Abstract
Translational biomarkers, such as prepulse inhibition (PPI) of the acoustic startle response, are playing an increasingly important role in the development of antipsychotic drugs for schizophrenia and related conditions. However, attempts to reliably induce a PPI deficit by psychotomimetic drugs have not been successful, leaving an unmet need for a cross-species psychosis model sensitive to this widely studied surrogate treatment target. Sleep deprivation (SD) might be such a model as it has previously been shown to induce PPI deficits in rats, which could be selectively prevented with antipsychotic but not anxiolytic or antidepressant compounds. Here, in a first proof-of-concept study we tested whether SD induces a deficit in PPI and an increase in psychosis-like symptoms in healthy humans. In two counterbalanced sessions, acoustic PPI and self-reported psychosis-like symptoms (Psychotomimetic States Inventory) were measured in 24 healthy human volunteers after a normal night's sleep and after a night of total SD. SD decreased PPI (p = 0.001) without affecting the magnitude or habituation of the startle response (all p > 0.13). SD also induced perceptual distortions, cognitive disorganization, and anhedonia (all p < 0.02). Thus, extending previous rodent work, we conclude that SD, in combination with the PPI biomarker, might be a promising translational surrogate model for psychosis as this method represents a possibility to partially and reversibly mimic the pathogenesis of psychotic states.
Keywords: model system, prepulse inhibition, schizophrenia, sensorimotor gating, sleep deprivation, startle
Introduction
Prepulse inhibition (PPI) refers to a reduction of response to a strong stimulus (pulse) if this is preceded with short stimulus onset asynchrony (SOA) by a weak stimulus (prepulse; Graham, 1975). PPI provides a simple operational measure of sensorimotor gating, serving to prevent the interruption of ongoing perceptual and early sensory analysis (Braff and Geyer, 1990), shows high test–retest reliability (Cadenhead et al., 1999; Ludewig et al., 2002a) and is amenable to cross-species comparisons (Swerdlow et al., 1994).
PPI is reliably reduced in schizophrenia (Braff et al., 1978, 2001a; Kumari et al., 2000, 2007b; Ludewig et al., 2003; Swerdlow et al., 2006, 2014), the prodrome of schizophrenia (Quednow et al., 2008), schizotypal personality disorder (Cadenhead et al., 2002), psychosis-prone healthy individuals (Swerdlow et al., 1995; Kumari et al., 1997, 2008), and unaffected relatives of schizophrenia patients (Cadenhead et al., 2000; Kumari et al., 2005). Corticostriato-pallido-thalamic circuitry modulates PPI in the rat (Swerdlow et al., 2001) and these neural substrates are implicated in the pathophysiology of schizophrenia and the therapeutic actions of antipsychotics (Geyer et al., 2001). These regions are also found active in functional imaging studies of healthy humans and show deficient activation in schizophrenia (Kumari et al., 2003, 2007a), making PPI a valuable biomarker to probe the pathophysiology and treatment of schizophrenia. PPI is often used to investigate the effects of putative antipsychotic compounds in animal research (Geyer et al., 2001). In healthy human volunteers, however, treatment efficacy, which should take the form of deficit correction, may not be determined meaningfully unless deficits are first induced by some pharmacological or experimental means (Kumari and Sharma, 2002).
In rats, sleep deprivation (SD) reduces PPI (Frau et al., 2008, Liu et al., 2011) and this reduction can be selectively reversed by antipsychotic (but not anxiolytic or antidepressant) drugs (Frau et al., 2008). To our knowledge, there are no studies on the effects of SD on PPI in humans. Here, we aim to fill this gap in the literature. If the PPI-decreasing effect of SD can be replicated in humans, the SD paradigm could prove a powerful model system of psychosis with strong clinical relevance.
Of relevance, sleep disturbance is a well documented symptom of schizophrenia and hallucinatory disorders (Benca et al., 1992). The severity of sleep disturbance is associated with the severity of psychotic symptoms and is reduced by antipsychotic treatments in schizophrenia (Monti and Monti, 2004). Severe insomnia is often seen during exacerbations of schizophrenia, and may precede the appearance of other symptoms (Monti and Monti, 2004). Insomnia is associated with paranoia even in the general population (Freeman et al., 2009). Furthermore, the SD method, if shown to work as a psychosis model, would provide a feasible method as SD-induced behavioral disturbances are fully reversible following restorative sleep (Ratcliff and Van Dongen, 2009).
Therefore, in this proof-of-concept study, we investigated the effects of 24 h SD on PPI in a sample of healthy human volunteers. We hypothesized to find SD-induced PPI deficits and a concurrent increase in psychosis-like symptoms.
Materials and Methods
Participants.
Healthy male and female volunteers (aged 18–40 years) were recruited via flyers and circular e-mails from the student population at the University of Bonn. After initial contact, potential participants were invited to a telephone screening in which the exclusion criteria were addressed: Pittsburgh Sleep Quality Index >5 (Backhaus et al., 2002), regular time of going to bed later than 1:00 A.M., any sleep disorder, irregular sleep–wake rhythm, shift-working, current consumption of any drugs or medication (other than contraceptive pill or vitamins), verbal IQ <85 estimated with a standardized German vocabulary test (Lehrl, 1989), diagnosis of a psychiatric disorder assessed with the Mini International Neuropsychiatric Interview (Lecrubier et al., 1997), Beck Depression Inventory >18 (Kühner et al., 2007), diagnosis of a neurological disorder, hearing impairments, visual impairment other than glasses or contact lenses, regular nicotine consumption, and body mass index >25. The study was approved by the ethics committee of the Department of Psychology, University of Bonn, and participants provided written informed consent before inclusion. Participants were compensated with either class credits or €80.
Procedure.
The study used a counterbalanced within-subjects design with each participant being tested on PPI and some other tasks starting ∼8:00 A.M. after a night of normal sleep or after a night of total SD. Testing sessions were scheduled 1 week apart. Procedures in both testing sessions are depicted in Figure 1.
Figure 1.
Timeline of the testing sessions. Solid line, normal night condition; dashed line, SD condition; Incl., including.
Both testing sessions started in the evening at 8:30 P.M. with a urine drug screening test (nal von minden) and the completion of the Psychotomimetic States Inventory (PSI; Mason et al., 2008). No positive drug tests were obtained at any point in the study. The PSI measures psychosis-like states and includes subscales of delusional thinking, perceptual distortions, cognitive disorganization, anhedonia, mania, and paranoia. At 9:00 P.M. Stanford Sleepiness Scale (SSS; Hoddes et al., 1973) was completed and a learning task was administered.
In the normal night condition, participants subsequently had some free time in which they were allowed to read or watch a movie before they had to go to bed shortly before 11:00 P.M. in a comfortable bed in the sleep laboratory. In the morning, participants were woken up at 6:55 A.M. and bedside assessments of SSS followed at 7:00 and 7:15 A.M. After getting up, another assessment of SSS followed at 7:30 A.M. before a light, standardized breakfast. At 8:00 A.M., SSS was measured again and a test battery (total duration ∼90 min) including PPI, oculomotor tasks (pro- and antisaccades, smooth pursuit eye movements), moral dilemmas, working memory, and creativity, was administered. The tests were presented in a pseudorandomized order (starting with either PPI or oculomotor tasks due to restricted access to startle and eye tracking equipment followed by a randomized order of the other tests). Testing always ended with assessment of SSS and the PSI. Participants were dismissed at ∼10:00 A.M.
In the SD condition, participants had to stay awake during the whole night in the presence of an examiner. In the evening, after completion of SSS and a learning task, they filled in some demographic and personality questionnaires. During the night, participants were allowed to read, watch movies, and play board games with the examiner. Participants had to abstain from eating and drinking except for water. In addition, the examiner took the participants for 15 min walks at 10:30 P.M., 1:30 A.M., 4:30 A.M., and 6:30 A.M. In the morning, the procedures were the same as in the normal night condition with the exception that SSS measurements at 7:00 and 7:15 A.M. were measured sitting upright. At dismissal, participants were reminded not to steer any vehicles or operate heavy machines and to have a good rest at home.
Startle response measurement.
An electromyographic (EMG) startle system (Mark II; SR Labs) was used for the delivery of acoustic startle stimuli and the recording and scoring of EMG activity (digital units; 1 unit = 2.62 μV). Recording and scoring methods followed those in previous articles (Quednow et al., 2006a,b). Each startle session began with a 3 min acclimation period of 70 dB background noise that continued throughout the session. The paradigm used a pulse (a 115 dB white-noise sound pulse, duration of 40 ms) presented either alone or following a prepulse (either a 78 dB, 20 ms white-noise burst or an 85 dB, 20 ms white-noise burst that preceded the pulse by an SOA of 30, 60, or 120 ms). Startle session began with the first block consisting of five pulse-alone (PA) trials. This block was followed by three blocks of 21 trials, which consisted of three trials each of the six prepulse conditions and three PA trials presented in a fixed, pseudorandom order. The paradigm concluded with the last (fifth) block consisting of five more PA trials for a total of 73 trials. The intertrial interval averaged 14 s (range, 9–22 s). The entire session lasted ∼21 min. PPI, startle reactivity, and habituation measures were calculated as previously described (Quednow et al., 2006a,b; Petrovsky et al., 2013). Due to habituation across the test session, PA trials from the middle blocks (i.e., blocks 2–4) were taken to calculate PPI (Cadenhead et al., 2000).
Statistical analyses.
Statistical analyses were performed using IBM SPSS Statistics 22 (IBM). Data were analyzed with multivariate ANOVA (MANOVA; Wilks lambda, Λ). The significance level was set at p < 0.05. P values of post hoc tests were Bonferroni-corrected.
Sleepiness (SSS scores) were analyzed with MANOVA with sleep condition (normal night, SD) and time of measurement (9:00 P.M., 7:00 A.M., 7:15 A.M., 7:30 A.M., 8:00 A.M., 10:00 A.M.) as within-subjects factors and order (normal night first, SD first) as a between-subjects factor.
Startle reactivity across blocks was examined with MANOVA on PA amplitude with sleep condition (normal night, SD) and block (1, 2, 3, 4, 5) as within-subjects factors and order (normal night first, SD first) as a between-subjects factor. Initial startle reactivity (amplitude over first 5 PA trials, block 1) and habituation measures (also calculated on PA amplitudes: early habituation, total habituation, and coefficient b) were analyzed with MANOVA with sleep condition (normal night, SD) as within-subjects factor and order (normal night first, SD first) as a between-subjects factor. PPI data were analyzed with MANOVA with sleep condition (normal night, SD), prepulse intensity (78 dB, 85 dB) and SOA (30 ms, 60 ms, 120 ms) as within-subjects factors, and order (normal night first, SD first) as a between-subjects factor.
Effects on PSI scores were examined with MANOVA with sleep condition (normal night, SD) and time of measurement (evening, morning) as within-subjects factors and order (normal night first, SD first) as a between-subjects factor.
Partial correlational analyses (Pearson's r) examined the relationships between PPI change scores (i.e., difference scores derived from SD minus normal night) and PSI change scores (SD minus normal night) while controlling for PA amplitude (change score of averaged PA trials from blocks 2–4) and sleepiness (change score of SSS at 8:00 A.M.). To restrict the number of correlations performed, only PSI and PPI scores that showed significant change with SD were examined (i.e., perceptual distortion, cognitive disorganization, and anhedonia with PPI data at the 30 and 60 ms SOAs, each averaged across intensity, hence a total of 6 correlations). Bonferroni-corrected threshold level of significance for correlation coefficients was set at p = 0.05/6 = 0.008.
Results
Demographic and psychophysiological data are shown in Tables 1 and 2.
Table 1.
Demographic information
| All participants (N = 24) | |
|---|---|
| Age | 24.1 (3.5) |
| Verbal IQ | 98.9 (9.6) |
| N (male:female) | 16:8 |
Data represent means (standard deviations) unless otherwise specified.
Table 2.
Psychophysiological parameters
| Normal night | Sleep deprivation | |
|---|---|---|
| Startle amplitude over pulse-alone trials | ||
| Block 1 | 508.74 (259.42) | 564.86 (366.25) |
| Block 2 | 339.96 (209.30) | 367.87 (346.77) |
| Block 3 | 268.00 (170.85) | 275.76 (293.92) |
| Block 4 | 199.85 (139.61) | 217.44 (193.26) |
| Block 5 | 236.29 (164.58) | 216.61 (172.92) |
| Percentage early habituation | 31.94 (31.82) | 35.93 (37.43) |
| Percentage total habituation | 50.92 (30.01) | 59.11 (20.32) |
| Habituation coefficient b | −68.50 (46.16) | −84.69 (63.19) |
| PPI | 46.48 (28.24) | 26.22 (33.58) |
Data represent means (standard deviations) unless otherwise specified. Startle amplitude is depicted in digital units (1 unit = 2.62 μV). Percentage PPI is the overall mean across prepulse intensities and SOA conditions.
SSS
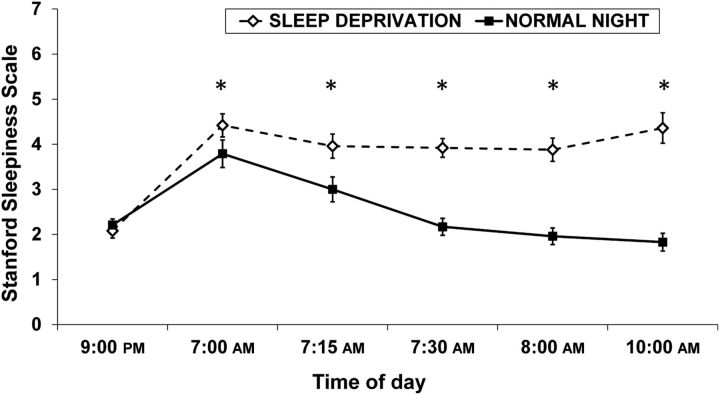
Participants' sleepiness was significantly higher after SD than after a normal night (Fig. 2). There were main effects of sleep condition (Λ = 0.30, F(1,22) = 44.58, p < 0.0001, ηp2 = 0.70) and time of measurement (Λ = 0.21, F(5,18) = 11.36, p < 0.0001, ηp2 = 0.79), as well as an interaction of sleep condition × time of measurement (Λ = 0.25, F(5,18) = 8.84, p < 0.001, ηp2 = 0.75) on sleepiness. Post hoc tests indicated that participants rated their sleepiness during the SD condition significantly higher than during the normal sleep condition for all five time points in the morning (all p < 0.04). Sleepiness ratings at 9:00 P.M. from the previous evening did not differ between conditions (p = 0.68).
Figure 2.
Mean SSS (±SEM). Sleepiness ratings did not differ before the intervention (p = 0.68). Following SD, sleepiness was significantly elevated (all p < 0.04).
Startle amplitude and habituation
No startle nonresponders were identified in this sample. For PA amplitude (i.e., startle reactivity) over the five blocks, there was a main effect of block (Λ = 0.23, F(4,19) = 15.62, p < 0.0001, ηp2 = 0.77), indicating startle habituation across blocks. The interaction of sleep condition × block was not significant (Λ = 0.88, F(4,19) = 0.64, p = 0.64, ηp2 = 0.12), suggesting equal habituation across blocks in normal night and SD conditions and. There were no further main or interaction effects (all F < 0.40, all p > 0.53).
Mean amplitude of PA trials from the first block as well as the habituation measures of PA trials (early habituation, total habituation, and coefficient b) did not reveal any main (all F < 2.01, all p > 0.17) or interaction (all F < 1.28, all p > 0.27) effects which also indicates no effects of SD on PA amplitudes.
PPI
There was a main effect of SOA (Λ = 0.23, F(2,21) = 35.22, p < 0.0001, ηp2 = 0.77) reflecting the well known effect of PPI to increase with rising SOA and showing the maximal magnitude at 120 ms (Braff et al., 2001b). The main effect of prepulse intensity did not reach significance (Λ = 0.87, F(1,22) = 3.42, p = 0.08, ηp2 = 0.13) although illustrating that stronger prepulses (i.e., 85 dB) tended to induce greater levels of PPI than weaker prepulses (i.e., 78 dB; Braff et al., 2001b).
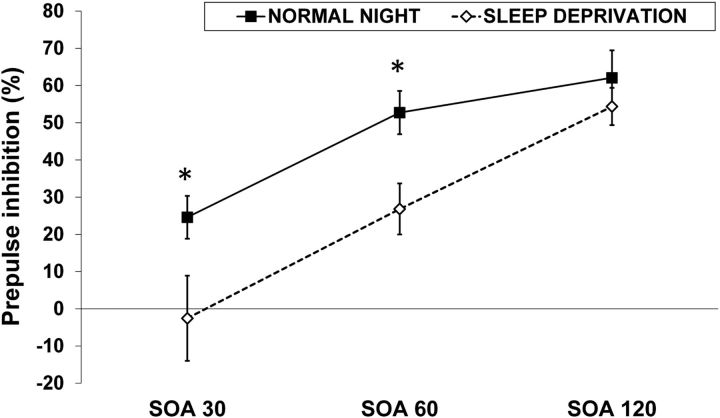
There was a main effect of sleep condition on PPI (Λ = 0.59, F(1,22) = 15.54, p = 0.001, ηp2 = 0.41) demonstrating a reduction of PPI with SD (Fig. 3). Furthermore, the interaction of sleep condition × SOA was significant (Λ = 0.59, F(2,21) = 7.22, p = 0.004, ηp2 = 0.41). Bonferroni-corrected post hoc comparisons revealed that the decrement of PPI by SD was formally significant for 30 ms SOA (p = 0.01) and 60 ms SOA (p < 0.0001), but not for 120 ms SOA (p = 0.12).
Figure 3.
Mean PPI (±SEM). SD decreased PPI across all prepulse intervals (p = 0.001). SD-induced disruption of PPI was more pronounced for the 30 and 60 ms prepulse intervals than for the 120 ms interval (interaction, p = 0.004).
The interaction of sleep condition × SOA × prepulse intensity was not significant (Λ = 0.89, F(2,21) = 1.29, p = 0.30, ηp2 = 0.11). There was neither a main effect of order (F = 1.11, p = 0.30), nor any further significant interactions (all F < 2.29, all p > 0.14).
PSI
PSI scores are depicted in Figure 4. For the subscales perceptual distortion (Λ = 0.72, F(1,22) = 8.77, p = 0.01, ηp2 = 0.28), cognitive disorganization (Λ = 0.55, F(1,22) = 18.16, p = 0.0003, ηp2 = 0.45), and anhedonia (Λ = 0.71, F(1,22) = 9.14, p = 0.01, ηp2 = 0.29), there were interaction effects of sleep condition and time of measurement. Post hoc tests, as expected, revealed that PSI scores did not differ between conditions at the evening (baseline) measurements (all p > 0.16) but showed increases with SD in morning measurements (perceptual distortion: p = 0.02, cognitive disorganization: p < 0.0001, anhedonia: p = 0.003; Fig. 4). The increase of psychosis-like symptoms after SD was further illustrated by significant post hoc comparisons of the morning and evening measurements within the SD condition (perceptual distortion: p = 0.006, cognitive disorganization: p < 0.0001, anhedonia: p < 0.0001; Fig. 4).
Figure 4.
Mean scores (+SEM) of the PSI. Three of six PSI scores were increased by SD: perceptual distortion, cognitive disorganization, and anhedonia (all p < 0.02).
Correlations between PPI and PSI change scores
There were no significant correlations between the PPI and PSI change scores (all r > −0.3, all p > 0.05).
Discussion
This proof-of-concept study investigated whether a night of total SD leads to deficits in PPI and increases in psychosis-like symptoms in healthy human volunteers. PPI was significantly decreased in the SD condition compared with the normal night condition, in agreement with evidence from rodent studies (Frau et al., 2008; Liu et al., 2011). Importantly, no effects of SD were observed for startle reactivity and habituation measures (i.e., no effects of SD for PA amplitudes were observed). Specifically, our pattern of results indicates that the SD-induced reduction of PPI stemmed from larger prepulse–pulse amplitudes rather than from decreased PA amplitudes, indicating true sensorimotor gating deficits following SD. Moreover, effect sizes of the SD-induced disruption of PPI were large, indicating a robust effect. We also replicated basic findings concerning the PPI phenomenon that are in line with previous studies (Braff et al., 2001b): startle reactivity was initially high and showed habituation across blocks, PPI increased with rising SOA with a maximal magnitude of inhibition at 120 ms, and stronger prepulses tended to induce greater levels of PPI.
The present results showed that SD led to an overall disruption of PPI suggesting that SD decreased PPI across all prepulse intervals. This strong main effect indicates that SD might be an alternative method to the approach to pharmacologically induce deficits in PPI in healthy volunteers. For instance, while the glutamatergic NMDA receptor antagonist ketamine demonstrated PPI-disruptive effects in rodents (Braff et al., 2001b), in healthy humans no effect (van Berckel et al., 1998; Duncan et al., 2001) or even PPI-increasing effects of ketamine (Abel et al., 2003) have been observed. Oranje et al. (2002) also found that ketamine alone did not disrupt PPI in healthy humans, although the combination of ketamine and haloperidol led to deficits in PPI. These results on ketamine exemplarily demonstrate that pharmacological findings in animals are not always easily transferable to humans. Thus, although cross-species translational drug studies of PPI remain important, it is of paramount importance to further pursue alternative cross-species models of psychosis such as SD.
Our results also revealed that the SD-induced disruption of PPI was stronger for the 30 and 60 ms prepulse intervals than for the 120 ms interval. These data suggest that acoustic PPI at short prepulse lead times (i.e., SOAs of 30 and 60 ms) might be more strongly affected by SD than PPI with a prepulse lead time of 120 ms which has been shown to be susceptible to attentional modulation (Li et al., 2009). In healthy humans, directing attention to the prepulse signal enhances PPI (Li et al., 2009), whereas attentional modulation of PPI is abnormal in schizophrenia patients (Dawson et al., 1993; Hazlett et al., 1998; for review, see Li et al., 2009) and individuals with schizotypal personality disorder (Hazlett et al., 2003). Inhibition of the acoustic startle reflex at the 60 ms prepulse interval might actually be regulated at the boundary between automatic and attentionally sensitive inhibition (Swerdlow et al., 2006) and this transitional zone between unconscious and consciously accessible processing may also be an epoch of particular vulnerability to disruption (Swerdlow et al., 2006). The 60 ms PPI is also most consistently deficient in schizophrenia (Swerdlow et al., 2006, 2014; Aggernaes et al., 2010) further underlining the susceptibility of this point in time in information processing.
Using the attention-to-prepulse paradigm (Hazlett et al., 2001) or a tactile PPI paradigm (Kumari et al., 2007a), functional magnetic resonance imaging (fMRI) studies in humans revealed that (as in rodents) a frontal-striatal-thalamic (FST) circuitry underlies PPI. In addition, another fMRI study investigating FST circuitry during an attention-to-prepulse paradigm revealed that this neural circuitry is aberrant in schizophrenia-spectrum patients (Hazlett et al., 2008). So far, it is unknown which structures of the FST circuitry underlying PPI are affected by SD; however, it is likely that all FST regions are affected by SD as illustrated by the following molecular imaging studies of SD.
In healthy humans, glucose metabolism of the prefrontal and parietal cortices and of the thalamus decreased at 48 and 72 h SD (Thomas et al., 2003), the same areas that showed decreases at 24 h SD (Thomas et al., 2000). Similarly, 29–34 h SD in healthy humans also decreased glucose metabolism of the frontal cortex, the thalamus, and the striatum (Wu et al., 2006). Furthermore, Volkow et al. (2008) demonstrated that 1 night of SD in healthy humans led to a reduction in D2/D3 receptors in striatum, suggesting increased dopamine release. Given that pharmacologically induced increases in striatal DA, e.g., by administration of amphetamine, are known to disrupt PPI (Geyer et al., 2001; Swerdlow et al., 2008), it is possible that the effects observed here stem from increased striatal DA. However, there is also evidence of cortical neurotransmitter changes following 1 night of SD in healthy humans, e.g., a global increase in mGluR5 binding (Hefti et al., 2013) and in 5-HT2A receptor binding (Elmenhorst et al., 2012). Overall, it is likely that SD induces both cortical and subcortical changes involving multiple neurotransmitter changes, which in combination led to the PPI deficit observed here.
In the present study, SD also induced psychosis-like phenomenology: The PSI subscales of perceptual distortions, cognitive disorganization, and anhedonia showed the expected increases after a night of total SD, whereas delusional thinking, mania, and paranoia did not. Our results regarding these self-reports thus suggest that one night of SD induces subclinical, psychosis-like symptoms in the three key symptom domains of schizophrenia, namely hallucinations, thought disorder, and negative symptoms. These increases were of small-to-moderate magnitude. These findings are in line with data by West et al. (1962) who demonstrated that >100 h (i.e., ∼5 d) of continuous insomnia must have passed for true psychotic symptoms, such as hallucinations, to emerge. Interestingly, evidence of delusions and paranoia did not emerge in our sample. Again, earlier studies (Berger and Oswald, 1962; Luby et al., 1962) observed full-blown delusions in healthy individuals after lengthier SD periods (of ∼100 h). Finally, we did not find any significant correlations between PSI and PPI change scores. This lack of covariation indicates that SD led to different reaction profiles in different individuals despite overall main effects, suggesting the operation of interindividual factors in the response to the intervention.
There are some limitations of our study that deserve attention. First, some participants had difficulty falling asleep in the unfamiliar bed in the sleep laboratory. Thus, actual sleep duration of a normal night in the laboratory might not have been the same as a normal night's sleep at home. Future studies might want to add a familiarization session in which participants sleep one additional night in the laboratory before the actual sleep testing session. Second, future studies should also assess mood and anxiety before and after SD as anxiety levels might influence startle reactivity and/or PPI (Ludewig et al., 2002b) and as SD is known to lead to affective changes as well (Kahn-Greene et al., 2007). Third, we tested our participants in the morning following SD. Future studies might want to aim for additional, later test sessions as SD-induced deficits might become more pronounced over time.
In conclusion, we demonstrated that SD induced sensorimotor gating deficits and elevated self-reported psychosis-like experiences in healthy humans. Extending previous rodent work, we conclude that SD, in combination with the PPI biomarker, might be a promising translational surrogate model for psychosis as this method represents a possibility to partially and reversibly mimic the pathogenesis of psychotic states.
Footnotes
V.K. was partially supported by the Biomedical Research Centre for Mental Health at the Institute of Psychiatry, King's College London, and the South London and Maudsley NHS Foundation Trust, UK. We thank Florian Toyka for assistance in data collection.
The authors declare no competing financial interests.
References
- Abel KM, Allin MP, Hemsley DR, Geyer MA. Low dose ketamine increases prepulse inhibition in healthy men. Neuropharmacology. 2003;44:729–737. doi: 10.1016/S0028-3908(03)00073-X. [DOI] [PubMed] [Google Scholar]
- Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months' treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13:1383–1395. doi: 10.1017/S1461145710000787. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/S0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–670. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Oswald I. Effects of sleep deprivation on behaviour, subsequent sleep, and dreaming. J Ment Sci. 1962;108:457–465. doi: 10.1192/bjp.108.455.457. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001a;49:171–178. doi: 10.1016/S0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001b;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45:360–364. doi: 10.1016/S0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL. Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? Am J Psychiatry. 2002;159:869–871. doi: 10.1176/appi.ajp.159.5.869. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and schizophrenia: impaired modulation of the startle reflex. J Abnorm Psychol. 1993;102:633–641. doi: 10.1037/0021-843X.102.4.633. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Madonick SH, Parwani A, Angrist B, Rajan R, Chakravorty S, Efferen TR, Szilagyi S, Stephanides M, Chappell PB, Gonzenbach S, Ko GN, Rotrosen JP. Clinical and sensorimotor gating effects of ketamine in normals. Neuropsychopharmacology. 2001;25:72–83. doi: 10.1016/S0893-133X(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Elmenhorst D, Kroll T, Matusch A, Bauer A. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep. 2012;35:1615–1623. doi: 10.5665/sleep.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Orrù M, Puligheddu M, Gessa GL, Mereu G, Marrosu F, Bortolato M. Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2008;11:947–955. doi: 10.1017/S1461145708008900. [DOI] [PubMed] [Google Scholar]
- Freeman D, Pugh K, Vorontsova N, Southgate L. Insomnia and paranoia. Schizophr Res. 2009;108:280–284. doi: 10.1016/j.schres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential address, 1974: the more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, Jimenez EA, Buchsbaum BR, Troyer BT. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. doi: 10.1111/1469-8986.3520186. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MB, Wei TC, Byne W, Haznedar MM. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry. 2001;50:281–291. doi: 10.1016/S0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Levine J, Buchsbaum MS, Silverman JM, New A, Sevin EM, Maldari LA, Siever LJ. Deficient attentional modulation of the startle response in patients with schizotypal personality disorder. Am J Psychiatry. 2003;160:1621–1626. doi: 10.1176/appi.ajp.160.9.1621. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, Haznedar MM, Chu KW, Nenadic I, Kemether EM, Tang CY, New AS, Siever LJ. Frontal-striatal-thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti K, Holst SC, Sovago J, Bachmann V, Buck A, Ametamey SM, Scheidegger M, Berthold T, Gomez-Mancilla B, Seifritz E, Landolt HP. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73:161–168. doi: 10.1016/j.biopsych.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone H, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–221. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Kühner C, Bürger C, Keller F, Hautzinger M. Reliability and validity of the revised beck depression inventory (BDI-II): results from German samples. Nervenarzt. 2007;78:651–656. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- Kumari V, Sharma T. Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology. 2002;162:97–101. doi: 10.1007/s00213-002-1099-x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Toone B, Gray JA. Habituation and prepulse inhibition of the acoustic startle reflex: effects of smoking status and psychosis-proneness. Pers Individ Dif. 1997;23:183–191. doi: 10.1016/S0191-8869(97)00045-7. [DOI] [Google Scholar]
- Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/S0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42:588–594. doi: 10.1111/j.1469-8986.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007a;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Sumich AL, Sharma T. Startle gating in antipsychotic-naive first episode schizophrenia patients: one ear is better than two. Psychiatry Res. 2007b;151:21–28. doi: 10.1016/j.psychres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA. Prepulse inhibition and “psychosis-proneness” in healthy individuals: an fMRI study. Eur Psychiatry. 2008;23:274–280. doi: 10.1016/j.eurpsy.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, Janavs J, Dunbar GC. The mini international neuropsychiatric interview (MINI): a short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224–231. doi: 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- Lehrl S. Multiple choice vocabulary test (form B) MWT-B. Erlangen: Perimed; 1989. [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Liu YP, Tung CS, Chuang CH, Lo SM, Ku YC. Tail-pinch stress and REM sleep deprivation differentially affect sensorimotor gating function in modafinil-treated rats. Behav Brain Res. 2011;219:98–104. doi: 10.1016/j.bbr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Luby ED, Grisell JL, Frohman CE, Lees H, Cohen BD, Gottlieb JS. Biochemical, psychological, and behavioral responses to sleep deprivation. Ann N Y Acad Sci. 1962;96:71–79. doi: 10.1111/j.1749-6632.1962.tb50102.x. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res. 2002a;55:129–137. doi: 10.1016/S0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/S0006-3223(02)01925-X. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety. 2002b;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res. 2008;103:138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8:133–148. doi: 10.1016/S1087-0792(02)00158-2. [DOI] [PubMed] [Google Scholar]
- Oranje B, Gispen-de Wied CC, Verbaten MN, Kahn RS. Modulating sensory gating in healthy volunteers: the effects of ketamine and haloperidol. Biol Psychiatry. 2002;52:887–895. doi: 10.1016/S0006-3223(02)01377-X. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Ettinger U, Kessler H, Mössner R, Wolfsgruber S, Dahmen N, Maier W, Wagner M, Quednow BB. The effect of nicotine on sensorimotor gating is modulated by a CHRNA3 polymorphism. Psychopharmacology (Berl) 2013;229:31–40. doi: 10.1007/s00213-013-3081-1. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kühn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry. 2006a;59:536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kühn KU, Beckmann K, Westheide J, Maier W, Wagner M. Attenuation of the prepulse inhibition of the acoustic startle response within and between sessions. Biol Psychol. 2006b;71:256–263. doi: 10.1016/j.biopsycho.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Kühn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Van Dongen HP. Sleep deprivation affects multiple distinct cognitive processes. Psychon Bull Rev. 2009;16:742–751. doi: 10.3758/PBR.16.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry. 1995;37:286–299. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Ray A, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophr Res. 2014;152:503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness: I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals RF, Wagner HN, Thorne DR, Popp KA, Rowland LM, Welsh AB, Balwinski SM, Redmond DP. Neural basis of alertness and cognitive performance impairments during sleepiness: II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat Syst. 2003;2:199–229. doi: 10.1017/S1472928803000207. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Oranje B, van Ree JM, Verbaten MN, Kahn RS. The effects of low dose ketamine on sensory gating, neuroendocrine secretion and behavior in healthy human subjects. Psychopharmacology (Berl) 1998;137:271–281. doi: 10.1007/s002130050620. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos PK, Ferré S, Jayne M. Sleep deprivation decreases binding of [11C] raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West LJ, Janszen HH, Lester BK, Cornelisoon FS., Jr The psychosis of sleep deprivation. Ann N Y Acad Sci. 1962;96:66–70. doi: 10.1111/j.1749-6632.1962.tb50101.x. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]