Short abstract
Review on mechanisms associated with functional loss of MAIT cells in HIV infection.
Keywords: CD8+ T cells, cytotoxicity, exhaustion, PD‐1, TCR iVα7.2
Abstract
MAIT cells represent an evolutionarily conserved, MR1‐restricted, innate‐like cell subset that express high levels of CD161; have a canonical semi‐invariant TCR iVα7.2; and may have an important role in mucosal immunity against various bacterial and fungal pathogens. Mature MAIT cells are CD161hiPLZFhiIL‐18Rα+iVα7.2+γδ‐CD3+CD8+ T cells and occur in the peripheral blood, liver, and mucosa of humans. MAIT cells are activated by a metabolic precursor of riboflavin synthesis presented by MR1 and, therefore, respond to many bacteria and some fungi. Despite their broad antibacterial properties, their functional role in persistent viral infections is poorly understood. Although there is an increasing line of evidence portraying the depletion of MAIT cells in HIV disease, the magnitude and the potential mechanisms underlying such depletion remain unclear. Recent studies suggest that MAIT cells are vulnerable to immune exhaustion as a consequence of HIV and hepatitis C virus infections and HIV/tuberculosis coinfections. HIV infection also appears to cause functional depletion of MAIT cells resulting from abnormal expression of T‐bet and EOMES, and effective ART is unable to completely salvage functional MAIT cell loss. Depletion and exhaustion of peripheral MAIT cells may affect mucosal immunity and could increase susceptibility to opportunistic infections during HIV infection. Here, we review some of the important mechanisms associated with depletion and functional loss of MAIT cells and also suggest potential immunotherapeutic strategies to restore MAIT cell functions, including the use of IL‐7 to restore effector functions in HIV disease.
Abbreviations
- 5‐A‐RU
= 5‐amino‐6‐d‐ribityl‐amino‐uracil
- 5‐OP‐RU
= 5‐(2‐oxopropylideneamino)‐6‐d‐ribitylaminouracil
- AICD
= activation‐induced cell death
- ART
= antiretroviral therapy
- BTLA
= B and T lymphocyte attenuator
- cART
= combination antiretroviral treatment
- CD
= cluster of differentiation
- DC
= dendritic cell
- DN
= double negative
- EC
= elite controller
- EOMES
= eomesodermin
- GrzB
= granzyme B
- HAVCR2
= hepatitis A virus cellular receptor 2
- HCV
= hepatitis C virus
- HLA‐DR
= human leukocyte antigen D‐related
- IBD
= inflammatory bowel disease
- iNKT
= invariant natural killer T lymphocyte
- LAG‐3
= lymphocyte activation gene‐3
- MAIT
= mucosal‐associated invariant T cell
- MeG
= methyl‐glyoxal
- MR1
= MHC‐Ib‐related protein
- NKG2D
= natural‐killer group 2, member D
- PD‐1
= programmed death‐1
- PD‐L
= PD‐1 ligand
- PLZF
= promyelocytic leukemia zinc finger
- Rα
= receptor‐α chain
- STAT
= signal transducer and activator of transcription
- TB
= tuberculosis
- T‐bet
= T‐box transcription factor
- TIM‐3
= T cell immunoglobulin mucin‐3
- TCR iVα7.2
= T cell receptor invariant α chain variable 7.2
Introduction
Human MAIT cells comprise 1–10% of the total T cells in the peripheral blood. MAIT cells represent a large subset of nonclassic, innate T cells predominantly occurring in the liver (15–39% of the T cell pool), gut mucosal tissues (3–5%), and circulating blood (1–10%) of healthy individuals [1, 2, 3–4]. Human MAIT cells express an evolutionarily conserved, semiinvariant TCR‐α chain. In humans, the iVα7.2 occurs together with restricted Jα segment usage (Jα33, Jα12, or Jα20) and limited Vβ repertoires [5, 6–7]. Human MAIT cells also express high levels of CD161, IL‐18Rα, and the transcription factor PLZF (or ZBTB16); mature MAIT cells can be defined as CD161hiPLZFhiIL‐18Rα+iVα7.2+γδ‐CD3+ lymphocytes [8, 9–10]. MAIT cells express high levels of NKG2D [11], which has a role as a cytotoxicity coreceptor in these cells [1, 8], whereas triggering of NKG2D stimulates effector functions in T cells [12]. At least 90% of MAIT cells are CD8+, expressing either CD8αα or CD8αβ, with minor CD4+ (<1%) or CD8/4 DN populations (∼7%) [7, 8, 10, 13, 14]. Peripheral MAIT cells display an effector memory (CD45RO+CD62L−CD95+) phenotype and are tissue‐homing cells (CCR2+CCR5+CCR6+CXCR6+CCR9+CCR7−).
MAIT cells recognize antigens presented by nonpolymorphic, highly evolutionarily conserved MR1 [2, 4]. MR1 presents unstable pyrimidine intermediates, formed by the nonenzymatic condensation of 5‐A‐RU, an early intermediate of vitamin B2 (riboflavin) synthesis, with glyoxal or MeG, derived from other metabolic pathways, to generate 5‐(2‐oxoethylideneamino)‐6‐d‐ribitylaminouracil or 5‐OP‐RU, respectively [15]. Binding of 5‐OP‐RU stabilizes the MR1 protein. The riboflavin synthetic pathway is present in many, but not all, bacteria and some fungi [16, 17]. Ligand‐bound MR1 is recognized by the MAIT cell TCR, leading to MAIT cell activation [16, 17–18]. Activated MAIT cells can promptly kill epithelial cells infected with invasive bacteria or B cell lines exposed to fixed bacteria [19, 20], inhibit intracellular microbial growth [21, 22], and produce proinflammatory cytokines, including IFN‐γ, TNF‐α, GM‐CSF, IL‐22, and IL‐17 [7, 13, 18, 23] but not Th2 cytokines.
MAIT cells can also be activated via exposure to the cytokines IL‐12 and IL‐18 in a TCR‐independent manner [24, 25], and this type of MAIT cell activation is seen in a range of infectious and noninfectious inflammatory diseases [26]. TLR signaling in professional APCs drives the expression of a range of proinflammatory cytokines, including IL‐12 and IL‐18, and hence, TLR8 agonists, because of their efficient stimulation of IL‐12 and IL‐18 production and their capacity to induce expression of IFN‐γ by MAIT cells [24, 25]. Therefore, in addition to MAIT cells’ antibacterial activity, they may also have a role in antiviral responses. The cytokine‐mediated activation of MAIT cells, which may contribute to antiviral activity, may also be involved in other inflammatory conditions, such as multiple sclerosis, experimental autoimmune encephalomyelitis, psoriasis, IBD, and arthritis [13, 27, 28, 29–30]. The phenotype of MAIT cells and the different mechanisms of activation are presented in Fig. 1 , and the proposed mechanism illustrating the crosstalk of APCs with MAIT cells in mucosal tissues in HIV infection is presented in Fig. 2 .
Figure 1.
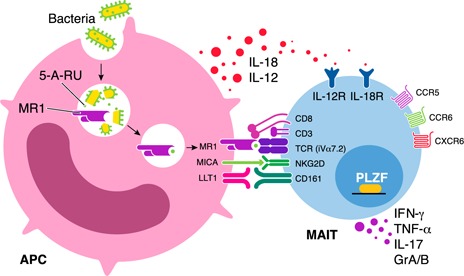
Mechanisms involved in the activation of MAIT cells. MAIT cells express a semi‐invariant TCR‐α chain, including an iVα7.2 segment combined with restricted Jα segments (Jα33, Jα12, or Jα20) and limited Vβ repertoires in humans. In addition, human MAIT cells express high levels of CD161, IL‐18Rα, the transcription factor PLZF, and the chemokine receptors CCR5, CCR6, and CXCR6. Mature MAIT cells are defined as CD161hiPLZFhiIL‐18Rα+iVα7.2+γδ−CD3+ lymphocytes. MAIT cells recognize unstable pyrimidine intermediates, formed by the nonenzymatic condensation of 5‐A‐RU, an early intermediate of vitamin B2 (riboflavin) synthesis, with glyoxal or MeG, derived from other metabolic pathways, presented by the highly evolutionarily conserved MR1 on APCs. This interaction leads to activation of the MAIT cell. After activation, MAIT cells can promptly kill infected cells, inhibit intracellular microbial growth, and produce proinflammatory cytokines, including IFN‐γ, TNF‐α, and IL‐17. It is noteworthy that MAIT cells can also be activated via exposure to the cytokines IL‐12 and IL‐18 in a TCR‐independent manner. MAIT cells also express high levels of NKG2D, which has a role as a cytotoxicity coreceptor.
Figure 2.
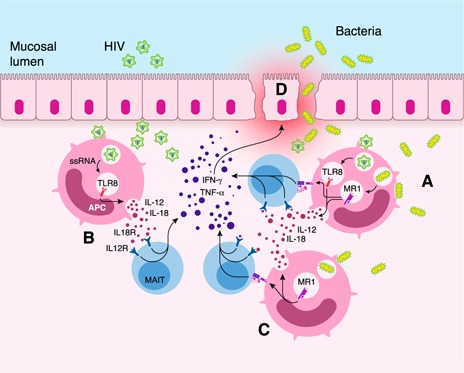
Proposed mechanism illustrating the crosstalk of APCs with MAIT cells across mucosal tissues in HIV infection. (A) Microbial translocation into the gut mucosa could release bacterial‐derived vitamin B metabolites that can activate MAIT cells in an MR1‐dependent manner. (B) APCs could also be exposed to HIV, which in turn can activate MAIT cells in an MR1‐independent fashion. (C) MAIT cells can also be activated through both MR1‐dependent and ‐independent pathways in which APCs are exposed not only to HIV but also to gut bacteria. (Bacteria can also activate the MR1‐independent pathway via stimulation of IL‐12 and IL‐18 secretion, and the type of TLR associated depends on the APC involved.) (D) Aberrant inflammatory responses can affect the mucosal epithelial integrity. Given that MAIT cells are known to produce IFN‐γ and TNF‐α along the mucosa by MR1‐dependent (A and B) and MR1‐independent (C) pathways of MAIT cell activation, IFN‐γ has been reported to induce cellular internalization of proteins associated with tight junctions, resulting in decreased transepithelial resistance in the gut epithelium [60, 61]. Furthermore, TNF‐α has also been reported to induce mucosal epithelial cell death resulting from tight‐junction changes [60, 61], leading to inflammation and mucosal damage (D).
Several studies have reported that MAIT cells recognize only bacterial‐ and yeast‐derived antigens presented via MR1 and that they do not have antiviral specificity [10, 31]. Interestingly, others have reported that influenza virus and CMV‐specific MHC tetramers bind to a minor fraction of the CD8+CD161hiIL‐18Rα+ T cells and that these cells could be expanded by means of peptide‐loaded APCs [32]. Nonetheless, the expression of iVα7.2 on tetramer‐positive cells was not determined in these studies, and hence, the relationship between antiviral CD161hiIL‐18Rα+CD8+ T cells and MAIT cells is still unclear [27]. Of note, T cells expressing CD161, including CD161hiiVα7.2−CD8+ T cells, have recently been shown to share a transcriptional and functional profile, including secretion of IFN‐γ in response to IL‐12 and IL‐18 [33].
The prominent homing capacity of MAIT cells to mucosal tissues, coupled with their ability to produce IL‐17A and IL‐22 [1, 34], key cytokines in functional mucosal immune responses [35, 36], suggests MAIT cells may play an important role in the immune response to mucosal pathogens [23], In mouse models, mucosal MAIT cells have been shown to be important in the control of pulmonary Francisella tularensis infection [21], pulmonary bacillus Calmette‐Guérin infection [22], and cystitis with uropathogenic Escherichia coli [11]. In humans, MAIT cells are increased in number in the lung of patients with tuberculosis [10, 31] and can be found in the urine of patients with cystitis [11]. MAIT cells may also play a role in gut mucosal responses as in patients with cholera, peripheral blood MAIT cells were activated 1 wk after infection and in children, but not adults, were depleted for up to 3 mo postinfection [37].
HIV‐infected patients are at increased risk of infection with mucosal pathogens including Mycobacterium tuberculosis, nontyphoidal Salmonella and Streptococcus pneumoniae [38, 39, 40–41]. It has recently been reported that CD161++ MAIT cells are lost from blood early in HIV infection and do not recover with ART [23, 42, 43–44]. Therefore, the loss of CD161++ MAIT cells may contribute to the increased susceptibility of HIV‐infected patients to these mucosal infections. The role of MAIT cells in HIV infection is the subject of this review.
LOSS OF MAIT CELLS IN HIV INFECTION
Several studies have reported the loss of circulating MAIT cells, defined by coexpression of iVα7.2 and CD161, and that the remaining MAIT cells existed in an activated and functionally exhausted state in HIV infection [23, 42, 44]. MAIT cell levels were already low by week 2–3 after the estimated date of HIV infection in some individuals, which indicates either a rapid drop or that the levels of MAIT cells were low in these patients before infection [44]. The reduction of CD161+ MAIT cells has been described as an early event in HIV infection that is independent of later stages of the disease [45]. The levels of CD161++iVα7.2+ MAIT cells in the lymph nodes are also decreased in HIV‐infected patients as compared with healthy subjects [45].
It has been suggested that, rather than being depleted, many MAIT cells, instead, have an altered phenotype, namely, the down‐regulation of CD161, leading to lower detection [42, 46]. Although Leeansyah et al. [42] observed a decrease in the size of the CD161++iVα7.2+ MAIT cell population, they found a concomitant increase in the frequency of CD161–Vα7.2+ T cells within the CD3+ T cell population and suggested that this was due to the down‐regulation of CD161 and the functional exhaustion of MAIT cells. It should be noted, however, that the antibody against iVα7.2 used in these investigations is not specific for the canonical MAIT cell TCR [8]. The MR1 tetramer does not bind CD161–Vα7.2+ T cells in healthy individuals [18] and, in a recent study, failed to bind to the Vα7.2+CD161– T cells that were observed during HIV infection [47]. Supporting this, iVα7.2−Jα33+ MAIT cells were found to be lost from the blood in HIV infection by quantitative real‐time PCR [14]. Together, these findings argue that the Vα7.2+CD161– T cell populations observed in HIV infection are not MAIT cells.
There are conflicting reports as to the fate of MAIT cells in ECs. One study reported similar numbers of MAIT cells in EC as in healthy controls [42], whereas another study observed a reduction in MAIT cells in ECs and a similar trend in long‐term nonprogressors [45]. The lower levels of MAIT cells in EC could be due to systemic immune activation, which occurs even in ECs [22, 45, 48, 49].
MAIT cells are present in the mucosa of the rectum and sigmoid colon in patients with chronic HIV infection, although there are conflicting reports as to their frequency [23, 42, 43]. Although one study found the frequencies of CD8+ and DN iVα7.2+CD161+ T cells in the rectal mucosa to be similar between HIV‐infected and healthy individuals [42], another study reported that MAIT cells were depleted from the sigmoid with similar kinetics to that of the blood [43]. Therefore, further studies are required in HIV infection to determine whether mucosal MAIT cells are unchanged in number, suggestive of either preservation of mucosal MAIT cells or migration of these cells from the peripheral blood (and possibly the liver), or whether they are depleted.
MAIT cells lost during HIV infection are reportedly reconstituted in the colon (rectum) following initiation of ART [43]. It is, however, not clear whether this reconstitution is due to a reduction of inflammation in the rectal mucosa of ART‐treated individuals and whether that reconstitution is a result of increased migration of MAIT cells into the mucosa from the blood or is caused by a proliferation of mucosal‐resident MAIT cells. It is also unknown why MAIT cells fail to reconstitute in blood within the time frames examined to date. The effect of HIV infection on different MAIT cell compartments and possible mechanisms of MAIT cell reconstitution in the colon following the initiation of ART are shown in Fig. 3 .
Figure 3.
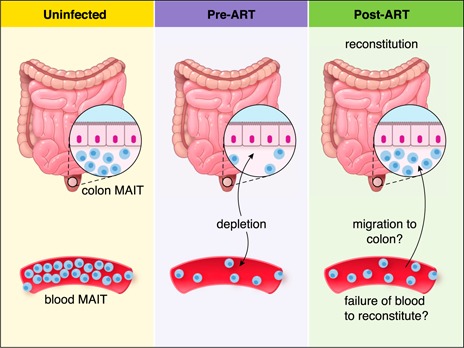
Proposed mechanism of MAIT cell restitution in the colon and blood following the initiation of ART. MAIT cells are lost from the colon during HIV infection [43], although the rate of MAIT loss may be markedly slower than it is in blood MAIT [42]. MAIT cell frequency is reconstituted in the colon following the initiation of ART [43]. Reconstitution may occur because of decreased mucosal inflammation and may be due to recruitment of MAIT cells from other compartments, such as the blood, or from local proliferation. MAIT‐cell reconstitution after initiation of ART is slower in blood than it is in the colon.
THEORIES ON THE DECLINE OF MAIT CELLS
There are several suggested explanations for the loss of MAIT cells in HIV infection: one is the down‐regulation of CD161 and functional exhaustion of MAIT cells, another is the recruitment of MAIT cells to mucosal tissues, and lastly the loss of MAIT cells due to activation‐induced apoptosis [23, 42]. There are studies reporting that MAIT cells down‐regulate CD161 upon activation in vitro [19, 42]. The mucosal immune system and barrier functions are compromised in early HIV‐1 infection [50, 51, 52–53], and activation of MAIT cells and the subsequent decrease of CD161 expression might occur when intestinal epithelial integrity is disrupted with resulting microbial translocation [23, 54, 55–56]. However, as discussed above, the decrease of iVα7.2+CD161++ MAIT cells in HIV‐infected individuals cannot be explained solely by down‐regulation of CD161. Recently, reduced levels of iVα7.2−Jα33 mRNA and genomic DNA and an unchanged frequency of Vα7.2+CD161– cells among CD3+CD4– lymphocytes in the blood of HIV‐infected patients were reported. There was a strong correlation of iVα7.2−Jα33 mRNA levels with the frequency of iVα7.2+CD161++ cells but not with total iVα7.2+ cells. Overall, these data indicate that MAIT cells are lost from the circulation [14].
MAIT cell recruitment to the mucosa could explain the loss of MAIT cells from blood in HIV infection and could be a possible mechanism for the immune system to compensate for the lack of CD4+ T cells to defend barrier integrity in the HIV‐1 infected mucosa [42]. The change in MAIT cell levels in blood in other conditions has been suggested to be due to an enrichment of the cells in tissues [10, 31]. MAIT cells express a number of chemokine receptors that have a role in trafficking to sites of inflammation. These include high levels of CCR6 and CXCR6 and intermediate levels of CCR9, CCR2, and CCR5 [1, 34, 42, 44]. The chemokine receptor CCR6 may have a role in recruiting and retaining CCR6+ T cells in secondary lymphoid tissues in HIV infection because of increased CCR6 ligand production [57]. The retained CCR6+ T cells undergo apoptosis in the secondary lymphoid organs leading to the gradual loss of these cells, and it has been suggested that CCR6 can be used as a marker to monitor HIV disease progression [57]. Surface expression of CCR6 is also one of the characteristic markers for a specific population of memory T cells that secrete TNF‐α, IL‐2, and IFN‐γ upon infection [57]. We and others have recently shown decreased CCR6 expression on MAIT cells from HIV‐infected patients, which could help explain why HIV‐infected patients have impaired immune responses in mucosal tissues, especially at the urogenital, respiratory, and intestinal mucosa [47, 58]. In contrast, we did not see any significant difference in the frequency of CCR5 expression by MAIT cells in HIV‐infected and HIV/TB‐coinfected patients as compared with healthy controls [58]. This finding is in accordance with a recent study in which CCR5 was increased on CD161++CD8+ T cells compared with CD161+CD8+ T cells, and that this did not change in patients with HIV and/or TB infection [44].
The gut‐homing receptors, CCR9 and β7 (CD49d) are expressed on MAIT cells in the jejunum of healthy individuals, and both these homing receptors are up‐regulated on circulating MAIT cells from HIV‐infected patients [45]. The frequency of CCR9+β7+ MAIT cells has a trend to inversely correlate with the total frequency of MAIT cells, supporting the model of partial homing of MAIT cells to the gut in HIV infection [45].
There may also be increased death of MAIT cells in HIV infection. Various mechanisms for the death of MAIT cells after their migration from blood to tissue have been investigated in vitro, including AICD, bystander activation, or direct HIV infection [23]. Although no evidence of preferential infection or bystander activation could be found, bacterially stimulated MAIT cells were found to be susceptible to apoptosis in vitro [23]. Given the amount of translocation of microbial products into the gut lamina propria in HIV infection, AICD is a plausible explanation for MAIT cell loss [23, 54, 55–56]. This is consistent with the proapoptotic predisposition, which is associated with expression of PLZF, in MAIT and iNKT cells [59]. Recent findings have underpinned the likelihood of MAIT‐cell depletion from AICD in HIV infection [45].
MAIT cells may have a role in mucosal inflammation in HIV infection, contributing to the disruption of mucosal epithelial integrity by the release of proinflammatory cytokines [43]. IFN‐γ has been reported to cause cells to internalize proteins associated with tight junctions leading to decreased trans‐epithelial resistance in the gut epithelium [60, 61]. Furthermore, TNF‐α has also been reported to induce mucosal epithelial cell death resulting from tight‐junction changes [62], possibly leading to microbial translocation (Fig. 2).
FUNCTIONAL IMPAIRMENT OF MAIT CELLS
MAIT cells are capable of secreting cytokines, including IL‐17A and IL‐22 [1, 34], which both have significant roles in mucosal immunity and control of HIV‐related opportunistic infections [23, 35, 36]. Recently, it was reported that individuals with loss‐of‐function mutations in STAT3 have reduced numbers of peripheral blood MAIT and NKT [63]; residual STAT3‐deficient MAIT cells were functionally impaired, with deficient secretion of IL‐17A, although they were able to secrete normal levels of IFN‐γ and TNF‐α. In HIV infection, iVα7.2+CD161+ MAIT cells were functionally impaired in ART‐naive individuals infected with HIV for ∼6–8 y when functionality was assessed with a whole‐bacterial stimulation assay [42]. Furthermore, impaired IFN‐γ and IL‐17A cytokine secretion by MAIT cells upon Escherichia coli stimulation was partially restored with cART, although cART failed to restore TNF‐α production and CD69 expression [42]. It is speculated, however, that down‐regulation of surface CD3/TCR on MAIT cells upon in vitro stimulation can make it difficult to accurately recognize MAIT cells in these assays [47]. In contrast with these previous reports, Fernandez et al. [47], reported that residual MAIT cells are functionally active in HIV‐infected individuals and may still be able to assist in controlling bacterial infection during HIV infection. Of note, the subjects in this study were ART‐naïve and recently infected (median, 4 mo) and were followed for a median of 25 mo [47] whereas, in the other study, patients had been infected for a mean of 85 mo at enrollment and were followed for a median of another 52 mo after the initiation of cART [42]. Impaired production of IL‐17A by MAIT cells was partially restored with 5 y of cART [42], whereas 2 y of cART failed to restore IL‐17A production [23], which points to a very slow recovery of functionality. Hence, it seems that functional impairment of MAIT cells occurs at a late stage in HIV infection and that cART could in part restore functionality. In HIV‐infected individuals, the functionally impaired MAIT cells exhibited abnormal T‐bet and EOMES expression patterns, which correlated with deficiency in cytotoxic capacity and cytokine production [64]. Effective ART did not fully restore these aberrations, including the ability of MAIT cells to arm with GrzB in response to bacterial antigen, suggesting that this deficiency may be largely irreversible in HIV‐infected individuals [64].
ACTIVATION AND IMMUNOSENESCENCE OF MAIT CELLS
Microbial translocation may stimulate innate immune cells via TLR pathways, as well as other pattern recognition receptor pathways, leading to secretion of proinflammatory cytokines and systemic immune activation in chronic HIV infection [54]. In liver, MAIT cells have a more‐activated phenotype compared with blood MAIT cells and express higher levels of activation markers CD69, CD38, and HLA‐DR, possibly indicating sustained antigen exposure [65]. The expression of CD38, HLA‐DR, and the immunosenescence marker CD57 were increased on MAIT cells in patients with chronic HIV infection in comparison with healthy controls [42]. In addition, the study also showed that the frequency of MAIT cells had a negative correlation with CD38 expression on MAIT cells as well as on total CD8+ T cells [42]. The study suggested that long‐term ART could decrease HLA‐DR expression on MAIT cells but did not affect the expression of CD38 and CD57 [42]. The up‐regulation of CD69 on MAIT cells has also been reported in HIV infection [64]. We recently showed that MAIT cells also have increased levels of HLA‐DR, CD38, and CD57 in chronic HCV disease [66]. Moreover, it has been also reported that MAIT cells expressing CD27 and CD127 (IL‐7Rα) are reduced in HIV‐infected individuals, although CD27, but not CD127, expression recovered after long‐term cART [42]. Future investigations should assess the correlation among markers of microbial translocation and MAIT‐cell activation and loss. The expression levels of functional molecules on MAIT cells are summarized in Table 1 .
Table 1.
Expression profile of functional molecules in MAIT cells
| Molecular expression | Healthy subjects | HIV infection | Post‐ART | References |
| CCR2 | + | ? | ? | [1, 34] |
| CCR5 | +++ | +++ | ? | [1, 23, 39, 42] |
| CCR6 | +++ | + | ? | [23, 39, 42] |
| CCR7 | − | − | − | [1] |
| CCR9 | ++ | +++ | ? | [1, 43] |
| CXCR4 | + | + | ? | [23] |
| CXCR6 | +++ | ? | ? | [1] |
| CD103 | + | + | ? | [39, 42] |
| β7 integrin | ++ | +++ | ? | [43] |
| CD161 | +++ | + | + | [23, 38, 39, 49] |
| PD‐1 | + | +++ | ? | [42] |
| HLA‐DR | + | +++ | ++ | [38] |
| CD38 | + | +++ | +++ | [38] |
| CD57 | + | +++ | +++ | [38] |
| CD27 | ++ | + | ++ | [38] |
| CD127 | ++ | + | + | [38] |
+, weak expression; ++, intermediate expression; +++, strong expression; ?, not known.
IMMUNE EXHAUSTION OF MAIT CELLS IN HIV INFECTION
Cellular immunity is controlled by different activating and inhibitory receptors, regulating the immune response toward infection. High expression of inhibitory receptors on T cells is associated with T cell exhaustion [67, 68–69]. PD‐1 (CD279), PD‐L1 (CD274), PD‐L2 (CD273), LAG‐3 (CD223), BTLA (CD272), TIM‐3 (HAVCR2), CD244, and CD160 are identified as coinhibitory surface receptors that are expressed on immune cells with inhibitory functions [70, 71–72]. Several studies, both in animal models and humans, have reported that immune suppression via inhibitory signaling pathways is involved in T cell impairment in chronic antigen‐exposure settings [73, 74, 75–76]. Initial reports have emphasized on the role of PD‐1, a major factor in T cell exhaustion and disease progression in HIV‐infected patients [73, 74–75]. Effective cART has been shown to down‐regulate PD‐1 on both CD4+ and CD8+ T cells [75, 77]. MAIT cells from patients with active TB showed increased expression of PD‐1, and blockade of the PD‐1 signaling pathway remarkably improved MAIT cell cytokine production in response to antigen activation [72]. We recently showed that PD‐1 is highly expressed on MAIT cells in the peripheral blood from HIV‐infected and HIV/TB coinfected patients but that cART +/− antituberculous drug treatment failed to reduce the elevated PD‐1 expression [58]. TIM‐3 expression is also elevated on MAIT cells from patients with chronic HIV infection compared with healthy control subjects, and long‐term cART was able to significantly lower the levels [42]. In chronic HCV infection, MAIT cells had increased expression levels of PD‐1, TIM‐3, and CTLA‐4 (CD152) [66]. This finding provides critical insights into the potential role of multiple coinhibitory receptors besides PD‐1; those receptors remain to be examined on MAIT cells from individuals at different stages of HIV infection.
We have previously reported that p38MAPK/STAT3 pathways were involved in HIV‐1–mediated up‐regulation of inhibitory receptors CTLA‐4, tumor necrosis factor‐related apoptosis‐inducing ligand, TIM‐3, LAG‐3, and CD160 and transcription factors B lymphocyte‐induced maturation protein‐1, deltex 1, and FoxP3; blockade of the p38MAPK/STAT3 pathways significantly ablated the expression of coinhibitory molecules and restored T cell proliferation in vitro [78]. It has been reported that expression of the inhibitory BTLA receptor was increased on blood MAIT cells in patients with IBD, compared with dimmer expression in controls [28]. It would be of interest to investigate the expression of these additional inhibitory receptors and transcription factors on MAIT cells in HIV infection.
The phenotype of exhausted MAIT cells is shown in Fig. 4 , and the proposed mechanisms leading to MAIT cell exhaustion are shown in Fig. 5 . As mentioned above, it has recently been reported that subjects with loss‐of‐function mutations in STAT3 had reduced numbers of peripheral blood MAIT and NKT cells [63]. It remains to be determined whether a change in STAT3 signaling is one of the underlying factors for MAIT cell exhaustion in HIV infection.
Figure 4.
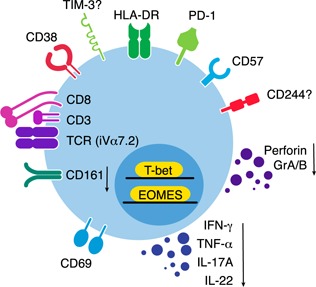
Phenotype of exhausted MAIT cells. PD‐1 is linked to exhaustion of MAIT cells in HIV, HIV/TB coinfection, and chronic HCV disease. Both exhausted and activated MAIT cells also down‐regulate CD161. The role of other coinhibitory molecules, such as TIM‐3 and CD244, and immune activation molecules, such as HLA‐DR, CD38, CD69, and CD57, are currently being investigated. Abnormal expression patterns of the transcription factors T‐bet and EOMES are believed to result in insufficiency of cytotoxic functions and cytokine production by MAIT cells.
Figure 5.
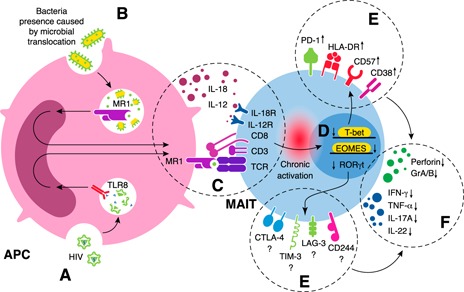
Proposed mechanism of long‐term activation of MAIT cells and their subsequent exhaustion during HIV infection. (A) HIV stimulates MAIT cells through the MR1‐independent pathway in which viral molecular patterns (e.g., ssRNA) are detected by pattern recognition receptors (e.g., TLR8) on APCs. (B) Microbial translocation results in MAIT‐cell activation through APCs via the MR1‐dependent pathway. (C) Long‐term activation of MAIT cells by MR1‐dependent and ‐independent pathways. (D) Decreased expression of transcription factors in MAIT cells occurs. (E) Increased expression of coinhibitory molecules and immunosenescence markers in MAIT cells also occurs. (F) Functional impairment of MAIT cells is caused by decreased production of IFN‐γ, TNF‐α, and IL‐17 as well as granzyme A/B and perforin.
MAIT CELL RESTORATION: PROMISING IMMUNOTHERAPEUTIC STRATEGIES
It is noteworthy that there is no significant recovery of circulating MAIT cells in HIV‐infected patients, despite years of otherwise effective cART [23, 42, 43, 44–45]. However, MAIT‐cell functionality is recovered, at least in part, by cART [42]. In addition, a recent report suggested that the colon MAIT cell population may be restored after cART [43]. There is a necessity to investigate MAIT cells in the initial stages of acute HIV infection to elucidate the kinetics of the loss of these cells and whether very early initiation of ART during primary HIV infection can restore the population and whether this has any effect on microbial translocation [45, 79]. The transcription factor expression profile of MAIT cells has been investigated in healthy controls as well as HIV‐infected patients [59, 80, 81, 82–83]. For instance, the ability to secrete IL‐17 is correlated with expression of the transcription factor retinoic acid–related orphan receptor‐γt [81]. In addition, the proapoptotic tendency of MAIT cells and iNKT cells is due to expression of the transcription factor PLZF [59]. Hence, the possibility of manipulating these transcription factors is an intriguing target for possible future therapeutic approaches. There are other theoretic therapeutic options, including the immunomodulatory blockade of circulating cytokines (e.g., IL‐18), the use of cytokines to expand MAIT cells or enhance their function (e.g., IL‐7), the blockade of coinhibitory receptors, or the use of probiotics to activate MAIT cell regeneration and proliferation by altering the indigenous gut microbiota [19, 64].
IL‐7 is a cytokine with pleiotropic effects that is produced by bone marrow and thymic stromal cells, DCs, hepatocytes, and epithelial cells. Besides functioning as a growth factor for cells of the lymphoid lineage, IL‐7 is believed to function as a growth factor for gut mucosal lymphocytes. IL‐7 confers strong survival signals to memory T cells [84, 85], with which MAIT cells show similarities. IL‐7 also appears to enhance the Th1 and Th17 cytokine‐production abilities of MAIT cells in response to polyclonal stimulation [65]. In addition, IL‐7 has been shown to turn resting MAIT cells from healthy donors into cytotoxic GrzB+ effector cells, and in HIV‐1 infected patients, this can partially reverse the defects in MAIT cell functions and their aberrant expression of transcription factors [64]. Furthermore, plasma IL‐7 levels appear to have a positive correlation with MAIT cell frequencies and functions in HIV‐infected patients, and IL‐7 treatment significantly restored MAIT cell effector functions in vitro, even in the absence of ART [64]. Hence, the immunotherapeutic credentials of IL‐7 to harness the protective functions of MAIT cells may be considered in HIV disease.
CONCLUSION AND FUTURE DIRECTIONS
Although a substantial amount of research has been conducted on circulating MAIT cells in HIV infection, there remains a need to characterize the frequency and function of MAIT cells isolated from different mucosal tissue compartments from HIV‐infected individuals (including ECs), from uninfected controls, and, significantly, from HIV/TB or HIV/HCV coinfected individuals. Further studies should also aim to address the cell types that could compensate for the functional loss of MAIT cells [86]. In addition, future analyses of MAIT cells would be assisted by the use of the MR1 tetramer technology. The mechanism of MAIT‐cell exhaustion and immunosenescence should be investigated closely, particularly in the context of coinhibitory molecules. The interaction of MAIT cells with different types of adaptive and innate cells has also been overlooked, especially in instances in which some of these cells could serve as potential sources of IL‐12 and IL‐18 after viral infection, leading to activation of MAIT cells in HIV infection. Lastly, the development of immunotherapeutic molecules to restore functional MAIT cell levels in the different tissue compartments in persistent viral infections, especially in HIV and HCV infections, is an area that requires in‐depth investigation.
AUTHORSHIP
A.S., Y.K.Y., H.Y.T., V.V., M.L., and E.M.S. wrote this review. R.E., J.E.U. and M.L. edited and provided critical inputs to the review.
DISCLOSURES
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by the University of Malaya and Ministry of Higher Education High Impact Research Grant UM.C/625/1/HIR/MOHE/MED/01 and the University of Malaya Research Grants RP021A‐13HTM and RG448‐12HTM) of the Health and Translational Medicine Research Cluster (to E.M.S.). M.L. was supported by Swedish Research Council Grant AI52731, the Swedish Physicians Against AIDS Research Foundation, the Swedish International Development Cooperation Agency, the Swedish International Development Cooperation Agency Special Assistant to the Resident Coordinator, VINNMER from VINNOVA, the Linköping University Hospital research fund, Governmental Funding of Clinical Research within National Health Service, and by the Swedish Society of Medicine. Y.K.Y. was supported by Research Officer Grant Scheme BR003‐2014. The authors also acknowledge the U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases Grant 1U19AI109633‐01 (to V.V.).
References
- 1. Dusseaux, M. , Martin, E. , Serriari, N. , Péguillet, I. , Premel, V. , Louis, D. , Milder, M. , Le Bourhis, L. , Soudais, C. , Treiner, E. , Lantz, O. (2011) Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood 117, 1250–1259. [DOI] [PubMed] [Google Scholar]
- 2. Huang, S. , Martin, E. , Kim, S. , Yu, L. , Soudais, C. , Fremont, D. H. , Lantz, O. , Hansen, T. H. (2009) MR1 antigen presentation to mucosal‐associated invariant T cells was highly conserved in evolution. Proc. Natl. Acad. Sci. U. S. A 106, 8290–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Bourhis, L. , Mburu, Y. K. , Lantz, O. (2013) MAIT cells, surveyors of a new class of antigen: development and functions. Curr. Opin. Immunol. 25, 174–180. [DOI] [PubMed] [Google Scholar]
- 4. Treiner, E. , Duban, L. , Bahram, S. , Radosavljevic, M. , Wanner, V. , Tilloy, F. , Affaticati, P. , Gilfillan, S. , Lantz, O. (2003) Selection of evolutionarily conserved mucosal‐associated invariant T cells by MR1. Nature 422, 164–169. [DOI] [PubMed] [Google Scholar]
- 5. Reantragoon, R. , Kjer‐Nielsen, L. , Patel, O. , Chen, Z. , Illing, P. T. , Bhati, M. , Kostenko, L. , Bharadwaj, M. , Meehan, B. , Hansen, T. H. , Godfrey, D. I. , Rossjohn, J. , McCluskey, J. (2012) Structural insight into MR1‐mediated recognition of the mucosal associated invariant T cell receptor. J. Exp. Med. 209, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepore, M. , Kalinichenko, A. , Colone, A. , Paleja, B. , Singhal, A. , Tschumi, A. , Lee, B. , Poidinger, M. , Zolezzi, F. , Quagliata, L. , Sander, P. , Newell, E. , Bertoletti, A. , Terracciano, L. , De Libero, G. , Mori, L. (2014) Parallel T‐cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire [published correction in Nat. Commun. 2014; 5:4493]. Nat. Commun. 5, 3866. [DOI] [PubMed] [Google Scholar]
- 7. Walker, L. J. , Kang, Y. H. , Smith, M. O. , Tharmalingham, H. , Ramamurthy, N. , Fleming, V. M. , Sahgal, N. , Leslie, A. , Oo, Y. , Geremia, A. , Scriba, T. J. , Hanekom, W. A. , Lauer, G. M. , Lantz, O. , Adams, D. H. , Powrie, F. , Barnes, E. , Klenerman, P. (2012) Human MAIT and CD8αα cells develop from a pool of type‐17 precommitted CD8+ T cells. Blood 119, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin, E. , Treiner, E. , Duban, L. , Guerri, L. , Laude, H. , Toly, C. , Premel, V. , Devys, A. , Moura, I. C. , Tilloy, F. , Cherif, S. , Vera, G. , Latour, S. , Soudais, C. , Lantz, O. (2009) Stepwise development of MAIT cells in mouse and human. PLoS Biol. 7, e1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beaulieu, A. M. , Sant'Angelo, D. B. (2011) The BTB‐ZF family of transcription factors: key regulators of lineage commitment and effector function development in the immune system. J. Immunol. 187, 2841–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Bourhis, L. , Martin, E. , Péguillet, I. , Guihot, A. , Froux, N. , Coré, M. , Lévy, E. , Dusseaux, M. , Meyssonnier, V. , Premel, V. , Ngo, C. , Riteau, B. , Duban, L. , Robert, D. , Huang, S. , Rottman, M. , Soudais, C. , Lantz, O. (2010) Antimicrobial activity of mucosal‐associated invariant T cells. [published correction in Nat. Immunol. 2010;11:969]. Nat. Immunol. 11, 701–708. [DOI] [PubMed] [Google Scholar]
- 11. Cui, Y. , Franciszkiewicz, K. , Mburu, Y. K. , Mondot, S. , Le Bourhis, L. , Premel, V. , Martin, E. , Kachaner, A. , Duban, L. , Ingersoll, M. A. , Rabot, S. , Jaubert, J. , De Villartay, J. P. , Soudais, C. , Lantz, O. (2015) Mucosal‐associated invariant T cell‐rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 125, 4171–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groh, V. , Rhinehart, R. , Randolph‐Habecker, J. , Topp, M. S. , Riddell, S. R. , Spies, T. (2001) Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus‐infected cells. Nat. Immunol. 2, 255–260. [DOI] [PubMed] [Google Scholar]
- 13. Miyazaki, Y. , Miyake, S. , Chiba, A. , Lantz, O. , Yamamura, T. (2011) Mucosal‐associated invariant T cells regulate Th1 response in multiple sclerosis. Int. Immunol. 23, 529–535. [DOI] [PubMed] [Google Scholar]
- 14. Ussher, J. E. , Phalora, P. , Cosgrove, C. , Hannaway, R. F. , Rauch, A. , Günthard, H. F. , Goulder, P. , Phillips, R. E. , Willberg, C. B. , Klenerman, P. (2015) Molecular analyses define Vα7.2‐Jα33+ MAIT cell depletion in HIV infection: a case‐control study. Medicine (Baltimore) 94, e1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corbett, A. J. , Eckle, S. B. , Birkinshaw, R. W. , Liu, L. , Patel, O. , Mahony, J. , Chen, Z. , Reantragoon, R. , Meehan, B. , Cao, H. , Williamson, N. A. , Strugnell, R. A. , Van Sinderen, D. , Mak, J. Y. , Fairlie, D. P. , Kjer‐Nielsen, L. , Rossjohn, J. , McCluskey, J. (2014) T‐cell activation by transitory neo‐antigens derived from distinct microbial pathways. Nature 509, 361–365. [DOI] [PubMed] [Google Scholar]
- 16. Kjer‐Nielsen, L. , Patel, O. , Corbett, A. J. , Le Nours, J. , Meehan, B. , Liu, L. , Bhati, M. , Chen, Z. , Kostenko, L. , Reantragoon, R. , Williamson, N. A. , Purcell, A. W. , Dudek, N. L. , McConville, M. J. , O'Hair, R. A. , Khairallah, G. N. , Godfrey, D. I. , Fairlie, D. P. , Rossjohn, J. , McCluskey, J. (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723. [DOI] [PubMed] [Google Scholar]
- 17. Patel, O. , Kjer‐Nielsen, L. , Le Nours, J. , Eckle, S. B. , Birkinshaw, R. , Beddoe, T. , Corbett, A. J. , Liu, L. , Miles, J. J. , Meehan, B. , Reantragoon, R. , Sandoval‐Romero, M. L. , Sullivan, L. C. , Brooks, A. G. , Chen, Z. , Fairlie, D. P. , McCluskey, J. , Rossjohn, J. (2013) Recognition of vitamin B metabolites by mucosal‐associated invariant T cells. Nat. Commun. 4, 2142. [DOI] [PubMed] [Google Scholar]
- 18. Reantragoon, R. , Corbett, A. J. , Sakala, I. G. , Gherardin, N. A. , Furness, J. B. , Chen, Z. , Eckle, S. B. , Uldrich, A. P. , Birkinshaw, R. W. , Patel, O. , Kostenko, L. , Meehan, B. , Kedzierska, K. , Liu, L. , Fairlie, D. P. , Hansen, T. H. , Godfrey, D. I. , Rossjohn, J. , McCluskey, J. , Kjer‐Nielsen, L. (2013) Antigen‐loaded MR1 tetramers define T cell receptor heterogeneity in mucosal‐associated invariant T cells. J. Exp. Med. 210, 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurioka, A. , Ussher, J. E. , Cosgrove, C. , Clough, C. , Fergusson, J. R. , Smith, K. , Kang, Y. H. , Walker, L. J. , Hansen, T. H. , Willberg, C. B. , Klenerman, P. (2015) MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 8, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Bourhis, L. , Dusseaux, M. , Bohineust, A. , Bessoles, S. , Martin, E. , Premel, V. , Coré, M. , Sleurs, D. , Serriari, N. E. , Treiner, E. , Hivroz, C. , Sansonetti, P. , Gougeon, M. L. , Soudais, C. , Lantz, O. (2013) MAIT cells detect and efficiently lyse bacterially‐infected epithelial cells. PLoS Pathog. 9, e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meierovics, A. , Yankelevich, W. J. , Cowley, S. C. (2013) MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. U. S. A 110, E3119–E3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chua, W. J. , Truscott, S. M. , Eickhoff, C. S. , Blazevic, A. , Hoft, D. F. , Hansen, T. H. (2012) Polyclonal mucosa‐associated invariant T cells have unique innate functions in bacterial infection. Infect. Immun. 80, 3256–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cosgrove, C. , Ussher, J. E. , Rauch, A. , Gärtner, K. , Kurioka, A. , Hühn, M. H. , Adelmann, K. , Kang, Y. H. , Fergusson, J. R. , Simmonds, P. , Goulder, P. , Hansen, T. H. , Fox, J. , Günthard, H. F. , Khanna, N. , Powrie, F. , Steel, A. , Gazzard, B. , Phillips, R. E. , Frater, J. , Uhlig, H. , Klenerman, P. (2013) Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 121, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ussher, J. E. , Bilton, M. , Attwod, E. , Shadwell, J. , Richardson, R. , de Lara, C. , Mettke, E. , Kurioka, A. , Hansen, T. H. , Klenerman, P. , Willberg, C. B. (2014) CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL‐12+IL‐18 in a TCR‐independent manner. Eur. J. Immunol. 44, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jo, J. , Tan, A. T. , Ussher, J. E. , Sandalova, E. , Tang, X. Z. , Tan‐Garcia, A. , To, N. , Hong, M. , Chia, A. , Gill, U. S. , Kennedy, P. T. , Tan, K. C. , Lee, K. H. , De Libero, G. , Gehring, A. J. , Willberg, C. B. , Klenerman, P. , Bertoletti, A. (2014) Toll‐like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 10, e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ussher, J. E. , Klenerman, P. , Willberg, C. B. (2014) Mucosal‐associated invariant T‐cells: new players in anti‐bacterial immunity. Front. Immunol. 5, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teunissen, M. B. , Yeremenko, N. G. , Baeten, D. L. , Chielie, S. , Spuls, P. I. , de Rie, M. A. , Lantz, O. , Res, P. C. (2014) The IL‐17A‐producing CD8+ T‐cell population in psoriatic lesional skin comprises mucosa‐associated invariant T cells and conventional T cells. J. Invest. Dermatol. 134, 2898–2907. [DOI] [PubMed] [Google Scholar]
- 28. Serriari, N. E. , Eoche, M. , Lamotte, L. , Lion, J. , Fumery, M. , Marcelo, P. , Chatelain, D. , Barre, A. , Nguyen‐Khac, E. , Lantz, O. , Dupas, J. L. , Treiner, E. (2014) Innate mucosal‐associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 176, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croxford, J. L. , Miyake, S. , Huang, Y. Y. , Shimamura, M. , Yamamura, T. (2006) Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat. Immunol. 7, 987–994. [DOI] [PubMed] [Google Scholar]
- 30. Illés, Z. , Shimamura, M. , Newcombe, J. , Oka, N. , Yamamura, T. (2004) Accumulation of Valpha7.2‐Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int. Immunol. 16, 223–230. [DOI] [PubMed] [Google Scholar]
- 31. Gold, M. C. , Cerri, S. , Smyk‐Pearson, S. , Cansler, M. E. , Vogt, T. M. , Delepine, J. , Winata, E. , Swarbrick, G. M. , Chua, W. J. , Yu, Y. Y. , Lantz, O. , Cook, M. S. , Null, M. D. , Jacoby, D. B. , Harriff, M. J. , Lewinsohn, D. A. , Hansen, T. H. , Lewinsohn, D. M. (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Havenith, S. H. , Yong, S. L. , Henson, S. M. , Piet, B. , Idu, M. M. , Koch, S. D. , Jonkers, R. E. , Kragten, N. A. , Akbar, A. N. , van Lier, R. A. , ten Berge, I. J. (2012) Analysis of stem‐cell‐like properties of human CD161++IL‐18Rα+ memory CD8+ T cells. Int. Immunol. 24, 625–636. [DOI] [PubMed] [Google Scholar]
- 33. Fergusson, J. R. , Smith, K. E. , Fleming, V. M. , Rajoriya, N. , Newell, E. W. , Simmons, R. , Marchi, E. , Björkander, S. , Kang, Y. H. , Swadling, L. , Kurioka, A. , Sahgal, N. , Lockstone, H. , Baban, D. , Freeman, G. J. , Sverremark‐Ekström, E. , Davis, M. M. , Davenport, M. P. , Venturi, V. , Ussher, J. E. , Willberg, C. B. , Klenerman, P. (2014) CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Reports 9, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billerbeck, E. , Kang, Y. H. , Walker, L. , Lockstone, H. , Grafmueller, S. , Fleming, V. , Flint, J. , Willberg, C. B. , Bengsch, B. , Seigel, B. , Ramamurthy, N. , Zitzmann, N. , Barnes, E. J. , Thevanayagam, J. , Bhagwanani, A. , Leslie, A. , Oo, Y. H. , Kollnberger, S. , Bowness, P. , Drognitz, O. , Adams, D. H. , Blum, H. E. , Thimme, R. , Klenerman, P. (2010) Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue‐homing properties. Proc. Natl. Acad. Sci. U. S. A 107, 3006–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung, D. R. , Kasper, D. L. , Panzo, R. J. , Chitnis, T. , Grusby, M. J. , Sayegh, M. H. , Tzianabos, A. O. (2003) CD4+ T cells mediate abscess formation in intra‐abdominal sepsis by an IL‐17‐dependent mechanism [published correction J. Immunol. 2003;170:4411]. J. Immunol. 170, 1958–1963. [DOI] [PubMed] [Google Scholar]
- 36. Sugimoto, K. , Ogawa, A. , Mizoguchi, E. , Shimomura, Y. , Andoh, A. , Bhan, A. K. , Blumberg, R. S. , Xavier, R. J. , Mizoguchi, A. (2008) IL‐22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leung, D. T. , Bhuiyan, T. R. , Nishat, N. S. , Hoq, M. R. , Aktar, A. , Rahman, M. A. , Uddin, T. , Khan, A. I. , Chowdhury, F. , Charles, R. C. , Harris, J. B. , Calderwood, S. B. , Qadri, F. , Ryan, E. T. (2014) Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl. Trop. Dis. 8, e3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huson, M. A. , Grobusch, M. P. , van der Poll, T. (2015) The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect. Dis. 15, 95–108. [DOI] [PubMed] [Google Scholar]
- 39. Buchacz, K. , Baker, R. K. , Palella, F. J. , Jr, Chmiel, J. S. , Lichtenstein, K. A. , Novak, R. M. , Wood, K. C. , Brooks, J. T., HOPS Investigators . (2010) AIDS‐defining opportunistic illnesses in US patients, 1994‐2007: a cohort study. AIDS 24, 1549–1559. [DOI] [PubMed] [Google Scholar]
- 40. Perbost, I. , Malafronte, B. , Pradier, C. , Santo, L. D. , Dunais, B. , Counillon, E. , Vinti, H. , Enel, P. , Fuzibet, J. G. , Cassuto, J. P. , Dellamonica, P. (2005) In the era of highly active antiretroviral therapy, why are HIV‐infected patients still admitted to hospital for an inaugural opportunistic infection? HIV Med. 6, 232–239. [DOI] [PubMed] [Google Scholar]
- 41. Sabin, C. A. , Smith, C. J. , Gumley, H. , Murphy, G. , Lampe, F. C. , Phillips, A. N. , Prinz, B. , Youle, M. , Johnson, M. A. (2004) Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS 18, 2145–2151. [DOI] [PubMed] [Google Scholar]
- 42. Leeansyah, E. , Ganesh, A. , Quigley, M. F. , Sönnerborg, A. , Andersson, J. , Hunt, P. W. , Somsouk, M. , Deeks, S. G. , Martin, J. N. , Moll, M. , Shacklett, B. L. , Sandberg, J. K. (2013) Activation, exhaustion, and persistent decline of the antimicrobial MR1‐restricted MAIT‐cell population in chronic HIV‐1 infection. Blood 121, 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greathead, L. , Metcalf, R. , Gazzard, B. , Gotch, F. , Steel, A. , Kelleher, P. (2014) CD8+/CD161++ mucosal‐associated invariant T‐cell levels in the colon are restored on long‐term antiretroviral therapy and correlate with CD8+ T‐cell immune activation. AIDS 28, 1690–1692. [DOI] [PubMed] [Google Scholar]
- 44. Wong, E. B. , Akilimali, N. A. , Govender, P. , Sullivan, Z. A. , Cosgrove, C. , Pillay, M. , Lewinsohn, D. M. , Bishai, W. R. , Walker, B. D. , Ndung'u, T. , Klenerman, P. , Kasprowicz, V. O. (2013) Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co‐infection [published correction in 2014;9:e95115]. PLoS One 8, e83474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eberhard, J. M. , Hartjen, P. , Kummer, S. , Schmidt, R. E. , Bockhorn, M. , Lehmann, C. , Balagopal, A. , Hauber, J. , van Lunzen, J. , Schulze zur Wiesch, J. (2014) CD161+ MAIT cells are severely reduced in peripheral blood and lymph nodes of HIV‐infected individuals independently of disease progression. PLoS One 9, e111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandberg, J. K. , Dias, J. , Shacklett, B. L. , Leeansyah, E. (2013) Will loss of your MAITs weaken your HAART [published correction in AIDS 2014;28:147], ? AIDS 27, 2501–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez, C. S. , Amarasena, T. , Kelleher, A. D. , Rossjohn, J. , McCluskey, J. , Godfrey, D. I. , Kent, S. J. (2015) MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol. Cell Biol. 93, 177–188. [DOI] [PubMed] [Google Scholar]
- 48. Krishnan, S. , Wilson, E. M. , Sheikh, V. , Rupert, A. , Mendoza, D. , Yang, J. , Lempicki, R. , Migueles, S. A. , Sereti, I. (2014) Evidence for innate immune system activation in HIV type 1‐infected elite controllers. J. Infect. Dis. 209, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pereyra, F. , Lo, J. , Triant, V. A. , Wei, J. , Buzon, M. J. , Fitch, K. V. , Hwang, J. , Campbell, J. H. , Burdo, T. H. , Williams, K. C. , Abbara, S. , Grinspoon, S. K. (2012) Increased coronary atherosclerosis and immune activation in HIV‐1 elite controllers. AIDS 26, 2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brenchley, J. M. , Schacker, T. W. , Ruff, L. E. , Price, D. A. , Taylor, J. H. , Beilman, G. J. , Nguyen, P. L. , Khoruts, A. , Larson, M. , Haase, A. T. , Douek, D. C. (2004) CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattapallil, J. J. , Douek, D. C. , Hill, B. , Nishimura, Y. , Martin, M. , Roederer, M. (2005) Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097. [DOI] [PubMed] [Google Scholar]
- 52. Sankaran, S. , George, M. D. , Reay, E. , Guadalupe, M. , Flamm, J. , Prindiville, T. , Dandekar, S. (2008) Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Veazey, R. S. , DeMaria, M. , Chalifoux, L. V. , Shvetz, D. E. , Pauley, D. R. , Knight, H. L. , Rosenzweig, M. , Johnson, R. P. , Desrosiers, R. C. , Lackner, A. A. (1998) Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431. [DOI] [PubMed] [Google Scholar]
- 54. Brenchley, J. M. , Price, D. A. , Schacker, T. W. , Asher, T. E. , Silvestri, G. , Rao, S. , Kazzaz, Z. , Bornstein, E. , Lambotte, O. , Altmann, D. , Blazar, B. R. , Rodriguez, B. , Teixeira‐Johnson, L. , Landay, A. , Martin, J. N. , Hecht, F. M. , Picker, L. J. , Lederman, M. M. , Deeks, S. G. , Douek, D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- 55. Jiang, W. , Lederman, M. M. , Hunt, P. , Sieg, S. F. , Haley, K. , Rodriguez, B. , Landay, A. , Martin, J. , Sinclair, E. , Asher, A. I. , Deeks, S. G. , Douek, D. C. , Brenchley, J. M. (2009) Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral‐treated HIV infection. J. Infect. Dis. 199, 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Estes, J. D. , Harris, L. D. , Klatt, N. R. , Tabb, B. , Pittaluga, S. , Paiardini, M. , Barclay, G. R. , Smedley, J. , Pung, R. , Oliveira, K. M. , Hirsch, V. M. , Silvestri, G. , Douek, D. C. , Miller, C. J. , Haase, A. T. , Lifson, J. , Brenchley, J. M. (2010) Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6, e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lécureuil, C. , Combadière, B. , Mazoyer, E. , Bonduelle, O. , Samri, A. , Autran, B. , Debré, P. , Combadière, C. (2007) Trapping and apoptosis of novel subsets of memory T lymphocytes expressing CCR6 in the spleen of HIV‐infected patients. Blood 109, 3649–3657. [DOI] [PubMed] [Google Scholar]
- 58. Saeidi, A. , Tien Tien, V. L. , Al‐Batran, R. , Al‐Darraji, H. A. , Tan, H. Y. , Yong, Y. K. , Ponnampalavanar, S. , Barathan, M. , Rukumani, D. V. , Ansari, A. W. , Velu, V. , Kamarulzaman, A. , Larsson, M. , Shankar, E. M. (2015) Attrition of TCR Vα7.2+ CD161++ MAIT cells in HIV‐tuberculosis co‐infection is associated with elevated levels of PD‐1 expression. PLoS One 10, e0124659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gérart, S. , Sibéril, S. , Martin, E. , Lenoir, C. , Aguilar, C. , Picard, C. , Lantz, O. , Fischer, A. , Latour, S. (2013) Human iNKT and MAIT cells exhibit a PLZF‐dependent proapoptotic propensity that is counterbalanced by XIAP. Blood 121, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bruewer, M. , Luegering, A. , Kucharzik, T. , Parkos, C. A. , Madara, J. L. , Hopkins, A. M. , Nusrat, A. (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis‐independent mechanisms. J. Immunol. 171, 6164–6172. [DOI] [PubMed] [Google Scholar]
- 61. Bruewer, M. , Utech, M. , Ivanov, A. I. , Hopkins, A. M. , Parkos, C. A. , Nusrat, A. (2005) Interferon‐gamma induces internalization of epithelial tight junction proteins via a macropinocytosis‐like process. FASEB J. 19, 923–933. [DOI] [PubMed] [Google Scholar]
- 62. Suzuki, T. (2013) Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 70, 631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilson, R. P. , Ives, M. L. , Rao, G. , Lau, A. , Payne, K. , Kobayashi, M. , Arkwright, P. D. , Peake, J. , Wong, M. , Adelstein, S. , Smart, J. M. , French, M. A. , Fulcher, D. A. , Picard, C. , Bustamante, J. , Boisson‐Dupuis, S. , Gray, P. , Stepensky, P. , Warnatz, K. , Freeman, A. F. , Rossjohn, J. , McCluskey, J. , Holland, S. M. , Casanova, J. L. , Uzel, G. , Ma, C. S. , Tangye, S. G. , Deenick, E. K. (2015) STAT3 is a critical cell‐intrinsic regulator of human unconventional T cell numbers and function. J. Exp. Med. 212, 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leeansyah, E. , Svärd, J. , Dias, J. , Buggert, M. , Nyström, J. , Quigley, M. F. , Moll, M. , Sönnerborg, A. , Nowak, P. , Sandberg, J. K. (2015) Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL‐7 and Defective in HIV‐1 Infection. PLoS Pathog. 11, e1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang, X. Z. , Jo, J. , Tan, A. T. , Sandalova, E. , Chia, A. , Tan, K. C. , Lee, K. H. , Gehring, A. J. , De Libero, G. , Bertoletti, A. (2013) IL‐7 licenses activation of human liver intrasinusoidal mucosal‐associated invariant T cells. J. Immunol. 190, 3142–3152. [DOI] [PubMed] [Google Scholar]
- 66. Barathan, M. , Mohamed, R. , Vadivelu, J. , Chang, L. Y. , Saeidi, A. , Yong, Y. K. , Ravishankar Ram, M. , Gopal, K. , Velu, V. , Larsson, M. , Shankar, E. M. (2016) Peripheral loss of CD8+ CD161++ TCRVα7md2+ mucosal‐associated invariant T cells in chronic hepatitis C virus‐infected patients. Eur. J. Clin. Invest. 46, 170–180. [DOI] [PubMed] [Google Scholar]
- 67. Wherry, E. J. (2011) T cell exhaustion. Nat. Immunol. 12, 492–499. [DOI] [PubMed] [Google Scholar]
- 68. Zinselmeyer, B. H. , Heydari, S. , Sacristán, C. , Nayak, D. , Cammer, M. , Herz, J. , Cheng, X. , Davis, S. J. , Dustin, M. L. , McGavern, D. B. (2013) PD‐1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 210, 757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shin, H. , Blackburn, S. D. , Intlekofer, A. M. , Kao, C. , Angelosanto, J. M. , Reiner, S. L. , Wherry, E. J. (2009) A role for the transcriptional repressor Blimp‐1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 31, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alvarez, I. B. , Pasquinelli, V. , Jurado, J. O. , Abbate, E. , Musella, R. M. , de la Barrera, S. S. , García, V. E. (2010) Role played by the programmed death‐1‐programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J. Infect. Dis. 202, 524–532. [DOI] [PubMed] [Google Scholar]
- 71. Jurado, J. O. , Alvarez, I. B. , Pasquinelli, V. , Martínez, G. J. , Quiroga, M. F. , Abbate, E. , Musella, R. M. , Chuluyan, H. E. , García, V. E. (2008) Programmed death (PD)‐1:PD‐ligand 1/PD‐ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181, 116–125. [DOI] [PubMed] [Google Scholar]
- 72. Jiang, J. , Wang, X. , An, H. , Yang, B. , Cao, Z. , Liu, Y. , Su, J. , Zhai, F. , Wang, R. , Zhang, G. , Cheng, X. (2014) Mucosal‐associated invariant T‐cell function is modulated by programmed death‐1 signaling in patients with active tuberculosis. Am. J. Respir. Crit. Care Med. 190, 329–339. [DOI] [PubMed] [Google Scholar]
- 73. Trautmann, L. , Janbazian, L. , Chomont, N. , Said, E. A. , Gimmig, S. , Bessette, B. , Boulassel, M. R. , Delwart, E. , Sepulveda, H. , Balderas, R. S. , Routy, J. P. , Haddad, E. K. , Sekaly, R. P. (2006) Upregulation of PD‐1 expression on HIV‐specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202. [DOI] [PubMed] [Google Scholar]
- 74. Petrovas, C. , Casazza, J. P. , Brenchley, J. M. , Price, D. A. , Gostick, E. , Adams, W. C. , Precopio, M. L. , Schacker, T. , Roederer, M. , Douek, D. C. , Koup, R. A. (2006) PD‐1 is a regulator of virus‐specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203, 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Day, C. L. , Kaufmann, D. E. , Kiepiela, P. , Brown, J. A. , Moodley, E. S. , Reddy, S. , Mackey, E. W. , Miller, J. D. , Leslie, A. J. , DePierres, C. , Mncube, Z. , Duraiswamy, J. , Zhu, B. , Eichbaum, Q. , Altfeld, M. , Wherry, E. J. , Coovadia, H. M. , Goulder, P. J. , Klenerman, P. , Ahmed, R. , Freeman, G. J. , Walker, B. D. (2006) PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature 443, 350–354. [DOI] [PubMed] [Google Scholar]
- 76. Velu, V. , Shetty, R. D. , Larsson, M. , Shankar, E. M. (2015) Role of PD‐1 co‐inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. D'Souza, M. , Fontenot, A. P. , Mack, D. G. , Lozupone, C. , Dillon, S. , Meditz, A. , Wilson, C. C. , Connick, E. , Palmer, B. E. (2007) Programmed death 1 expression on HIV‐specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 179, 1979–1987. [DOI] [PubMed] [Google Scholar]
- 78. Che, K. F. , Shankar, E. M. , Muthu, S. , Zandi, S. , Sigvardsson, M. , Hinkula, J. , Messmer, D. , Larsson, M. (2012) p38 Mitogen‐activated protein kinase/signal transducer and activator of transcription‐3 pathway signaling regulates expression of inhibitory molecules in T cells activated by HIV‐1‐exposed dendritic cells. Mol. Med. 18, 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fidler, S. , Porter, K. , Ewings, F. , Frater, J. , Ramjee, G. , Cooper, D. , Rees, H. , Fisher, M. , Schechter, M. , Kaleebu, P. , Tambussi, G. , Kinloch, S. , Miro, J. M. , Kelleher, A. , McClure, M. , Kaye, S. , Gabriel, M. , Phillips, R. , Weber, J. , Babiker, A. , Babiker, A.; SPARTAC Trial Investigators . (2013) Short‐course antiretroviral therapy in primary HIV infection. N. Engl. J. Med. 368, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chang, P. P. , Barral, P. , Fitch, J. , Pratama, A. , Ma, C. S. , Kallies, A. , Hogan, J. J. , Cerundolo, V. , Tangye, S. G. , Bittman, R. , Nutt, S. L. , Brink, R. , Godfrey, D. I. , Batista, F. D. , Vinuesa, C. G. (2011) Identification of Bcl‐6‐dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43. [DOI] [PubMed] [Google Scholar]
- 81. Constantinides, M. G. , Bendelac, A. (2013) Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol. 25, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gordon, S. M. , Carty, S. A. , Kim, J. S. , Zou, T. , Smith‐Garvin, J. , Alonzo, E. S. , Haimm, E. , Sant'Angelo, D. B. , Koretzky, G. A. , Reiner, S. L. , Jordan, M. S. (2011) Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate‐like CD8+ T cells. J. Immunol. 186, 4573–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alonzo, E. S. , Sant'Angelo, D. B. (2011) Development of PLZF‐expressing innate T cells. Curr. Opin. Immunol. 23, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li, J. , Huston, G. , Swain, S. L. (2003) IL‐7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kondrack, R. M. , Harbertson, J. , Tan, J. T. , McBreen, M. E. , Surh, C. D. , Bradley, L. M. (2003) Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hartjen, P. , Meyer‐Olson, D. , Lehmann, C. , Stellbrink, H. J. , van Lunzen, J. , Schulze zur Wiesch, J. (2013) Vγ2Vδ2 T cells are skewed toward a terminal differentiation phenotype in untreated HIV infection. J. Infect. Dis. 208, 180–182. [DOI] [PubMed] [Google Scholar]


