Abstract
Apathy is one of the most common and debilitating nonmotor manifestations of Parkinson's disease (PD) and is characterized by diminished motivation, decreased goal-directed behavior, and flattened affect. Despite its high prevalence, its underlying mechanisms are still poorly understood, having been associated with executive dysfunction, and impaired emotional processing and decision making. Apathy, as a syndrome, has recently been associated with reduced activation in the ventral striatum, suggesting that early- to middle-stage Parkinson's disease patients with this manifestation may have a compromised mesocorticolimbic dopaminergic pathway and impaired incentive processing. To test this hypothesis, we measured the amplitude of the feedback-related negativity, an event-related brain potential associated with performance outcome valence, following monetary gains and losses in human PD patients (12 women) and healthy controls (6 women) performing a gambling task. Early- to middle-stage PD patients presenting clinically meaningful symptoms of apathy were compared with nonapathetic PD patients and healthy controls. Patients with cognitive impairment, depression, and other psychiatric disturbances were excluded. Results showed that the amplitude of the feedback-related negativity, measured as the difference wave in the event-related brain potential between gains and losses, was significantly reduced in PD patients with apathy compared with nonapathetic patients and healthy controls. These findings indicate impaired incentive processing and suggest a compromised mesocorticolimbic pathway in cognitively intact PD patients with apathy.
Keywords: apathy, feedback-related negativity, Parkinson's disease, incentive processing
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder mainly characterized by dopamine-dependent motor features, such as resting tremor, bradykinesia, and rigidity. However, a wide range of cognitive, behavioral, and other nonmotor complications, such as psychotic symptoms, impulse control disorders (ICDs), and apathy, are presently recognized as characteristic of the disease (Aarsland et al., 2009).
Apathy is one of the most common neuropsychiatric features of PD, with a prevalence ranging from 17% to 70% (Pluck and Brown, 2002; Aarsland et al., 2009; Pedersen et al., 2009). Clinically, apathy is identified as a reduction of goal-directed behavior because of a lack of feeling, interest, emotional reactivity, and motivation (Marin, 1991).
Altered goal-directed behavior is commonly found in neurodegenerative and neuropsychiatric conditions where the functionality of the basal ganglia is compromised, such as in PD, Huntington's disease, and progressive supranuclear palsy (Aarsland et al., 1999, 2001; Hamilton et al., 2003; Levy, 2012). Although the compromise of the basal ganglia may be a major contributing factor to these behavioral alterations, the exact physiopathological mechanism underlying the development of apathetic symptoms in PD remains unclear. In addition to executive dysfunction, depression, dementia, and disease progression have also been proposed (Dujardin et al., 2007, 2009; Aarsland et al., 2009).
Recently, defects involving the mesocorticolimbic system and reward processing have been proposed as possible etiopathogenic factors for apathy in PD. Although the classical pattern of disease progression in PD assumed the relative preservation of the mesocorticolimbic pathway in the early and middle stages of the disease (Braak et al., 2004), recent findings have shown that this pathway can be impaired in de novo drug-naive PD patients (van der Vegt et al., 2013). It is therefore reasonable to assume that impaired incentive processing could lead to a disruption of motivation and to the presence of apathetic symptoms in these patients. This impairment would manifest as an inability to differentiate between favorable and unfavorable outcomes and to properly adjust subsequent behavior (Cohen and Ranganath, 2007).
Research of incentive processing using event-related potentials (ERPs) has identified a signal that is sensitive to the valence of performance outcomes. The feedback-related negativity (FRN) has a frontocentral distribution and reaches a maximum within 350 ms of feedback presentation. This wave is larger after unfavorable outcomes than after favorable outcomes, such as negative versus positive feedback or monetary losses versus monetary gains (Gehring and Willoughby, 2002; Holroyd et al., 2002). The FRN has been associated with reinforcement learning (Holroyd et al., 2002; Cohen and Ranganath, 2007), and dipole modeling studies have located its generators in the anterior cingulate cortex (ACC) (Gehring and Willoughby, 2002; Luu et al., 2003), a key structure within the mesocorticolimbic pathway (Alexander et al., 1986).
Here we wished to assess whether incentive processing, as measured by the FRN, is abnormal in cognitively intact PD patients showing apathy, compared with nonapathetic patients. We postulated that decreased FRN amplitudes would be measured in this patient subpopulation
Materials and Methods
Study participants.
A sample 40 outpatients (12 women) regularly attending the Movement Disorders Unit at Sant Pau Hospital and fulfilling diagnostic criteria for PD participated in the study. The Starkstein Apathy Scale (SAS; proposed score of ≥ 14;) (Starkstein et al., 2009) was used to identify clinically significant symptoms of apathy in patients at an early or middle stage of the disease. The SAS is an instrument recommended by the Movement Disorders Society to screen and assess severity of apathy in PD. It consists of 14 items, phrased as questions to be answered by the patient on a 4 point Likert scale. Exclusion criteria were patients presenting fluctuating response to l-DOPA, advanced PD, clinically meaningful anxiety, and/or depression (ruled out by a semistructured psychiatric interview based on DSM-IV-R criteria), clinically relevant cognitive impairment, focal abnormalities in neuroimaging studies, alterations in blood tests, or noncompensated systemic disease (i.e., diabetes, hypertension), and patients taking antipsychotic or other drugs that could interfere with performance and ERP morphology.
Criteria for absence of dementia and clinically relevant cognitive impairment were set at a score of 0 on the Clinical Dementia Rating scale (Morris, 1993) and a score >26 on the Mini-Mental State Examination (Folstein et al., 1975). The Mattis Dementia Rating scale was also administered to more precisely assess global cognitive functioning (Llebaria et al., 2008). The whole sample was assessed in pharmacological “on” condition.
Each patient was interviewed regarding disease onset and medication history, including type of motor response to l-DOPA. All study participants in the nonapathetic and apathetic groups were taking l-DOPA and dopaminergic agonists. Current medications and dosages were calculated for l-DOPA daily dose, and equivalent l-DOPA daily dose using Tomlinson et al. formulae (Tomlinson et al., 2010). PD patients were required to have received stable doses of dopaminergic drugs for the last 12 weeks and to show a stable response to medications. Motor status and disease stage were assessed by experienced movement disorder neurologists (J.P. and J.K.) using the Unified Parkinson's Disease Rating Scale and Hoehn and Yahr stages (Hoehn and Yahr, 1967).
Stimuli and procedure.
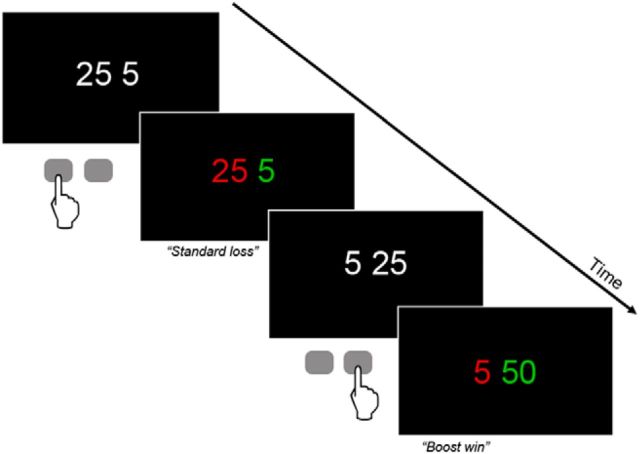
The experimental paradigm was based on Gehring's gambling task (Gehring and Willoughby, 2002), modified by Marco-Pallarés et al. and Riba et al. (Marco-Pallares et al., 2008; Riba et al., 2008) (Fig. 1). Each trial began with a fixation sign (asterisk). After 500 ms, two numbers, 25 or 5, were presented in white against a black background. Participants had to bet on one of these two numbers to increase a starting amount of 1000 euro cents. They were instructed to choose one of the two numbers by pressing a button. Immediately after the selection, the numbers changed color. One of the numbers changed to red, whereas the other changed to green. If the selected number turned green, it indicated a win (i.e., 5 or 25), whereas if it turned red, it indicated a loss (i.e., − 5 or −25). This feedback was shown for 1 s. A new trial was initiated after 3 s. The experimental session comprised 4 runs of 92 trials each. In 60 of the 92 trials (65%), the feedback indicated a standard win or a standard loss. Additionally, in 16 of the 92 trials (17.5%), the so-called “boost trials,” wins and losses were doubled and participants won or lost 10 cents after choosing 5 (a green or red “10” was shown as feedback on the screen) and 50 cents after choosing 25 (a green or red “50” was shown as feedback on the screen). Finally, to provide an adequate comparison condition for the boost trials and to tease apart the effect of increased absolute magnitude of the feedback from the effect of their reduced likelihood, in 16 of the 92 trials (17.5%), additional unexpected gains and losses were shown. However, this time the additional increase or decrease was minimal. Participants won or lost 7 cents after choosing 5 (a green or red “7” was shown as feedback on the screen) and 27 cents after choosing 25 (a green or red “27” was shown as feedback on the screen). Thus, the eight possible outcomes were as follows: win 5, win 25, lose 5, lose 25, win 10 (“boost+10”), lose 10 (“boost-10”), win 50 (“boost+50”), lose 50 (“boost-50”), win 7, lose 7, win 27, and lose 27.
Figure 1.
Schematic representation of the gambling task. Each trial of the gambling task involved the presentation of two numbers, 5 and 25. Participants had to choose one of the two numbers by button press (left button for left number). One second after the participant's selection, the numbers changed color. Green indicated a win, red a loss. Thus, in the first (top) example, the subject incurred a loss of 25 euro cents. The second (bottom) example shows an infrequent “boost” trial in which gains were doubled. For further details, see Materials and Methods.
Participants were told that they should adjust their choices based on outcomes in each trial to increase their gains. However, the task was programmed to yield wins in 50% of the trials and losses in the other 50%. This meant that there were no specific strategies that would have yielded better outcomes than others. Participants were simply encouraged to maximize the initial sum of 1000 euro cents. At three different time points during each run, participants were informed about the money they had won.
Electrophysiological recording.
The electroencephalogram (EEG) was recorded from 19 standard scalp sites (Fp1/2, F3/4, C3/4, T3/4, T5/6, P3/4, O1/2, F7/8, Fz, Cz, Pz) using tin electrodes mounted in an elastic cap and referenced to the two mastoid leads. Vertical eye movements were monitored using a bipolar montage with two electrodes linked together and placed below each eye referenced to a third electrode placed centrally above the eyes. Horizontal eye movements were monitored using two electrodes placed on the external canthi of each eye. Electrode impedances were kept <5 kOhm. The electrophysiological signals were filtered with a bandpass of 0.1–35 Hz and digitized at a rate of 250 Hz.
To maximize the information available for the subsequent ERP analysis, raw EEG signals were subjected to an ocular artifact minimization process based on Blind Source Separation. This technique expresses a set of signals as a linear combination of statistically independent component signals. For this purpose, the SOBI algorithm (Belouchrani et al., 1997) was used. This algorithm is based on an eigenvalue decomposition of time-delayed covariance matrices. After identifying the source signals associated with eye movements, corrected EEG signals were obtained from the remaining components. Identification of ocular signal sources was based on frequency and scalp topography analyses as described by Romero et al. (2008).
Feedback-locked ERPs were averaged from 200 ms before until 1000 ms after the feedback stimulus. Epochs exceeding ±75 μV were removed from further analysis. The mean amplitude value in the interval between −50 and 0 ms was used as baseline. Averages were calculated for each of the 12 conditions. The resulting ERPs were filtered with a low pass filter (12 Hz half amplitude cutoff).
Statistical analysis.
The behavioral data obtained included reaction times and the probability (between 0 and 1) of making a “risky” choice (i.e., choosing “25”). This probability was calculated using the following ratio: n25/(n25 + n5), n25 being the number of times “25” was chosen and n5 the number of times “5” was chosen. For each participant, an overall probability was calculated for the whole experiment. To study how previous outcomes influenced choices in subsequent trials, the ratio was also calculated as a function of type of outcome in the immediately preceding trial (each of the 12 possibilities described above). These probability ratios were subjected to repeated-measures ANOVAs with the within-subject factors “type of outcome” (win vs loss in the preceding trial), “magnitude of previous outcome” (25, 5, 10, and 50), and the between-subjects factor “group” (apathetic/nonapathetic).
ERP effects were quantified measuring the mean amplitude of the ERP between 250 and 450 ms after feedback presentation. This measure was obtained for the three midline electrodes (Fz, Cz, Pz) and subjected to repeated-measures ANOVAs with within-subject factors, such as “electrode” (Fz, Cz, Pz) and “condition” (win/lose), and a between-subjects factor “group” (apathetic/nonapathetic).
p values for the ANOVAs were calculated after Greenhouse–Geisser correction. Pairwise comparisons were performed using Student's t test. Results were considered significant for p values <0.05
Results
Demographic and clinical data
The study included 40 nondemented PD patients: 20 with clinically meaningful symptoms of apathy (7 women) and 20 without (5 women). As shown in Table 1, groups were carefully matched for age, education, and main clinical characteristics.
Table 1.
Clinical and sociodemographic characteristics of the samplea
| Apathetic (n = 20) |
Nonapathetic (n = 20) |
p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 67 | 6.5 | 64 | 6.9 | 0.192 |
| Education (years) | 11.2 | 4.2 | 12.6 | 4.5 | 0.531 |
| Disease duration (years) | 9.2 | 6.6 | 9.6 | 5.1 | 0.855 |
| Hoehn and Yahr | 2.1 | 0.3 | 2 | 0.4 | 0.392 |
| UPDRS-III motor score | 18.7 | 6.4 | 18.3 | 5.3 | 0.832 |
| l-DOPA daily doseb | 489 | 277 | 450 | 264 | 0.649 |
| DA-LEDDc | 127 | 98 | 105 | 83 | 0.454 |
| MMSE | 28.5 | 1 | 28.8 | 0.9 | 0.260 |
| MDRS | 134.8 | 3.9 | 136.6 | 6.3 | 0.239 |
| SAS | 19.8 | 5.7 | 8.3 | 2.5 | 0.000 |
aUPDRS, Unified Parkinson's Disease Rating Scale; MMSE, Mini-Mental State Examination; MDRS, Mattis Dementia Rating Scale; SAS, Starsktein's Apathy Scale.
bl-DOPA daily equivalent dose in milligrams.
cDopamine agonist l-DOPA equivalent daily dose.
Behavior
Reaction times (choosing “5” or “25”) were significantly slower for apathetic patients than for nonapathetic patients (t(38) = −2.12, p = 0.041). Mean ± SD. reaction time was 1149 ± 366 ms for apathetic patients and 918 ± 323 ms for nonapathetic patients.
Participants consistently chose “25” more often than “5.” The percentage of trials (expressed as mean ± SD) in which participants chose “25” was 63.8 ± 11.5%, and the percentage of trials in which they chose “5” was 36.2 ± 11.5%. Nonapathetic Parkinson's patients chose “25” and “5” in 64.3% and 35.7% of trials, respectively, and apathetic patients chose “25” and “5” in 63.3 and 36.8% of trials, respectively. A two-way ANOVA with the within-subjects factor choice (“25” vs “5”) and the between-subjects factor patient group (nonapathetic vs apathetic) showed a significant effect of choice (F(1,38) = 56.44, p = 0.000) but no statistically significant differences between patient groups (F(1,38) < 1).
To test how previous outcomes influenced current choices, we analyzed the relationship between a given choice and the outcome on the preceding trial. The probability of making a “risky” choice (i.e., choosing “25”) was measured in relation to the outcome in the immediately preceding trial. The analysis was conducted separately for standard, “similar” and “boost trials.” The analysis of the standard trials using a three-way ANOVA with type of previous outcome (win vs loss), magnitude of previous outcome (25 vs 5) as within-subjects factor, and patient group (nonapathetic vs apathetic) as between-subjects factor showed a marginally significant triple interaction (F(1,38) = 4.12, p = 0.049). However, no significant effect was found when analyzing potential behavioral differences between patient groups as a function of gain magnitude (25 vs 5) (F(1,38) = 0.62, not significant) or loss magnitude (F(1,38) = 1.62, not significant).
Analysis of the “similar” trials did not yield a significant interaction between previous outcome (win vs loss), magnitude (27 vs 7), and patient group (nonapathetic vs apathetic).
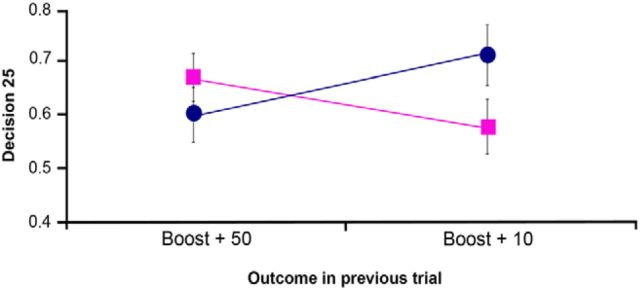
On the other hand, analysis of the infrequent “boost trials” showed a significant interaction between type of previous outcome (win vs loss), magnitude (50 vs 10) and patient group (nonapathetic vs apathetic) (F(1,38) = 15.03, p = 0.000). A detailed analysis showed that this effect was not driven by the “boost” losses − outcome by magnitude by patient group: (F(1,38) = 1.57, not significant) − but by “boost” gains (50 vs 10). Thus, the magnitude of the gain showed a significant interaction with patient group (F(1,38) = 11.18, p = 0.002). Apathetic patients made significantly more conservative choices after unexpected high wins (50) than after unexpected low wins (10), choosing “25” in 62% and 71% of the trials, respectively (t(19) = 2.2, p = 0.040). Nonapathetic patients showed the reverse pattern, making riskier choices after unexpected high wins than after unexpected low wins. They chose “25” in 68% of the trials following “50” and in 57% of the trials following “10.” This effect was significant (t(19) = 2.52, p = 0.021). Figure 2 shows this interaction.
Figure 2.
Behavioral results. The relationship between outcome in the previous trial (unexpected high wins vs unexpected low wins) and the probability of making a risky choice (choosing 25) are shown for the nonapathetic (square) and apathetic (circle) patient groups. A significant interaction was found between magnitude of the gain in the boost trial and patient group (F(1,38) = 11.18, p = 0.002). Error bars indicate SEM.
ERP analysis
The number of EEG epochs (mean ± SD) included in the averages did not differ between patient groups for any of the 6 main outcomes: (1) “standard wins”: nonapathetic patients 108 ± 17, apathetic patients 115 ± 15 (t(38) = −1.52, p > 0.1); (2) “standard losses”: nonapathetic patients 113 ± 17, apathetic patients 114 ± 13 (t(38) < 1, not significant); (3) “boost wins”: nonapathetic patients 30 ± 4, apathetic patients 30 ± 4 (t(38) < 1, not significant); (4) “boost losses”: nonapathetic patients 30 ± 4, apathetic patients 30 ± 4 (t(38) < 1, not significant); (5) “similar wins”: nonapathetic patients 29 ± 4, apathetic patients 31 ± 4 (t(38) < 1, not significant); and (6) “similar losses”: nonapathetic patients 30 ± 5, apathetic patients 30 ± 4, (t(38) < 1, not significant).
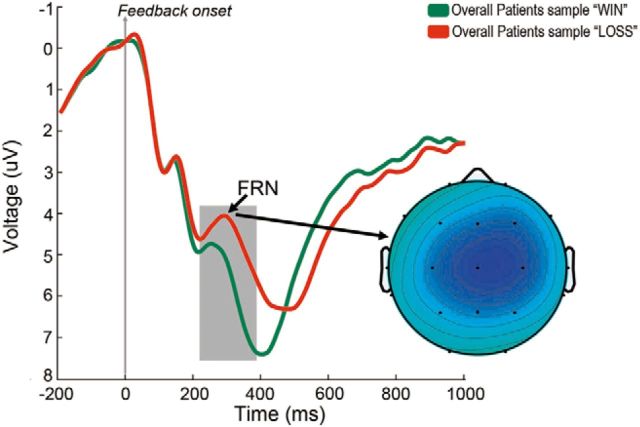
Feedback-locked averages after standard wins (5 and 25 combined) and losses (−5 and −25 combined) for the entire patient sample are shown in Figure 3. After monetary losses, a negative-going deflection was observed in the ERP, starting ∼250 ms after feedback presentation, peaking ∼300 ms and remaining differentiated from that associated with monetary wins until ∼450 ms after feedback. An ANOVA on the mean amplitude of the ERP between 250 and 450 ms with outcome (win vs loss) and electrode site (Fz, Cz, Pz) as factors showed the main effects of outcome (F(1,39) = 12.8, p = 0.001), electrode (F(2,78) = 16.7 p = 0.000) and the interaction outcome by electrode (F(2,78) = 22.7, p = 0.000). As shown in the topographic map of the difference wave loss-win, the negativity associated with monetary losses showed a frontocentral distribution.
Figure 3.
ERPs associated with monetary wins and losses. Grand average feedback-locked ERPs at Cz for monetary standard wins (25 or 5 combined) and losses (−25 and −5 combined) for the whole patient sample (apathetic and nonapathetic PD patients). The topographical map shows the FRN as the difference wave (loss-win) using isovoltage spline interpolation for the peak of activity. Relative scaling was used. Minimum and maximum values: −2/2 μV.
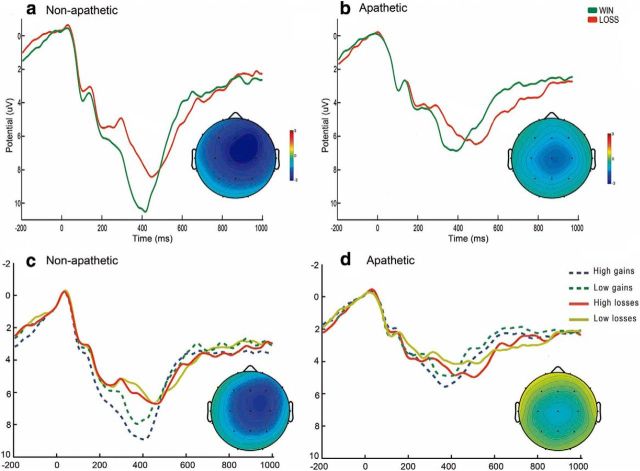
As shown in Figure 4, the difference between wins and losses was larger for nonapathetic patients than for apathetic patients. Statistically, this was shown as a significant interaction between patient group and outcome at Fz (F(1,38) = 4.3, p = 0.046). This effect was driven by the difference between maximum wins (25) and maximum losses (−25), as indicated by the interaction between outcome (maximum win vs maximum loss) and patient group, which was significant at both the Fz (F(1,38) = 8.4, p = 0.006) and Cz (F(1,38) = 4.4, p = 0.044) leads. Statistical comparison of the difference waves yielded significant results between patient groups at Fz (t(38) = 2.9, p = 0.007) and Cz (t(38) = −2.1, p = 0.045). Figure 4 (bottom) shows the average waves for each patient group and outcome magnitude (25, 5, −5, −25). To assess whether the decreases in ERP amplitude observed selectively reflected an impairment of the reward (wins) or punishment (losses) aspects of incentive processing, we conducted valence-specific tests. Pairwise comparison of ERP amplitude after high wins between the apathetic PD patients and the nonapathetic group yielded nonsignificant results at both Fz and Cz. The pairwise comparisons of ERP amplitude after high losses between the two patient groups were also nonsignificant.
Figure 4.
ERPs after standard wins and losses for each patient group. Top panels, Grand average feedback-locked ERPs at Cz for monetary standard wins (25 and 5 combined) and losses (−25 and −5 combined) for nonapathetic Parkinson's patients (a) and apathetic Parkinson's patients (b). Increased negativity peaks ∼300 ms after feedback observed for monetary losses. Bottom panels, Grand averages at Cz for monetary wins and losses separated by feedback magnitude (25, 5, −5, −25) for nonapathetic (c) and apathetic PD patients (d). Topographical maps in all four panels show the FRN as the respective difference wave (loss-win) using isovoltage spline interpolation for the peak of activity. Relative scaling was used. Minimum and maximum values: −3/3 μV.
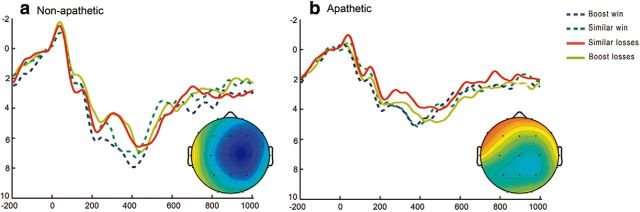
Additional differences between groups were found for the infrequent “boost trials.” As indicated in Materials and Methods, to separate the effect of increased absolute magnitude of the feedback from the effect of reduced likelihood, boost trials were compared with the so-called “similar” trials, that is, infrequent trials showing the same likelihood (17.5%) as the boost trials but yielding only marginally higher wins and losses than the standard trials. To increase the signal-to-noise ratio in the ERPs used in this analysis, we pooled high and low wins (“50” and “10” for the “boost trials”; and “27” and “7” for the “similar trials”) and high and low losses (“−50” and “−10” for the “boost trials”; and “−27” and “−7” for the “similar trials”) together in each trial type. The ERP graphs for the boost and similar conditions are shown in Figure 5. A three-way ANOVA with type of trial (“similar” vs “boost”) and outcome (win vs loss) and patient group as factors yielded a significant triple interaction at Fz (F(1,38) = 8.9, p = 0.005).
Figure 5.
ERPs after nonstandard “similar” and “boost” wins and losses for each patient group. Grand average feedback-locked ERPs at Fz for monetary wins and losses in “similar” and “boost” trials. a, Traces for nonapathetic patients. b, Traces for apathetic patients. Topographical maps show the FRN as the respective difference wave (loss-win) using isovoltage spline interpolation for the peak of activity. Relative scaling was used. Minimum and maximum values: −3/3 μV.
The difference waves (boost win-boost loss; and similar win-similar loss) were calculated and their mean amplitude compared between patient groups. This comparison showed that the effect of the triple interaction was driven by differences in the boost trials. Thus, the difference wave for the boost trials at Fz was larger in the nonapathetic than in the apathetic patient group (t(38) = −2.24, p = 0.31), but the difference wave for the similar trials was not different between groups (t(38) = 0.37, not significant). The averages are shown in Figure 5.
Control experiment
To compare behavior and ERP amplitudes in each subgroup of PD patients with those of the general population, we conducted a control experiment in which we administered the same gambling task to a group of healthy individuals. We recruited and tested a control sample of 11 healthy volunteers (6 women) with a mean (SD) age of 65.7 (3.7) years, and 11.9 (2.7) years of education. All volunteers were free of somatic, neurological, and psychiatric disorders and were not using any medication. No statistical differences were found between groups for age (F(2,48) > 1, not significant) or years of education (F(2,48) = 1.34, p > 0.1).
The behavioral analysis in the main study showed that apathetic patients made significantly more conservative choices after unexpected high wins (50) than after unexpected low wins (10). In this control experiment (Fig. 6) we subjected the differential probability of making a risky choice between these two conditions [probability of choosing 25 after an unexpected high win (50) minus the probability of choosing 25 after an unexpected low win (10)] to a one-way ANOVA with group as between-subjects factor (nonapathetic PD patients, apathetic PD patients, and healthy controls). The results showed a significant effect of group (F(2,48) = 4.88, p = 0.012). Pairwise comparisons showed significant differences between the apathetic and nonapathetic PD patients (t(38) = 3.31, p = 0.002), but no significant differences between the controls and either patient group.
Figure 6.
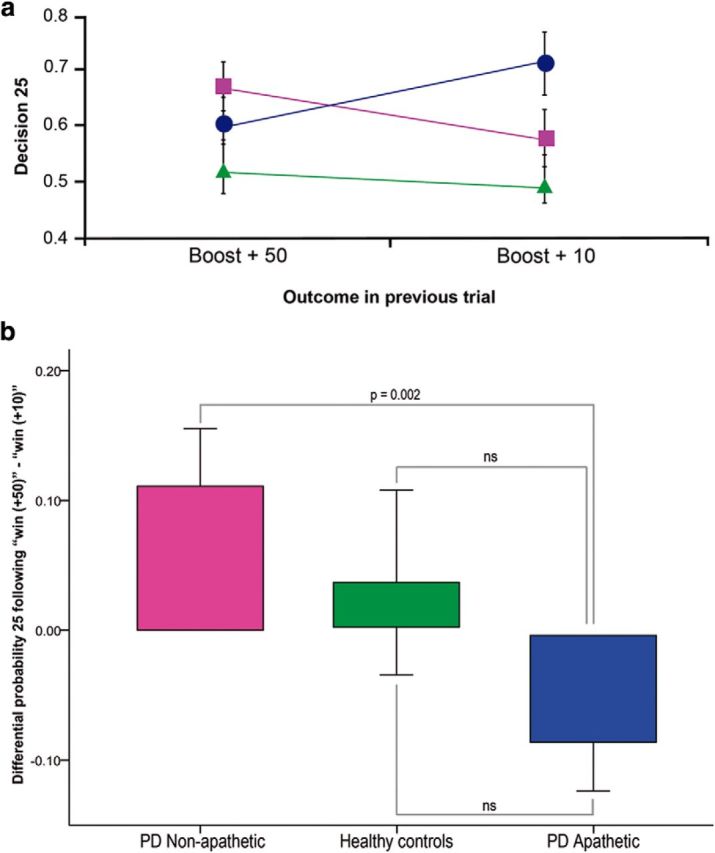
Control experiment: Behavioral results. a, The relationship between outcome in the previous trial (unexpected high wins vs unexpected low wins) and the probability of making a risky choice (choosing 25) is shown for the nonapathetic patients (square), apathetic patients (circle), and healthy controls (triangle). b, The probability of making a risky choice (25) after a “boost high” trial (50) minus the probability of making a risky choice (25) after a “boost low” trial (10) is shown for each participant group. ns, Not significant.
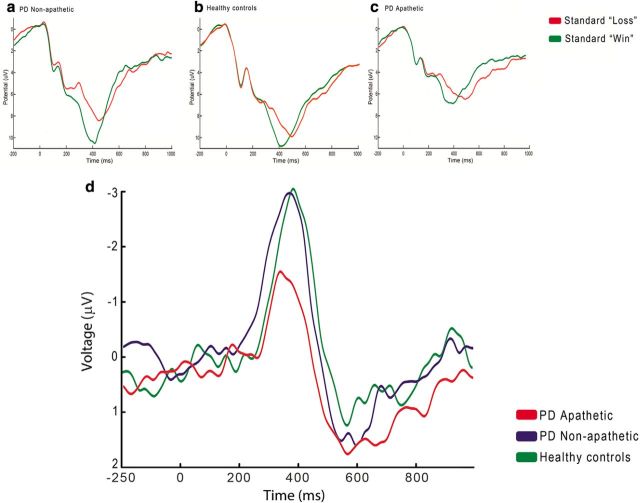
Regarding the ERPs, the main study showed a statistically significant interaction between outcome in the standard trials (wins vs losses) and group (apathetic vs nonapathetic). This effect was further modulated by the high wins (25) and high losses (−25). In this control experiment, we therefore subjected the difference wave (standard losses − standard wins) to a one-way ANOVA with group as the between-subjects factor (nonapathetic PD patients, apathetic PD patients, and healthy controls). The number of EEG epochs (mean ± SD) included in the averages was 115 ± 8 for the “standard wins” (25 and 5) and 118 ± 7 for the “standard losses” (−25 and −5). No differences were found in the number of epochs between the controls and any of the two patient groups.
Results in the ERP analysis showed a significant group effect at Fz (F(2,48) = 3.40, p = 0.042) and Cz (F(2,48) = 3.35, p = 0.044). Pairwise comparisons showed significant differences between the apathetic PD patients and the control group at Fz (t(29) = 2.62, p = 0.014) and Cz (t(29) = 2.25, p = 0.032), but not between the nonapathetic patients and the controls either at Fz (t(29) = 0.62, p > 0.1) or Cz (t(29) = 0.73, p > 0.1). The grand average waveforms for standard wins and losses and the corresponding difference waves are shown for each of the three participant groups in Figure 7.
Figure 7.
Control experiment: ERPs. Grand average feedback-locked ERPs at Cz for monetary standard wins and losses in the three participant groups: a, nonapathetic PD patients; b, healthy controls; c, apathetic PD patients. Lower right panel, Difference waves (standard loss − standard win) at Cz for each of the three participant groups (d).
We also tested whether the above effect was driven by the high or the low wins and losses. We therefore subjected the difference wave of the high outcomes (high losses − high wins) to a one-way ANOVA with group as the between-subjects factor (nonapathetic PD patients, apathetic PD patients, and healthy controls). Results showed a significant group effect at Fz (F(2,48) = 4.80, p = 0.013) and Cz (F(2,48) = 3.35, p = 0.044). Pairwise comparisons showed significant differences between the apathetic PD patients and the control group at Fz (t(29) = 2.83, p = 0.008) and Cz (t(29) = 2.11, p = 0.043), but not between the nonapathetic patients and the controls either at Fz (t(29) = 0.060, p > 0.1) or Cz (t(29) = 0.177, p > 0.1). The same analysis was conducted for the difference wave of the low outcomes (low losses − low wins). The one-way ANOVA with group as factor was not significant either at Fz (F(2,48) = 1.47, p > 0.1) or at Cz (F(2,48) = 2.04, p > 0.1).
The main experiment showed an effect of group on the ERPs associated with the boost trials. To compare values in each patient group with the healthy controls, the difference waves (boost loss − boost win) were calculated and subjected to a one-way ANOVA with group as the between-subjects factor (nonapathetic PD patients, apathetic PD patients, and healthy controls). Only a trend effect was observed (F(2,48) = 2.83, p = 0.069). Pairwise comparison showed the significant difference between the apathetic and nonapathetic PD patients (t(38) = −2.24, p = 0.031), but no differences between either of the patient groups and the controls.
An additional analysis was conducted to test whether the decreases in ERP amplitude observed in the apathetic sample selectively reflected the reward (wins) or punishment (losses) aspects of incentive processing. In these valence-specific tests, we performed a two-way ANOVA with outcome (high win vs high loss) and group (apathetic vs controls) as factors. A main effect of outcome was observed (F(1,29) = 14.31, p = 0.001) and a trend interaction between outcome and group (F(1,29) = 3.14, p = 0.087). Pairwise comparison of ERP amplitude after high wins between apathetic PD patients and healthy controls showed only trend effects at Fz (mean ± SD amplitude: apathetic patients 4.81 ± 4.49; controls 8.01 ± 4.34; t(29) = −1.94, p = 0.066) and Cz (mean ± SD amplitude: apathetic patients 4.28 ± 4.06; controls 7.05 ± 4.03; t(29) = −1.82, p = 0.079). We then performed the pairwise comparisons between groups for the ERP amplitudes after high losses. No significant differences or trend differences were found.
Discussion
Our results showed decreased incentive processing in apathetic PD patients compared with nonapathetic PD patients and healthy controls, as measured by a neurophysiological marker, the FRN. The ERP showed an increased negativity after monetary losses compared with monetary wins in the whole sample, replicating studies by others (Gehring and Willoughby, 2002; Nieuwenhuis et al., 2004; Nieuwenhuis et al., 2005). However, amplitude of the difference wave was significantly lower in the PD patients with apathy than in the other two participant groups. We also observed that behavior differed between the two patient subgroups, both of which had been carefully selected to rule out any potential confounds, such as concomitant dementia or depression. Together, these data strongly support the impairment of incentive processing as a distinctive trait of apathy in PD. This patient subpopulation would thus show diminished capacity to process, identify, and differentiate between favorable and unfavorable outcomes and to adjust subsequent behaviors accordingly (Holroyd et al., 2002; Cohen and Ranganath, 2007).
Neuropsychiatric features in PD have received increasing attention in recent years (Aarsland et al., 2009). In nondemented PD patients, these alterations have been mainly associated with the concurrent progression of executive dysfunction resulting from progressive dopaminergic denervation along the frontal–subcortical circuitry and/or from pharmacological treatment (Starkstein et al., 1992; Aarsland et al., 1999; Isella et al., 2002; Pluck and Brown, 2002). However, recent research in the field of apathy as a syndrome has shown impaired emotion and reward processing in the absence of deficits of higher cognition (Bowers et al., 2006; Rowe et al., 2008; Martínez-Corral et al., 2010; Lawrence et al., 2011).
The present study adds to the increasing corpus of data indicating that alterations in incentive processing − rather than just executive dysfunction − may underlie the blunted motivation and goal-directed behavior observed in apathetic PD patients. More specifically, the trend decrease in ERP amplitude after high wins and the absence of differences after high losses observed in the apathetic subgroup suggests a more selective impairment of reward compared with punishment processing. In line with this possibility, Rowe et al. (2008) have found increasing alterations in reward circuits in PD as a function of disease progression. Furthermore, Künig et al. (2000) reported decreased activation in the striatum after monetary rewards in PD patients compared with healthy controls. In a study directly comparing PD patients scoring high on apathy with those scoring low, Lawrence et al. (2011) found decreased responsivity after monetary gains in an extensive circuit involving the ventromedial prefrontal cortex, the amygdala, the striatum, and the midbrain.
Although it is commonly assumed that the mesocorticolimbic pathway is not specifically affected by dopamine depletion in PD, our results suggest that some degree of degeneration at this level is present, in line with a recent neuroimaging study in de novo drug-naive PD patients (van der Vegt et al., 2013). The dopaminergic projections from the ventral tegmental area in the midbrain to the striatum and the prefrontal cortex play a key role in reward, reinforcement learning and motivation (Hollerman and Schultz, 1998; Salamone and Correa, 2012; Schultz, 2013). Lesions in the striatum in non-PD patients have been found to cause a profound apathetic state, supporting the involvement of the mesocorticolimbic pathway in apathy (Schmidt et al., 2008; Adam et al., 2013). This apathetic state was reversed with the administration of levodopa and the dopaminergic agonist ropirinole (Adam et al., 2013).
At the other end of the behavioral continuum (Sinha et al., 2013), we find ICDs, a common finding in PD, especially in patients receiving dopaminergic agonists (Weintraub et al., 2010). Dopaminergic drugs impair behavioral performance in reward-related tasks, such as decision making and probabilistic reversal learning (Frank et al., 2004; Poletti et al., 2010). This impairment has been interpreted to result from the “overdosing” effects of these drugs on a relatively intact mesocorticolimbic pathway (Gotham et al., 1986; Cools et al., 2002, 2006; Aarts et al., 2012). The FRN has been shown to be abnormal in conditions, such as pathological gambling (Oberg et al., 2011) and impulsivity (Onoda et al., 2010). Future studies should investigate the FRN in PD patients with ICDs.
In conclusion, in the present study, we demonstrate impaired incentive processing in cognitively preserved apathetic PD patients in the early and middle stages of the disease. Amplitude of the FRN, a neurophysiological correlate of valence outcome, was found to be decreased in this sample. These findings strongly support the compromise of the mesocorticolimbic dopaminergic pathway in the pathogenesis of apathy in PD.
Footnotes
This work was supported in part by Fundació La Marató de TV3 (060310) and CIBERNED (Fundación CIEN, Instituto de Salud Carlos III, Spain) public research grants.
The authors declare no competing financial interests.
References
- Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Litvan I, Larsen JP. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2001;13:42–49. doi: 10.1176/appi.neuropsych.13.1.42. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Brønnick K, Alves G, Tysnes OB, Pedersen KF, Ehrt U, Larsen JP. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009;80:928–930. doi: 10.1136/jnnp.2008.166959. [DOI] [PubMed] [Google Scholar]
- Aarts E, Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Cools R. Aberrant reward processing in Parkinson's disease is associated with dopamine cell loss. Neuroimage. 2012;59:3339–3346. doi: 10.1016/j.neuroimage.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Adam R, Leff A, Sinha N, Turner C, Bays P, Draganski B, Husain M. Dopamine reverses reward insensitivity in apathy following globus pallidus lesions. Cortex. 2013;49:1292–1303. doi: 10.1016/j.cortex.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Belouchrani A, Cardoso JF, Moulines E. A blind source separation technique using second-order statistics. IEEE Trans Signal Process. 1997;45:434–444. doi: 10.1109/78.554307. [DOI] [Google Scholar]
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, Foote K, Okun M. Startling facts about emotion in Parkinson's disease: blunted reactivity to aversive stimuli. Brain. 2006;129:3356–3365. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. J Neurosci. 2007;27:371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Sockeel P, Devos D, Delliaux M, Krystkowiak P, Destée A, Defebvre L. Characteristics of apathy in Parkinson's disease. Mov Disord. 2007;22:778–784. doi: 10.1002/mds.21316. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Sockeel P, Delliaux M, Destée A, Defebvre L. Apathy may herald cognitive decline and dementia in Parkinson's disease. Mov Disord. 2009;24:2391–2397. doi: 10.1002/mds.22843. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Levodopa treatment may benefit or impair “frontal” function in Parkinson's disease. Lancet. 1986;2:970–971. doi: 10.1016/S0140-6736(86)90617-3. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Corey-Bloom J, Gamst A, Paulsen JS, Jerkins S, Jacobson MW, Peavy G. Behavioural abnormalities contribute to functional decline in Huntington's disease. J Neurol Neurosurg Psychiatry. 2003;74:120–122. doi: 10.1136/jnnp.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002;296:1610–1611. doi: 10.1126/science.296.5573.1610. [DOI] [PubMed] [Google Scholar]
- Isella V, Melzi P, Grimaldi M, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I. Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson's disease. Mov Disord. 2002;17:366–371. doi: 10.1002/mds.10041. [DOI] [PubMed] [Google Scholar]
- Künig G, Leenders KL, Martin-Sölch C, Missimer J, Magyar S, Schultz W. Reduced reward processing in the brains of Parkinsonian patients. Neuroreport. 2000;11:3681–3687. doi: 10.1097/00001756-200011270-00019. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Apathy blunts neural response to money in Parkinson's disease. Soc Neurosci. 2011;6:653–662. doi: 10.1080/17470919.2011.556821. [DOI] [PubMed] [Google Scholar]
- Levy R. Apathy: a pathology of goal-directed behavior. A new concept of the clinic and pathophysiology of apathy. Rev Neurol (Paris) 2012;168:585–597. doi: 10.1016/j.neurol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Llebaria G, Pagonabarraga J, Kulisevsky J, García-Sánchez C, Pascual-Sedano B, Gironell A, Martínez-Corral M. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson's disease. Mov Disord. 2008;23:1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, García R, Andrés-Pueyo A, Münte TF, Rodríguez-Fornells A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Martínez-Corral M, Pagonabarraga J, Llebaria G, Pascual-Sedano B, García-Sánchez C, Gironell A, Kulisevsky J. Facial emotion recognition impairment in patients with Parkinson's disease and isolated apathy. Parkinsons Dis. 2010;2010:930627. doi: 10.4061/2010/930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb Cortex. 2004;14:741–747. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci. 2005;21:3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Oberg SA, Christie GJ, Tata MS. Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia. 2011;49:3768–3775. doi: 10.1016/j.neuropsychologia.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Onoda K, Abe S, Yamaguchi S. Feedback-related negativity is correlated with unplanned impulsivity. Neuroreport. 2010;21:736–739. doi: 10.1097/WNR.0b013e32833bfd36. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Alves G, Aarsland D. Prevalence and clinical correlates of apathy in Parkinson's disease: a community-based study. Parkinsonism Relat Disord. 2009;15:295–299. doi: 10.1016/j.parkreldis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Pluck GC, Brown RG. Apathy in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson's disease. Mov Disord. 2010;25:1432–1436. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- Riba J, Krämer UM, Heldmann M, Richter S, Münte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PloS One. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Mañanas MA, Barbanoj MJ. A comparative study of automatic techniques for ocular artifact reduction in spontaneous EEG signals based on clinical target variables: a simulation case. Comput Biol Med. 2008;38:348–360. doi: 10.1016/j.compbiomed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM. Parkinson's disease and dopaminergic therapy: differential effects on movement, reward and cognition. Brain. 2008;131:2094–2105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, d'Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schüpbach M, Hartmann A, Lévy R, Dubois B, Pessiglione M. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Manohar S, Husain M. Impulsivity and apathy in Parkinson's disease. J Neuropsychol. 2013;7:255–283. doi: 10.1111/jnp.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Merello M, Jorge R, Brockman S, Bruce D, Power B. The syndromal validity and nosological position of apathy in Parkinson's disease. Mov Disord. 2009;24:1211–1216. doi: 10.1002/mds.22577. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- van der Vegt JP, Hulme OJ, Zittel S, Madsen KH, Weiss MM, Buhmann C, Bloem BR, Münchau A, Siebner HR. Attenuated neural response to gamble outcomes in drug-naive patients with Parkinson's disease. Brain. 2013;136:1192–1203. doi: 10.1093/brain/awt027. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]