Abstract
Nicotine addiction is characterized by repetitive drug taking and drug seeking, both tightly controlled by cannabinoid CB1 receptors. The responsiveness of neurons of the bed nucleus of the stria terminalis (BNST) to infralimbic cortex (ILCx) excitatory inputs is increased in rats with active, but not passive, nicotine taking. Therefore, we hypothesize that acquisition of the learned association between nicotine infusion and a paired cue light permits the strengthening of the ILCx–BNST synapses after ILCx tetanic stimulation. We exposed rats to intravenous nicotine self-administration for 2 months. Using a combination of in vivo protocols (electrical stimulations, extracellular recordings, and pharmacological manipulations), we characterized the effects of 10 Hz stimulation of the ILCx on BNST excitatory responses, under different conditions of exposure to nicotine. In addition, we tested whether the effects of the stimulation were CB1 receptor-dependent. The results show that nicotine self-administration supports the induction of evoked spike potentiation in the BNST in response to 10 Hz stimulation of ILCx afferents. Although not altered by nicotine abstinence, this cellular adaptation was blocked by CB1 receptor antagonism. Moreover, blockade of BNST CB1 receptors prevented increases in time-out responding subsequent to ILCx stimulation and decreased cue-induced reinstatement. Thus, the synaptic potentiation within the BNST in response to ILCx stimulation seems to contribute to the cue-elicited responding associated with nicotine self-administration and is tightly controlled by CB1 receptors.
Keywords: addiction, bed nucleus of the stria terminalis, CB1 receptor, in vivo electrophysiology, intravenous self-administration, nicotine
Introduction
The ventromedial prefrontal cortex (infralimbic cortex [ILCx] in rodents) is involved in behavioral inhibition, attentional processes, and goal-directed behaviors (Smith et al., 2012), suggesting a crucial role in drug-related behaviors, such as learning and relapse (Peters et al., 2009; Lüscher and Malenka, 2011). Furthermore, the ILCx sends major excitatory projections to the bed nucleus of the stria terminalis (BNST) (Massi et al., 2008), a structure responsive to drug rewards (Carboni et al., 2000; Dumont et al., 2005; Krawczyk et al., 2013). In agreement with these findings, we previously showed that the hyperactivity of VTA dopamine neurons after extended nicotine taking is driven by changes in the ILCx–BNST excitatory afferent circuit to the VTA (Caillé et al., 2009). However, it is not known whether ILCx–BNST synapses are more prone to developing synaptic plasticity subsequent to the acquisition of nicotine self-administration behavior. The first aim of our study was to investigate whether the stimulation of ILCx inputs at physiologically relevant frequencies could reveal cellular adaptations at ILCx–BNST synapses in rats trained to self-administer nicotine. Thus, at different time points of nicotine self-administration training, 10 Hz stimulation of the ILCx (Jackson et al., 2001) was applied and in vivo recordings were performed in the BNST. Based on the hypothesis that long-term adaptations of synapses may support persistent drug seeking, we also investigated whether the effect of ILCx stimulation on ILCx–BNST synapses would resist either extinction or 1 month of abstinence to nicotine. Moreover, a challenging question was whether the LTP protocol imposed on the glutamatergic ILCx projection to the BNST had consequences on operant behavior for nicotine and nicotine-paired cues. Finally, cannabinoid CB1 receptors play an important role in nicotine-related behaviors (Maldonado et al., 2006; Simonnet et al., 2013), and blocking CB1 receptors has been proposed for the treatment of nicotine addiction (Le Foll et al., 2008). Preclinical studies have demonstrated that CB1 knock-out mice do not develop nicotine-induced hyperactivity (Castañé et al., 2002) or nicotine-induced conditioned place preference (Castañé et al., 2002; Merritt et al., 2008). CB1 receptor antagonism has been shown to decrease nicotine self-administration (Cohen et al., 2002) as well as nicotine seeking (Cohen et al., 2005; Shoaib, 2008). Importantly, CB1 receptors in the BNST control cortical excitation of BNST neurons (Massi et al., 2008). Therefore, we also tested whether the neuroplastic changes characterized at ILCx–BNST synapses and their effect on nicotine-related behaviors were modulated by CB1 receptors.
Materials and Methods
Animals
Male Sprague Dawley rats weighing 175–200 g (Charles River) were housed collectively at 20°C–22°C with a reversed 12 h light/dark cycle (lights off from 9:30 A.M.). One week before the start of the experiment, rats were placed on a restricted diet of 20 g/d laboratory chow, sufficient to maintain body weight and growth. Water was available ad libitum, and food was given daily after the self-administration session. All procedures were conducted in accordance with the European Directive 2010-63-EU on the protection of animals used for scientific purposes and with approval of the Bordeaux University Animal Care and Use Committee (# 5012058-A).
Surgery
Intravenous surgery was performed under anesthesia with ketamine (75 mg/kg) and xylazine (7.5 mg/kg) (i.p.). Chronic indwelling jugular catheters were implanted as described previously (Caillé et al., 2009). During postoperative recovery, catheters were flushed daily with 0.2 ml ampicillin (0.1 g/ml; Coophavet) in heparinized saline (300 IU heparin per ml 0.9% NaCl) for 6 d. Subsequently, catheters were flushed daily with heparinized saline. Catheter patency was verified with an infusion of the short-acting barbiturate hypnomidate (2 mg/ml, Janssen-Cilag), when necessary.
Stereotaxic surgery for in vivo electrophysiology experiments was performed under isoflurane anesthesia as described previously (Georges and Aston-Jones, 2002). Stimulation electrodes and recording and injection pipettes were inserted into the ILCx or BNST at the following coordinates (Paxinos and Watson, 1998): ILCx: 3.0 mm from bregma, 0.5 mm from midline, 4.5 mm from brain surface; BNST: −0.3 mm from bregma, 1.5 mm from midline, 6.0–7.5 mm from brain surface.
Drugs
Nicotine hydrogen tartrate salt (Sigma-Aldrich) and AP5 (Sigma Aldrich) were dissolved in sterile 0.9% saline and stored at room temperature. The CB1 receptor antagonists O-2050 (Tocris Bioscience) and AM251 (Tocris Bioscience) were dissolved in a vehicle containing ethanol (Sigma-Aldrich), cremophor (Sigma-Aldrich), and saline at a ratio of 1:1:18, and stored at 4°C. Nicotine (30 and 60 μg/kg/0.1 ml), AM251 (2 mg/kg), and O-2050 (0.5 mg/kg) were infused intravenously. AM251 (400 μm) and AP5 (100 μm) were microinjected into the BNST using brief pulses of pneumatic pressure (Picospritzer; General Valve).
Intravenous nicotine self-administration
Experiments started at the beginning of the dark phase. Rats were tested in standard operant chambers (Imetronic) equipped with two nose-poke detectors (“active” and “inactive”). Daily 2 h self-administration sessions started with illumination of the house light and a single noncontingent infusion. “Active” nose-poking resulted in the delivery of a nicotine infusion (30 μg base/kg/100 μl first, increasing to 60 μg base/kg/100 μl when stabilized on FR5) or saline over 4 s. Each infusion was paired with a cue-light for 4 s and followed by a 20 s time-out period during which visits to the active nose-poke hole were recorded but had no consequences. “Inactive” nose-pokes were recorded but had no programmed consequences. Infusions were earned on a fixed-ratio (FR) schedule of reinforcement (10 d FR1; 2 d FR2; stabilizing on FR5). Self-administration chambers were equipped with infrared activity detectors, allowing general activity measures (number of beam breaks) when necessary.
Extinction and reinstatement.
Extinction commenced by removal of nicotine delivery and associated cue-light, and continued for 2 d. Reinstatement testing started with 30 min no cue/no nicotine presentation, immediately followed by a 30 min period during which “active” nose-pokes resulted in illumination of the nicotine associated cue-light but nicotine remained unavailable. Thus, seeking behavior was measured by the number of visits to the active hole.
Oral saccharin self-administration
Experiments started at the beginning of the dark phase. Rats were tested in standard operant chambers for at least 6 weeks, as described above. Daily 30 min sessions started with illumination of the house light. “Active” nose-poking resulted in the delivery of 112 μl of saccharin 0.13% (Sigma-Aldrich) over 4 s. Each reinforcer was paired with a cue-light for 4 s. “Inactive” nose-pokes were recorded but had no programmed consequences. Reinforcers were earned on an FR schedule (10 d FR1; 2 d FR2; stabilized on FR5).
Electrical stimulation of the ILCx
Bipolar electrical stimulation of the ILCx was conducted with a concentric electrode (Phymep) and a stimulus isolator (500 μs, 0.2–1 mA, DS3; Digitimer). First, a 10 min baseline was established when recording evoked spikes in the BNST during a 2 × 100 pulse train (0.5 Hz), after which tetanic stimulation was performed (1 min, 10 Hz).
BNST recordings and pharmacological microinfusion
Extracellular single-unit recordings were used for electrophysiological experiments. A glass micropipette (tip diameter, 1–2 μm; 10–15 mΩ) filled with a 2% pontamine sky blue solution in 0.5 m sodium acetate was lowered into the BNST. The extracellular potential was recorded with an Axoclamp2B amplifier and filtered (300 Hz/0.5 kHz) via a differential AC amplifier (Georges and Aston-Jones, 2002). Single-neuron spikes were discriminated and collected online (CED 1401, SPIKE 2; Cambridge Electronic Design).
Double-barreled pipettes (Georges and Aston-Jones, 2002) were used to record BNST activity while microinjecting drugs. A total volume of 60 nl was infused into the BNST, using pneumatic pressure (Picospritzer; General Valve). One minute after microinfusion into the BNST, the ILCx was electrically stimulated for 1 min at 10 Hz. Post-tetanic evoked responses were recorded for at least 20 min while stimulating the ILCx (100 pulses; 0.5 Hz).
Experimental design
Nicotine-dependent LTP induction in the BNST.
The first aim here was to determine the conditions under which 10 Hz stimulation of the ILCx would induce LTP within the BNST. Moreover, the stimulation protocol was run at several time points to examine whether the experimenter-induced LTP was correlated with the acquisition of nicotine self-administration (see Fig. 1A). We used five groups of rats: (1) single session (NIC-1 d, n = 5); (2) eight sessions (NIC-8 d, n = 8); (3) extended training for nicotine (NIC-60 d, n = 8); (4) extended training for saline (SAL-60 d, n = 6); and (5) nicotine yoked rats (n = 5), in which nicotine infusions were matched to those of NIC-60 d rats, so that yoked rats never controlled the nicotine delivery associated with the visual stimulus. A last control group, trained for oral saccharin 0.13% (n = 5), was added to show that the electrical stimulation of ILCx induced LTP specifically in the BNST of nicotine-taking rats. Electrophysiological recordings were performed 24 h after the last access to the operant chambers.
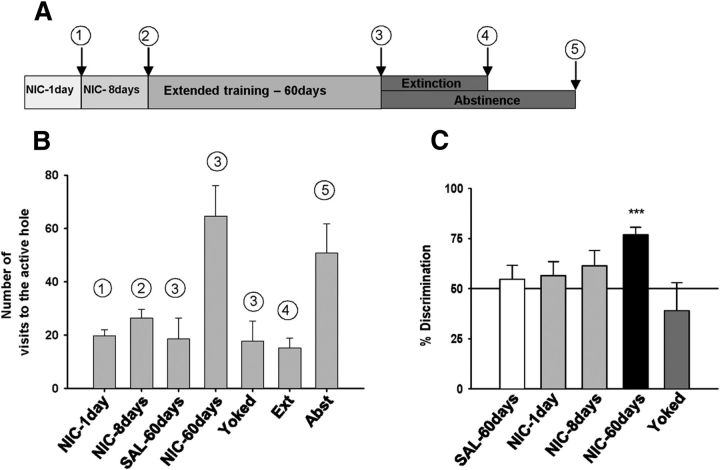
Figure 1.
Operant behavior before electrophysiological recordings. A, Timeline of experiment. Animals were recorded 24 h after the following: (1) a single nicotine self-administration session (NIC-1 d, n = 5), (2) eight sessions of nicotine self-administration (NIC-8 d, n = 8), (3) extended training (NIC-60 d, n = 8; SAL-60 d, n = 7; yoked, n = 5), (4) extinction (Ext, n = 6), or (5) abstinence (Abst, n = 7). B, Number of visits in the active hole. Each bar represents the average number over the 3 last days before electrophysiological recording for each experimental group (except the “1 day” group). C, NIC-60 d rats showed a strong preference for the active hole. ***p < 0.001. Data are mean + SEM.
Persistence of induced LTP in the BNST.
We examined whether the neuroadaptations of ILCx–BNST synapses were sensitive to passive abstinence or to active suppression of acquired responses (extinction training). A group of animals was trained for nicotine self-administration for 60 d. Half of the group (Abst, n = 7) was confined to home cages for 30 d, until electrophysiological recordings were performed. The other half (Ext, n = 6) performed extinction training where active nose-poking had no programmed consequences: no drug delivery and no cue presentation. For each rat, extinction training took place until the active responding level was 30% of baseline (for 3 consecutive days); then electrophysiological recordings were performed 24 h after the last extinction session.
Characterization of induced LTP in the BNST. NMDA-dependent plasticity.
Rats received 60 d of nicotine self-administration training. Twenty-four hours after the last session, each rat was subjected to in vivo electrophysiology. One minute before tetanic stimulation, either the NMDA receptor antagonist AP5 (NIC-60 d+AP5, 100 μm, 60 nl, n = 5) or saline vehicle (NIC-60 d+Veh, 60 nl; n = 8) was microinfused into the BNST.
CB1 receptor-dependent plasticity.
Rats received 60 d of nicotine self-administration training and were recorded 24 h after the last session. Three groups were tested, with each rat receiving an intravenous injection of either AM251 (NIC-60 d+AM251, n = 5), O-2050 (NIC-60 d+O-2050, n = 4), or vehicle (NIC-60 d+Veh, n = 3) 60 min (O-2050) or 15 min (AM251 or vehicle) before tetanic stimulation.
Two additional groups were tested with intra-BNST infusion of AM251 (NIC-60 d+AM251, n = 5) or vehicle (NIC-60 d+Veh; n = 5).
Effect of electrical stimulation of the ILCx on nicotine taking (self-administration) and nicotine seeking (cue-induced reinstatement): control by CB1 receptors.
Rats received 60 d of nicotine self-administration training. Twenty-four hours after the last self-administration session, electrodes were bilaterally inserted into the ILCx as described above. Electrical stimulation (1 min, 10 Hz) was administered once, using a square pulse stimulator and stimulus isolator (DS3; Digitimer). One minute before tetanic stimulation, rats received an intra-BNST infusion (60 nl) of either AM251 (400 μm, n = 13) or vehicle (n = 13). Control groups received the same protocols with sham stimulation (vehicle, n = 8; AM251, n = 7). After a 48 h recovery period, rats were placed back in the operant cages and nicotine self-administration was monitored for four consecutive sessions. Then rats from the group stimulated (AM251, n = 8; Veh, n = 8) and the group sham stimulated (AM251, n = 7; Veh, n = 8) performed extinction training for 2 d and were tested for cue-induced reinstatement on the third day (Deroche-Gamonet et al., 2004).
Data analysis
Behavioral data.
Nicotine self-administration data were subjected to ANOVAs, with nicotine exposure (NIC-1 d, NIC-8 d, NIC-60 d, yoked, saline) or saccharin exposure as the between-subject factor and self-administration session as the within-subject factor. For reinstatement data, between-subjects factors were the treatment with the CB1 antagonist (vehicle or AM251) and the stimulation of the ILCx (sham or stim). Whenever main factor effects were found, post hoc comparisons were performed using Fisher's protected least significant difference test. The discrimination rate ([active nose-pokes/total nose-pokes] × 100) was compared with chance (50%) with Student's t test. Statistical significance was set at p < 0.05.
Electrophysiological recordings.
During electrical stimulation of the ILCx, cumulative peristimulus time histograms (5 ms bin width) of evoked-spike activity were generated for each neuron recorded in the BNST. Peristimulus time histograms were analyzed to determine excitatory epochs as described previously (Georges and Aston-Jones, 2002). Excitatory magnitudes (Rmag values) were normalized for different levels of baseline impulse activity, allowing comparison of effects of stimulus intensity on evoked responses independent of those on baseline activity. Rmag values for excitation were calculated using the following equation: Excitation Rmag = (counts in excitatory epoch) − (mean counts per baseline bin × number of bins in excitatory epoch). For multiple comparisons, values were subjected to a one-way ANOVA followed by Bonferroni post hoc analysis. Where two means were compared, the two-tailed paired Student's t test was used.
Results
Acquisition of nicotine self-administration in rats allows the induction of LTP at ILCx–BNST synapses in response to 10 Hz stimulation of the ILCx
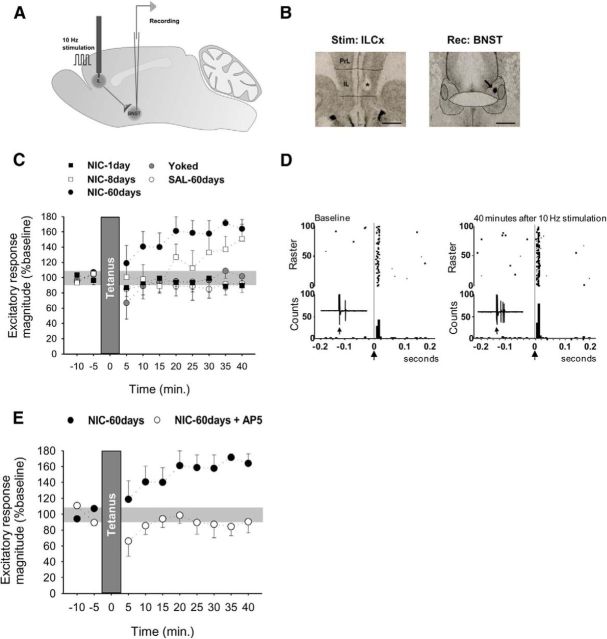
Rats had access to nicotine self-administration by nose-poke operant responding (60 μg base/kg/0.1 ml) for 1 d (NIC-1 d), 8 d (NIC-8 d), or 60 d (NIC-60 d; timeline, Fig. 1A). Control rats were trained with saline (SAL-60 d), and the volitional aspect was examined using a yoked group (Palmatier et al., 2007). Depending on drug access, rats developed different levels of responding for the active nose-poke (Fig. 1B). NIC-60 d rats developed robust nicotine intake with good discrimination between the “active” and “inactive” holes (Fig. 1C; one-way ANOVA, F(3,21) = 4.28, p < 0.05; discrimination rate vs chance, t(7) = 7.33, p < 0.001). At the end of the 2 month training period, NIC-60 d and yoked rats had similar total nicotine intake (NIC-60 d, 31.75 ± 1.63 mg/kg; yoked, 36.30 ± 4.74 mg/kg). In vivo electrophysiological extracellular single-unit recordings of BNST neurons were performed 24 h after the last self-administration session. Evoked spike potentiation in response to electrical stimulation of the ILCx (1 min, 10 Hz; Fig. 2A,B) in NIC-60 d rats was greater than in all other conditions of exposure (Fig. 2C,D; two-way ANOVA, interaction time × treatment, F(36,153) = 1.60, p = 0.02; effect of treatment, F(4,17) = 4.87, p = 0.008). The average magnitude of the 20–40 min excitatory responses was significantly different from baseline in NIC-60 d (t(7) = 3.42, p < 0.05) but not in NIC-1 d (t(4) = 0.87, p > 0.05), NIC-8 d (t(5) = 0.89, p > 0.05), yoked (t(4) = 0.08, p > 0.05), or SAL-60 d rats (t(6) = 0.65, p > 0.05). Stimulation of the ILCx induced potentiation of evoked spikes, which was NMDA-dependent (Fig. 2E; 20–40 min after tetanic stimulation, magnitude 88 ± 9%; p > 0.5), indicating induction of LTP at ILCx–BNST synapses. No differences were observed between the control groups (no discrimination, no LTP). Detailed analysis of the discrimination rate for each rat in the NIC-8 d group showed that only 50% of the rats had acquired nicotine IVSA. Interestingly, only these rats developed LTP in response to ILCx stimulation (Spearman correlation, r = 0.7421, p < 0.01). Saccharin-trained animals showed a strong preference for the reinforced nose-poke (discrimination between active and inactive, 83.1%). However, oral self-administration of saccharin did not induce synaptic changes within the BNST (excitatory response magnitude 20–40 min after tetanic stimulation, 129 ± 33%; p > 0.5). These data suggest that the acquisition of self-administration of nicotine, but not saccharin, produces experience-dependent modifications of ILCx–BNST synapses, which can be revealed by the stimulation of ILCx afferents.
Figure 2.
Effect of the 10 Hz ILCx stimulation onto BNST neurons at different stages of nicotine acquisition and maintenance. A, Stimulation and recording protocol. B, Histological control of stimulation (ILCx, *) and recording (BNST, arrow) sites. Scale bar, 1 mm. C, LTP seen in NIC-60 d rats but not in NIC-1 d, NIC-8 d, SAL-60 d, or yoked rats. D, Baseline and post-tetanic stimulation responses of ILCx–BNST projection neurons in NIC-60 d rats. Stimulus delivered at time = 0 (arrow). Each histogram consists of 100 trials individually illustrated in the associated raster. Bin width, 5 ms. E, The 10 Hz ILCx stimulation induces NMDA-dependent LTP within the BNST. Microinjection of the NMDA antagonist AP5 (400 μm, 60 nl) blocks the induction of LTP. Data are mean ± SEM.
Persistence of the synaptic changes within the BNST
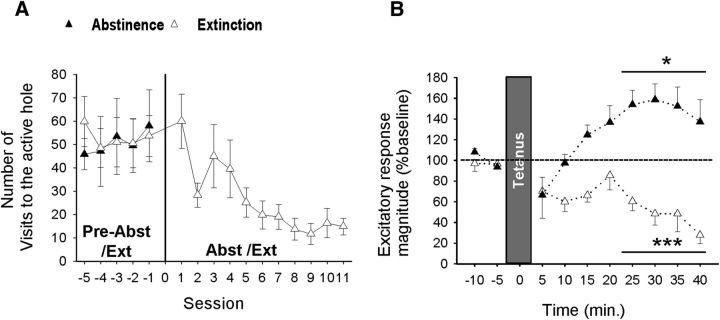
To determine the persistence of the observed synaptic changes, we either imposed abstinence or performed extinction training in rats previously exposed to nicotine self-administration (Fig. 3A). Across extinction training sessions (no drug, no cue), active responses declined (t(5) = 3.97, p = 0.02), whereas responses at the inactive hole remained stable (data not shown). Extinction training suppressed LTP in response to 10 Hz ILCx stimulation, which instead induced long-term depression after abstinence (Fig. 3B; two-way ANOVA, F(9,74) = 4.91, p < 0.0001, 20–40 min; Abst, t(5) = 2.94, p < 0.05; Ext, t(4) = 14.84, p < 0.001), indicating that the synaptic alteration is resistant to a passive drug-free period but sensitive to new learning.
Figure 3.
Effect of the 10 Hz ILCx stimulation onto BNST neurons after abstinence versus extinction. A, Decreasing active responses during extinction training. B, Extinction (Ext, n = 6) but not abstinence (Abst, n = 7) blocks LTP. *p < 0.05. ***p < 0.001. Data are mean ± SEM.
BNST LTP in response to ILCx 10 Hz stimulation is CB1 receptor-dependent
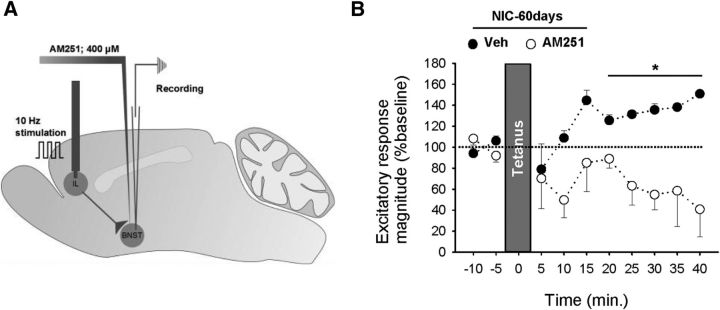
Because the endocannabinoid system plays an important role in nicotine reward (Simonnet et al., 2013) and in the modulation of synaptic plasticity in the BNST (Massi et al., 2008; Puente et al., 2011), we investigated the ability of the CB1 receptor to control the potentiation of ILCx–BNST excitatory synapses. Induction of LTP in the BNST of NIC-60 d rats was blocked by peripheral injection of the CB1 antagonists (excitatory response magnitude 20–40 min: Veh, 131.6 ± 4.4%; AM251, 78 ± 10%, p > 0.05; O-2050, 129 ± 33%; p > 0.5). More specifically, LTP induction was blocked after infusion of AM251 within the BNST (Fig. 4A,B; two-way ANOVA, F(1,48) = 31.33, p < 0.0001, 20–40 min; Veh, 131 ± 4%, AM251, 64 ± 13%; p > 0.05). This suggests that nicotine self-administration permits the induction of CB1-dependent LTP at BNST synapses in response to 10 Hz stimulation of ILCx afferents.
Figure 4.
In Nic-60 d rats, 10 Hz stimulation of ILCx inputs evokes LTP that is CB1 receptor mediated. A, In vivo ILCx stimulation (1 min, 10 Hz) in anesthetized rats. CB1 antagonist AM251 (60 nl, 400 μm) or vehicle was infused into the BNST before stimulation. B, AM251 blocks electrically induced LTP in NIC-60 d rats (Veh, n = 5; AM251, n = 5). *p < 0.05. Data are mean ± SEM.
Effect of ILCx stimulation on nicotine self-administration and cue-induced nicotine seeking
Next, the challenging question was whether electrical stimulation of the ILCx may alter future operant behaviors for nicotine (Fig. 5A, timeline) and whether it could be prevented by local microinjection of AM251.
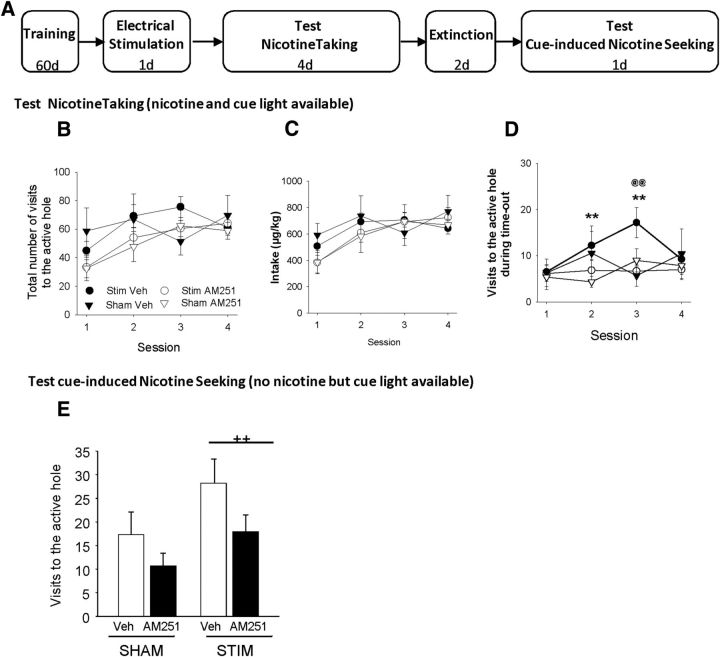
Figure 5.
Effects of 10 Hz ILCx stimulation on active responding during nicotine self-administration and on cue-induced reinstatement. A, Timeline of experiment. Rats were: (1) trained for nicotine self-administration (60 d), (2) anesthetized, received ILCx electrical stimulation 24 h after the last session (1 d) and CB1 antagonist pretreatment (Veh or AM251, 400 μm; 60 nl), (3) tested for nicotine taking (4 d), (4) trained for extinction (2 d), and (5) tested for cue-induced nicotine seeking. Effects of ILCx and sham stimulation in vehicle and AM251-treated rats on the following: B, Total number of visits in active hole during the whole session. C, Total nicotine intake over the whole session. D, Total number of visits in active hole during time-out. E, Total number of visits in the active hole during the cue-induced reinstatement period. Post hoc active hole Veh Session 1 versus Session 2 and Veh Session 1 versus Session 3, **p < 0.01. @@p < 0.01, main effect stimulation versus sham stimulation. ++p < 0.02. B–D, Data are mean ± SEM. E, Data are mean + SEM.
Our results showed that neither 10 Hz stimulation of the ILCx nor the AM251 pretreatment altered operant responding for nicotine; all groups respond by a similar number of visits to the active hole (Fig. 5B; ANOVA, p > 0.05). Moreover, nicotine intake was similar in all groups whatever the session considered (Fig. 5C; ANOVA, p > 0.05). However, after extended training, the stimulation altered responding at the active hole during time-out (Fig. 5D; ANOVA, F(3,108) = 3.75, p < 0.01). Rats that underwent stimulation with vehicle pretreatment showed a progressive increase in responses (vs Session 1, p < 0.01). This was absent in sham stimulated rats and completely prevented by BNST CB1 receptor antagonism in stimulated animals (Stim Veh vs Stim AM251, p < 0.01). No differences were observed between these two groups in general activity (stim + Veh, 1241 ± 106 beam breaks; stim × AM251, 1254 ± 140 beam breaks; Student's t test, t(21) = 0.08, p > 0.05). These data suggest that the stimulation increased responding for the cue light.
To test this hypothesis, all groups were tested for cue-induced reinstatement (Fig. 5E). ANOVA revealed a significant effect of the cue presentation compared with no cue presentation (F(1,27) = 20.48, p < 0.001). The no cue period resulted in a low number of visits to the active hole for all groups (interaction F(1,27) = 2.42, p > 0.05). Stimulation of the ILCx increased the cue-induced visits to the active hole (main effect stimulation, F(1,27) = 5.50, p < 0.02). There was no significant interaction stimulation × CB1 antagonist treatment (ANOVA, p > 0.05), suggesting that AM251 tends to decrease visits to the active hole in both stimulated and nonstimulated rats. The level of responding in the inactive hole was not altered by cue presentation (ANOVA, p > 0.05).
Discussion
The aim of the present study was to determine whether acquisition of nicotine taking, and then cue-induced nicotine seeking, involved cellular adaptations at ILCx–BNST synapses. To reveal alterations in this neuronal circuit, we applied electrical stimulation to the ILCx at frequencies similar to physiological activation. Our results indicate that 60 d of nicotine self-administration facilitates the induction of LTP in the BNST in response to 10 Hz ILCx stimulation. The occurrence of this stimulation-induced LTP correlates with the degree of acquisition of self-administration and is dependent on BNST CB1 receptors. In addition, it resists 30 d of forced abstinence. Finally, 10 Hz stimulation increased time-out responding during nicotine self-administration as well as cue-induced nicotine seeking. Results suggest that blockade of BNST CB1 receptors reduced aberrant responding.
Electrical stimulation of the ILCx at 10 Hz mimics the physiological activation observed during goal-oriented behaviors (Jackson et al., 2001). This physiologically relevant stimulation triggered neuroplastic changes in BNST neurons only in rats that had acquired nicotine self-administration behavior, but not after passive exposure to nicotine. We propose that synaptic plasticity is the cellular mechanism underlying the change in responsiveness of BNST neurons to prefrontal inputs after 10 Hz ILCx stimulation in NIC-60 d rats. This conclusion is supported by our results showing that the mechanism depends on the activation of NMDA receptors. Alternative mechanisms for evoked spike potentiation in the BNST could be a change in intrinsic excitability of BNST neurons after extended training with nicotine or a change in the level of tonic activity at inhibitory inputs. However, these scenarios are unlikely as the reduction in threshold current for spike initiation in the BNST of NIC-60 d rats is not associated with changes in the basal activity of BNST neurons (Caillé et al., 2009). Further work will be needed to determine whether increased spike firing in response to 10 Hz stimulation in NIC-60 d rats is the result of the enhancement of synaptic strength or a change in intrinsic excitability of the BNST neurons.
Acquisition of self-administration of nicotine, but not of a natural reinforcer, promotes cellular adaptations at ILCx–BNST excitatory synapses. Rats that did not respond for nicotine (saline or saccharin) and rats that did not meet the learning criterion (yoked or NIC-1 d) did not show LTP in response to electrical stimulation of the ILCx. Importantly, in the NIC-8 d group, we report a correlation between the development of a preference for the cue and nicotine-paired hole, and the emergence of synaptic potentiation in the ILCx–BNST pathway. Rats that self-administered the drug by chance did not show facilitation of LTP induction, whereas all rats with extended training did (Kasanetz et al., 2013). Our findings suggest that the ILCx–BNST pathway is involved in aberrant stimulus–response behavior and might contribute to the established habitual behaviors observed in drug addiction (Everitt and Robbins, 2005). The development of habits is involved in the transition from drug use to drug abuse; therefore, an interesting investigation would be to examine how the stimulus–response association (Zapata et al., 2010; Smith et al., 2012) controls nicotine seeking as well as the neuroadaptations at ILCx–BNST synapses.
Smoking-related cues have increased significance in smokers (Rose and Corrigall, 1997) and in animal models of nicotine addiction (Chaudhri et al., 2006). We show here that LTP induction and suppression are both directly linked to the learning of a specific relationship between motor actions and cue-associated reinforcement, rather than to the presence (yoked condition) or absence (extinction condition) of the drug. Thus, although nicotine enhances the reinforcing properties of discrete and contextual stimuli (Palmatier et al., 2007), reexposing rats with a long history of nicotine taking to such stimuli may reactivate the strengthened projections of the ILCx (Bossert et al., 2011) and subsequently induce nicotine seeking. In accordance with this idea, we show here that 10 Hz stimulation of the ILCx promotes excessive visits to the nicotine- and cue-paired nose-poke hole while the drug is not available.
Much evidence implicates the BNST in stress and anxiety (Glangetas et al., 2013; Jennings et al., 2013). Therefore, an additional explanation of our results might be that the induction of LTP and the increase in responding during time-out are both produced by the experience of the negative/anxiogenic effects of nicotine (George et al., 2007). Consistent with this hypothesis, previous studies have demonstrated that extended access to nicotine produced escalation of nicotine intake only when animals experienced intermittent withdrawal periods (O'Dell and Koob, 2007; Cohen et al., 2012). In our experiment, the ILCx stimulation protocol (Fig. 5A) introduced a period of abstinence, which may have subsequently led to the development of a nicotine withdrawal-induced negative drive. Therefore, one cannot discard the possibility that the increase in active responding during the time-out period is the expression of increased incentive value of nicotine acting as a negative reinforcer (Koob and Le Moal, 2001) and that this increase modified nicotine intake.
In the present study, we have demonstrated that extinction training facilitates inhibition of the ILCx–BNST pathway, whereas the synaptic strengthening remains intact after forced abstinence. The extinction protocol used in this study resulted in an average of 17.2 d to meet extinction criteria. However, the forced abstinence period was 30 d, which might suggest that LTP found after abstinence is the result of an incubation effect (Grimm et al., 2001), rather than the persistence of synaptic modifications through a drug-free period. We have not subjected rats to various durations of forced abstinence to rule out the incubation effect of LTP over time, but such an effect is unlikely as the period of extinction training ranged from 11 to 27 d, and all rats showed induction of long-term depression.
In rats trained to self-administer nicotine for 60 d, the induction of LTP in response to 10 Hz stimulation of ILCx afferents depends on CB1 receptors expressed within the BNST. Notably, we can discard the possibility that this was a consequence of CB1 receptor inverse agonist properties of AM251 because the LTP was also blocked by the administration of O-2050, a CB1 receptor neutral antagonist (Dubreucq et al., 2013). In naive rats, ex vivo electrophysiological studies support the existence of endocannabinoid-dependent forms of synaptic plasticity in the BNST (Puente et al., 2011). Interestingly, nicotine self-administration increases 2-arachidonoyl glycerol as well as anandamide levels in the VTA of behaving animals (Buczynski et al., 2013). Although the increased VTA anandamide levels correlate with the volitional nature of drug exposure, the specific blockade of CB1 receptors in the VTA strongly decreases nicotine self-administration in rats (Simonnet et al., 2013). One can therefore propose that, similar to the VTA mechanisms, chronic nicotine consumption might lead to changes in endocannabinoid tone within the BNST and contribute to cellular adaptations at ILCx–BNST synapses. However, the nature of the endocannabinoid involved here remains unknown.
Peripheral administration of a CB1 antagonist strongly decreases nicotine self-administration (Cohen et al., 2002; Shoaib, 2008; Simonnet et al., 2013), suggesting that stimulation of CB1 receptors is important for nicotine reinforcement. In the present study, AM251 injected within the BNST selectively blocked excessive responding during time-out but did not change nicotine intake. In line with this observation, previous studies have indicated that the BNST is involved in cue-induced drug seeking rather than in the control of drug taking. For instance, increased activity of neurons in the BNST is associated with increased drug seeking during protracted withdrawal and in opiate-induced conditioned place preference (Harris and Aston-Jones, 2003). Moreover, inactivation of the BNST specifically blocks cocaine seeking elicited by drug-paired cues (Buffalari and See, 2011). Together, these findings strongly support the hypothesis that extended training to self-administer nicotine and sustained activation of the ILCx induce CB1 receptor-dependent neuroplastic changes in the BNST that might be involved in the stimulus tracking behavior paired with nicotine taking (Palmatier et al., 2013; Paolone et al., 2013).
In conclusion, our findings indicate that acquisition of nicotine self-administration allows the induction of evoked-spike potentiation in the BNST in response to 10 Hz stimulation of ILCx afferents. This neuroadaptation resists abstinence and seems to contribute to a maladaptive stimulus–response behavior controlled by CB1 receptors (Marsicano et al., 2002) in the BNST, suggesting that it might be responsible for vulnerability to cue-induced relapse. Further investigation will be needed to fully elucidate the key role of the ILCx–BNST pathway in the attribution of salience to nicotine-paired cues.
Footnotes
This work was supported by Centre National de la Recherche Scientifique, University of Bordeaux, Agence Nationale de la Recherche ANR-2010-BLAN-1439-01 to S.C. and F.G., and Region Aquitaine. We thank Florence Darlot, Emilie Noe, and Delphine Girard for technical assistance; Drs. Cyril Herry, Giovanni Marsicano, and Francis Chaouloff for valuable comments on the manuscript; and Julia Slone-Murphy (www.neuroedit.com) for editorial assistance.
The authors declare no competing financial interests.
References
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology. 2013;38:574–584. doi: 10.1038/npp.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Guillem K, Cador M, Manzoni O, Georges F. Voluntary nicotine consumption triggers in vivo potentiation of cortical excitatory drives to midbrain dopaminergic neurons. J Neurosci. 2009;29:10410–10415. doi: 10.1523/JNEUROSCI.2950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Rolando MT, Silvagni A, Di Chiara G. Increase of dialysate dopamine in the bed nucleus of stria terminalis by clozapine and related neuroleptics. Neuropsychopharmacology. 2000;22:140–147. doi: 10.1016/S0893-133X(99)00085-8. [DOI] [PubMed] [Google Scholar]
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/S0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37:2153–2160. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrié P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Durand A, Matias I, Bénard G, Richard E, Soria-Gomez E, Glangetas C, Groc L, Wadleigh A, Massa F, Bartsch D, Marsicano G, Georges F, Chaouloff F. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73:895–903. doi: 10.1016/j.biopsych.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glangetas C, Girard D, Groc L, Marsicano G, Chaouloff F, Georges F. Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:19657–19663. doi: 10.1523/JNEUROSCI.3175-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem. 2001;78:920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, Di Prospero C, Chiang C, Martinez A, Jones AA, Doudnikoff É, Caillé S, Bézard E, Georges F, Dumont ÉC. D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. J Neurosci. 2013;33:11960–11971. doi: 10.1523/JNEUROSCI.1784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict Biol. 2008;13:239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 2007;195:235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013;226:247–259. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54:438–444. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Simonnet A, Cador M, Caillé S. Nicotine reinforcement is reduced by cannabinoid CB1 receptor blockade in the ventral tegmental area. Addict Biol. 2012;18:930–936. doi: 10.1111/j.1369-1600.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]