mouse neutrophil migration toward fMLP, e.g., bone marrow neutrophils that were sensitized for several hours with dinitrofluorobenzene [10] or neutrophils that were collected from the peritoneal cavity after peritoneal injection with thioglycollate [35]. Second, the mouse blood assay uses concentrations of fMLP compatible with those used for human neutrophils and exposes known differences in the affinity of the mouse formyl peptide receptor 1 for fMLP, which are between 100 and 500 times less sensitive than in humans [36, 37]. Third, the design of our assay is the utmost specific for chemotaxing cells. Moving neutrophils have to navigate through several bifurcations before reaching the FCC, and the neutrophils that lack directionality have only a very low chance of reaching the FCC. Such specificity is absent in Transwell assays, where randomly moving cells could cross the membrane and are counted together with chemotaxing cells. Finally, our results that use LTB4 show comparable neutrophil migration in the whole‐blood and in the Transwell assays. These results further substantiate the claim that the differences in mouse neutrophil migration toward fMLP between the whole‐blood and previously reported Transwell assays are likely the reflection of differences in the biologic status of the cells, which are revealed by the new assay.
The differences of neutrophil migration among mouse, rat, and human neutrophils are consistent with other differences in innate immune responses. Examples include the in vivo differences in the sensitivity to proinflammatory stimuli, such as endotoxin (<0.015 mg/kg in humans compared with 5 mg/kg in mice [33]), and/or the lower numbers of bacteria in the bloodstream during severe infections (<100 colonies/ml in humans [38] compared with 100,000 to 1 million in mice [39]). Neutrophil migratory phenotype differences between humans and certain strains of mice might partially explain why some drugs were successful in C57BL/6 mouse models of sepsis but later failed in human clinical trials [6]. These results further emphasize the immune‐system differences known to exist between mouse strains [15, 16]. Among hundreds of laboratory mouse strains available, 2/3 of all murine research is undertaken with the C57BL/6 strain (compared with 1/100 with Sv129S6 [40]), mainly because of its robustness and availability of congenic strains. As therapies for human diseases become specifically targeted, it is increasingly important to measure and take into account differences in innate‐immune function between humans and various species and strains of animal models. Such measurements may eventually help make more accurate predictions for how the findings from animal models should translate to the human response to disease or therapeutic interventions [41]. The “next generation” of humanized mouse models [2, 42], with the ability to engraft hematopoietic stem cells as well as functional human lymphoid cells and tissues to recapitulate the human immune system in immunodeficient mice, is an exciting development that warrants further studies [42].
In conclusion, a new assay is available to perform single‐cell resolution measurements of neutrophil migration from humans, mice, and rats, directly from whole‐blood, droplet‐sized samples. The assay revealed significant differences in neutrophil migratory responses, not only among species but also among common laboratory mouse model strains. These quantitative measurements should be useful when comparing the performance of various animal models of human infection or inflammation.
AUTHORSHIP
C.N.J., A.N.H., L.D., A.M., Y.I., N.K., and M.Y. designed and performed experiments. J.M.M., B.H., and D.I. designed microfluidic devices. C.N.J., A.N.H., and N.K. analyzed data. C.N.J., A.N.H., M.K., H.S.W., D.E.B., and D.I. conceived of the experimental plan and wrote the manuscript.
DISCLOSURES
The authors declare no competing financial interests relevant to work described in this manuscript.
Supporting information
Supplementary data
Supplementary data
ACKNOWLEDGMENTS
Microfabrication work was performed at the BioMEMS Research Center (EB002503). This work was supported by grants from the U.S. National Institutes of Health (GM092804; to D.I.), Shriners Burns Institute (8700; to H.S.W.), and Defense Advanced Research Projects Agency (DARPA; W911NF‐13‐1; to H.S.W.).
REFERENCES
- 1. Wipke, B. T. , Allen, P. M. (2001) Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167, 1601–1608. [DOI] [PubMed] [Google Scholar]
- 2. Brehm, M. A. , Wiles, M. V. , Greiner, D. L. , Shultz, L. D. (2014) Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods 410, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao, J. , Li, K. , Wohlford‐Lenane, C. , Agnihothram, S. S. , Fett, C. , Zhao, J. , Gale, M. J. , Jr, Baric, R. S. , Enjuanes, L. , Gallagher, T. , McCray, P. B. , Jr, Perlman, S. (2014) Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. USA 111, 4970–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodcock, J. , Woosley, R. (2008) The FDA critical path initiative and its influence on new drug development. Annu. Rev. Med. 59, 1–12. [DOI] [PubMed] [Google Scholar]
- 5. Haley, P. J. (2003) Species differences in the structure and function of the immune system. Toxicology 188, 49–71. [DOI] [PubMed] [Google Scholar]
- 6.Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osterburg, A. R. , Hexley, P. , Supp, D. M. , Robinson, C. T. , Noel, G. , Ogle, C. , Boyce, S. T. , Aronow, B. J. , Babcock, G. F. (2013) Concerns over interspecies transcriptional comparisons in mice and humans after trauma. Proc. Natl. Acad. Sci. USA 110, E3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mestas, J. , Hughes, C. C. (2004) Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738. [DOI] [PubMed] [Google Scholar]
- 9. Petkova, S. B. , Yuan, R. , Tsaih, S. W. , Schott, W. , Roopenian, D. C. , Paigen, B. (2008) Genetic influence on immune phenotype revealed strain‐specific variations in peripheral blood lineages. Physiol. Genomics 34, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao, J. L. , Lee, E. J. , Murphy, P. M. (1999) Impaired antibacterial host defense in mice lacking the N‐formylpeptide receptor. J. Exp. Med. 189, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hajjar, E. , Broemstrup, T. , Kantari, C. , Witko‐Sarsat, V. , Reuter, N. (2010) Structures of human proteinase 3 and neutrophil elastase—so similar yet so different. FEBS J. 277, 2238–2254. [DOI] [PubMed] [Google Scholar]
- 12. Condliffe, A. M. , Davidson, K. , Anderson, K. E. , Ellson, C. D. , Crabbe, T. , Okkenhaug, K. , Vanhaesebroeck, B. , Turner, M. , Webb, L. , Wymann, M. P. , Hirsch, E. , Ruckle, T. , Camps, M. , Rommel, C. , Jackson, S. P. , Chilvers, E. R. , Stephens, L. R. , Hawkins, P. T. (2005) Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106, 1432–1440. [DOI] [PubMed] [Google Scholar]
- 13. Barrick, C. J. , Rojas, M. , Schoonhoven, R. , Smyth, S. S. , Threadgill, D. W. (2007) Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal‐ and background‐dependent development of concentric left ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 292, H2119–H2130. [DOI] [PubMed] [Google Scholar]
- 14. Marques, S. M. , Campos, P. P. , Castro, P. R. , Cardoso, C. C. , Ferreira, M. A. , Andrade, S. P. (2011) Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc. Res. 82, 246–252. [DOI] [PubMed] [Google Scholar]
- 15. Brown, D. E. , Libby, S. J. , Moreland, S. M. , McCoy, M. W. , Brabb, T. , Stepanek, A. , Fang, F. C. , Detweiler, C. S. (2013) Salmonella enterica causes more severe inflammatory disease in C57/BL6 Nramp1G169 mice than Sv129S6 mice. Vet. Pathol. 50, 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sellers, R. S. , Clifford, C. B. , Treuting, P. M. , Brayton, C. (2012) Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 49, 32–43. [DOI] [PubMed] [Google Scholar]
- 17. Simon, M. M. , Greenaway, S. , White, J. K. , Fuchs, H. , Gailus‐Durner, V. , Wells, S. , Sorg, T. , Wong, K. , Bedu, E. , Cartwright, E. J. , Dacquin, R. , Djebali, S. , Estabel, J. , Graw, J. , Ingham, N. J. , Jackson, I. J. , Lengeling, A. , Mandillo, S. , Marvel, J. , Meziane, H. , Preitner, F. , Puk, O. , Roux, M. , Adams, D. J. , Atkins, S. , Ayadi, A. , Becker, L. , Blake, A. , Brooker, D. , Cater, H. , Champy, M. F. , Combe, R. , Danecek, P. , di Fenza, A. , Gates, H. , Gerdin, A. K. , Golini, E. , Hancock, J. M. , Hans, W. , Hölter, S. M. , Hough, T. , Jurdic, P. , Keane, T. M. , Morgan, H. , Müller, W. , Neff, F. , Nicholson, G. , Pasche, B. , Roberson, L. A. , Rozman, J. , Sanderson, M. , Santos, L. , Selloum, M. , Shannon, C. , Southwell, A. , Tocchini‐Valentini, G. P. , Vancollie, V. E. , Westerberg, H. , Wurst, W. , Zi, M. , Yalcin, B. , Ramirez‐Solis, R. , Steel, K. P. , Mallon, A. M. , de Angelis, M. H. , Herault, Y. , Brown, S. D. (2013) A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 14, R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyden, S. (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 115, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugawara, T. , Miyamoto, M. , Takayama, S. , Kato, M. (1995) Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J. Pharmacol. Toxicol. Methods 33, 91–100. [DOI] [PubMed] [Google Scholar]
- 20. Kurihara, T. , Jones, C. N. , Yu, Y. M. , Fischman, A. J. , Watada, S. , Tompkins, R. G. , Fagan, S. P. , Irimia, D. (2013) Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 27, 2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones, C. N. , Dalli, J. , Dimisko, L. , Wong, E. , Serhan, C. N. , Irimia, D. (2012) Microfluidic chambers for monitoring leukocyte trafficking and humanized nano‐proresolving medicines interactions. Proc. Natl. Acad. Sci. USA 109, 20560–20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim, D. , Haynes, C. L. (2013) On‐chip evaluation of neutrophil activation and neutrophil‐endothelial cell interaction during neutrophil chemotaxis. Anal. Chem. 85, 10787–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boxio, R. , Bossenmeyer‐Pourié, C. , Steinckwich, N. , Dournon, C. , Nüsse, O. (2004) Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75, 604–611. [DOI] [PubMed] [Google Scholar]
- 24. Hasenberg, M. , Köhler, A. , Bonifatius, S. , Borucki, K. , Riek‐Burchardt, M. , Achilles, J. , Männ, L. , Baumgart, K. , Schraven, B. , Gunzer, M. (2011) Rapid immunomagnetic negative enrichment of neutrophil granulocytes from murine bone marrow for functional studies in vitro and in vivo. PLoS One 6, e17314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agrawal, N. , Toner, M. , Irimia, D. (2008) Neutrophil migration assay from a drop of blood. Lab Chip 8, 2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sackmann, E. K. , Berthier, E. , Young, E. W. , Shelef, M. A. , Wernimont, S. A. , Huttenlocher, A. , Beebe, D. J. (2012) Microfluidic kit‐on‐a‐lid: a versatile platform for neutrophil chemotaxis assays. Blood 120, e45–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoang, A. N. , Jones, C. N. , Dimisko, L. , Hamza, B. , Martel, J. , Kojic, N. , Irimia, D. (2013) Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology (Singap World Sci) 1, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones, C. N. , Hoang, A. N. , Dimisko, L. , Hamza, B. , Martel, J. , Irimia, D. (2014) Microfluidic platform for measuring neutrophil chemotaxis from unprocessed whole blood. J. Vis. Exp. 88, 51215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Everitt, E. A. , Malik, A. B. , Hendey, B. (1996) Fibronectin enhances the migration rate of human neutrophils in vitro. J. Leukoc. Biol. 60, 199–206. [DOI] [PubMed] [Google Scholar]
- 30. Singh, R. K. , Furze, R. C. , Birrell, M. A. , Rankin, S. M. , Hume, A. N. , Seabra, M. C. (2014) A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 15, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golde, W. T. , Gollobin, P. , Rodriguez, L. L. (2005) A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim. (NY) 34, 39–43. [DOI] [PubMed] [Google Scholar]
- 32. Jacobelli, J. , Friedman, R. S. , Conti, M. A. , Lennon‐Dumenil, A. M. , Piel, M. , Sorensen, C. M. , Adelstein, R. S. , Krummel, M. F. (2010) Confinement‐optimized three‐dimensional T cell amoeboid motility is modulated via myosin IIA‐regulated adhesions. Nat. Immunol. 11, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warren, H. S. , Fitting, C. , Hoff, E. , Adib‐Conquy, M. , Beasley‐Topliffe, L. , Tesini, B. , Liang, X. , Valentine, C. , Hellman, J. , Hayden, D. , Cavaillon, J. M. (2010) Resilience to bacterial infection: difference between species could be due to proteins in serum. J. Infect. Dis. 201, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boneschansker, L. , Yan, J. , Wong, E. , Briscoe, D. M. , Irimia, D. (2014) Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat. Commun. 5, 4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moniaga, C. S. , Watanabe, S. , Honda, T. , Nielsen, S. , Hara‐Chikuma, M. (2015) Aquaporin‐9‐expressing neutrophils are required for the establishment of contact hypersensitivity. Sci. Rep. 5, 15319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao, J. L. , Murphy, P. M. (1993) Species and subtype variants of the N‐formyl peptide chemotactic receptor reveal multiple important functional domains. J. Biol. Chem. 268, 25395–25401. [PubMed] [Google Scholar]
- 37. Ye, R. D. , Boulay, F. , Wang, J. M. , Dahlgren, C. , Gerard, C. , Parmentier, M. , Serhan, C. N. , Murphy, P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yagupsky, P. , Nolte, F. S. (1990) Quantitative aspects of septicemia. Clin. Microbiol. Rev. 3, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inflammation and the Host Response to Injury Investigators. (2005) Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson, M. (2012) Laboratory mice and rats. Mater. Methods 2, 113. [Google Scholar]
- 41. Efron, P. A. , Mohr, A. M. , Moore, F. A. , Moldawer, L. L. (2015) The future of murine sepsis and trauma research models. J. Leukoc. Biol. 98, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coughlan, A. M. , Freeley, S. J. , Robson, M. G. (2012) Humanised mice have functional human neutrophils. J. Immunol. Methods 385, 96–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data


