Abstract
Human cortical thickness and surface area are genetically independent, emerge through different neurobiological events during development, and are sensitive to different clinical conditions. However, the relationship between changes in the two over time is unknown. Additionally, longitudinal studies have almost invariably been restricted to older adults, precluding the delineation of adult life span trajectories of change in cortical structure. In this longitudinal study, we investigated changes in cortical thickness, surface area, and volume after an average interval of 3.6 years in 207 well screened healthy adults aged 23–87 years. We hypothesized that the relationships among metrics are dynamic across the life span, that the primary contributor to cortical volume reductions in aging is cortical thinning, and that magnitude of change varies with age and region. Changes over time were seen in cortical area (mean annual percentage change [APC], −0.19), thickness (APC, −0.35), and volume (APC, −0.51) in most regions. Volume changes were primarily explained by changes in thickness rather than area. A negative relationship between change in thickness and surface area was found across several regions, where more thinning was associated with less decrease in area, and vice versa. Accelerating changes with increasing age was seen in temporal and occipital cortices. In contrast, decelerating changes were seen in prefrontal and anterior cingulate cortices. In conclusion, a dynamic relationship between cortical thickness and surface area changes exists throughout the adult life span. The mixture of accelerating and decelerating changes further demonstrates the importance of studying these metrics across the entire adult life span.
Keywords: aging, trajectory, cortex, volume, thickness, area
Introduction
Individual differences in cortical volume have been attributed to variability mainly in surface area rather than thickness (Pakkenberg and Gundersen, 1997; Im et al., 2008). However, although developmental evidence suggests different change rates among measures (Raznahan et al., 2011; Wierenga et al., 2014), little is known about the dynamics of these cortical characteristics in longitudinal change across the adult life span. While volumetric decreases in aging have been firmly established (Resnick et al., 2003; Fjell et al., 2009b; Raz et al., 2010; Pfefferbaum et al., 2013), the relative contribution of thickness and area effects is unknown, and area change has rarely been studied longitudinally. In humans, the primary processes driving cortical surface area and thickness—cortical column generation and genesis of neurons within columns, respectively (Rakic, 1988)—are restricted to prenatal and perinatal life (Bhardwaj et al., 2006). Later changes are prominent, however, and may in part be driven by alterations at the levels of synapses, dendrites, and spines. A number of studies have identified widespread age differences in cortical thickness (Fjell et al., 2009a), indicating that this could drive age-dependent volumetric decrease. Particularly prominent changes have been observed in prefrontal and temporal regions, with a possible acceleration of medial temporal decline with age (Raz et al., 2010; Fjell et al., 2013a, 2014; Pfefferbaum et al., 2013). However, no study has systematically investigated the relative dynamics of cortical area and thickness change across the adult life span, which due to their different underlying biology may be selectively affected through aging.
Cortical thickness and area show unique regional variations across the cortical surface (White et al., 2010) and are thought to be genetically essentially unrelated (Panizzon et al., 2009), suggesting they should be considered separate morphometric features of neurodevelopment, aging, and disease (Im et al., 2008; Dickerson et al., 2009; Ostby et al., 2009; Panizzon et al., 2009; White et al., 2010; Winkler et al., 2010; Eyler et al., 2011; Lemaitre et al., 2012). Recently, the relationship between cortical thickness and surface area was characterized cross-sectionally across adult life (Hogstrom et al., 2013), both showing strong negative age relationships. However, independently of age, thickness and area were negatively correlated, indicating increasing local arealization with decreasing thickness (Hogstrom et al., 2013). It is unknown whether this may apply to age changes in area and thickness, as its investigation requires longitudinal measurement.
In this study, we asked how much cortical thickness and area change with age, and where and when the most prominent changes occur. We hypothesized, based on neurobiological knowledge as well as previous cross-sectional data, (1) that the primary contributor to cortical volume reductions is thickness reductions, (2) that a negative relationship exists between changes in area and thickness, (3) that longitudinal changes are strongest in temporal and prefrontal cortices, and (4) that accelerating change occurs with increasing age in selected regions (Raz et al., 2005). Based on previous neuroanatomical (Fjell et al., 2014) and cognitive investigations (Rönnlund et al., 2005), a break point around the age of 60 years was hypothesized for temporal cortices.
Materials and Methods
Sample.
The longitudinal sample was drawn from the ongoing project Cognition and Plasticity through the Lifespan, run by the Research Group for Lifespan Changes in Brain and Cognition, Department of Psychology, University of Oslo (Fjell et al., 2008; Westlye et al., 2010, 2011). All procedures were approved by the Regional Committee for Medical and Health Research Ethics, and written informed consent was obtained from all participants. For the first wave of data collection, participants were recruited mainly through newspaper ads. Recruitment for the second wave was by written invitation to the original participants. At both time points (Tp1, Tp2), participants were screened with standardized health interviews. Participants were required to be right handed, fluent Norwegian speakers, and have normal or corrected to normal vision and hearing. Exclusion criteria were history of injury or disease known to affect CNS function, including neurological or psychiatric illness or serious head trauma, being under psychiatric treatment, use of psychoactive drugs known to affect CNS functioning, and MRI contraindications. Participants were required to score ≥26 on the Mini Mental State Examination (MMSE; Folstein et al., 1975), have a Beck Depression Inventory (BDI; Beck and Steer, 1987) score of ≤16, and score ≥85 on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
At follow-up, an additional set of inclusion criteria was used: MMSE (Folstein et al., 1975) score of ≥26; MMSE change from Tp1 to Tp2 of <10%; California Verbal Learning Test II—Alternative Version (CVLT II; Delis et al., 2000) immediate delay and long delay T-score of >30; CVLT II immediate delay and long delay change from Tp1 to Tp2 of <60%. At both time points all scans were evaluated by a neuroradiologist and were required to be deemed free of significant injuries or conditions.
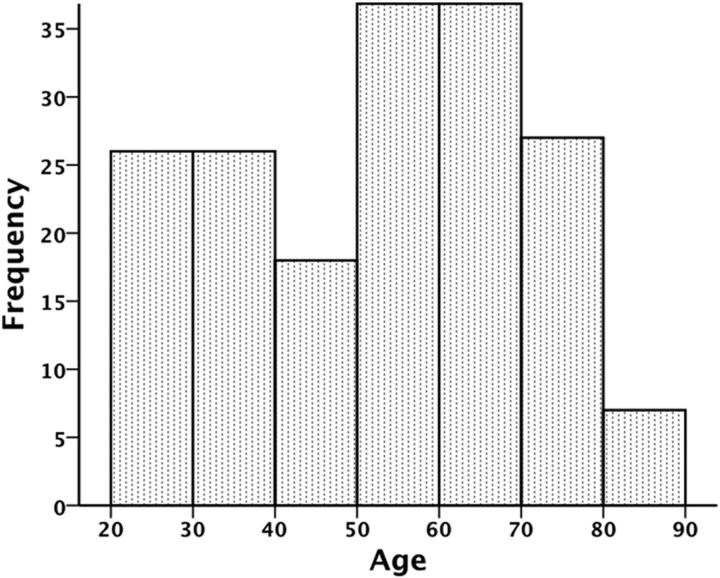
Two hundred and eighty-one participants completed Tp1 assessment. For the follow-up study, 42 opted out, 18 could not be located, 3 did not participate due to health reasons (the nature of these were not disclosed), and 3 had MRI contraindications, yielding a total of 66 dropouts [35 females; mean (SD) age, 47.3 (20.0) years]. Independent samples t tests revealed that dropouts had significantly lower full-scale intelligence quotient (t = −3.92, p < 0.001) but comparable BDI, CVLT, and MMSE scores (p's > 0.05). Of the 215 participants that completed MRI and neuropsychological testing at both time points, 8 failed to meet ≥1 of the additional inclusion criteria for the follow-up study described above. This resulted in a final follow-up sample of 207 participants (122 females) aged 20–84 years at Tp1 and 23–87 years at Tp2. Mean (SD) age was 50.2 (16.5) years at Tp1 and 53.8 (16.5) years at Tp2. Mean (SD) scan interval was 3.6 (0.5) years (range, 2.7–4.8 years). Age distribution at Tp2 is displayed in Figure 1. Few participants under the age of 25 (n = 7) and over the age of 80 (n = 7) completed testing at both time points, meaning that age trajectories in these age groups may be somewhat less reliable than for other age groups. It should be noted, however, that excluding participants above the age of 80 did not significantly alter the results of the present study. An independent samples t test revealed no age (t = −1.62, p = 0.11) or scan interval (t = −0.31, p = 0.76) differences between males and females. Sample characteristics are presented in Table 1.
Figure 1.
Histogram displaying the age distribution at Tp2 in the current sample.
Table 1.
Characteristics of participants from the first wave of the present study (Tp1) that did (stayers) or did not (dropouts) take part in the follow-up study, as well as final sample characteristics at follow-up (Tp2)
| Tp1 |
Diff D–Sa | Tp2 final sample (n = 207) | Diff Tp2–Tp1b | ||
|---|---|---|---|---|---|
| Dropouts (n = 66) | Stayers (n = 207) | ||||
| Gender | 35 females | 122 females | 122 females | ||
| Age in years at MR scan | 47.3 (20.0) | 50.2 (16.5) | −3.0 | 53.8 (16.5) | 3.6i |
| Years of educationc | 15.2 (2.7) | 15.9 (2.6) | −0.7 | 3.3 (0.7) | Not applicable |
| MMSEd | 29.3 (0.9) | 29.4 (0.7) | −0.1 | 29.1 (1.0) | −0.3i |
| CVLT II IRe | 54.6 (12.7) | 56.4 (11.7) | −1.8 | 58.1 (11.0) | 1.7i |
| CVLT II SDRf | 11.7 (3.4) | 12.3 (2.9) | −0.6 | 12.9 (2.9) | 0.6i |
| CVLT II DRg | 12.4 (3.2) | 12.8 (2.9) | −0.4 | 13.1 (2.7) | 0.3 |
| Full-scale intelligence quotienth | 110.5 (8.7) | 115.8 (8.6) | −5.3i | 119.1 (9.8) | 3.3i |
| BDI | 3.6 (3.0) | 4.5 (3.9) | −0.9 | Not applicable | Not applicable |
aComparison of dropouts and stayers at Tp1.
bObserved changes within the final sample from Tp1 to Tp2.
cAt Tp2, a categorical classification system on a four-point scale was introduced: 1, primary school (9 years); 2, high school (12 years); 3, bachelor's degree; 4, master's degree or higher.
dMaximum score, 30.
eCVLT II immediate recall; maximum score, 80.
fCVLT II short delay recall (5 min); maximum score, 16.
gCVLT II delayed recall (30 min); maximum score, 16.
hFour-component Wechsler Abbreviated Scale of Intelligence at Tp1 and two-component Wechsler Abbreviated Scale of Intelligence at Tp2.
ip < 0.01.
Participants included in analyses performed well above average on cognitive tests and passed a thorough screening procedure, including health interview, cognitive assessments, and radiological evaluation, thus minimizing the likelihood of current psychological or neurological diagnoses confounding results. Furthermore, although preclinical conditions cannot be completely ruled out, recent reports suggest that morphometric changes in aging exist, including in the temporal lobes, the prime area of affection in early AD, likely independent of incipient disease (Driscoll et al., 2009; Fjell et al., 2013b). However, it should also be noted that although our primary focus has been to retain a healthy sample, the high level of functioning generally displayed by our participants means that it cannot be considered representative of the population of adults as a whole. For example, given that general cognitive ability is associated with a thicker cortex (Narr et al., 2007; Schnack et al., 2014) and larger surface area (Fjell et al., 2013b; Vuoksimaa et al., 2014), it is possible that the present results to some extent overestimate absolute thickness and surface area values compared with a more representative sample. It is unclear, however, whether this sample bias toward higher-functioning individuals could translate to an underestimation of cortical change, compared with a more representative sample. Although cognitive training has been shown to protect against cortical atrophy in older adults (Engvig et al., 2010), the question of to what extent naturally occurring higher cognitive abilities protect against longitudinal reductions in cortical thickness and surface area in adulthood is not yet fully understood. However, to the extent that commonly occurring medical conditions increase brain aging, the present well screened sample may lead to an underestimation of the age trajectories in the population.
MRI acquisition.
Imaging data were collected using a 12-channel head coil on a 1.5 T Siemens Avanto scanner (Siemens Medical Solutions) at Rikshospitalet, Oslo University Hospital. The same scanner and sequences were used at both time points. The pulse sequence used for morphometric analyses were two repeated 160-slice sagittal T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequences with the following parameters: repetition time, 2400 ms; echo time, 3.61 ms; time to inversion, 1000 ms; flip angle, 8°; matrix, 192 × 192; field of view, 240; voxel size, 1.25 × 1.25 × 1.20 mm) per participant per visit. To increase the signal-to-noise ratio, the two runs were averaged at both time points. Scanning time for each MPRAGE sequence was 7 min 42 s.
MRI analysis.
Image processing and analyses were performed at the Neuroimaging Analysis Laboratory, Research Group for Lifespan Changes in Brain and Cognition, Department of Psychology, University of Oslo. The raw data were reviewed for quality and automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al., 2006) and B1 field inhomogeneity (Sled et al., 1998). For all participants, the two image volumes collected at each time point were coregistered, averaged to improve the signal-to-noise ratio, and resampled to isotropic 1 mm voxels. Images were first automatically processed cross-sectionally (independently) for each time point with the FreeSurfer software package (version 5.1.0; Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA), which is documented and freely available online (http://surfer.nmr.mgh.harvard.edu/). This processing includes motion correction, removal of nonbrain tissue, automated Talairach transformation, intensity correction, volumetric segmentation (Fischl et al., 2002), and cortical surface reconstruction (Dale et al., 1999; Fischl et al., 1999a; Fischl and Dale, 2000) and parcellation (Fischl et al., 2004; Desikan et al., 2006). All volumes were inspected for accuracy and minor manual edits were performed where needed by a trained operator, usually restricted to removal of nonbrain tissue included within the cortical boundary. To extract reliable longitudinal cortical volume, thickness and area change estimates, the cross-sectionally processed images were subsequently run through the longitudinal stream in FreeSurfer (Reuter et al., 2012). Here, an unbiased within-subject template volume based on the two cross-sectional images was created for each participant, and processing of both time points were then initialized using common information from this template. This increased sensitivity and robustness of the longitudinal analysis and ensured inverse consistency (Reuter et al., 2010), meaning that the inverse transform was obtained when registering Tp2–Tp1 as opposed to Tp1–Tp2 (Reuter and Fischl, 2011), which is critical in longitudinal analyses (Thompson et al., 2011). In addition, new probabilistic methods (temporal fusion) were applied to further reduce the variability across time points. Surface maps were resampled, mapped to a common surface, smoothed using a circularly symmetric Gaussian kernel with a full-width half-maximum of 15 mm (Fischl et al., 1999b), and submitted to statistical analyses.
Statistical analyses.
Statistical analyses were performed by use of FreeSurfer 5.1.0 (http://surfer.nmr.mgh.harvard.edu/) and IBM SPSS Statistics 20.0. Spaghetti plots and longitudinal curve fitting were performed using functions freely available through the statistical environment R (http://www.r-project.org/). Longitudinal change in cortical area, thickness, and volume in each hemisphere was calculated as symmetrized APC (i.e., the annual rate of change with respect to the average volume/thickness/area measure across the two time points). A series of general linear models (GLMs), as implemented in FreeSurfer, were used to perform cortical analyses across the surface, and correction for multiple comparisons was ensured by either (1) thresholding significance maps by a conventional criterion for correction for multiple comparisons [false discovery rate (FDR) at 5% level; Genovese et al., 2002], or (2) testing results against an empirical null distribution of maximum cluster size across 10,000 iterations using Z Monte Carlo simulations as implemented in FreeSurfer (Hayasaka and Nichols, 2003; Hagler et al., 2006), synthesized with a cluster-forming threshold of p < 0.05 (two-sided). FDR corrections were used for the analyses of whether change was different from zero and of the relationships among different metrics. Monte Carlo simulations were used to correct the results for the age–change relationship analyses, since this approach in our experience may be somewhat more sensitive to detect effects that are not of great magnitude in terms of extension or intensity. Results from these GLMs were displayed on a semi-inflated template brain. We first tested whether APCs in cortical area, in thickness, and in volume were significantly different from zero. Areas that reached significance were then used as a mask within which APC values were displayed. The relationships among changes in cortical thickness, area, and volume were then examined by entering each map as a per-vertex regressor of interest to each other. Furthermore, using per-vertex GLMs we investigated whether there were any significant effects of sex, scan interval, intracranial volume (ICV), and age on cortical change. Finally, the significance of the effects of the interaction term age × sex on cortical area, thickness, and volume change was tested, with age and sex included as covariates.
In addition to the vertex-wise analyses, post hoc region of interest (ROI) analyses were run. Based on the cortical parcellation in FreeSurfer (Desikan et al., 2006), longitudinal change in the left and right hemispheres was compared with paired one-sample t tests in SPSS. ROI area is calculated as the sum of the area of each vertex, and thus dependent on the size of the ROI, while vertex-wise area depends on the area of each vertex only. Because volume is the product of area and thickness, the area and volume values in ROIs versus the vertex-wise analyses will not be identical, though nearly the same. To reduce the number of ROIs in subsequent analyses, mean change values across the right and left hemisphere were used. To illustrate cortical change within selected ROIs, spaghetti plots of absolute cortical area, thickness, and volume change by age were created. As global fits, such as quadratic models, may be affected by irrelevant factors, e.g., the sampled age range (Fjell et al., 2010), an assumption-free longitudinal nonparametric general additive mixed model (GAMM) for each ROI as a function of age was fitted to accurately describe changes across the studied age range. One-sample t tests were used to test whether longitudinal change was significantly different from zero for each of the 34 ROIs. All ROI results were Bonferroni corrected by a factor of 34 (reflecting the number of ROIs), approximately corresponding to a corrected α of p = 0.0015.
Results
Longitudinal cortical change across age
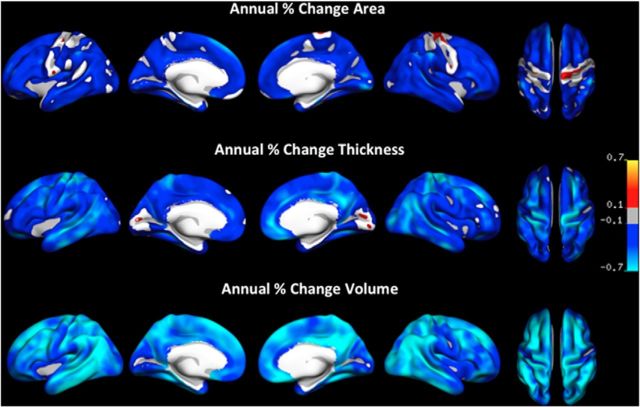
Figure 2 depicts mean longitudinal APC across the entire adult age range for the three cortical measures—area, thickness, and volume—using a common scale to illustrate between-measure differences in change and thresholded by a conventional criterion for correction for multiple comparisons (FDR at 5% level). Significant reductions with time were found across most of the cortical mantle for all measures. Greatest annual change was evident for cortical volume (mean APC, −0.51), next thickness (mean APC, −0.35), with area changes being smallest (mean APC, −0.19). Table 2 displays change in each ROI for each measure to illustrate within-measure differences in change between different cortical regions. Relatively strong volume reductions were found medially in posterior temporal, posterior cingulate/retrosplenial, and orbitofrontal cortices, and laterally in temporal, temporoparietal, superior, and caudal middle frontal, and inferior and superior parietal areas. Relatively weak volume effects were found in lateral prefrontal and medial anterior temporal, insula and occipital cortices, with no changes observed in precuneus. Thickness effects appeared to be most pronounced laterally in temporal and superior and caudal middle frontal cortices, and medially in posterior cingulate, precuneus, and orbitofrontal cortices. No changes in cortical thickness were observed in medial occipital and entorhinal cortices, and relatively weak changes occurred in medial temporal and lateral prefrontal cortices. As for cortical area, changes were most prominent in medial temporal, occipital, and posterior cingulate, and no significant reductions were seen in portions of precentral and postcentral structures. Although vertex-wise GLMs were not run to formally test differences in age slopes across regions and measures, this overall pattern of results was supported by a repeated-measures GLM of all FreeSurfer parcellations, which revealed significant main effects of measure (FGG(1.2,246.5) = 30.2, p < 0.0001), due to differential change rates between measures, and brain region (FGG(9.7,1994.5) = 9.62, p < 0.0001), due to different regions changing at different rates, and a significant interaction between measure and brain region (FGG(14.1,2904.8) = 5.51, p < 0.001), due to patterns of regional change rates differing across measures.
Figure 2.
Annual percentage change in cortical area, thickness, and volume, shown on the same scale to highlight variations in change magnitude across the three measures. Only effects that survived FDR correction for multiple comparisons at the 0.05 level are displayed. Blue-cyan reflects decreases in area/thickness/volume and red-yellow reflects increases.
Table 2.
Mean (SD) annual percentage volume, area, and thickness change in the respective cortical regions
| Cortical region | Percentage longitudinal annual cortical change |
|||||
|---|---|---|---|---|---|---|
| Volume |
Area |
Thickness |
||||
| Mean percentage | SD | Mean percentage | SD | Mean percentage | SD | |
| Cingulate | ||||||
| Caudal anterior cingulate | −0.59a | (0.97) | −0.24a | (0.54) | −0.39a | (0.93) |
| Rostral anterior cingulate | −0.31a | (1.04) | −0.09 | (0.61) | −0.32a | (0.86) |
| Posterior cingulate | −0.71a | (0.86) | −0.37a | (0.47) | −0.41a | (0.72) |
| Retrosplenial cortex | −0.53a | (0.81) | −0.17a | (0.64) | −0.35a | (0.68) |
| Frontal | ||||||
| Superior frontal | −0.60a | (0.82) | −0.22a | (0.36) | −0.40a | (0.76) |
| Caudal middle frontal | −0.71a | (1.09) | −0.30a | (0.38) | −0.46a | (1.00) |
| Rostral middle frontal | −0.49a | (1.00) | −0.27a | (0.39) | −0.29a | (0.89) |
| Pars opercularis | −0.51a | (0.93) | −0.52a | (0.75) | −0.58a | (1.62) |
| Pars triangularis | −0.38a | (1.06) | −0.21a | (0.44) | −0.22a | (0.97) |
| Pars orbitalis | −0.55a | (0.93) | −0.34a | (0.66) | −0.23a | (0.85) |
| Lateral orbital frontal | −0.51a | (0.79) | −0.21a | (0.41) | −0.32a | (0.80) |
| Medial orbital frontal | −0.63a | (1.04) | −0.18a | (0.64) | −0.48a | (0.89) |
| Frontal pole | −0.30a | (1.10) | −0.12 | (1.37) | −0.20 | (0.98) |
| Parietal | ||||||
| Insula | −0.32a | (0.60) | −0.15a | (0.41) | −0.26a | (0.48) |
| Precentral | −0.49a | (1.12) | −0.07 | (0.41) | −0.41a | (1.14) |
| Postcentral | −0.47a | (1.09) | −0.07 | (0.35) | −0.37a | (1.09) |
| Paracentral | −0.52a | (1.12) | −0.12a | (0.41) | −0.36a | (1.12) |
| Superior parietal | −0.61a | (1.19) | −0.17a | (0.29) | −0.40a | (1.10) |
| Inferior parietal | −0.70a | (0.95) | −0.27a | (0.29) | −0.45a | (0.86) |
| Supramarginal | −0.57a | (0.93) | −0.21a | (0.28) | −0.37a | (0.84) |
| Precuneus | −0.64a | (1.04) | −0.21a | (0.28) | −0.45a | (0.91) |
| Temporal | ||||||
| Parahippocampal | −0.59a | (0.85) | −0.31a | (0.42) | −0.32a | (0.80) |
| Entorhinal | −0.34a | (1.18) | −0.24a | (1.03) | −0.19 | (0.87) |
| Temporal pole | −0.27a | (0.79) | −0.11 | (0.92) | −0.20a | (0.62) |
| Superior temporal | −0.46a | (0.70) | −0.12a | (0.27) | −0.39a | (0.61) |
| Middle temporal | −0.60a | (0.78) | −0.24a | (0.39) | −0.42a | (0.69) |
| Inferior temporal | −0.68a | (0.79) | −0.29a | (0.40) | −0.41a | (0.69) |
| Transverse temporal | −0.56a | (1.04) | −0.40a | (0.67) | −0.25a | (1.04) |
| Banks sup temporal | −0.64a | (0.97) | −0.30a | (0.33) | −0.46a | (0.89) |
| Fusiform | −0.57a | (0.83) | −0.25a | (0.39) | −0.35a | (0.73) |
| Occipital | ||||||
| Lateral occipital | −0.52a | (0.93) | −0.26a | (0.43) | −0.30a | (0.81) |
| Pericalcarine | −0.04 | (1.37) | −0.16a | (0.51) | −0.01 | (1.06) |
| Lingual | −0.54a | (0.90) | −0.34a | (0.48) | −0.28a | (0.69) |
| Cuneus | −0.41a | (1.15) | −0.32a | (0.51) | −0.14 | (0.98) |
ap < 0.05 (Bonferroni corrected, factor of 34).
Comparisons between right and left hemisphere change for each ROI showed no significant hemisphere differences, after correcting for multiple comparisons. Thus, to reduce the number of comparisons, the mean values of the two hemispheres were used in all subsequent ROI analyses. Table 2 shows APC for each ROI and confirms the pattern of cortical change indicated by the continuous surface maps. Furthermore, surface GLMs as well as separate ROI analyses revealed no significant effects of sex, Tp1–Tp2 scan interval, or ICV on APC in cortical area, thickness, or volume change. The age–sex interaction term was also not significant. Cortical GLMs for effects that did not survive correction for multiple comparisons are not shown.
Relationship between cortical area, thickness, and volume change
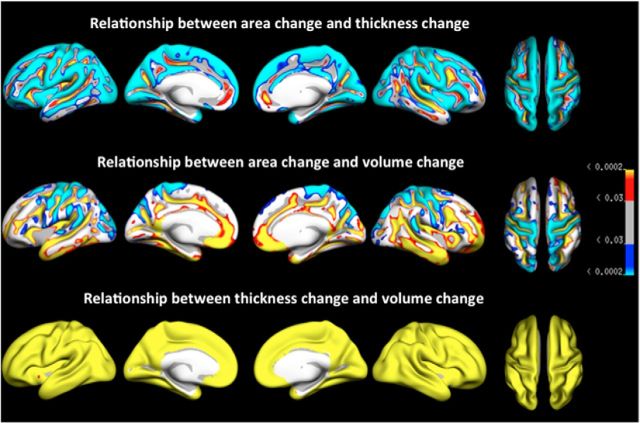
Figure 3 displays the results of GLMs testing the relationships among percentage annual cortical area, thickness, and volume changes across the cortical surface, thresholded at 5% FDR level. The results show that for large portions of the cortical mantle there was either no significant relationship or a negative relationship between thickness and area measures, such that relatively large reductions in cortical thickness are associated with relatively low reductions in cortical area. Positive thickness–area relationships were generally found within sulci. A mix of negative and positive relationships was found between area change and volume change, with positive associations predominantly in frontotemporal regions, and negative associations in occipital-parietal regions. As expected, thickness change–volume change relationships were highly positive, indicating increasing cortical thinning with cortical volume loss. To further illustrate these types of effects, Figure 4 provides scatterplots of the relationship between change in the different measures from the superior parietal cortex, in which there is a very strong positive association between volume and thickness changes (r = 0.97), but negative volume–area (r = −0.23) and thickness–area (r = −0.39) associations.
Figure 3.
p value map for the area–thickness, area–volume, and thickness–volume relationships. Blue-cyan reflects a negative relationship, in which a relatively large decrease on one measure is associated with a relatively small decrease on the other measure. Red-yellow reflects a positive relationship, where a relatively large decrease in one measure is associated with a relatively large decrease in the other measure. Only effects that survived FDR correction for multiple comparisons at the 0.05 level are displayed.
Figure 4.
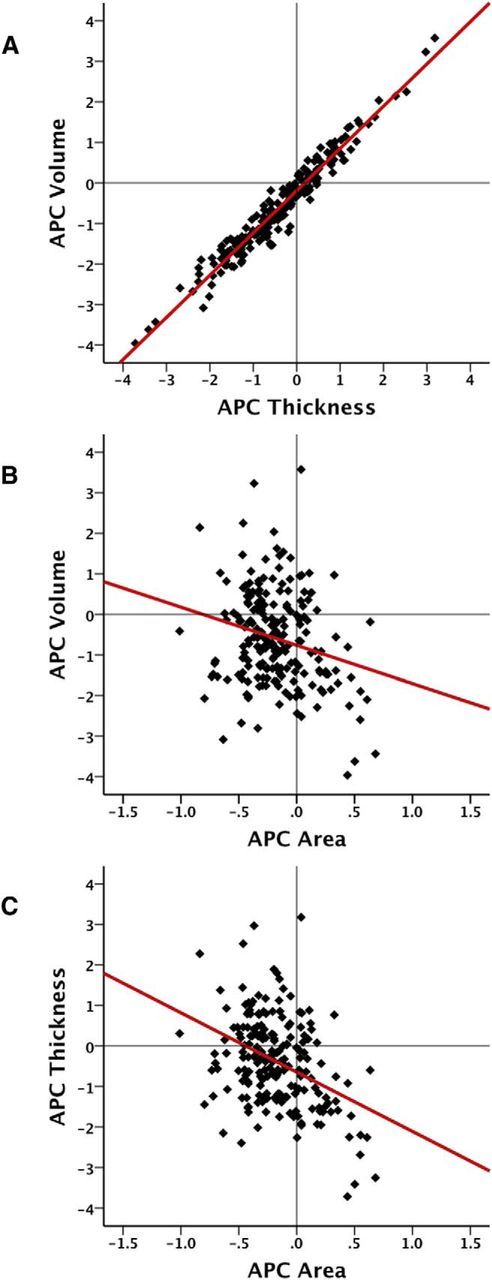
A–C, Scatter plots with added linear best fit line, showing the relationships between annual percentage change (APC) in volume and thickness (A), volume and area (B), and thickness and area (C), respectively, in the superior parietal cortex.
Table 3 displays correlations between cortical area, thickness, and volume APC for each ROI, and confirms the relationship between longitudinal changes in these measures observed in the vertex-wise analyses. Furthermore, analyses of hemispheric asymmetries in interrelationships by Fisher's r-to-z transformations revealed very few differences between the right and left hemisphere after correcting for multiple comparisons. However, significant asymmetries were found in the caudal anterior cingulate (p = 0.001 for thickness–area relationship; p < 0.0001 for volume–area relationship) and the medial orbitofrontal cortex (p = 0.0002 for thickness–area relationship; p < 0.0001 for volume–thickness relationship).
Table 3.
Correlations between annual percentage cortical change in volume and thickness, volume and area, and thickness and area in cortical regions
| Cortical region | Relationships among changes in volume, thickness, and area |
|||||
|---|---|---|---|---|---|---|
| Volume–thickness |
Volume–area |
Thickness–area |
||||
| r | p | r | p | r | p | |
| Cingulate | ||||||
| Caudal anterior cingulate | 0.81a | <10−6 | 0.36a | <10−6 | −0.10 | 0.166 |
| Rostral anterior cingulate | 0.79a | <10−6 | 0.47a | <10−6 | 0.01 | 0.840 |
| Posterior cingulate | 0.87a | <10−6 | 0.34a | <10−6 | −0.01 | 0.912 |
| Retrosplenial cortex | 0.68a | <10−6 | 0.45a | <10−6 | −0.27a | <10−4 |
| Frontal | ||||||
| Superior frontal | 0.93a | <10−6 | 0.15 | 0.029 | −0.11 | 0.105 |
| Caudal middle frontal | 0.95a | <10−6 | 0.03 | 0.674 | −0.19 | 0.006 |
| Rostral middle frontal | 0.92a | <10−6 | 0.38a | <10−6 | 0.18 | 0.008 |
| Pars opercularis | 0.93a | <10−6 | 0.09 | 0.203 | −0.14 | 0.041 |
| Pars triangularis | 0.93a | <10−6 | 0.12 | 0.084 | −0.14 | 0.042 |
| Pars orbitalis | 0.86a | <10−6 | 0.24a | 0.001 | −0.17a | 0.012 |
| Lateral orbital frontal | 0.89a | <10−6 | 0.33a | <10−6 | 0.05 | 0.449 |
| Medial orbital frontal | 0.81a | <10−6 | 0.61a | <10−6 | 0.16 | 0.020 |
| Frontal pole | 0.66a | <10−6 | 0.27a | <10−3 | −0.45a | <10−6 |
| Parietal | ||||||
| Insula | 0.78a | <10−6 | 0.55a | <10−6 | 0.04 | 0.550 |
| Precentral | 0.97a | <10−6 | −0.20 | 0.003 | −0.41a | <10−6 |
| Postcentral | 0.97a | <10−6 | −0.38a | <10−6 | −0.58a | <10−6 |
| Paracentral | 0.96a | <10−6 | −0.20 | 0.004 | −0.42a | <10−6 |
| Superior parietal | 0.97a | <10−6 | −0.23a | 0.001 | −0.39a | <10−6 |
| Inferior parietal | 0.96a | <10−6 | 0.16 | 0.024 | −0.01 | 0.846 |
| Supramarginal | 0.96a | <10−6 | 0.05 | 0.508 | −0.13 | 0.070 |
| Precuneus | 0.97a | <10−6 | 0.15 | 0.028 | 0.00 | 0.975 |
| Temporal | ||||||
| Parahippocampal | 0.89a | <10−6 | 0.42a | <10−6 | 0.10 | 0.146 |
| Entorhinal | 0.56a | <10−6 | 0.56a | <10−6 | −0.15 | 0.030 |
| Temporal pole | 0.65a | <10−6 | 0.36a | <10−6 | −0.35a | <10−6 |
| Superior temporal | 0.96a | <10−6 | 0.39a | <10−6 | 0.19 | 0.005 |
| Middle temporal | 0.94a | <10−6 | 0.38a | <10−6 | 0.16 | 0.018 |
| Inferior temporal | 0.91a | <10−6 | 0.53a | <10−6 | 0.27a | <10−4 |
| Transverse temporal | 0.90a | <10−6 | −0.15 | 0.028 | −0.53a | <10−6 |
| Banks sup temporal | 0.94a | <10−6 | 0.44a | <10−6 | 0.26a | <10−3 |
| Fusiform | 0.95a | <10−6 | 0.41a | <10−6 | 0.22a | 0.001 |
| Occipital | ||||||
| Lateral occipital | 0.93a | <10−6 | −0.06 | 0.426 | −0.35a | <10−6 |
| Pericalcarine | 0.93a | <10−6 | 0.57a | <10−6 | 0.29a | <10−4 |
| Lingual | 0.89a | <10−6 | 0.37a | <10−6 | −0.04 | 0.541 |
| Cuneus | 0.90a | <10−6 | 0.09 | 0.193 | −0.29a | <10−4 |
ap < 0.05 (Bonferroni-corrected, factor of 34).
Temporal patterns of longitudinal cortical change rates
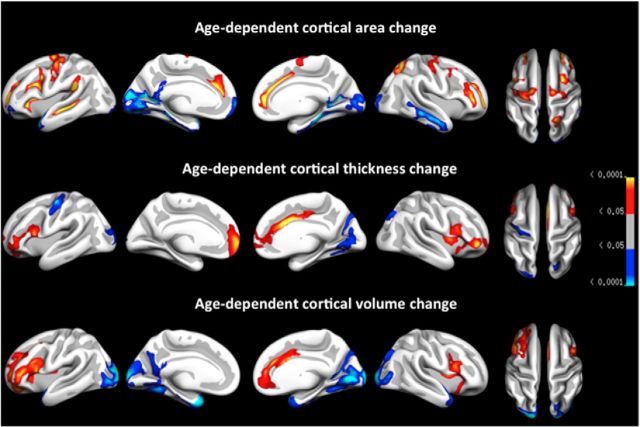
To examine the relationship between brain atrophy and age, GLMs with annual percentage area, thickness, and volume change at each vertex entered as separate dependent variables and age entered as the independent variable were run. Corrections for multiple comparisons were performed using Z Monte Carlo simulations. Figure 5 shows the clusters that survived correction for multiple comparisons for each cortical measure in each hemisphere. Mean symmetrized APC in the significant clusters was extracted to allow for post hoc analyses. Given that age effects are overlapping across area, thickness, and volume, clusters identified for volume change were used as a reference point from which the relative contributions of area change and thickness change with age are described. In the left hemisphere, the following four clusters were identified as having a nonconstant rate of volume change across the sampled age range (APC–age correlations are given in brackets): accelerating volume change was seen bilaterally in occipital (left hemisphere, r = −0.29; right hemisphere, r = −0.29) and temporal (left hemisphere, r = −0.29; right hemisphere, r = −0.26) clusters, as well as in a left hemisphere cluster covering part of fusiform, retrosplenial, cuneus, and precuneus cortices (r = −0.24). Decelerating change was seen in a right inferior prefrontal cluster (r = 0.25), and in the left hemisphere in clusters covering part of the pars opercularis/precentral and insula cortices (r = 0.24, as well as the anterior cingulate, r = 0.22). Inspection of age-dependent area and thickness change suggests that age-dependent volume effects were predominantly driven by changes in area in posterior/occipital and temporal regions (acceleration of change with age). Deceleration of change with age in frontal and anterior cingulate regions was seen across all measures.
Figure 5.
Cortical regions (clusters) showing evidence of nonconstant levels of atrophy (area, thickness, volume) across age, following correction for multiple comparisons using cluster size correction by means of Monte Carlo simulations (p values displayed). Red-yellow reflects deceleration of atrophy with increasing age and blue-cyan reflects acceleration of atrophy with increasing age.
Figures 6 (lateral cortices), 7 (medial cortices), and 8 (frontal cortices) present spaghetti plots (raw values at tp1 and tp2 for each participant) for selected cortical regions and further highlight the complex relationship between age and cortical change. The GAMM fit line represents the best local fit of the data and is often more sensitive to deviations from linearity than the global fit surface analyses (age–change) presented in Figure 5. As expected from the surface analyses, overall reductions in cortical area, thickness, and volume with age, both on the individual (longitudinal) and group (cross-sectional) level, was observed, and cortical area remained more stable than did cortical thickness across the sampled age range. Figure 6 indicates a negative linear relationship between age and total cortical area, thickness, and volume. This linear trend is reflected in most lateral regions, but with lateral occipital cortex showing an acceleration of change with age for thickness and volume and superior temporal showing accelerated thickness reduction from approximately age 60. Figure 7 shows a more complex picture for selected medial cortices. While the rate of decline for cortical area remained linear across most structures, with the exception of posterior cingulate and parahippocampal cortices, which showed tendencies toward accelerated reductions after age 60, measures of thickness and volume change predominantly displayed nonlinear trajectories. Precuneus, posterior cingulate, rostral anterior cingulate, and especially entorhinal cortices showed accelerated reduction in thickness late in life (≈60 years of age), a pattern that is preceded by relatively little change/deceleration of change midlife. While thickness reduction in early adulthood (age 20–40) was evident in most structures, entorhinal cortex thickness appeared to remain more or less stable before showing steep thickness reduction from age 60. Although this effect did not survive Monte Carlo correction in the surface GLM, acceleration of entorhinal change with age became apparent with slightly more liberal correction criteria. The apparent discrepancy between the trajectory for area in entorhinal cortex from the quadratic function (surface plot) versus the GAMM fit (scatterplot) may have arisen partly due to different fit functions and partly due to the differences in calculation of area in FreeSurfer in ROIs versus vertex-wise analyses (see above). As expected, given the strong relationship between changes in thickness and changes in volume, volume followed a similar but less pronounced trajectory to that of thickness for most cortical regions. Figure 8 shows change trajectories for frontal cortices. Area appears to decline linearly for all ROIs except possible minor deceleration with age for superior and rostral middle frontal. Small deviations from linearity in thickness can be seen for pars orbitalis (decelerates), pars opercularis (decelerates), rostral middle frontal (deceleration followed by acceleration), and medial orbitofrontal (deceleration followed by acceleration). All displayed some volume change deceleration midlife, with medial orbitofrontal showing subsequent acceleration in late life.
Figure 6.
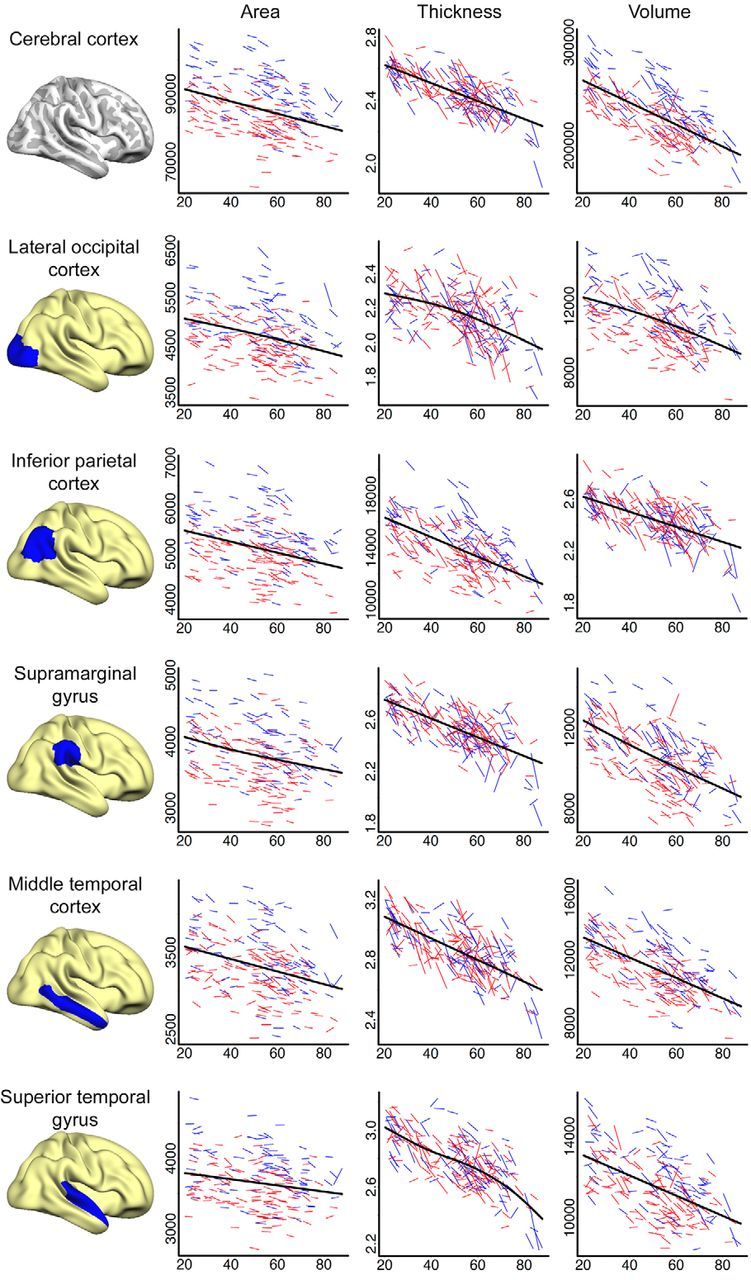
Spaghetti plots of area (mm2), thickness (mm), and volume (mm3) for cerebral cortex and selected lateral cortical regions. Average values of left and right hemisphere are displayed. For each region, an assumption-free general additive model as a function of age was fitted to accurately describe changes across the studied age range. Males are represented by blue lines, females by red lines.
Figure 7.
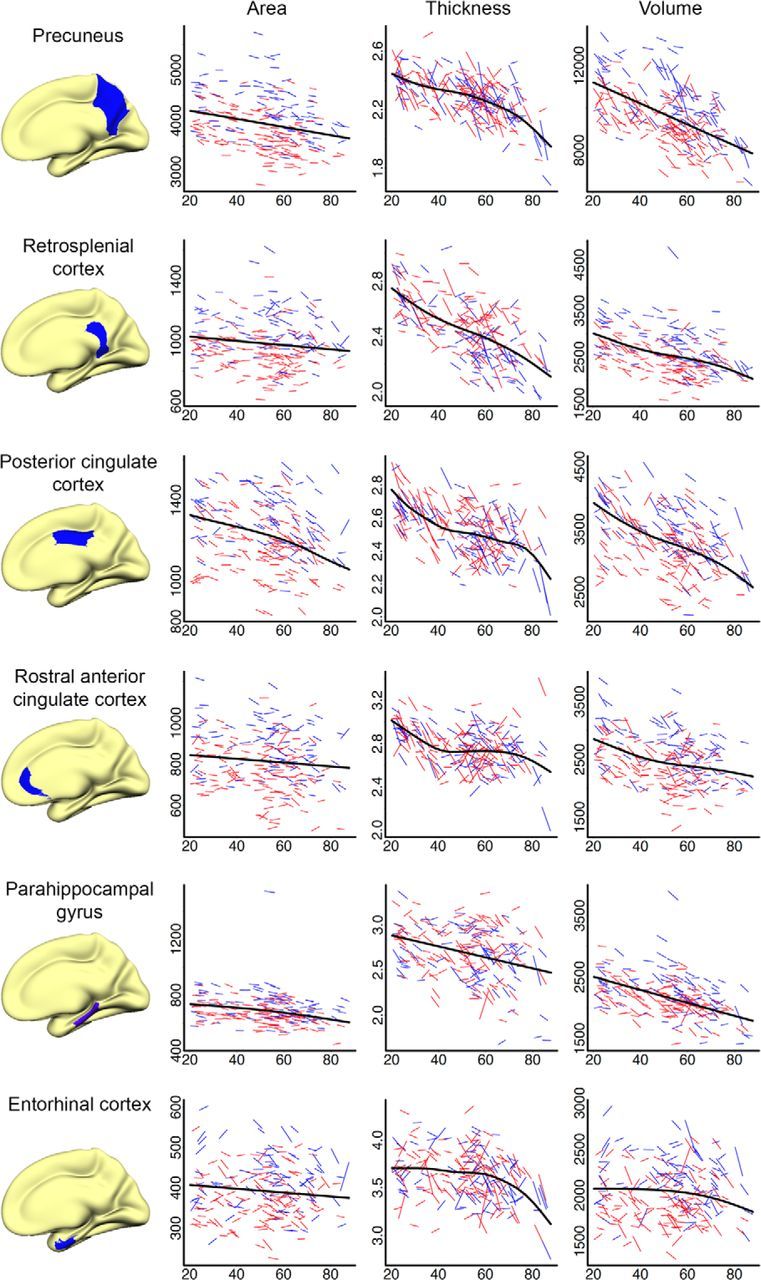
Spaghetti plots of area (mm2), thickness (mm), and volume (mm3) for selected medial cortical regions. Average values of left and right hemisphere are displayed. For each region, an assumption-free general additive model as a function of age was fitted to accurately describe changes across the studied age range. Males are represented by blue lines, females by red lines.
Figure 8.
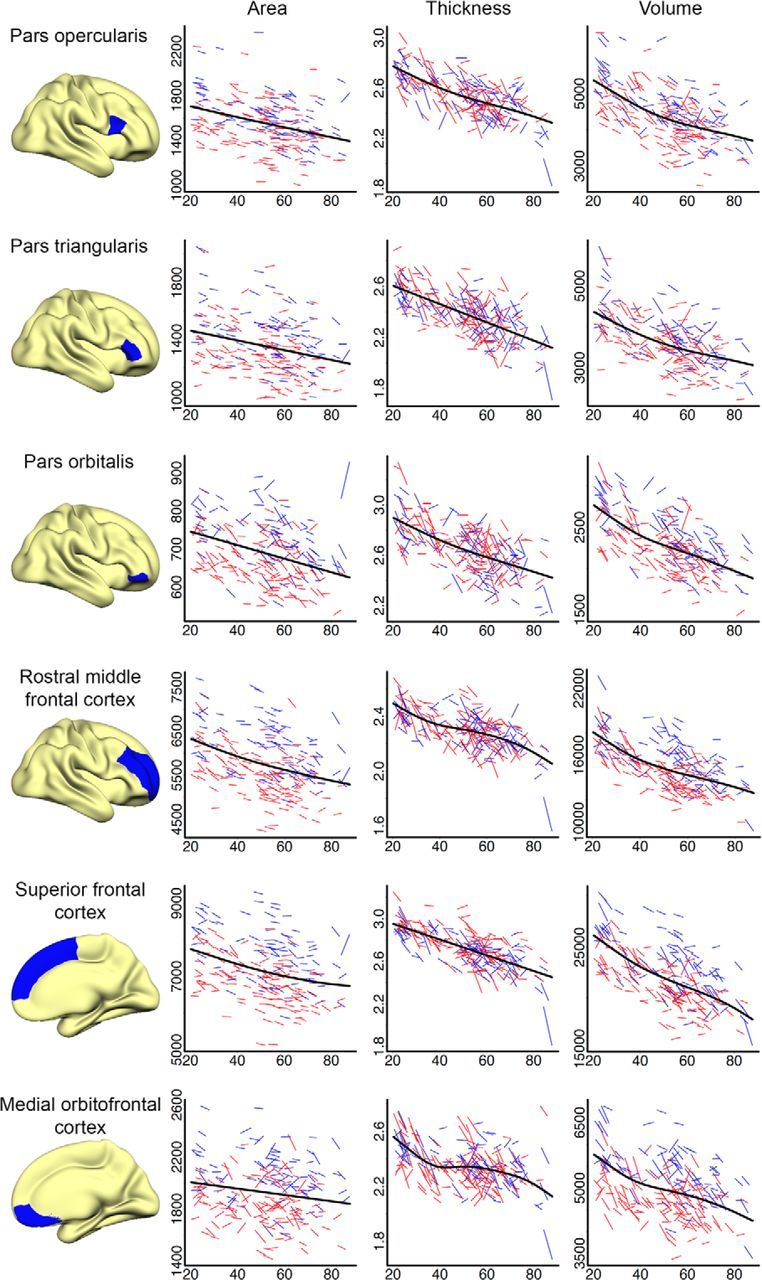
Spaghetti plots of area (mm2), thickness (mm), and volume (mm3) for selected frontal cortical regions. Average values of left and right hemisphere are displayed. For each region, an assumption-free general additive model as a function of age was fitted to accurately describe changes across the studied age range. Males are represented by blue lines, females by red lines.
Discussion
In the present study, we observed marked changes over time in cortical area, thickness, and volume, which were not uniform across regions or age. Further, complex relationships among changes in the different metrics were observed, underscoring the fundamental differences among thickness, area, and volume as measures of cortical structural change. The results are discussed in relation to our initial hypotheses.
Hypotheses 1 and 2: the primary contributor to cortical volume reductions is thickness reductions, and there is a negative relationship between age-dependent changes in area and thickness
The current study highlights the need to consider cortical thickness and area change separately. Previous studies have established that area and thickness are genetically independent (Panizzon et al., 2009), but longitudinal studies have not investigated how changes in cortical volume, thickness, and area are related. In support of Hypothesis 1, a strong positive relationship between thickness change and volume change was found across the entire cortical surface, whereas area change showed a mix of positive, negative, and null relationships with volume change, with a pronounced dorsal (negative)–ventral (positive) gradient of correlations. Although a mix of positive, negative, and null relationships were found also between cortical thickness and area change, the relatively large number of vertices displaying a negative relationship between these measures provides at least partial support for Hypothesis 2 and extends the findings of a previously reported cross-sectional study performed on a partly overlapping sample (Hogstrom et al., 2013), where adults with a larger surface area tended to have thinner cortices.
Most of the individual variation in human cortical volumes is due to variation in surface area, rather than thickness (Im et al., 2008). However, the present study suggests that the main driver of cortical volume change is thickness change. The fundamental mechanisms underlying the observed negative relationship between cortical thickness and area are possibly related to interactions between early neurodevelopmental processes, including pruning, life-long reshaping, and neurodegenerative processes related to aging. It has been suggested that cortical area expansion might be more efficient in terms of facilitating brain connectivity than increasing cortical thickness (Ruppin et al., 1993; Murre and Sturdy, 1995), and that pruning during early stages of life is a prerequisite for optimal area increases (White et al., 2010). As such, increases in surface area may be related to cortical thinning, which should result in a negative relationship between these two measures in healthy development that is also evident in aging. Another explanation relates to the gray matter–white matter (GM–WM) border becoming fuzzier with increasing age. We have previously shown that the GM–WM contrast directly underneath and above the WM surface is reduced in healthy aging (Westlye et al., 2009), likely partly due to changes in myelin content intracortically and in the superficial WM (Grydeland et al., 2013). This could have opposite effects on thickness and area estimates. As area is measured along the WM surface to avoid confounding effects of GM atrophy, a shift of the GM–WM border further into WM would result in a reduction of surface area estimates and at the same time partly counter the age-related thickness reductions. This way, a negative correlation between the two measures could appear both in cross-sectional and longitudinal data, although both area and thickness are reduced with time and negatively correlated with age. Future studies should address this hypothesis directly by relating GM–WM contrast changes to degree of area shrinkage. However, regardless of the exact interpretation of the complex relationship between the different metrics, it appears that changes are driven by partly independent underlying neurobiological mechanisms that are differentially affected over time, highlighting the need to consider these as separate measures of cortical structural change.
Hypothesis 3: longitudinal changes are strongest in temporal and prefrontal cortices
Significant reductions over time were found across most of the cortical mantle for all measures. The greatest magnitude of change was evident for cortical volume, next thickness, with area changes being relatively minor in comparison. Rather than being predominantly restricted to temporal and prefrontal cortices, as predicted in Hypothesis 3, strong volume effects were found across the cortical mantle; bilaterally medially especially in posterior temporal, posterior cingulate/retrosplenial, superior parietal/precuneus, and superior frontal cortices, and laterally in temporal, superior frontal, and inferior parietal areas. This pattern corresponds well with previous cross-sectional and more age-restricted longitudinal (Resnick et al., 2003; Fjell et al., 2009b, 2013a; Raz et al., 2010; Pfefferbaum et al., 2013) investigations of volume change in healthy older adults. The present results extend previous findings in showing that the volume reductions in occipital cortices are primarily due to area decreases medially. Interestingly, area and thickness reduction also seems to be pronounced in regions involved in the default mode network (Buckner et al., 2008), a collection of structures involved in episodic memory, including the temporal lobes, supramarginal gyrus (predominantly thickness reductions), precuneus cortex (predominantly thickness reductions), and posterior cingulate cortex. Reductions were not restricted to these regions, however, but rather affected regions known to support a number of different cognitive functions. As for changes in cortical area, no significant reductions were seen in large portions of precentral and postcentral structures, in line with previous cross-sectional results (Hogstrom et al., 2013). In these structures, marked thinning was, however, observed, resulting in significant, yet not overall the strongest volume reduction, in line with another recent longitudinal study on these areas (Pfefferbaum et al., 2013). It should be noted, however, that GM–WM contrast tends to be poorer in these regions, which can affect the thickness estimation and possibly the estimated longitudinal change.
Hypothesis 4: there are accelerating changes with increasing age, with a break point around the age of 60 for temporal cortices
Accelerating cortical volume change with age was found bilaterally in occipital and temporal cortices, in support of Hypothesis 4, with especially marked age effects found in entorhinal cortex, as well as in a left hemisphere cluster covering part of fusiform, retrosplenial, and precuneus cortices. Increased atrophy rates with age in the temporal cortices, including the entorhinal cortex, and in the occipital cortices have previously been reported cross-sectionally (Fjell et al., 2014), but findings from ROI-driven or more age-restricted longitudinal samples are mixed, with some studies reporting linear trends for temporal cortices (Raz et al., 2005, 2010) and others showing nonlinear, accelerating temporal (Fjell et al., 2009b) and occipital (Pfefferbaum et al., 2013) change. Interestingly, decelerating change was seen in a cluster in the right prefrontal area, and left in clusters covering part of the pars opercularis/precentral and insula cortices as well as the anterior cingulate. This pattern is inconsistent with some previous longitudinal studies (Raz et al., 2005, 2010; Pfefferbaum et al., 2013), but in line with recent cross-sectional thickness data (Fjell et al., 2014), and could also help explain a previous failure to detect significant longitudinal annual atrophy or atrophy–age correlations in selected frontal (e.g., pars orbitalis) and anterior cingulate (e.g., rostral anterior cingulate) cortices in a healthy sample 60–91 years of age (Fjell et al., 2009b, 2014). A previous report showed that volume differences between younger and older adults in entorhinal and parahippocampal cortices were predominantly due to cortical surface area differences rather than differences in cortical thickness (Dickerson et al., 2009). This is consistent with our finding that area effects contribute to volume decline in temporal cortices, but the fit curves still indicate that thickness change was partly responsible for the acceleration of volumetric reduction in the last part of life. Interestingly, a recent study found that heritability for area and thickness differed across the cortex (Eyler et al., 2012). Surface area in medial temporal lobe regions was found to have lower genetic influences than did frontal areas (Eyler et al., 2011).
The relative contributions of area and thickness to age-dependent acceleration and deceleration of volume change has not been described previously, and the current results suggest that volume change with age is not a uniform process but rather is the result of separate mechanisms in separate anatomical regions, in line with cross-sectional heritability studies. This further highlights the need to take multiple measures of cortical atrophy into consideration to gain a better understanding of the neurobiological processes that characterize normal aging. To this end, future studies should also aim to describe the present pattern of accelerating and decelerating cortical changes in relation to possibly corresponding trajectories of cognitive functions.
Concluding remarks
The current study showed that age-related atrophy is a general feature of normal aging. Further, although thickness change was most closely related to volume change, area and thickness change appeared to have both overlapping and differential effects on volume change across the adult life span. The patterns of regional accelerating versus decelerating area and thickness change in temporal and occipital cortices versus prefrontal and anterior cingulate cortices should be further investigated, as this could have important implications for our understanding of which biomarkers are most sensitive to the effects of aging in healthy adults.
Footnotes
This work was supported by the Department of Psychology, University of Oslo (A.B.S., K.B.W., C.K.T., L.T.W.), the Norwegian Research Council (K.B.W., A.M.F., L.T.W.), and the European Research Council's Starting Grant scheme (K.B.W., A.M.F.).
The authors declare no competing financial interests
References
- Beck AT, Steer R. Beck depression inventory scoring manual. New York: Psychological; 1987. [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisén J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test—second edition (CVLT–II) San Antonio, TX: Psychological; 2000. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Prom-Wormley E, Panizzon MS, Kaup AR, Fennema-Notestine C, Neale MC, Jernigan TL, Fischl B, Franz CE, Lyons MJ, Grant M, Stevens A, Pacheco J, Perry ME, Schmitt JE, Seidman LJ, Thermenos HW, Tsuang MT, Chen CH, Thompson WK, et al. Genetic and environmental contributions to regional cortical surface area in humans: a magnetic resonance imaging twin study. Cereb Cortex. 2011;21:2313–2321. doi: 10.1093/cercor/bhr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Chen CH, Panizzon MS, Fennema-Notestine C, Neale MC, Jak A, Jernigan TL, Fischl B, Franz CE, Lyons MJ, Grant M, Prom-Wormley E, Seidman LJ, Tsuang MT, Fiecas MJ, Dale AM, Kremen WS. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: a magnetic resonance imaging twin study. Twin Res Hum Genet. 2012;15:304–314. doi: 10.1017/thg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bjørnerud A, Due-Tønnessen P, Walhovd KB. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009a;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009b;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Ostby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer's disease. J Neurosci. 2013a;33:8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Tamnes CK, Grydeland H, Engvig A, Espeseth T, Reinvang I, Lundervold AJ, Lundervold A, Walhovd KB. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb Cortex. 2013b doi: 10.1093/cercor/bht201. Advance online publication. Retrieved May 13, 2014. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB, Walhovd KB. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 2014;24:919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 2013;33:18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient nonlinearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:617.e1–617.e9. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre JM, Sturdy DP. The connectivity of the brain: multi-level quantitative analysis. Biol Cybern. 1995;73:529–545. doi: 10.1007/BF00199545. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. doi: 10.1002/(SICI)1096-9861(19970728)384:2<312::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Ruppin E, Schwartz EL, Yeshurun Y. Examining the volume efficiency of the cortical architecture in a multi-processor network model. Biol Cybern. 1993;70:89–94. doi: 10.1007/BF00202570. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2014 doi: 10.1093/cercor/bht357. Advance online publication. Retrieved May 13, 2014. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Thompson WK, Holland D, Holland D. Bias in tensor based morphometry Stat-ROI measures may result in unrealistic power estimates. Neuroimage. 2011;57:1–4. doi: 10.1016/j.neuroimage.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen C-H, Fiecas M, Eyler LT, Fennema-Notestine C, Hagler DJ, Fischl B, Franz CE, Jak A, Lyons MJ, Neale MC, Rinker DA, Thompson WK, Tsuang MT, Dale AM, Kremen WS. The genetic association between neocortical volume and general cognitive ability is driven by global surface area rather than thickness. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu018. Advance online publication. Retrieved May 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological; 1999. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Espeseth T, Reinvang I, Raz N, Agartz I, Greve DN, Fischl B, Fjell AM. Increased sensitivity to effects of normal aging and Alzheimer's disease on cortical thickness by adjustment for local variability in gray/white contrast: a multi-sample MRI study. Neuroimage. 2009;47:1545–1557. doi: 10.1016/j.neuroimage.2009.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52:172–185. doi: 10.1016/j.neuroimage.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. Unique development trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]