Abstract
Spatial working memory is one of the most studied cognitive functions, serving as a model system to decipher computational principles of the brain. Although neuronal mechanisms for remembering a single location have been well elucidated, little is known about memory for multiple locations. Here, we examined the activities of prefrontal neurons during monkeys remembered positions of one or two visual cue(s). When the two cues were presented across the left and right visual fields, neurons exhibited a comparable response to the activity for the preferred cue presented alone. When the two cues were presented within the same hemifield, neurons exhibited an intermediate response between those to the individual cues. Subsequent computer simulations predicted a lower signal-to-noise ratio in the latter condition, which was further verified by behavioral experiments. Considering the separation of contralateral and ipsilateral visual processing, the lateral inhibition in local circuits might implicitly determine different neuronal computations and memory capacities for bilateral and unilateral displays.
Keywords: memory capacity, prefrontal cortex, primate, single-unit recording, working memory
Introduction
Working memory, the ability to selectively maintain task-relevant information for subsequent behavior and thoughts, constitutes a fundamental building block of cognition (Goldman-Rakic, 1995). Exploration of its neuronal correlates has been remarkably advanced through the development of delayed-response paradigms (Fuster and Alexander, 1971; Kubota and Niki, 1971), in particular, the memory-guided saccade task (Hikosaka and Wurtz, 1983). In the cortical network that includes the prefrontal cortex (PFC) (Bruce and Goldberg, 1985; Gnadt and Andersen, 1988; Funahashi et al., 1989), many neurons show sustained, enhanced activity after a brief presentation of a visual cue. Because the delay-period activities are spatially tuned, the remembered location can be encoded as the preferred location of the most active neurons in the population, which is thought to be an efficient neuronal code for spatial working memory.
However, it remains unanswered how the brain maintains information about multiple locations simultaneously. Based on previous results, there are three possibilities. First, each neuron might solely signal the presence of a visual cue in the preferred location to be remembered, ignoring any other cues presented previously (Edin et al., 2009; Wei et al., 2012). Second, there might be neurons encoding multiple locations as a single packet. In line with this, when monkeys remembered the identities of two visual objects, prefrontal neurons nonlinearly combined a representation of each item to respond to a specific pair (Warden and Miller, 2007). Finally, neuronal activity might reflect the competitive interaction of multiple representations. The biased competition model of selective attention suggests that responses to individual stimuli are linearly integrated with biases toward attended objects (Desimone and Duncan, 1995; Reynolds and Heeger, 2009), which has much empirical support (Moran and Desimone, 1985; Treue and Maunsell, 1996). Because computations within individual neurons must limit the representational capacity of the whole population (Rigotti et al., 2013), it is of great importance to elucidate how each neuron encodes multiple locations.
In the present study, we examined the activities of prefrontal neurons while monkeys remembered the location of one or two visual cue(s). Our data show that each neuron represents multiple locations differently depending on whether the stimuli were presented across or within visual hemifield(s). We suggest that the inherent, anatomical separation of contralateral and ipsilateral information might differentiate neuronal computations for bilateral and unilateral displays, imposing physiological constraints on the memory capacity.
Materials and Methods
Animal preparation.
Experiments were conducted on three Japanese macaques (Macaca fuscata, 6–7 kg female, Monkeys J, L, and O). All animal protocols were approved by the Animal Care and Use Committee of Hokkaido University and were in accord with the Guide for the Care and Use of Laboratory Animals. The animal preparation procedure was described previously in detail (Tanaka, 2005). Briefly, a pair of head holders was implanted on the skull using titanium screws and dental acrylic under general isoflurane anesthesia. A coil of stainless steel wire was implanted under the conjunctiva. During subsequent training and experimental sessions, the monkey's head was secured to the primate chair, and eye positions were continuously recorded using the search coil technique (MEL-25; Enzanshi Kogyo). After training on behavioral tasks, a recording cylinder was installed over a small craniotomy, allowing electrode penetrations into the prearcuate PFC. Animals received analgesia after each surgery. The monkeys' water intake was controlled daily so that they were motivated to perform the tasks.
Visual stimuli and behavioral paradigms.
Experiments were controlled by a Windows-based real-time data acquisition system (TEMPO; Reflective Computing). Visual stimuli were presented on a 24-inch cathode-ray tube monitor (60 Hz) positioned 38 cm from the eyes and subtending 64 × 44° of visual angle. Experiments were performed in a darkened booth. Each trial began with the appearance of a fixation point (FP, 0.5° red square) at the center of the screen. Monkeys were required to maintain fixation for >300 ms to proceed with the trial. Correct performance was reinforced with a drop of liquid reward at the end of each trial.
During recording sessions, we presented two memory-guided saccade tasks. In the multiple memory-guided saccade (MMS) task (see Fig. 1A), two sample (200 ms) and three test stimuli were presented 12° eccentrically across a 2 s delay (1° white squares). One test stimulus was presented at the same location as either sample (matched stimulus), whereas the others were presented elsewhere (nonmatched stimuli). The location of the matched stimulus was chosen randomly from the two sample locations so that the animals had to remember both locations. Monkeys were required to keep their eyes within 5° of the central FP during the sample and delay periods, then to make a saccade to the matched stimulus in response to the FP offset and the simultaneous appearance of test stimuli (<400 ms). As a control, we also presented the single memory-guided saccade (SMS) task with only one sample stimulus (see Fig. 1B). In all recording sessions, the deviation of eye position from the FP was much less than the window size (mean ± SD, 0.51 ± 0.11°, 0.50 ± 0.14°, and 0.64 ± 0.14°, for Monkeys J, L, and O, respectively). Both sample and test stimuli appeared either at four oblique (45°, 135°, 225°, or 315° measured from rightward) or four cardinal (0°, 90°, 180°, or 270°) polar angles. Different tasks were presented pseudo-randomly within a block that usually consisted of the SMS trials in four oblique and/or four cardinal (90° increments) directions, and the MMS trials with every combination of them (10 or 20 cases).
Figure 1.
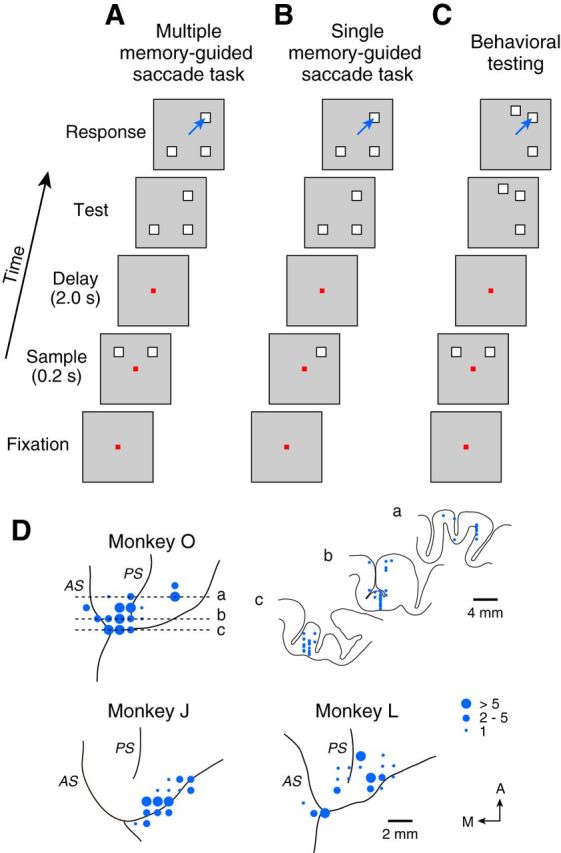
Behavioral paradigms and recording sites. A, Sequence of events in the MMS task. Monkeys remembered two sample locations during the delay and made a saccade to one of three test stimuli presented at either sample location. Both the sample and test stimuli were chosen from four possible locations (cardinal or oblique locations). B, In the SMS task, only one sample was presented. C, For the behavioral experiment, test stimuli were chosen from eight possible locations. D, Recording sites. The size of each circle indicates the number of recorded neurons. Coronal sections were shown only for Monkey O at the level indicated by broken lines. PS, Principal sulcus; AS, arcuate sulcus.
We performed an additional behavioral experiment for the analysis shown in Figure 5. Locations of test stimuli were randomly assigned to one of eight directions (0–315° with 45° increments), whereas the samples were presented at two of four oblique directions in the MMS trials (see Fig. 1C) or presented at one of eight directions in the interleaved SMS trials. Although the two nonmatched stimuli were not always equidistant from the matched stimulus in individual trials because of the random assignment (e.g., see Fig. 1C, “Across” trial), the overall angular difference did not differ between the Across and Within conditions in all monkeys (unpaired t test, p > 0.6).
Figure 5.
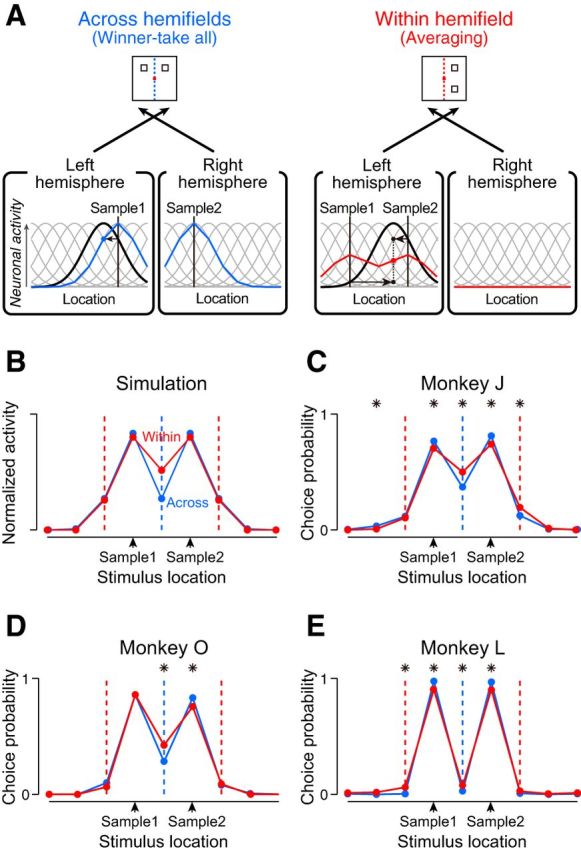
Model prediction and behavioral performance. A, Representations of two sample locations in simulated population activities. The model assumes that the visual inputs from opposite visual fields are processed independently while those from the same visual field are processed interactively. Only contralaterally responsive neurons are shown for clarity. The neuronal modulation is large in the Across condition (blue curve) but small in the Within condition (red curve). In both conditions, the sample locations are encoded as the two peaks in the population activity. B, Comparison of the population activities between the Across and Within conditions. Population activity for each condition was normalized so that the areas under the curves became equal. Blue and red vertical dashed lines indicate the vertical meridian in the Across and Within conditions, respectively. C–E, Choice probability for each test stimulus was plotted as a function of its location relative to the samples, for individual monkeys. *Significant difference between the choice probabilities in the Across and Within conditions (both-side z test, p < 0.05). All animals made more errors in choosing the test stimulus presented at the midpoint of the samples in the Within condition.
Physiological procedures.
Neuronal activity was recorded through tungsten electrodes (Alpha-Omega Engineering) lowered into the PFC though a 23-gauge guide tube using a micromanipulator (MO-97S; Narishige). Neuronal signals were amplified (Model 1800; A-M Systems), filtered (Model 3625; NF), and monitored online using oscilloscopes and an audio device. The waveforms of single neuronal activity were isolated using a real-time spike sorter with template-matching algorithms (MSD or ASD; Alpha-Omega Engineering). Occurrences of action potentials were time stamped and saved in files along with the eye movement and stimulus location data during the experiments (sampling rate: 1 kHz).
Histological procedures.
A postmortem examination of recording sites was performed in all monkeys (see Fig. 1D). At the end of the experiments, the animals were deeply anesthetized with a lethal dose of sodium pentobarbital (>50 mg/kg, intraperitoneally), and several landmark pins were penetrated at known coordinates. The animals were then perfused with an 0.1 m phosphate buffer followed by 3.5% formalin. The brain was removed, blocked, and fixed with the same solution overnight. Once the brain was equilibrated with an 0.1 m phosphate buffer containing 30% sucrose, 100-μm-thick coronal sections were cut using a freezing microtome. Sections were stained with cresyl violet, and location of each task-related neuron was reconstructed according to the coordinates of electrode penetrations relative to the landmark pins.
Data analysis.
Data were analyzed offline using MATLAB (MathWorks). For the population analysis (see Fig. 3), we only considered neurons with greater excitatory response to the preferred sample than those presented 90° away from it, in the SMS trials (t test, p < 0.01). Individual neuronal activities were normalized by the peak activity during a 2300 ms period starting at the sample onset and averaged to see the time course of population activities (see Fig. 3A,B). To assess linear weights for the population activities in the SMS trials with preferred (RESPpref) or nonpreferred (RESPnon-pref) cue to explain the activity in the MMS trials (RESPdouble), we computed the value (RESPdouble − RESPnon-pref)/(RESPpref − RESPnon-pref). To calculate it reliably, we used the data during the last 500 ms of the delay when most neurons were highly activated. Neurons were resampled using the bootstrap procedure (1000 repetition) to estimate the 95% confidence interval (CI). For the computer simulation shown in Figure 5, we used 16 model neurons with Gaussian directional tuning (σ = 30°) centered at different directions (22.5° increment, Fig. 5A, gray curves).
Figure 3.
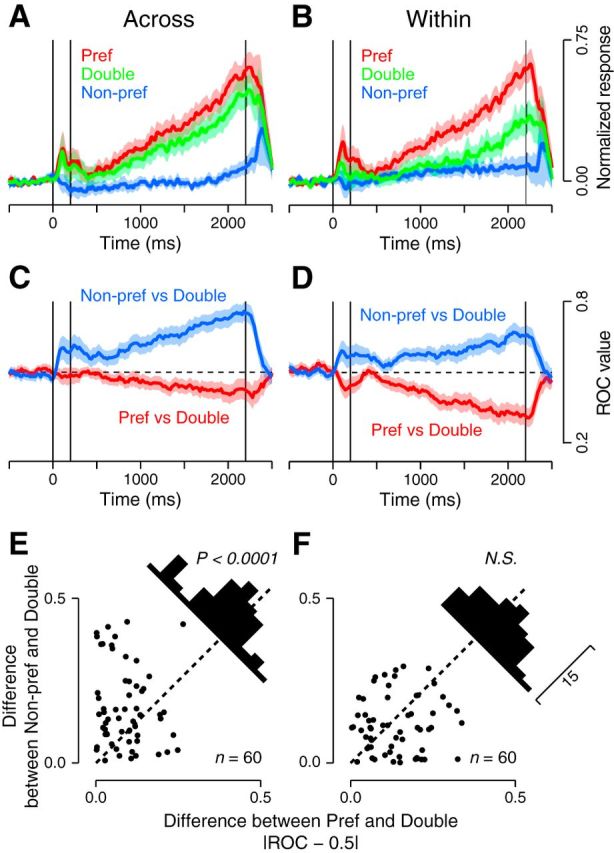
Comparison between the Across and Within conditions. A, B, Population activities in the MMS trials with two samples presented across (A) or within (B) visual field(s) (green traces) are compared with those in the SMS trials with a sample at either the preferred (red) or nonpreferred (blue) location. Shaded areas represent 95% confidence intervals. C, D, Population averages of the ROC values computed between the activity in the MMS and SMS trials. ROC values were taken to be 1 or 0 when activities in MMS trials were consistently higher or lower than those in any SMS trials, respectively. E, F, Differences in neuronal responses in the MMS and SMS trials were assessed by comparing the deviation of the ROC values from 0.5. In the Across condition (E), the response to the two samples was significantly closer to the response to the single sample presented at the preferred location (paired t test, p < 0.0001). In the Within condition (F), the response to the two samples was in between the responses to each sample presented alone (p > 0.1). N.S., Not significant.
Results
We recorded from single neurons in the macaque PFC (Fig. 1D). In the MMS task (Fig. 1A), two sample and three test stimuli were presented across a 2 s delay. Monkeys maintained a central fixation throughout the cue and delay periods and then made a saccade to a test stimulus presented at the same location as either sample (matched stimulus). Because the matched stimulus was randomly assigned to one of the two sample locations, monkeys had to remember both locations during the delay. Two samples were always presented 90° apart from each other in the MMS trials examined in the current study. As a control, we also used the SMS task (Fig. 1B), in which only one sample was presented. All three monkeys performed very well in the SMS (mean ± SD of the rate of correct choice: 98 ± 2%, 98 ± 3%, and 98 ± 2%, for Monkeys J, L, and O, respectively) and MMS (90 ± 7%, 94 ± 6%, and 98 ± 2%, for Monkeys J, L, and O, respectively) tasks.
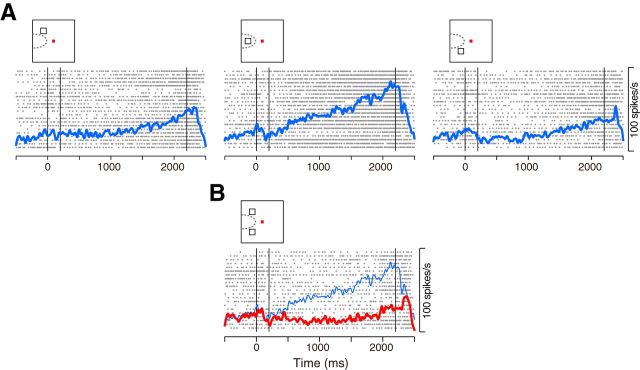
Consistent with previous studies (Funahashi et al., 1989; Matsushima and Tanaka, 2012), we found neurons exhibiting a sustained response to the visual cues. A representative neuron shown in Figure 2 exhibited an elevated activity throughout the delay period after a single sample presented in the upper-left receptive field (RF, Fig. 2A). When the cue was located 90° away from the RF, however, the delay-period activity disappeared (Fig. 2B,C). In the MMS trials with two samples presented across the left and right visual fields (Fig. 2D, Across condition), the same neuron exhibited strong activity (green trace), comparable with the response to a single cue presented in the RF (red trace, same data as in Fig. 2A). On the other hand, when the two cues were presented within the same hemifield (Fig. 2E, Within condition), the neuron exhibited an intermediate response between those to the preferred (red) and nonpreferred (blue) stimulus presented alone.
Figure 2.
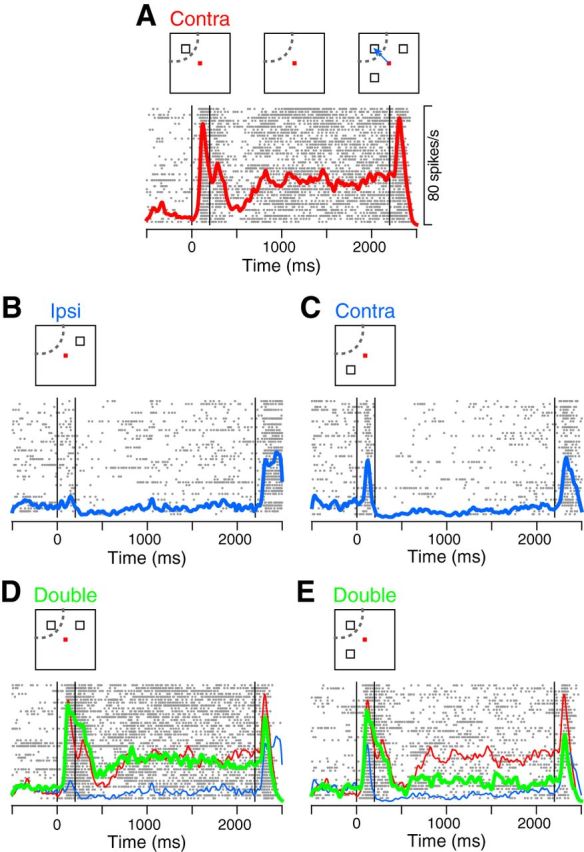
Activity of a representative neuron. A, Activity in the SMS trials with a sample in the RF. From left to right, vertical lines indicate the onset and offset of the sample, and the onset of test stimuli. B, C, Activity in the SMS trials with a sample presented 90° away from the RF. D, When two samples were presented across the left and right visual fields (green), the activity was similar to that for the single sample presented in the RF (red, the same data as in A). E, When two samples were presented within the same visual field (green), the neuron exhibited an intermediate response to those for each stimulus presented alone (red and blue traces, the same data as in A and C).
Neuronal responses in the two conditions were consistently different in the sampled population. In the Across condition, the population activity for the two cues in the MMS trials (Fig. 3A, green trace) was similar to that for the preferred cue presented alone (Fig. 3A, red trace), but far stronger than that for the nonpreferred cue (Fig. 3A, blue trace). On the contrary, in the Within condition, the population activity was intermediate between the activities for the individual cues (Fig. 3B). The linear weight of population activities was significantly biased toward the preferred cue in the Across condition (0.75, 95% CI = [0.66 0.84], bootstrap repetition = 1000), but neither cue in the Within condition (0.40, 95% CI = [0.29 0.52]). To assess the separation of responses between the MMS and SMS trials in individual neurons, we computed ROC values for every 100 ms (20 ms step). The population average shows that the ROC values for the Across condition were closer to 0.5 when responses in the MMS trials were compared with those in the SMS trials for the preferred (Fig. 3C, red trace), rather than the nonpreferred sample (Fig. 3C, blue trace), indicating that the response to the two cues was closer to the response to the single cue presented at the preferred location. On the other hand, ROC values for the Within condition were equally separated from 0.5 when compared with either sample (Fig. 3D), indicating that the response to the two cues differed evenly from the response to the preferred or nonpreferred cue presented alone. When we computed the mean deviation of the ROC values from 0.5 in the last 1500 ms of the delay, we found a significant difference between those for the preferred and nonpreferred samples in the Across condition (paired t test, p < 0.001, Fig. 3E), but not in the Within condition (p > 0.1, Fig. 3F). We also examined trial-by-trial response variability by computing the Fano factor during the delay but failed to find differences between the task configurations for the Across (two samples, 1.2 ± 0.5; single preferred, 1.1 ± 0.5; single nonpreferred, 1.2 ± 0.5, one-way ANOVA, n = 60, p > 0.5) and Within conditions (1.3 ± 0.5, 1.1 ± 0.5, 1.2 ± 0.4, p > 0.4). These results suggest that multiple locations might be represented by different firing rates of neurons depending on the relative stimulus locations. In the Across condition, neuronal activity is dominated by the preferred stimulus, almost in a winner-take-all manner. In the Within condition, neuronal activity is equally affected by individual stimuli, resulting in the average response.
Different neuronal modulations could not be attributed to other factors than the relative location across the visual fields. First, one might argue that monkeys attended to a specific sample in the Across condition. However, this is unlikely because monkeys performed equally well when the matched stimulus was presented at the preferred or nonpreferred location in the Across (preferred vs nonpreferred, 97.9 ± 4.8% vs 99.0 ± 3.1%, paired t test, p > 0.1) as well as in the Within condition (97.9 ± 5.8% vs 99.2 ± 2.7%, p > 0.1). Second, the nonpreferred stimulus might be presented inside the RF in the Within condition so that it competed with the preferred stimulus. However, as we fitted Gaussian tuning curves to delay-period activities in the SMS trials, the nonpreferred cues presented in both conditions were located 3.1 SD away from the RF center (mean ± SD, Across, 3.1 ± 1.8; Within, 3.1 ± 2.1, n = 60, paired t test, p > 0.7). Third, multiple locations might be remembered as a single entity encompassing the two samples in the Within condition. Incompatible with this, neurons tuned to the midpoint of the samples were suppressed when monkeys remembered the two locations simultaneously (Fig. 4). Furthermore, the rate of choosing the test stimuli when presented in between the two samples was much lower than the rate at the cued locations (see below, Fig. 5C–E). These results were analogous to those for multifocal attention; visual responses to a distractor flanked by attended objects were suppressed in the extrastriate visual area (Niebergall et al., 2011). Because the response variability was comparable between the MMS and SMS trials, the focus of working memory seemed to be divided, rather than fluctuating, between the two locations.
Figure 4.
Activity of a neuron tuned to the midpoint of samples. A, Activities in the SMS trials. This neuron exhibited a strong response to a single sample presented in the left RF (middle), but not to samples presented 45° away from the RF (left, right). B, Activity in the MMS trials. The same neuron exhibited a much lower response to two samples presented within the left visual field (red thick trace) than to a single sample presented at the midpoint of them (blue thin trace; the same data as in A, middle).
Based on the results of the neuronal recordings, we next attempted to simulate population activities during the delay in the MMS trials. In the Across condition where neurons responded in a “winner-take-all” manner (Fig. 5A, left), a neuron tuned to a specific location (black solid curve) would exhibit a comparable response to the activity for a single cue presented around its preferred location (blue dot at the level of intercept with vertical line indicating Sample 1). In the Within condition (Fig. 5A, right), however, the same neuron would exhibit an “Averaging” response of those to individual samples (red dot at the mean level of two intercepts). Repetition of similar computations for 16 spatially tuned neurons yielded a population coding with maximal activities at the two sample locations, both in the Across (blue) and Within conditions (Fig. 5A, red solid curves). This result indicates that cued locations are encoded by the most active neurons in the array, just like the previous computational model (Compte et al., 2000). However, the quality of the signal represented in the neuronal population appeared to differ between the two conditions, as further clarified by overlaying the normalized population activities (Fig. 5B); the activity contrast between the cued and the intermediate locations was reduced in the Within condition compared with the Across condition.
To see the corresponding behavioral outcomes, we conducted an additional experiment. In the behavioral test, one of the nonmatched stimuli was occasionally presented in between the two sample locations (Fig. 1C). As the choice probability was calculated for each test stimulus (Fig. 5C–E), we found that all monkeys made more errors in choosing the midpoint of two samples in the Within than in the Across condition (both-side z test, p < 0.05), consistent with the simulation results (Fig. 5B). Together, these results suggest that the interaction between neuronal representations of multiple locations might hinder the individuation of each spatial memory and ultimately could constrain behavioral performance.
Discussion
In the present study, we probed the neuronal correlates of working memory for multiple locations. When monkeys remembered two cues presented across the left and right visual fields, their neuronal activities were comparable with those for a single cue presented at the preferred location. When monkeys remembered two cues presented within the same hemifield, their neuronal activities were intermediate between the responses to individual cues. Our data might reflect an inherent, anatomical separation of contralateral and ipsilateral information processing along the visual pathways.
After the optic chiasm, visual inputs from the right visual field are transmitted to the left side of the brain, whereas those from the left visual field are transmitted to the right side. This laterality is especially evident in the early stages of cortical processing, in which RFs are confined to contralateral visual fields (Bullier, 2004). Reflecting the division into hemispheres, visual stimuli presented in opposite visual fields are more difficult to compare than those presented in the same hemifield (Banich and Belger, 1990; Sergent, 1990).
Even in the PFC, where neurons responding to contralateral and ipsilateral stimuli coexist (Rainer et al., 1998; Lennert and Martinez-Trujillo, 2013), inputs from different visual fields are known to reach distinct cortical columns (Goldman-Rakic and Schwartz, 1982). Related to this, behavioral performance is strongly influenced by the spatial arrangement of visual items even in tasks requiring higher order processing; humans can attend to (Alvarez and Cavanagh, 2005) or remember (Delvenne, 2005) more objects when presented bilaterally than unilaterally. As for the neural correlates, Buschman et al. (2011) recently showed that neuronal information about object identity is more reduced by the presence of other objects in the same compared with the opposite visual field. Our data might provide a reasonable explanation for these previous observations from the view of neuronal computation within local circuits. Signals from the same visual field are processed highly competitively and averaged through recurrent connections, whereas signals from opposite hemifields are processed almost independently and spared in distinct cortical columns.
Differences in neuronal computations were further verified by monkeys' performance. Based on the observed firing modulations, we simulated the population activities during the delay in the Across and Within conditions. The simulation can be viewed as a specific form of the normalization model proposed by Reynolds and Heeger (2009), where individual neuronal activities are normalized by the total activity in each neuronal ensemble responsible for either visual hemifield. As suggested by a previous computational model (Compte et al., 2000), we found that the cued locations could be represented by the most active neurons in the population. Thus, in our task configuration, the matched stimulus would be simply read out by detecting a peak of activity when visual responses to test stimuli were added to the population activity. However, the generally noisy activities of neurons seemed to produce an accidental peak at uncued locations, causing errors. Corresponding to the relatively higher activity at the midpoint of the two sample locations, monkeys erroneously reported the midpoint as a cued location more often in the Within than in the Across condition. These results demonstrate that the interaction of multiple representations within each neuron determines the signal-to-noise ratio in the population activity and ultimately constrains the behavioral performance. Considering the columnar organization of contralateral and ipsilateral neurons in the PFC (Goldman-Rakic and Schwartz, 1982), the interactions might be mediated by interneurons constructing recurrent network with nearby functionally related pyramidal neurons (Gabbott and Bacon, 1996; Rao et al., 1999). Because there also exist long-range horizontal connections in the cortex (Stepanyants et al., 2009), signal processing in each cortical column might not be completely independent so that the magnitude of intracolumnar and intercolumnar interactions might be relatively, rather than absolutely, different. Nonetheless, our data suggest that the relative difference is sufficient to alter the neuronal signals and behavioral performance in the Across and Within conditions. Because the balanced inhibition to excitation within local circuits is essential to prevent epileptic activity (Turrigiano, 2011), the working memory capacity limited by the recurrent inhibition might be computationally (Usher and Cohen, 1999) and evolutionarily (Hultsch, 1992) inevitable.
Footnotes
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labour and Welfare of Japan, the Japan Society for the Promotion of Science, the Smoking Research Foundation, and the Takeda Science Foundation. Animals were provided by the National Bio-Resource Project. We thank T. Mori and A. Hironaka for the animal care, M. Suzuki for administrative help, and all laboratory members for comments and discussions.
The authors declare no competing financial interests.
References
- Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Banich MT, Belger A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex. 1990;26:77–94. doi: 10.1016/S0010-9452(13)80076-7. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields: I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bullier J. Communications between cortical areas of the visual system. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: Massachusetts Institute of Technology; 2004. pp. 522–540. [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proc Natl Acad Sci U S A. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Delvenne JF. The capacity of visual short-term memory within and between hemifields. Cognition. 2005;96:B79–B88. doi: 10.1016/j.cognition.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegnér J, Compte A. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a-c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Schwartz ML. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982;216:755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulate: III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Hultsch H. Time window and unit capacity: dual constraints on the acquisition of serial information in songbirds. J Comp Physiol Sensory Neural Behav Physiol. 1992;170:275–280. [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Lennert T, Martinez-Trujillo JC. Prefrontal neurons of opposite spatial preference display distinct target selection dynamics. J Neurosci. 2013;33:9520–9529. doi: 10.1523/JNEUROSCI.5156-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Tanaka M. Neuronal correlates of multiple top-down signals during covert tracking of moving objects in macaque prefrontal cortex. J Cogn Neurosci. 2012;24:2043–2056. doi: 10.1162/jocn_a_00265. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Niebergall R, Khayat PS, Treue S, Martinez-Trujillo JC. Multifocal attention filters targets from distracters within and beyond primate MT neurons' receptive field boundaries. Neuron. 2011;72:1067–1079. doi: 10.1016/j.neuron.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Memory fields of neurons in the primate prefrontal cortex. Proc Natl Acad Sci U S A. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J. Furtive incursions into bicameral minds: integrative and coordinating role of subcortical structures. Brain. 1990;113:537–568. doi: 10.1093/brain/113.2.537. [DOI] [PubMed] [Google Scholar]
- Stepanyants A, Martinez LM, Ferecskó AS, Kisvárday ZF. The fractions of short- and long-range connections in the visual cortex. Proc Natl Acad Sci U S A. 2009;106:3555–3560. doi: 10.1073/pnas.0810390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Involvement of the central thalamus in the control of smooth pursuit eye movements. J Neurosci. 2005;25:5866–5876. doi: 10.1523/JNEUROSCI.0676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD. Connectionist models in cognitive neuroscience. London: Springer; 1999. Short term memory and selection processes in a frontal-lobe model; pp. 78–91. [Google Scholar]
- Warden MR, Miller EK. The representation of multiple objects in prefrontal neuronal delay activity. Cereb Cortex. 2007;17(Suppl 1):i41–i50. doi: 10.1093/cercor/bhm070. [DOI] [PubMed] [Google Scholar]
- Wei Z, Wang XJ, Wang DH. From distributed resources to limited slots in multiple-item working memory: a spiking network model with normalization. J Neurosci. 2012;32:11228–11240. doi: 10.1523/JNEUROSCI.0735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]