Abstract
Prolonged limb immobilization deprives sensorimotor cortical areas of an important source of excitatory input, as well as of motor output. Previous work has described effects on motor excitability but it is unclear whether motor plasticity is also influenced. In two groups of eight healthy human subjects, the left hand was immobilized for 8 h to induce sensorimotor deprivation of the cortical representation of the abductor pollicis brevis muscle. We used transcranial magnetic stimulation protocols to evaluate motor excitability with motor-evoked potentials, input–output (IOcurve) and short-latency intracortical inhibition (SICI) recruitment curves, as well as long-term potentiation (LTP)/long-term depression (LTD)-like plasticity with paired-associative stimulation (PAS) of the median nerve and motor cortex using an interstimulus interval of 25 ms (PAS25) or 10 ms (PAS10), respectively, in two sessions at least 7 d apart (baseline and after immobilization). After immobilization, the slope of the IOcurve decreased, and SICI at lower conditioning pulse intensities was reduced. The LTP-like effects of PAS25 and the LTD-like effect of PAS10 were both significantly enhanced. The effects differed among individuals: the more IOslope decreased after immobilization, the greater the increase of PAS25 and the smaller the increase of PAS10 effects. We suggest that sensorimotor deprivation has two effects. It increases the sensitivity to remaining sensory inputs and therefore increases the effectiveness of both PAS protocols. In addition, it reduces neuronal excitability to an individually different level, as reflected in the reduced IOslope and leads to an interdependent modulation of synaptic plasticity as such as it shifts the threshold of LTP/LTD-like plasticity induction.
Keywords: human, plasticity, sensorimotor deprivation, transcranial magnetic stimulation
Introduction
The functional organization of the motor cortex is dynamic and shaped by movement as well as by the sensory feedback it evokes. For example, we have recently shown that practicing a proprioceptive discrimination task that focuses attention on the hand temporarily changes sensorimotor cortical organization and influences behavior by facilitating motor learning (Rosenkranz and Rothwell, 2012). Indeed, it is well known from the animal and human literature that reorganization of the sensory (Kaas et al., 1983; Merzenich et al., 1983; Coq and Xerri, 1999; Weiss et al., 2004) and motor cortices (Liepert et al., 1995; Ridding and Rothwell, 1995, 1997; Hallett et al., 1999) can be produced not only by increasing sensory input but also by removing ongoing inputs and restricting movements.
In humans, the two most frequently used paradigms to investigate relatively short-term effects of decreasing sensory feedback and motor activation are ischemic deafferentation and limb immobilization. Ischemic nerve block (INB) increases the excitability of corticospinal projections to muscles proximal to the block (Ziemann et al., 1998a,b). However, because INB paralyzes the muscles distal to the block, it is not possible to evaluate plasticity within their representations in the motor cortex. Experiments in which limb immobilization has been used to evaluate the effect of reducing sensory input and motor output have shown that this intervention changes corticospinal excitability, as assessed with transcranial magnetic stimulation (TMS). However, the details of the results appear to vary depending on the duration of the immobilization: shorter periods of immobilization (12 h and 4 d; Facchini et al., 2002; Huber et al., 2006; Ngomo et al., 2012) reduce excitability, whereas longer periods (10 or 30–40 d) increase excitability (Zanette et al., 1997, 2004; Roberts et al., 2007). Very long lasting immobilization (80 d or more; Liepert et al., 1995) reduces excitability but the effects are difficult to interpret because of concomitant peripheral muscle atrophy (Yue et al., 1997).
Thus, most studies of the effect of limb immobilization so far are descriptive and do not probe the underlying mechanisms that drive change to occur. The aim of the present experiments was to provide preliminary evidence that sensorimotor deprivation as induced by immobilization not only alters measures of cortical excitability but also changes the sensitivity of basic processes of synaptic plasticity that could then promote more lasting changes in neural organization.
In our study, we examined motor excitability and LTP/LTD-like plasticity in the motor cortical representation of the left thumb abductor muscle, after the hand was immobilized for 8 h by use of an adaptable splint. The immobilization procedure particularly restricted motion of the left thumb, including abduction movements, and therefore restricted contractions of the thumb abductor muscle and generation of movement feedback. Our hypothesis was that immobilization would lead to potentially interdependent changes in motor excitability and plasticity with the latter having the potential to change motor cortical organization over the longer-term.
Materials and Methods
Subjects
Two groups of subjects, consisting of eight subjects each (Group 1: 5 females, mean age 27 ± 5 years; Group 2: 4 females, mean age 27 ± 3 years) were tested. All subjects were right-handed according to the Oldfield questionnaire of handedness. The study was approved by the joint ethics committee of the Institute of Neurology and National Hospital for Neurology and neurosurgery, London, UK, and by the ethics committee of the Medical Faculty Mannheim of the University of Heidelberg, Germany. All experiments conform to the Declaration of Helsinki.
Group 1 was studied in the laboratory of the Sobell Department, Institute of Neurology, London, UK, and Group 2 was studied in the laboratory of the Central Institute of Mental Health, Medical Faculty Mannheim of the University of Heidelberg, Germany. Both laboratories were equipped with matching technical equipment (magnetic stimulator, stimulating coil, electrical stimulator, EMG amplifier, and software to control the experiment and collect data).
TMS
Transcranial magnetic stimulation (TMS) was performed using a Magstim 200 stimulator connected to a figure-eight-shaped coil with an internal wing diameter of 7 cm (Magstim). The coil was held with the handle pointing backward and laterally 45° to the interhemispheric line to evoke anteriorly directed current in the brain and was optimally positioned to obtain motor-evoked potentials (MEPs) in the abductor pollicis brevis (APB) muscle. Stimulation intensities are quoted in Table 1 as a percentage of maximal stimulator output (±SE).
Table 1.
Subjects' age and TMS parameters determined in the APB (±SE)
| Age (years) | Baseline |
After immobilization |
|||
|---|---|---|---|---|---|
| aMT | SI1mV | aMT | SI1mV | ||
| PAS25 group | 27.0 ± 1.9 | 28.9 ± 1.3 | 47.0 ± 2.8 | 30.0 ± 1.6 | 49.3 ± 2.2 |
| PAS10 group | 27.3 ± 1.0 | 29.0 ± 0.6 | 42.4 ± 1.9 | 31.8 ± 1.3 | 44.3 ± 1.6 |
| t test (group comparison) | 0.89 | 0.94 | 0.19 | 0.39 | 0.09 |
TMS parameters are given in percentage stimulator output.
EMG recording
Surface electromyographic (EMG) recordings in a belly-to-tendon montage were made from the APB, the first dorsal interosseus (FDI), and the abductor digiti minimi (ADM) muscle of the left hand. The raw signal was amplified and filtered with a bandpass filter of 30 Hz to 1 kHz (Digitimer). Signals were digitized at 2 kHz (CED Power1401, Cambridge Electronic Design) and stored on a laboratory computer for off-line analysis.
Experimental parameters
Motor excitability.
At the beginning of each experiment, the stimulus intensity (SI) needed to evoke an MEP of ∼1 mV peak-to-peak amplitude (SI1mV) was defined. SI1mV was used to record 15 MEPs before and after PAS (Fig. 1; see Experimental protocol). The mean amplitude of these responses was calculated in each subject. In addition, we measured the input–output relationship of MEP amplitude to stimulus intensity (IOcurve). The intensities of single TMS stimuli were expressed as a percentage of SI1mV. Ten MEPs each were recorded with 50, 70, 80, 90, 100 (equal to SI1mV), 110, 120, 130, and 150% of SI1mV. The mean MEP amplitude per stimulus intensity was calculated for each subject.
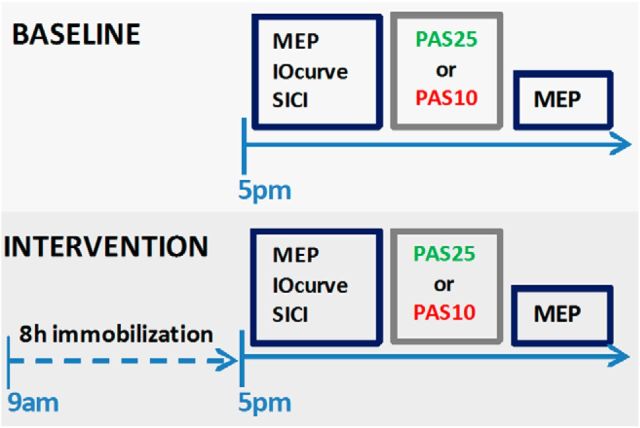
Figure 1.
Experimental protocol. The subjects participated first in the baseline session, which was followed by the interventional session within 7 d minimal.
SICI.
The input–output relation for short-interval intracortical inhibition (SICI curve; Orth et al., 2003; Rosenkranz et al., 2007) was measured at baseline and after 8 h of immobilization (see Experimental protocol) using subthreshold conditioning stimulus intensities of 70, 80, and 90% of active motor threshold (aMT). The aMT was defined as the minimum intensity needed to evoke an MEP of ≥200 μV in 5 of 10 trials in the tonically active APB (∼20% of maximal contraction as assessed visually on an oscilloscope) and was tested before each SICI measurement. The conditioning stimulus preceded the suprathreshold test stimulus (intensity set at SI1mV) by 3 ms (Kujirai et al., 1993). Ten trials were recorded for each conditioning pulse intensity. Before, in between, and after the blocks, five single test pulses were given to ensure that the unconditioned MEP size was stable. In case the MEP was out of the 0.7–1.3 mV range, the test stimulus intensity was readjusted and the experiment restarted. The peak-to-peak amplitude of the conditioned and test MEPs was measured for each single trial to calculate the mean amplitude and percentage SICI (conditioned MEP: test) for the three different conditioning stimulus intensities. This approach allowed us to measure the level of SICI at a single conditioning intensity as well as the recruitment of SICI (SICI curve) defined as the increase in SICI with increasing intensities of conditioning stimulus. Due to technical limitations that were present when the subjects of the PAS10 group were tested, the SICI curve was measured in the subjects of the PAS25 group only.
PAS
Paired-associative stimulation (PAS) consisted of 200 electrical stimuli of the left median nerve at the wrist paired with a single TMS pulse over the hot spot of the APB area of the right hemisphere with a rate of 0.25 Hz. Electrical stimulation (Digitimer DS7A) was applied through a bipolar electrode (cathode proximal), using square-wave pulses (duration 0.2 ms) at an intensity of three times the perceptual threshold. TMS was delivered through a figure-eight coil (diameter of each wing 70 mm) connected to a Magstim 200 magnetic stimulator and was held in the same position as described above.
Stimulation was applied at an intensity adjusted to evoke an MEP of SI1mV in the relaxed APB. Subjects of each group took part in two experimental sessions that were separated by at least 1 week. In Group 1 (PAS25 group), the effect of PAS given with an interstimulus interval of 25 ms (PAS25) between peripheral and TMS stimulus was tested, which has been shown previously to induce long-lasting MEP increase (Stefan et al., 2000, 2002). In Group 2 (PAS10 group), the effect of PAS given with an interstimulus interval of 10 ms (PAS10) between peripheral and TMS stimulus was tested, which has previously been shown to induce an MEP decrease (Wolters et al., 2003). Subjects were instructed to look at their stimulated hand and count the peripheral electrical stimuli they perceived; they were asked the actual count by the experimenter approximately three to four times during the PAS protocol (Stefan et al., 2004). The MEPs evoked in the APB, FDI, and ADM were displayed on-line during the intervention to control for the correct coil position and stored for off-line analysis.
The effect of the PAS protocols was measured as change of the MEP amplitude in the APB. Using the stimulus intensity that evokes an MEP of 1 mV peak-to-peak amplitude (SI1mV) in the APB, defined before the PAS protocol, 15 MEPs were recorded before and after each PAS protocol, and the mean amplitude was calculated for the data obtained before and after PAS in each single subject.
Immobilization procedure
The subject's left forearm was immobilized in a splint which ensured a complete immobilization of the wrist, and carpometacarpal and metacarpophalangal joints of the thumb and fingers, and restricted movements in the interphalangeal joint of the thumb, and the proximal and distal interphalangeal joints of the fingers. This procedure also restricted contractions of the left APB, and consequently the generation of proprioceptive feedback from this muscle. To prevent any discomfort induced by pressure of the splint, the hand was wrapped with a soft bandage and the splint was adjusted to fit the individual's hand size and shape before it was put on and additionally secured by an elastic bandage. The tip of the thumb and the other fingers remained visible to control for any restriction in blood circulation or swelling.
The splint was put on between 8:00 and 9:00 A.M. and subjects wore it for 8 h. They were instructed to move their left arm as little as possible during these 8 h and to place their left forearm in a pronated position and flexed at the elbow on a small cushion on top of their work desk. Because all subjects were right-handed and worked in academia (staff members or students of the institutes in London or Mannheim) it was possible for them to arrange a “study day” for reading which enabled them to follow the instructions quite well with minimal interruptions (e.g., lunch break). During the immobilization the subjects were regularly checked for any signs of numbness or tingling in the left hand, as well as restriction in blood flow at least once per hour. An occurrence of any of these symptoms would have let to an immediate end to the immobilization and consequently the experiment.
Experimental protocol
Figure 1 shows which interventions were performed and which experimental parameters were measured. In the baseline experiment, the motorcortical excitability, tested with the IOcurve, and the effect of either PAS25 or PAS10, for the PAS25 and PAS10 groups, respectively, were tested. These measurements were repeated at least a week later after 8 h of immobilization of the left thumb and wrist. Both measurements, baseline and after immobilization, were performed in the late afternoon (∼4 to 5 P.M.) to control for circadian influences on motor excitability and plasticity.
Data analysis and statistics
The subjects' age and TMS parameters (aMT and SI1mV) expressed as percentages of stimulator output are given in Table 1. The comparability of the PAS25 and PAS10 groups in terms of age and TMS parameters were tested by unpaired t tests, after testing for normal distribution by use of the Kolmogorov–Smirnov test.
The input–output curve data were analyzed using ANOVA with the main factors “stimulus intensity” and “immobilization condition,” which refers to the two experimental conditions “at baseline” and “after immobilization.” To simplify the dataset obtained by measuring the IOcurves, the slopes defined as the steepness of the linear regression line through the given data points between 90% and 130% SI1mV were calculated. The IOslopes measured at baseline and after immobilization were compared within each group by using paired t tests.
The SICI data were analyzed using ANOVA with “conditioning pulse intensity” and “immobilization condition” as main factors. In addition, the data obtained at each intensity of conditioning pulse were compared between the baseline and after immobilization conditions by use of paired t tests.
To control for correct adjustment of MEP size to 1 mV peak-to-peak amplitude, the MEPs measured before PAS in the baseline and immobilization measurement were compared within each group by means of paired t tests; furthermore, the MEPs measured either at baseline or after immobilization were compared between the groups by means of unpaired t tests. Within the PAS25 and PAS10 groups, ANOVAs were performed on the raw data of MEPs with the factors immobilization condition and “MEP amplitude before PAS: after PAS.” For further analysis, the MEP raw data were normalized and expressed as percentage of MEPs (MEPs after PAS: MEPs before PAS). Using these normalized data, the effect of PAS at baseline and after immobilization was compared within the PAS25 and PAS10 groups by use of paired t tests.
The change in IOslope after immobilization was expressed as a percentage of the IOslope at baseline (IOslope after immobilization: IOslope at baseline; percentage). This was then correlated with two other variables: (1) the change in PAS effect after immobilization expressed as a percentage of the PAS effect at baseline (PAS after immobilization: PAS at baseline), and (2) with immobilization-induced changes of SICI (SICI after immobilization: SICI at baseline; percentage). In addition, baseline motor excitability (baseline IOslope) was correlated with the immobilization-induced changes to the PAS effects. Pearson's r was calculated for all correlations.
Significance levels for the statistical tests are set to p < 0.01 to correct for multiple comparisons and small sample size. All ANOVAs were tested for sphericity using Mauchly's test. In case of significant sphericity, Greenhouse–Geisser corrections were performed. Corrected ANOVAs are marked with an asterisk (*). All data are given as mean ± SEM.
Results
None of the subjects experienced any discomfort during the 8 h of immobilization, nor any side effects of TMS testing.
There was no significant difference between the age and TMS parameters for the PAS25 and PAS10 groups (Table 1); in addition, a two-way ANOVA with the factors “immobilization condition” and “PAS25group/PAS10group” did not show any significant interaction or main effects for either aMT or SI1mV parameters. Similarly, the number of peripheral nerve stimuli (201 ± 2 on average) that were counted by participants during the PAS protocols did not differ between either the PAS25 and PAS10 groups or between the experimental conditions (at baseline and after immobilization).
Motor excitability
Figure 2 shows the IOcurve measured at baseline and after immobilization, before the PAS protocols were administered, for the PAS25 (A) and PAS10 groups (B), and Table 2 summarizes the results of the statistical analysis. All IOcurves showed a significant increase of MEP amplitudes with increasing TMS intensity (Table 2, one-way ANOVAs).
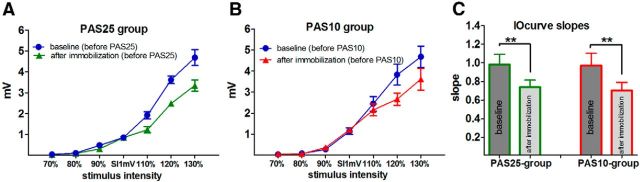
Figure 2.
IOcurves and IOcurve slopes in the APB. The mean MEP amplitude (in mV± SE) as given on the y-axis against the stimulus intensity given on the x-axis (in percentage of SI1mV). A, B, IOcurves measured at baseline and after immobilization in the PAS25 group (A) and the PAS10 group (B). In both groups, the IOcurves are less steep after immobilization compared with baseline. C, The slopes of the IOcurves have been calculated for the approximately linear part between 90 and 130% SI1mV. The decrease of IOcurve slopes after immobilization is significant in both groups (paired t test; p < 0.001).
Table 2.
Statistical results of ANOVAs on IOcurves
| df/Error df | F | p | |
|---|---|---|---|
| Two-way interaction | |||
| Stimulus intensity × immobilization condition | |||
| PAS25 | 4; 28 | 3.9 | 0.012 |
| PAS10 | 2.1; 14.4* | 10.0 | 0.002 |
| Stimulus intensity | |||
| PAS25 | 4; 28 | 48.3 | <0.001 |
| PAS10 | 1.2; 8.6* | 52.7 | <0.001 |
| Immobilization condition | |||
| PAS25 | 1; 7 | 9.9 | 0.016 |
| PAS10 | 1; 7 | 5.9 | 0.045 |
| One-way interaction | |||
| Stimulus intensity | |||
| PAS25 baseline | 4; 28 | 36.0 | <0.001 |
| PAS25 after immobilization | 2.0; 14.3* | 38.9 | <0.001 |
| PAS10 baseline | 1.4; 9.6* | 45.5 | <0.001 |
| PAS10 after immobilization | 1.3; 8.9* | 45.7 | <0.001 |
Separate two-way ANOVAs were performed on the data of the IOcurves of the PAS25 and PAS10 groups (measured before PAS) at baseline and after immobilization (but before PAS) with the within-group factors stimulus intensity and immobilization condition. Separate one-way ANOVAs with the factor stimulus intensity were performed on the IOcurves at baseline and after immobilization of the PAS25 and PAS10 groups, respectively. The degrees-of-freedom (df) and error of degrees-of-freedom (error df), and the F and p values are given. For significant results in Mauchly's test for sphericity (p < 0.05), Greenhouse–Geisser corrections were performed, and the corrected degree-of-freedom are given (*).
After immobilization (and before PAS), the IOcurves were less steep than in the baseline condition before PAS for both the PAS25 and PAS10 groups, indicating that immobilization reduced the steepness of the IOcurves in both groups. The statistical analysis of the IOcurves showed a significant interaction of the factors “immobilization condition” and “stimulus intensity” (see two-way ANOVA in Table 2), with significant main effects of both factors.
Further analysis of the IOslopes (Fig. 2C) indicated that there was no difference between the PAS25 and PAS10 groups at baseline (before PAS). Immobilization significantly reduced the IOslopes in both groups (paired t test; p < 0.009).
PAS induced plasticity
There was no difference in the amplitude of MEPs (∼1 mV) measured at baseline and after immobilization in either the PAS25 or the PAS10 group (within-group comparison; paired t test; n.s.); similarly, there were no significant between-group differences (unpaired t test; n.s.).
Figure 3 shows that the PAS25 protocol increased, and the PAS10 protocol decreased MEP, amplitudes, at baseline and after immobilization. However, both effects were stronger after immobilization. The results of the statistical analysis using ANOVAs with the main factors “MEP amplitude before and after PAS” and “immobilization condition” are summarized in Table 3.
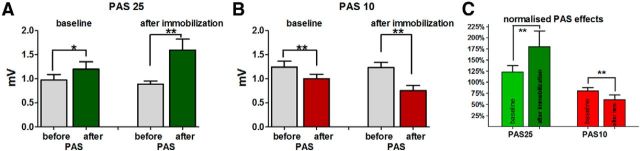
Figure 3.
Mean MEP (±SE) in the APB in the PAS25 and PAS10 groups. A, B, The mean MEP in millivolts measured at baseline and after immobilization; the MEPs measured before PAS are shown in gray columns and after PAS in colored columns (green for PAS25; red for PAS10). There was no difference in MEP size before PAS in the experiments performed at baseline and after immobilization, neither in the PAS25 nor in the PAS10 group. Therefore, the MEPs were normalized (MEP after PAS/ MEP before PAS) and expressed as percentages (C). After immobilization, both PAS protocols were significantly more effective: PAS25 increased whereas PAS10 decreased MEPs more than at baseline. Statistical results are given in the figure (paired t tests; *p < 0.05; **p < 0.01).
Table 3.
Statistical results of ANOVAs on PAS
| Two-way interaction | df/Error df | F | p |
|---|---|---|---|
| Immobilization condition × before and after PAS | |||
| PAS25 | 2; 14 | 8.9 | 0.021 |
| PAS10 | 2; 14 | 15.2 | 0.006 |
| Immobilization condition | |||
| PAS25 | 1; 7 | 1.6 | 0.25 |
| PAS10 | 1; 7 | <0.01 | 0.9 |
| Before and after PAS | |||
| PAS25 | 1; 7 | 18.9 | 0.003 |
| PAS10 | 1; 7 | 77.6 | <0.001 |
Separate two-way ANOVAs were performed on the raw MEP data of the PAS25 and PAS10 groups measured before and after PAS either at baseline or after immobilization with the within-group factors “immobilization condition” and “before and after PAS.” The degrees-of-freedom (df) and error of degrees-of-freedom (error df), and the F and p values are given. As Mauchly's test for sphericity was nonsignificant, a correction of the degree-of-freedom was not necessary.
For the PAS25 group (Fig. 3A) there was a significant interaction of the main factors “MEP amplitude before and after PAS” and “immobilization condition,” indicating that immobilization influenced the PAS25 effect. Post hoc paired t tests showed that PAS25 significantly increased MEPs in the baseline condition as well as after immobilization (paired t test, p < 0.001; Fig. 3A). When the effect of PAS25 was expressed as a percentage change in MEP amplitude (normalized PAS effect), it became clear that the PAS25 effect after immobilization was stronger than at baseline (paired t test, p = 0.001; Fig. 3C).
PAS10 significantly decreased MEPs at baseline as well as after immobilization (paired t test, p < 0.001; Fig. 3B). As with the results in the PAS25 group, there was a significant interaction of the factors “MEP amplitude before and after PAS10” and “immobilization condition,” which highlights the influence of immobilization on the PAS10 effect. Using normalized data (MEPs after PAS10/before PAS10), post hoc paired t tests showed that PAS10 reduced MEPs more after immobilization than at baseline (paired t test, p = 0.006; Fig. 3C).
Correlation between the effect of immobilization on IOcurve and PAS-induced plasticity
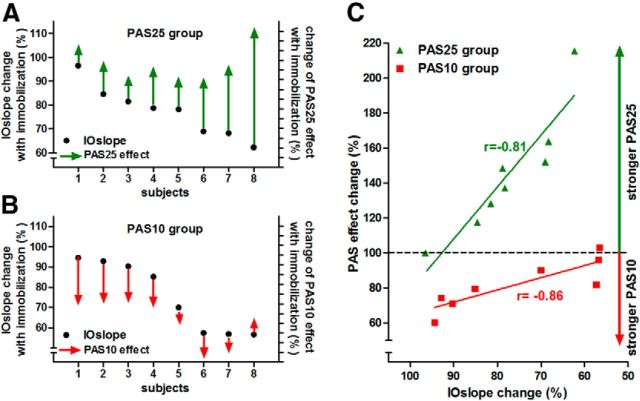
Participants in whom immobilization produced the largest changes in IOcurve had the largest changes in response to the PAS25 and the smallest changes in response to PAS10. This is shown in Figure 4. The percentage change in the IOslope (IOslope after immobilization: IOslope at baseline) in each person is plotted as filled circles; the percentage difference in the PAS response (PAS before: PAS after immobilization) is indicated by the vertical arrows. Arrows going upward indicate an increase of PAS25 effect with immobilization (Fig. 4A), and arrows going down indicate an increase in the PAS10 effect with immobilization (Fig. 4B). The length of the arrow indicates the amount of change.
Figure 4.
Changes and correlations of IOslopes and PAS effects after immobilization in the individual subjects. A, B, Plot for each individual subject of the PAS25 (A) and PAS10 (B) groups the IOslope change (IOslope after immobilization: IOslope at baseline; percentage) on the left y-axis, as well as the change of the normalized PAS effect (PAS effect after immobilization: PAS effect at baseline; percentage) on the right y-axis, which displays the relative PAS effect change in percentage with ticks indicating steps of 10%. In both groups, the IOslope decreases after immobilization below the values measured at baseline (all black dots are <100%). However, the extent of the decrease varied in the individual subjects. The change in PAS effect is plotted as arrows going up (PAS25) or down (PAS10) to indicate the physiological direction of the PAS effects (LTP-like effect of PAS25; LTD-like effect of PAS10) which both show an increase after immobilization (note: subject 8 in the PAS10 group shows a decreased effect). In the PAS25 group, stronger decrease of the IOslope was associated with a stronger increase of the PAS25 effect after immobilization; whereas in the PAS10 group, stronger decrease of the IOslope was associated with a weaker increase of the PAS10 effect after immobilization (except subject 8 in the PAS10 group). C, The linear regression between the IOslope change (IOslope after immobilization: IOslope at baseline; percentage; x-axis) and the PAS effect change (PAS effect after immobilization: PAS effect at baseline; percentage; y-axis) for the PAS25 (green symbols) and PAS10 (red symbols) groups. In both groups, the immobilization-induced changes of IOslope and PAS effects are significantly correlated (PAS25 group: p < 0.002; PAS10 group: p < 0.013); Pearson's r is given in the figure.
For PAS25, subjects in whom immobilization produced the greatest decrease in IOslope were those who had the greatest increase in the effect of PAS25. Conversely, for PAS10, subjects in whom immobilization produced the greatest decrease in IOslope were those who had the least change in the effect of PAS10.
Figure 4C displays the same data in a different way, plotting the change in IOslope against the change in PAS effect. There is a statistically significant correlation for both the PAS25 (Pearson's r: −0.81; p = 0.015) and PAS10 group (Pearson's r: −0.86; p = 0.006).
SICI
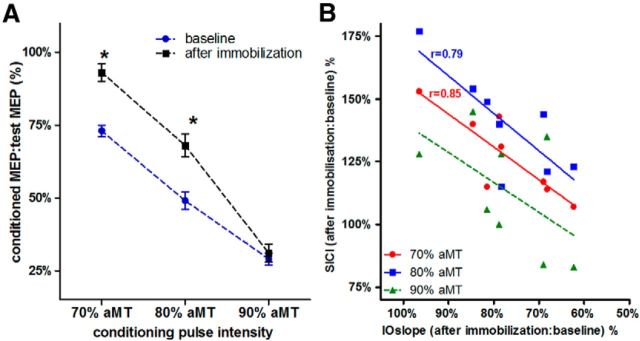
Figure 5A shows the results of the SICI protocol, measured with conditioning pulse intensities of 70, 80, and 90% aMT at baseline and after immobilization (for the 8 subjects of the PAS25 group only). SICI increased with stronger conditioning stimuli intensities. Although at 90% aMT the level of SICI was similar at baseline and after immobilization, it was significantly reduced after immobilization when conditioning stimuli of 70 and 80% aMT were used (t tests; p < 0.002). Two-way ANOVA with the factors “conditioning pulse intensity” and “immobilization condition” showed a significant interaction (F(2,14) = 4.3; p = 0.019), while both factors had a significant main effect (F > 25,6; p < 0.001).
Figure 5.
SICI and correlation of SICI and IOslope changes after immobilization. A, SICI obtained with a conditioning pulse intensity of 70, 80, and 90% aMT. The y-axis plots the amplitude of the conditioned MEP as the percentage of MEP evoked by the test pulse alone (±SE). With a conditioning stimulus of 70 and 80% aMT, SICI was significantly lower after immobilization than at baseline (paired t tests; *p < 0.02). B, Correlation of the changes in IOslope (x-axis) and SICI (y-axis) induced by immobilization. The SICI changes measured with 70 and 80% aMT are significantly correlated with the change of IOslope: the more the IOslope decreases after immobilization, the smaller is the reduction in SICI. Pearson's r is given for the significant correlations.
Correlation between changes in SICI and IOslope
Figure 5B displays the correlation between the change of IOslope and the change of SICI (SICI after immobilization: SICI at baseline; percentage) and shows that the more the IOslope decreased after immobilization, the smaller was the reduction of SICI. The correlations were significant for SICI measured using a conditioning pulse intensity of 70 and 80% aMT (p < 0.007, p < 0.02, respectively; Fig. 5B, Pearson's r), but not 90% aMT.
Correlation between baseline motor excitability and changes in response to PAS and SICI
Given the significant correlations of the immobilization-induced changes in IOslopes to the changes in the PAS effects and SICI, we also tested whether the immobilization effects on plasticity (PAS25 and PAS10 effects) and intracortical inhibition (SICI) were associated with baseline motor excitability as measured with the IOcurves. Marginal effects were found in the PAS25 group only. There was a positive correlation of the IOslope at baseline and the change of PAS25 effect (p = 0.0113, r = 0.83), but not the PAS25 effect at baseline. No other effects were found.
Discussion
Sensorimotor deprivation induced by short-term immobilization of the thumb reduces the excitability (IOslope) of the corticospinal projection to APB. The new findings are that this is accompanied by an increase of the LTP/LTD-like effects of PAS25 and PAS10. The effects vary between individuals and are correlated: individuals who show the largest reduction in IOslope have the largest increase in the PAS25 effect, and the smallest increase in the PAS10 effect. SICI measures with low conditioning pulse intensities (70 and 80% aMT) are also reduced; however, the change is smallest in individuals with the largest change in IOslope.
The interdependence of these findings is compatible with a model in which there are concomitant changes of corticospinal excitability, GABAergic intracortical inhibition, and synaptic plasticity.
Immobilization as a model for sensorimotor deprivation
Hand immobilization for only 12 h changes motor excitability (Huber et al., 2006). We shortened this duration to 8 h to test immobilization in a form that is feasible and easy to apply in potential clinical studies. The splint we used fixed the left thumb tightly and restricted especially abduction movements produced by APB, as target muscle, and reduced phasic activation of cutaneous receptors. This procedure temporarily reduced the amount of efferent muscle activation as well as the sensory input from proprioceptors in the muscle. Given that immobilization of two fingers for 4 d did not change spinal excitability as measured with M- and F-waves (Facchini et al., 2002), it is unlikely that our short procedure affected spinal or muscular excitability in any way. Instead, we hypothesize the effects of immobilization in the present experiments to occur at supraspinal, probably cortical level and to result from reduced efferent output to the muscle as well as sensory afferent input from the muscle. The latter has relatively direct effects on the motor cortex because proprioceptive input from muscles is known to project via thalamus directly to area 3a and area 4 (Huffman and Krubitzer, 2001a,b; Golaszewski et al., 2002; Cooke et al., 2012).
Motor cortical effects of immobilization
Corticospinal excitability and recruitment
The effects of immobilization on motor thresholds are ambiguous and described as increased (Facchini et al., 2002) or unchanged (Ngomo et al., 2012). In the present study the active motor threshold, which controls for baseline levels of excitability and is thought to depend on excitability of axons in the cortex (Terao and Ugawa, 2002; Di Lazzaro et al., 2004; Peterchev et al., 2013), was not significantly changed by immobilization.
IOcurves depend on the distribution of excitability in the corticospinal projection which is activated trans-synaptically by TMS (Amassian et al., 1987; Day et al., 1989; Ridding and Rothwell, 1997). If axonal thresholds do not change, then a given TMS intensity increase will recruit the same extra number of axons at baseline and after immobilization. A reduction in slope of the IOcurves therefore indicates that the synaptic effect of these activated axons on corticospinal neurons is reduced: either because immobilization may depress synaptic transmission of excitatory inputs to corticospinal output (Kirkwood et al., 1996; Allen et al., 2003); or, postsynaptic neurons may be less excitable because of loss of ongoing excitatory input.
However, there is also experimental evidence that when the time averaged activity of a neuron is reduced, e.g., by reducing sensory input, mechanisms of homeostatic plasticity are activated to keep the output stable (Turrigiano et al., 1998; Burrone and Murthy, 2003; Watt and Desai, 2010; Hengen et al., 2013; Keck et al., 2013). These mechanisms include (1) “synaptic scaling” that controls the total synaptic strength of a neuron, (2) the modulation of intrinsic excitability as a function of average activity, and (3) the ability of synapses to undergo Hebbian modification depending on their history of use (“metaplasticity”). They occur at a longer time scale than spike-time-dependent plasticity (STDP), although initial changes in synaptic scaling have been observed as early as 4 h after activity is reduced (Ibata et al., 2008).
Given that, it might indeed be possible that during the 8 h of immobilization mechanisms of homeostatic plasticity are increasingly activated to prevent too large a decrease of corticospinal output. The individual variability of the IOslope decrease after immobilization could then indicate individual differences in either the level of reduction in neuronal output or the amount of homeostatic compensation.
Short-latency intracortical inhibition
SICI, as measure of GABAa-ergic inhibition (Di Lazzaro et al., 2006; Florian et al., 2008), was reduced after immobilization although only when lower conditioning pulse intensities were used. Conditioning stimuli of 90% aMT are less specific for inhibitory interneurons and activate superimposed intracortical facilitatory pathways, which complicates interpretation of the effects (Ilić et al., 2002). At the lower intensities (70 and 80% aMT) there was a greater reduction in SICI in individuals with the smallest reduction in IOslope, and vice versa. Individuals who show the greatest reduction in SICI are those who most successfully compensate for the reduction in excitatory inputs and hence have the smallest change in IOslope. Thus, the changes in GABAa-ergic inhibition could be interpreted as one mechanism to compensate reduction of neuronal excitability after immobilization, with the aim of keeping overall motor excitability within certain limits.
LTP- and LTD-like plasticity
At baseline all subjects responded to PAS by a similar amount (Ridding and Ziemann, 2010). On average, immobilization increased the effectiveness of both the LTP-like PAS25, as well as the LTD-like PAS10 protocol. The findings of increased LTP-like plasticity with PAS25 are consistent with the idea that excitatory synaptic plasticity is upregulated following a period of reduced neuronal activity. Enhanced LTP has been described in animal studies after visual or whisker deprivation (Kirkwood et al., 1996; Allen et al., 2003). Indeed, the idea that LTP is more likely to occur after a period of reduced activity is consistent with the Bienenstock-Cooper-Munro (BCM) concept which states that whether a synapse is strengthened or weakened by presynaptic activity depends upon whether the postsynaptic activity has been below or above a threshold (Bienenstock et al., 1982).
However, the effects on PAS10 are at first sight less expected: on average LTD-like plasticity also increased after immobilization. The BCM theory suggests that the opposite should happen: after a period of reduced activity, LTD-like plasticity should be less likely to occur. So why should immobilization increase the effectiveness of both LTP- and LTD-like protocols? We propose that our results are best explained by the presence of a second factor.
By reducing sensory input immobilization reduces the activity of postsynaptic neurones in the motor cortex, which as a consequence may activate mechanisms of homeostatic plasticity to prevent neuronal output from decreasing too much (see discussion of IOslope decrease in Corticospinal excitability and recruitment, above). These homeostatic mechanisms (e.g., synaptic scaling) modulate all synapses of the neuron (Watt and Desai, 2010). Thus the consequence of reduced activity of postsynaptic neurones is to increase the effectiveness of all inputs to the neuron (Ibata et al., 2008). In the context of the present experiments this will mean that during PAS, the effectiveness of the median nerve sensory input will increase, and thus will tend to increase the effectiveness of both PAS25 and PAS10.
PAS follows the principles of STDP in which the timing between the sensory stimulus and the TMS pulse, but also the significance of the sensory stimulus determine whether synaptic potentiation or depression is induced and how strong the effects are (Stefan et al., 2004; Kamke et al., 2014).
The significant correlations of immobilization-induced changes in IOslope and PAS suggest that the reduced activity of the postsynaptic motor neurones, as expressed in the decrease of IOslope, determines two things: (1) the direction of plasticity that can be induced with PAS following the BCM model, and (2) the significance of the sensory stimulus in PAS that modulates the strength of the STDP effect.
For PAS25, the increase in the LTP-like plasticity induction was strongest in individuals who showed the strongest reduction in IOslope and presumably strongest effect of sensory input during PAS. This finding is in line with the prediction of the BMC model and with strengthening of the STDP effect.
In contrast, for PAS10 the BCM rule predicts a decrease of LTD-like plasticity. However, this is balanced by the strengthening of the PAS10 effect: individuals who had the largest reduction in IOslope showed the smallest change in the amount of LTD-like plasticity. A large reduction in IOslope indicates a large reduction in postsynaptic excitability, and hence should lead to a greater increase in the effectiveness of synaptic inputs and a greater tendency to undergo LTD-like effects according to the STDP rule. This outbalances the expectation from the BCM rule that reduced excitability should lead to reduced LTD. Thus in these individuals, there is little change in the measured LTD-like effect of PAS10.
In summary, the analysis of the individually different findings revealed complex and interdependent changes of motor excitability and plasticity induced by sensorimotor deprivation. It is important to consider these physiological differences when describing and interpreting the effects of plasticity inducing protocols, especially with the aim of devising and stratifying potential clinical approaches.
Footnotes
This work was funded by the Dystonia Medical Research Foundation, and the European Research Council.
The authors declare no competing financial interests.
References
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2012;22:1959–1978. doi: 10.1093/cercor/bhr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq JO, Xerri C. Tactile impoverishment and sensorimotor restriction deteriorate the forepaw cutaneous map in the primary somatosensory cortex of adult rats. Exp Brain Res. 1999;129:518–531. doi: 10.1007/s002210050922. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini S, Romani M, Tinazzi M, Aglioti SM. Time-related changes of excitability of the human motor system contingent upon immobilisation of the ring and little fingers. Clin Neurophysiol. 2002;113:367–375. doi: 10.1016/S1388-2457(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex: a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golaszewski SM, Siedentopf CM, Baldauf E, Koppelstaetter F, Eisner W, Unterrainer J, Guendisch GM, Mottaghy FM, Felber SR. Functional magnetic resonance imaging of the human sensorimotor cortex using a novel vibrotactile stimulator. Neuroimage. 2002;17:421–430. doi: 10.1006/nimg.2002.1195. [DOI] [PubMed] [Google Scholar]
- Hallett M, Chen R, Ziemann U, Cohen LG. Reorganization in motor cortex in amputees and in normal volunteers after ischemic limb deafferentation. Electroencephalogr Clin Neurophysiol. 1999;51:183–187. [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Thalamo-cortical connections of areas 3a and M1 in marmoset monkeys. J Comp Neurol. 2001a;435:291–310. doi: 10.1002/cne.1031. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001b;11:849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Kamke MR, Ryan AE, Sale MV, Campbell ME, Riek S, Carroll TJ, Mattingley JB. Visual spatial attention has opposite effects on bidirectional plasticity in the human motor cortex. J Neurosci. 2014;34:1475–1480. doi: 10.1523/JNEUROSCI.1595-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hübener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–334. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG. The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1993;89:227–234. doi: 10.1016/0168-5597(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980X(95)00194-P. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Ngomo S, Leonard G, Mercier C. Influence of the amount of use on hand motor cortex representation: effects of immobilization and motor training. Neuroscience. 2012;220:208–214. doi: 10.1016/j.neuroscience.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/S1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Peterchev AV, Goetz SM, Westin GG, Luber B, Lisanby SH. Pulse width dependence of motor threshold and input-output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:1364–1372. doi: 10.1016/j.clinph.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Reorganisation in human motor cortex. Can J Physiol Pharmacol. 1995;73:218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–344. doi: 10.1016/S0924-980X(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Ricci R, Funke FW, Ramsey P, Kelley W, Carroll JS, Ramsey D, Borckardt JJ, Johnson K, George MS. Lower limb immobilization is associated with increased corticospinal excitability. Exp Brain Res. 2007;181:213–220. doi: 10.1007/s00221-007-0920-5. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci. 2012;32:9000–9006. doi: 10.1523/JNEUROSCI.0120-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27:5200–5206. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Desai NS. Homeostatic plasticity and STDP: keeping a neuron's cool in a fluctuating world. Front Synaptic Neurosci. 2010;2:5. doi: 10.3389/fnsyn.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Miltner WH, Liepert J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci. 2004;20:3413–3423. doi: 10.1111/j.1460-9568.2004.03790.x. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Yue GH, Bilodeau M, Hardy PA, Enoka RM. Task-dependent effect of limb immobilization on the fatigability of the elbow flexor muscles in humans. Exp Physiol. 1997;82:567–592. doi: 10.1113/expphysiol.1997.sp004048. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol. 1997;105:269–279. doi: 10.1016/S0924-980X(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–1275. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998a;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998b;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]