Abstract
Maladaptive memories elicited by exposure to environmental stimuli associated with drugs of abuse are often responsible for relapse among addicts. Interference with the reconsolidation of drug memory can inhibit drug seeking. Previous studies have indicated that the dephosphorylation of the eukaryotic initiation factor 2 α-subunit (eIF2α) plays an important role in synaptic plasticity and long-term memory consolidation, but its role in the reconsolidation of drug memory remains unknown. The amygdala is required for the reconsolidation of a destabilized drug memory after retrieval of drug-paired stimuli. Here, we used conditioned place preference (CPP) and self-administration procedures to determine whether amygdala eIF2α dephosphorylation is required for the reconsolidation of morphine and cocaine memories in rats. We found that the levels of eIF2α phosphorylation (Ser51) and activating transcription factor 4 (ATF4) were decreased after reexposure to a previously morphine- or cocaine-paired context (i.e., a memory retrieval procedure) in the basolateral amygdala (BLA) but not in the central amygdala. Intra-BLA infusions of Sal003, a selective inhibitor of eIF2α dephosphorylation, immediately after memory retrieval disrupted the reconsolidation of morphine- or cocaine-induced CPP, leading to a long-lasting suppression of drug-paired stimulus-induced craving. Advanced knockdown of ATF4 expression in the BLA by lentivirus-mediated short-hairpin RNA blocked the disruption of the reconsolidation of morphine-induced CPP induced by Sal003 treatment. Furthermore, inhibition of eIF2α dephosphorylation in the BLA immediately after light/tone stimulus retrieval decreased subsequent cue-induced heroin-seeking behavior in the self-administration procedure. These results demonstrate that eIF2α dephosphorylation in the BLA mediates the memory reconsolidation of drug-paired stimuli.
Introduction
Addiction is characterized by compulsive drug use despite adverse consequences. Relapse is a major challenge to the treatment of drug addiction, even after prolonged drug-free periods (Grant et al., 1996; Childress et al., 1999). Environmental conditioned cues that are repeatedly associated with drugs are primary contributors to relapse (Stewart et al., 1984; O'Brien et al., 1992). The memory of these cues that maintain powerful conditioned reinforcing properties through predictive association with the drug's rewarding and aversive effects (Gawin, 1991; Di Ciano and Everitt, 2004) is highly resistant to disruption in abstinent addicts (Gawin, 1991; Ehrman et al., 1992) and in experimental animals (de Wit and Stewart, 1981; Fuchs et al., 1998). The disruption of drug memory has become a potential strategy for relapse prevention (Milton and Everitt, 2010).
Reconsolidation is characterized as a de novo protein-synthesis-dependent and time-dependent process in which consolidated memory returns to a labile state shortly after retrieval (Nader et al., 2000; Dudai, 2006; Tronson et al., 2006). Disruption of reconsolidation by pharmacological interventions produces amnesia for drug-associated memories in animals (Wang et al., 2008; Sanchez et al., 2010; Barak et al., 2013; Wells et al., 2013), indicating that targeting the reconsolidation process of drug memory has therapeutic potential for the treatment of addiction. Although the regulation of mRNA translation in the initiation process has been reported to interfere with memory reconsolidation (Blundell et al., 2008; Hoeffer et al., 2011; Stoica et al., 2011), little is currently known about the specific mechanisms that underlie the reconsolidation of drug-paired stimuli.
Eukaryotic initiation factor 2 α-subunit (eIF2α) plays a critical role in the regulation of protein synthesis (Klann and Dever, 2004; Costa-Mattioli et al., 2009a; Costa-Mattioli et al., 2009b; Gkogkas et al., 2010; Trinh and Klann, 2013). eIF2α phosphorylation at residue Ser51 prevents the formation of the eIF2-GTP-Met-tRNAiMet ternary complex, resulting in the repression of general translation, but selectively triggering the translation of activating transcription factor 4 (ATF4), a repressor of CRE-mediated gene transcription (Ameri and Harris, 2008; Jackson et al., 2010). eIF2α dephosphorylation is involved in synaptic plasticity and the formation of long-term memory (LTM; (Costa-Mattioli and Sonenberg, 2008; Costa-Mattioli et al., 2009a). eIF2α dephosphorylation in eIF2α+/S51A knock-in mice has been shown to decrease the threshold of late-phase long-term potentiation (L-LTP) and enhance the consolidation of LTM. In contrast, preventing eIF2α dephosphorylation by Sal003, an eIF2α phosphatase inhibitor, impaired the formation of both L-LTP and LTM (Costa-Mattioli et al., 2007). However, the role of eIF2α dephosphorylation in the reconsolidation of drug memories remains unclear.
The amygdala is a critical brain region for the convergence of conditioned and unconditioned stimuli in associative learning (Johansen et al., 2011) and is necessary for regulating the reconsolidation of drug memory (Li et al., 2010; Sanchez et al., 2010; Luo et al., 2013). In the present study, we used Pavlovian conditioned place preference (CPP) and self-administration procedures in animals to determine whether eIF2α dephosphorylation in the amygdala is required for the reconsolidation of drug memory.
Materials and Methods
Subjects.
Male Sprague Dawley rats (weighing 220–240 g upon arrival) were obtained from the Laboratory Animal Center, Peking University, Health Science Center. They were housed five per cage in a temperature (23 ± 2°C)- and humidity (50 ± 5%)-controlled animal facility with ad libitum access to food and water. They were maintained on a reverse 12 h/12 h light/dark cycle (lights off at 8:00 A.M.). All of the experimental procedures were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and the procedures were approved by the Biomedical Ethics Committee for animal use and protection of Peking University.
Intracranial and intravenous surgery.
Sodium pentobarbital (60 mg/kg, i.p.) was used to anesthetize the rats (weighing 280–320 g at the time of surgery) before they were bilaterally implanted with permanent guide cannulae (23 gauge; Plastics One) 1 mm above the basolateral amygdala (BLA) or central amygdala (CeA). The coordinates for the BLA were the following: anterior/posterior, −2.9 mm; medial/ lateral, ±5.0 mm from bregma; dorsal/ventral, −8.5 mm from the skull surface; the coordinates for the CeA were the following: anterior/posterior, −2.9 mm; medial/lateral, ±4.2 mm from bregma; dorsal/ventral, −7.8 mm from the skull surface. These coordinates were based on previous work (Lu et al., 2005; Paxinos et al., 2005; Lu et al., 2007; Wang et al., 2008; Li et al., 2010). The cannula was anchored to the skull with stainless-steel screws and dental cement was applied to secure the cannula in position. A stainless-steel stylet blocker was inserted into each cannula to prevent blockage and infection.
For intravenous surgery, catheters were inserted into the right jugular vein with the tip terminating at the opening of the right atrium, as described previously (Wang et al., 2010; Xue et al., 2012). After surgery, the rats were housed individually with ad libitum access to food and water and allowed to recover for 5–7 d before training began.
CPP.
CPP was assessed using an unbiased procedure, as described in our previous work (Li et al., 2008; Li et al., 2010). In the baseline preference test, the rats were individually placed in the middle chamber with the doors removed for 15 min. Rats that showed strong unconditioned preference for any chamber (i.e., >540 s spent in each chamber) were excluded from the experiments. The conditioning sessions were then conducted once per day for 8 consecutive days. On alternating days, the rats were given injections of morphine (10 mg/kg, s.c.) or saline (1 ml/kg, s.c.) immediately before being confined to the morphine-paired or saline-paired chamber for 45 min, and were then returned to their home cages. The postconditioning session, which was conducted 24 h after the last training session, was identical to the initial baseline preference assessment. The CPP score was defined as the time spent (in seconds) in the morphine-paired chamber minus the time spent in the saline-paired chamber during the CPP test. The training and testing procedures for cocaine-induced CPP were identical, with the exception that cocaine (10 mg/kg, i.p.) was injected instead of morphine. Twenty-four hours after the CPP test, the rats were reexposed to the drug-paired chamber for 10 min without drug injections, serving as a memory retrieval trial, and then received the different experimental treatments.
Intravenous heroin self-administration.
The procedures for heroin self-administration training were based on our previous studies (Lu et al., 2005; Xue et al., 2012). The rats were trained to self-administer heroin hydrochloride (0.05 mg/kg/infusion) during three 1 h sessions daily separated by 5 min over 10 d. A fixed-ratio 1 schedule of reinforcement was used, with a 40 s timeout period after each infusion. Each session began with illumination of a house light that remained on for the entire session. The number of heroin infusions was limited to 20 per hour. At the end of the training phase, the groups in the different experimental conditions were matched for their heroin intake during training. The session that assessed the retrieval of heroin cue memories was conducted 24 h after the last heroin self-administration training session. The rats underwent the retrieval session for 15 min, during which time nose poke responses led to a 5 s tone-light cue without heroin delivery. The drug-seeking test conditions were the same as those during training, with the exception that active nose pokes were not reinforced by heroin. The duration of the test was 1 h.
Drugs and injection procedures.
Morphine hydrochloride, cocaine hydrochloride, and heroin hydrochloride were purchased from Qinghai Pharmaceutical and dissolved in 0.9% physiological saline. Sal003 (Tocris Bioscience) was dissolved in dimethylsulfoxide (DMSO) and further diluted in 0.9% NaCl (saline) to a final DMSO concentration of 0.1%. For intracerebral microinfusions, the rats were gently held and the stylets were removed from the guide cannulae. Sal003 (0.5 μl) or vehicle (0.1% DMSO in saline) was infused bilaterally in the BLA or CeA using Hamilton syringes connected to 30 gauge injectors (Plastics One) that were lowered 1 mm below the guide cannula. The drug was injected bilaterally over 1 min. The injection needle was kept in place for an additional minute to allow diffusion of the drug into the tissue. The injectors were then removed and the stylets were replaced (Lu et al., 2005).
Lentiviral vector and virus injection.
ATF4 knockdown in the BLA was established using a lentiviral-vector-based shRNA system according to the manufacturer's instructions (catalog #LPP-RSH050838-3-LVRH1GP-0100; GeneCopoeia). For the detection of ATF4, the hairpin loop sequence was TCAAGAG. The targeted sequence was 5′-ACCATGCCAGATGAGCTTT-3′. The sequences for the primers were the following: 5′-TAATACGACTCACTATAGGG-3′ (forward); 5′-CTGGAATAGCTCAGAGGC-3′ (reverse). For lentiviral-mediated gene transfer, these sequences were cloned into the hairpin structures under the control of the H1 promoter in the psiHIV-H1 vector (Siouda et al., 2012).
The rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). Using Hamilton syringes connected to 30 gauge injectors (Plastics One) that reached 1 mm below the guide cannula, 0.5 μl of virus (7.84 × 109 TU/ml) was injected at a constant rate over a 5 min period and then allowed to diffuse for 5 min. The needle was then raised slowly and the stylets were replaced.
Histology.
The animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.01 m PBS, followed by 4% paraformaldehyde in 0.2 m PB. The brain was extracted, postfixed overnight at 4°C, and cryoprotected in 30% sucrose in 0.2 m PB.
The cannula placements were confirmed in 40-μm-thick sections using Nissl staining under light microscopy. Rats with misplaced cannulae were excluded from the statistical analysis. The locations of the cannula tips are shown in Figure 1. For enhanced green fluorescent protein expression in lentiviral-vector-injected animals, consecutive 10 μm coronal brain sections were examined using an Olympus BX53 fluorescence microscope (Xue et al., 2014).
Figure 1.
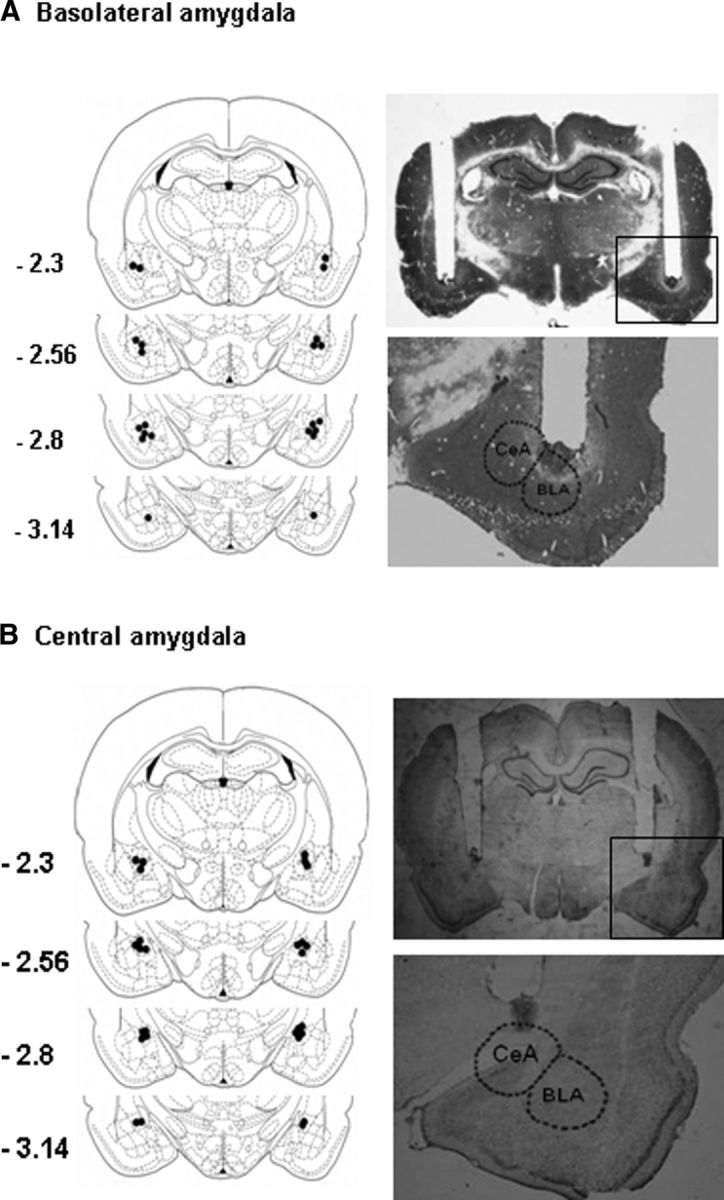
Schematic representation and photomicrographs of injection sites and potential damage in the basolateral amygdala (A) and central amygdala (B). The schematic representation (left column) begins with the most anterior injection site associated with the amygdala, showing that most of the needle tracks terminated within the amygdala and tissue damage was generally confined to 0.1 mm around the needle tracks. The photomicrographs (right column) show no significant tissue damage (characterized by gliosis and cell-poor areas) around the needle tracks.
Tissue sample preparation.
The procedure was based on our previous study (Lu et al., 2005; Li et al., 2010; He et al., 2011; Xue et al., 2012). The brains were harvested by rapid decapitation without anesthesia after the tests. After decapitation, the brains were rapidly extracted, frozen in −60°C N-hexane and transferred to a −80°C freezer. Bilateral tissue punches (16 gauge) of the CeA and BLA were taken using a freezing cryostat (−20°C) from 1-mm-thick coronal sections located ∼2.5–3.0 mm from bregma. The tissue punches were homogenized 3× for 10–15 s with 5 s intervals using an electrical disperser (Wiggenhauser) after 30 min in RIPA lysis buffer protease and phosphatase inhibitors (Beyotime Biotechnology; 20 mm Tris, pH 7.5, 150 mm NaCl, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm ethylenediamine tetraacetic acid, 1% Na3VO4, 0.5 μg/ml leupeptin, and 1 mm phenylmethanesulfonyl fluoride). The tissue homogenates were then subjected to 12,000 × g centrifugation for 8 min at 4°C to remove any insoluble materials, including nuclei and large debris, and the supernatant containing cytosolic proteins and light membrane fraction was collected based on previous studies (Jiang et al., 2010; Devi and Ohno, 2013). All of the procedures were performed at 0–4°C. The protein concentrations of all of the samples were determined using the bicinchoninic acid assay (Beyotime Biotechnology). The samples were further diluted in RIPA lysis buffer to equalize the protein concentrations. Aged-matched naive rats were subjected to daily handling during the behavioral experiments. The brains of these naive rats were taken to assess basal protein levels, which were used as a reference for the graphical presentation of the molecular data (see figures; Li et al., 2008; Li et al., 2010).
Western blot assays.
The Western blot procedures were based on our previous studies (Xue et al., 2012; Ren et al., 2013). Four-times loading buffer (16% glycerol, 20% mercaptoethanol, 2% SDS, and 0.05% bromophenol blue) was added to each sample (3:1; sample:loading buffer) before being boiled for 3 min. The samples were cooled and loaded to SDS-PAGE (10% acrylamide/0.27% N,N′-methylenebisacryalamide resolving gel) for ∼40 min at 80 V in stacking gel and for ∼1 h at 120 V in resolving gel. Proteins were electrophoretically transferred to Immobilon-P transfer membranes (Millipore) at 0.25 A for 2.5 h. The blots were blocked for 2 h with blocking buffer (5% BSA in TBST) at room temperature. They were then incubated overnight at 4°C with the following primary antibodies: total eIF2α (1:1000; Cell Signaling Technology), p-eIF2α (Ser51; 1:1000; Cell Signaling Technology), β-actin (1:1000; Cell Signaling Technology), ATF4 (1:200; Santa Cruz Biotechnology), Fos (1:1000; Santa Cruz Biotechnology), and Zif268 (1:1000; Santa Cruz Biotechnology) in TBST plus 5% BSA. After 3 5 min washes in TBST buffer, the blots were incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG; 1:5000; Santa Cruz Biotechnology) for 45 min at room temperature on a shaker. The blots then underwent 5 5 min washes with TBST and the immunostaining was visualized using the EZ-ECL chemiluminescence detection kit. The immunoblots were quantified with the Gel Doct EZ system (Bio-Rad). The values for total eIF2α, p-eIF2α, ATF4, Fos, and Zif268 protein levels were normalized to β-actin and analyzed with Quantity One Version 4.4.0 software (Bio-Rad).
Statistical analysis.
The results are expressed as mean ± SEM. ANOVA was used with appropriate between- and within-subjects factors for the different experiments (see Results). Significant main effects and interactions (p < 0.05) in the factorial ANOVAs were further analyzed using Tukey's post hoc test as appropriate. We only report significant effects that are critical for the interpretation of the data in the Results.
Results
Effect of retrieval of drug-induced CPP on eIF2α phosphorylation in the BLA and CeA
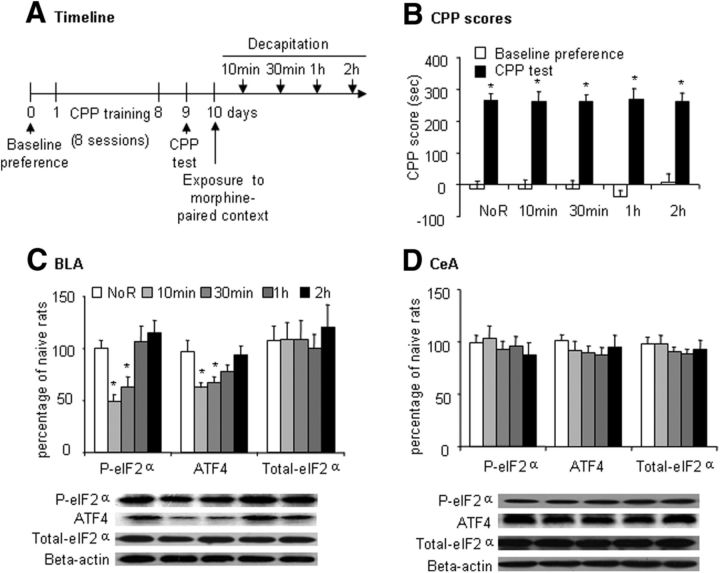
In the first experiment, different groups of rats (n = 6–8 per group) were used to assess the effect of retrieval of morphine-induced CPP on eIF2α phosphorylation (Ser51) and ATF4 levels in the BLA and CeA. Rats underwent CPP training for 8 d and the CPP test on day 9. On day 10, 4 groups of rats underwent a memory retrieval trial, during which they were exposed to the morphine-paired chamber for 10 min without a morphine injection and then decapitated 10 min, 30 min, 1 h, and 2 h after the retrieval trial. In the no-retrieval group, the rats remained in their home cages without undergoing a memory retrieval trial and then were decapitated. The rats in the naive group that was subjected only to daily handling in the home cage were decapitated at the same time as the no retrieval group. Their brains were removed for the subsequent determination of eIF2α phosphorylation (Ser51) and ATF4 levels (Fig. 2A). The repeated-measures ANOVA of the behavioral data revealed a significant main effect of test (F(1,35) = 269.19, p < 0.01; Fig. 2B) but no significant effect of group (F(4,35) = 0.13, p > 0.1; Fig. 2B). The p-elF2α level was decreased 10 and 30 min after the retrieval trial and returned to baseline level 2 h after retrieval in the BLA (one-way ANOVA, F(4,29) = 7.15, p < 0.05; Fig. 2C) but not CeA (Fig. 2D). The phosphorylation of eIF2α inhibits general mRNA translation but selectively stimulates the translation of ATF4 mRNA (Costa-Mattioli et al., 2007). We observed the same alteration in ATF4 expression as in eIF2α dephosphorylation in the BLA (one-way ANOVA, F(4,29) = 4.16, p < 0.05; Fig. 2C) but not CeA (Fig. 2D).
Figure 2.
Retrieval of morphine memory induced dephosphorylation of eIF2α (Ser51) in the BLA but not CeA. A, Timeline of the experiment. B, CPP scores (mean ± SEM) in rats during the baseline preference and CPP test phases. Rats in the morphine groups acquired significant place preference. The data are shown as preference scores (in seconds) during the CPP preference tests (n = 8 per group). *p < 0.05 compared with baseline preference in morphine group. C, D, Phosphorylated eIF2α, ATF4, and total eIF2α protein levels and representative Western blots in the BLA and CeA after exposure to morphine-paired context. The data are expressed as a percentage of phosphorylated eIF2α, ATF4, and total eIF2α in naive control rats (n = 6 per group). In the BLA but not CeA, exposure to the morphine-paired context decreased phosphorylated eIF2α and ATF4 in the morphine group, which time-dependently recovered. *p < 0.05 compared with the no-retrieval (NoR) group.
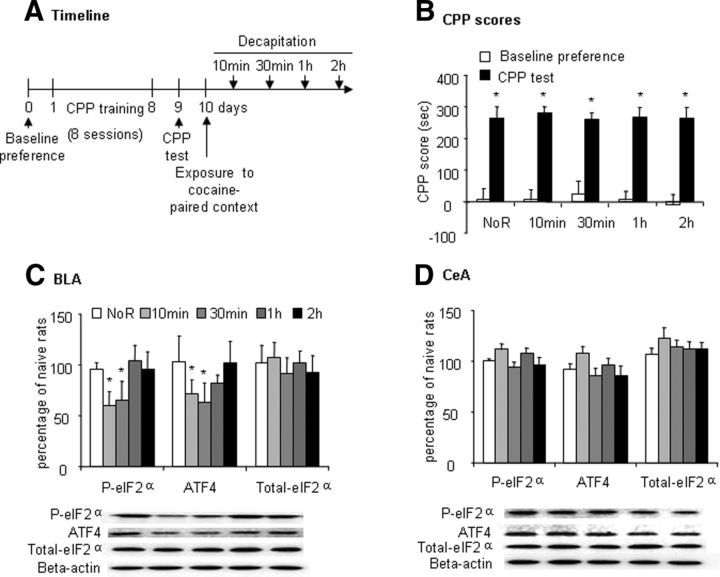
We also used an identical protocol to determine the effect of retrieval of cocaine-induced CPP on eIF2α phosphorylation (Ser51) in the amygdala (n = 6–9 per group; Fig. 3A). Cocaine CPP data revealed a significant main effect of test (F(1,39) = 159.12, p < 0.01; Fig. 3B) but no significant effect of group (F(4,39) = 0.08, p > 0.05; Fig. 3B). The p-eIF2α level (one-way ANOVA, F(4,29) = 10.97, p < 0.05; Fig. 3C) and ATF4 expression (one-way ANOVA, F(4,29) = 5.44, p < 0.05; Fig. 3C) were decreased 10 and 30 min after the retrieval trial and returned to baseline levels 2 h after the retrieval trial in the BLA but not CeA (Fig. 3D).
Figure 3.
Retrieval of cocaine memory induced dephosphorylation of eIF2α (Ser51) in the BLA but not CeA. A, Timeline of the experiment. B, CPP scores (mean ± SEM) in rats during the baseline preference and CPP test phases. Rats in the cocaine groups acquired significant place preference. The data are shown as preference scores (in seconds) during the CPP preference tests (n = 8–9 per group). *p < 0.05 compared with baseline preference in cocaine group. C, D, Phosphorylated eIF2α, ATF4, and total eIF2α protein levels and representative Western blots in the BLA and CeA after exposure to cocaine-paired context. The data are expressed as a percentage of phosphorylated eIF2α, ATF4, and total eIF2α in naive control rats (n = 6 per group). In the BLA but not CeA, exposure to the cocaine-paired context decreased phosphorylated eIF2α and ATF4 in the cocaine group, which time-dependently recovered. *p < 0.05 compared with no-retrieval (NoR) group.
These results show that the retrieval of drug-memory-induced eIF2α dephosphorylation and decreased ATF4 levels in the BLA but not CeA.
Effect of preventing eIF2α dephosphorylation by Sal003 in the BLA or CeA on the reconsolidation of morphine reward memory
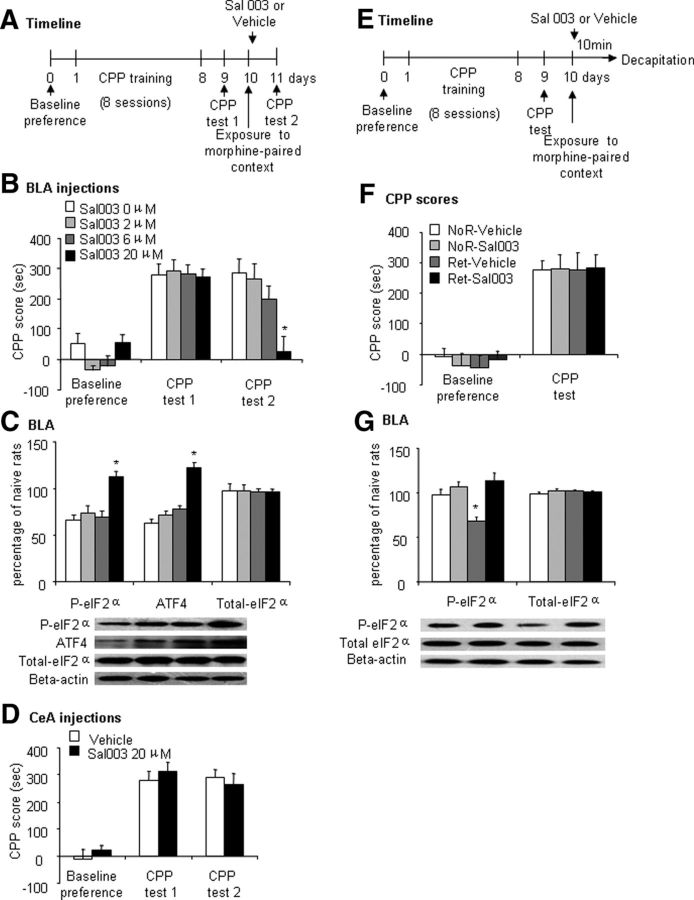
After the observation that the retrieval of morphine reward memory induced eIF2α dephosphorylation in the BLA, we then determined the effect of local infusion of Sal003, a selective inhibitor of eIF2α dephosphorylation, in the BLA and CeA immediately after retrieval on the reconsolidation of morphine reward memory. Rats were trained for morphine-induced CPP. On day 10, 4 groups of rats (n = 6–10 per group) were given different doses of Sal003 (0, 2, 6, and 20 μm/side) bilaterally in the BLA immediately after the memory retrieval trial. Twenty-four hours later, the rats underwent CPP Test 2 and were decapitated 10 min later to determine eIF2α phosphorylation and ATF4 levels in the BLA (Fig. 4A). The repeated-measures ANOVA of CPP scores revealed significant effects of Sal003 dose (F(3,30) = 3.68, p < 0.05; Fig. 4B) and test phase (F(2,60) = 59.52, p < 0.01; Fig. 4B) and a significant Sal003 dose × test phase interaction (F(6,60) = 5.24, p < 0.01; Fig. 4B). The post hoc analysis showed that all of the groups acquired CPP after morphine-induced CPP training (p < 0.01), with no significant differences in CPP scores among groups during CPP Test 1 (p > 0.05). Compared with the group of rats that received Sal003 (0 μm/side), CPP scores significantly decreased in the group of rats that received Sal003 (20 μm/side) in CPP Test 2 (p < 0.01). We also found that p-eIF2α level (one-way ANOVA, F(3,23) = 9.99, p < 0.01; Fig. 4C) and ATF4 expression (one-way ANOVA, F(3,23) = 31.83, p < 0.01; Fig. 4C) significantly increased in the group of rats treated with Sal003 (20 μm/side). Furthermore, we used 2 additional groups of rats (n = 7–8 per group) to determine the effect of intra-CeA infusion of Sal003 on the reconsolidation of morphine reward memory (Fig. 4A). We found that local microinjection of Sal003 (20 μm/side) in the CeA after morphine memory retrieval had no effect on the reconsolidation of morphine reward memory (Fig. 4D). The repeated-measures ANOVA revealed a significant main effect of test phase (F(2,26) = 54.60, p < 0.01) but no main effect of Sal003 dose (F(1,13) = 0.21, p > 0.1) and no test phase × Sal003 dose interaction (F(2,26) = 0.58, p > 0.1). These results revealed that microinjection of Sal003 in the BLA but not CeA immediately after the retrieval trial impaired the reconsolidation of morphine reward memory.
Figure 4.
Intra-BLA infusion of Sal003 prevented retrieval-related eIF2α dephosphorylation in the BLA and impaired the reconsolidation of morphine-induced CPP. The data are expressed as mean ± SEM. A, Timeline of the experiment. B, CPP scores during baseline preference and tests for the expression of morphine-induced CPP in rats injected with Sal003 (0, 2, 6, and 20 μm/side) in the BLA immediately after exposure to the morphine-paired context on day 10 (n = 8–10 per group). *p < 0.05 compared with CPP Test 1 in the Sal003 group (20 μm/side) and compared with Sal003 (0 μm/side) in CPP Test 2. C, Phosphorylated eIF2α, ATF4, and total eIF2α protein levels and representative Western blots in the BLA after CPP Test 2. The data are expressed as a percentage of phosphorylated eIF2α, ATF4, and total eIF2α in naive control rats (n = 6 per group). In the BLA, Sal003 (20 μm/side) increased phosphorylated eIF2α and ATF4 after CPP Test 2. *p < 0.05 compared with Sal003 (0 μm/side) group. D, CPP scores during baseline preference and tests for the expression of morphine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the CeA immediately after exposure to the morphine-paired context on day 10 (n = 7–8 per group). E, Timeline of the experiment. F, CPP scores during baseline preference and a test for the expression of morphine-induced CPP in rats decapitated 10 min after exposure to the morphine-paired context on day 10 (n = 5 per group). G, Western blot analysis of phosphorylated and total eIF2α in the BLA in the no-retrieval (NoR)-Vehicle, NoR-Sal003, retrieval (Ret)-Vehicle, and Ret-Sal003 groups. The phosphorylation level of eIF2α decreased in the Ret-Vehicle group (n = 5 per group). *p < 0.05 compared with NoR-Vehicle group.
Another 4 groups of rats (n = 5 per group) were used to determine the effect of Sal003 infusion in the BLA on retrieval-induced changes in eIF2α phosphorylation. The between-subjects factors were CPP memory retrieval session (present or absent) and Sal003 dose (0 or 20 μm/side). The rats that did not undergo the memory retrieval trial received Sal003 or vehicle in the BLA without context exposure at the same time as the rats that were infused with Sal003 or vehicle after a 10 min memory retrieval trial on day 10. All of the rats were decapitated 10 min after Sal003 or vehicle infusions and their brains were extracted to determine eIF2α phosphorylation in the BLA (Fig. 4E). The repeated-measures ANOVA revealed a significant main effect of test (F(1,16) = 83.68, p < 0.01; Fig. 4F) but no significant effect of group (F(3,16) = 0.09, p > 0.1; Fig. 4F), indicating that all of the groups acquired equivalent morphine-induced CPP. The one-way ANOVA of eIF2α phosphorylation revealed a main effect of group (F(3,19) = 11.15, p < 0.01; Fig. 4G). The post hoc analysis showed that eIF2α phosphorylation level in the group of rats that received an intra-BLA infusion of Sal003 (20 μm/side) immediately after retrieval was significantly higher than the group of rats that received an intra-BLA infusion of vehicle immediately after retrieval (p < 0.05) but did not differ from the group that received a vehicle injection without retrieval (p > 0.1). No difference was observed in the total protein levels of eIF2α (one-way ANOVA, F(3,19) = 0.59, p > 0.1; Fig. 4G).
Therefore, preventing retrieval-induced eIF2α dephosphorylation in the BLA but not CeA immediately after the retrieval trial impaired the reconsolidation of morphine reward memory.
Effect of preventing eIF2α dephosphorylation by Sal003 in the BLA without exposure or 6 h after exposure to a morphine-paired context on the expression of morphine reward memory
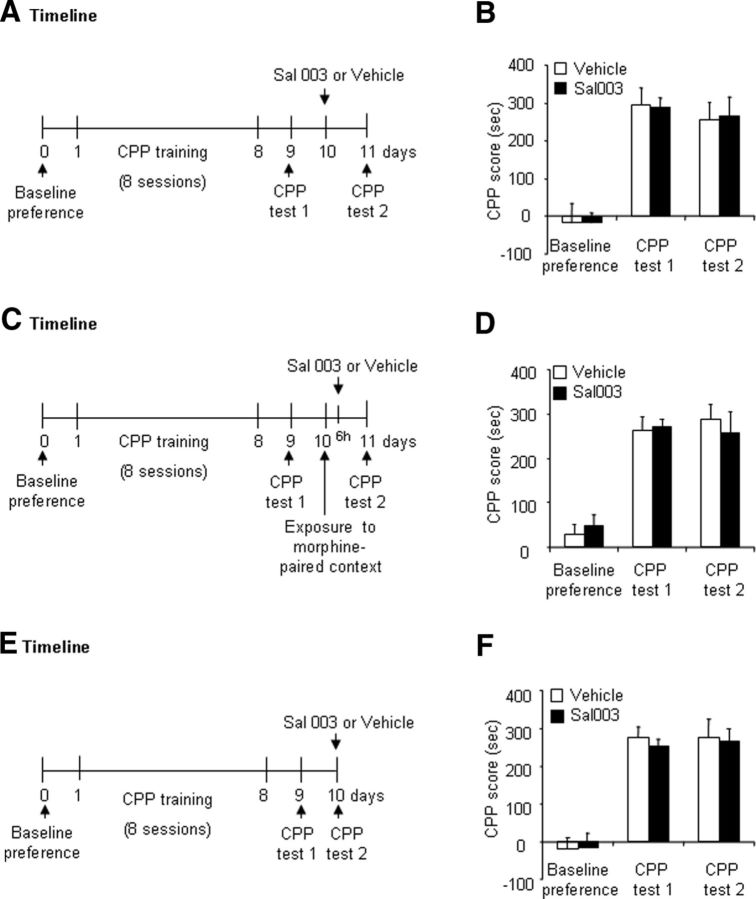
We further determined whether the effect of Sal003 on the reconsolidation of morphine reward memory is retrieval dependent and temporally specific and whether Sal003 has an effect on retrieval itself. Two groups of rats (n = 7 per group) were trained for morphine-induced CPP and then received a microinjection of vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA without undergoing a morphine memory retrieval trial (Fig. 5A). The repeated-measures ANOVA of CPP scores revealed a significant main effect of test phase (F(2,24) = 28.58, p < 0.01; Fig. 5B) but no main effect of Sal003 dose (F(1,12) = 0.00, p > 0.1; Fig. 5B) and no significant test phase × Sal003 dose interaction (F(2,24) = 0.02, p > 0.1; Fig. 5B). We found that preventing eIF2α dephosphorylation by Sal003 in the BLA had no effect on the subsequent expression of morphine-induced CPP in the absence of morphine-paired context reexposure.
Figure 5.
Preventing eIF2α dephosphorylation by Sal003 in the BLA without exposure or 6 h after exposure to a morphine-paired context had no effect on the expression of morphine reward memory. A, Timeline of the experiment. B, CPP scores (mean ± SEM) during baseline preference and tests for the expression of morphine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA on day 10 without exposure to the morphine-paired context (n = 7 per group). C, Timeline of the experiment. D, CPP scores (mean ± SEM) during baseline preference and tests for the expression of morphine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA 6 h after exposure to the morphine-paired context on day 10 (n = 7 per group). E, Timeline of the experiment. F, CPP scores (mean ± SEM) during baseline preference and tests for the expression of morphine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA immediately before CPP Test 2 (n = 7–8 per group).
We then determined the effect of intra-BLA injection of Sal003 (20 μm/side) 6 h after the morphine memory retrieval trial on the expression of morphine reward memory (n = 7 per group; Fig. 5C). The repeated-measures ANOVA of CPP scores revealed a significant main effect of test phase (F(2,24) = 52.73, p < 0.01; Fig. 5D) but no main effect of Sal003 dose (F(1,12) = 0.00, p > 0.1; Fig. 5D) and no test phase × Sal003 dose interaction (F(2,24) = 0.51, p > 0.1; Fig. 5D). These results indicate that the effect of preventing eIF2α dephosphorylation by Sal003 in the BLA on the reconsolidation of morphine reward memory was temporally specific.
Finally, we used 2 groups of rats (n = 7–8 per group) that received a microinjection of vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA 5 min before CPP Test 2 to determine whether preventing eIF2α dephosphorylation by Sal003 affects memory retrieval (Fig. 5E). The repeated-measures ANOVA of CPP scores with test phase (baseline preference, CPP Test 1, and CPP Test 2) as the within-subjects factor and Sal003 dose (0 and 20 μm/side) as the between-subjects factor revealed a significant main effect of test phase (F(2,26) = 29.85, p < 0.01; Fig. 5F) but no main effect of Sal003 dose (F(1,13) = 0.99, p > 0.1; Fig. 5F) and no test phase × Sal003 dose interaction (F(2,26) = 0.48, p > 0.1; Fig. 5F), indicating that the prevention of eIF2α dephosphorylation by Sal003 had no effect on the retrieval of morphine reward memory.
Long-lasting effect of eIF2α preventing dephosphorylation by Sal003 in the BLA on the reconsolidation of drug reward memory
Previous studies have shown that the disruption of drug reward memory by interfering with the reconsolidation process produces enduring inhibition of the responses to conditioned cues that are not sensitive to spontaneous recovery and reinstatement induced by a drug-priming injection (Li et al., 2010; Wu et al., 2011). In the present study, we tested the long-lasting effect of preventing eIF2α dephosphorylation by microinjecting Sal003 in the BLA after morphine memory retrieval on the reconsolidation of morphine reward memory (n = 7–8 per group). The rats underwent the CPP Test 24 h (CPP Test 2) and 14 d (CPP Test 3) after Sal003 treatment and then the morphine-priming-induced reinstatement test was conducted (priming test; Fig. 6A). The repeated-measures ANOVA of CPP scores revealed main effects of Sal003 dose (F(1,13) = 22.87, p < 0.01; Fig. 6B) and test phase (F(4,52) = 11.18, p < 0.01; Fig. 6B) and a significant Sal003 dose × test phase interaction (F(4,52) = 5.52, p < 0.01; Fig. 6B). The post hoc tests showed that CPP scores in the group of rats that received Sal003 (20 μm/side) were significantly different from the vehicle group in the CPP Test 3 (p < 0.05) and the priming test (p < 0.05).
Figure 6.
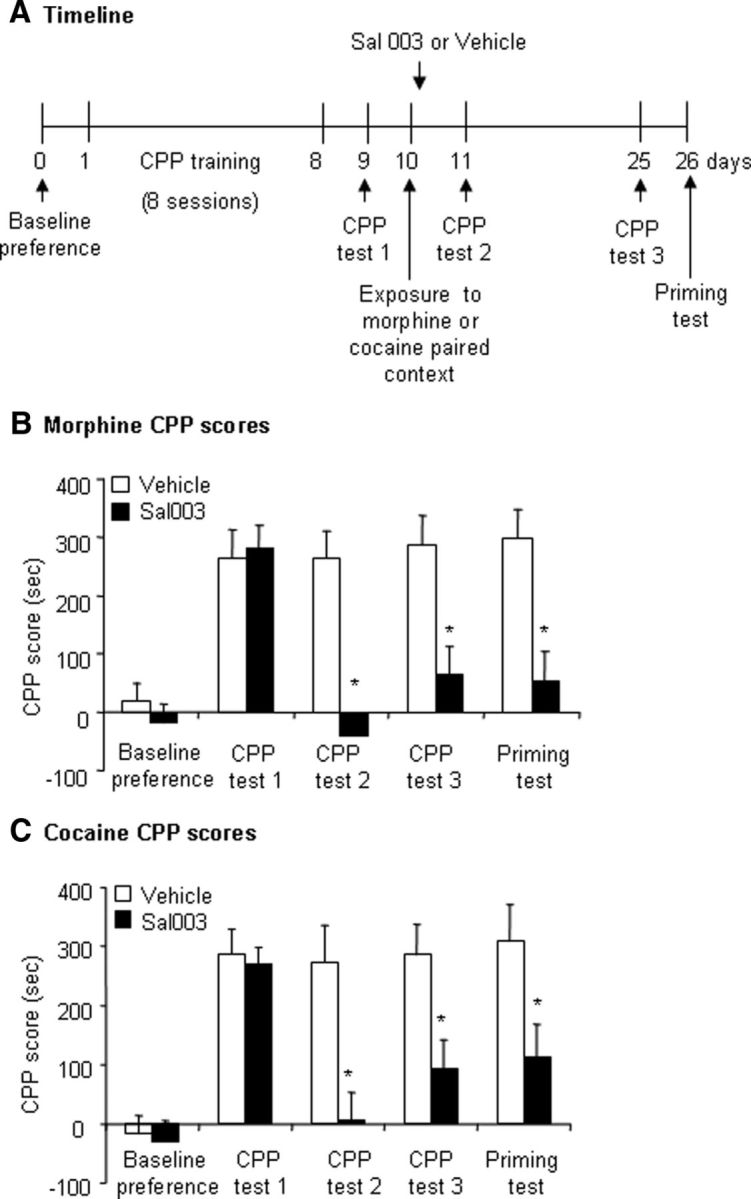
Preventing eIF2α dephosphorylation by Sal003 in the BLA immediately after morphine or cocaine memory retrieval caused long-lasting impairment of the expression of drug reward memory, which was not restored by acute priming injections. A, Timeline of the experiment. B, CPP scores (mean ± SEM) during baseline preference and tests for the expression of morphine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA immediately after exposure to the morphine-paired context on day 10. Five minutes before the last CPP test (termed the priming test in A), the rats were injected with morphine (5 mg/kg, s.c.; n = 7–8 per group). *p < 0.05 compared with Vehicle group. C, CPP scores (mean ± SEM) during baseline preference and tests for the expression of cocaine-induced CPP in rats injected with vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA immediately after exposure to the cocaine-paired context on day 10. Five minutes before the last CPP test, the rats were injected with cocaine (10 mg/kg, i.p.; n = 8–9 per group). *p < 0.05 compared with Vehicle group.
We also found the same long-lasting inhibitory effect of Sal003 on the reconsolidation of learned cocaine-induced CPP (n = 8–9 per group). The repeated-measures ANOVA of CPP scores revealed main effects of Sal003 dose (F(1,15) = 20.06, p < 0.01; Fig. 6C) and test phase (F(4,60) = 12.98, p < 0.01; Fig. 6C) and a significant Sal003 dose × test phase interaction (F(4,60) = 3.64, p < 0.05; Fig. 6C). The post hoc tests showed that CPP scores in the group of rats that received Sal003 (20 μm/side) were significantly different from the vehicle group in the CPP Test 3 (p < 0.05) and the priming test (p < 0.05). Altogether, these results indicate that the inhibitory effect of preventing eIF2α dephosphorylation by Sal003 in the BLA after the retrieval trial on the reconsolidation of drug reward memory lasted at least 2 weeks.
Effect of preventing eIF2α dephosphorylation by Sal003 in the BLA on the reconsolidation of morphine reward memory in ATF4 knockdown rats
Given that Sal003 failed to suppress L-LTP in slices from ATF4 knock-out mice compared with wild-type mice (Costa-Mattioli et al., 2007), we hypothesized that Sal003 disrupts the reconsolidation of drug memory based on the increased expression of ATF4. To test this hypothesis, we tested the effect of knockdown of ATF4 expression before Sal003 treatment on the reconsolidation of morphine reward memory. First, we examined the efficiency of ATF4 knockdown by lentivirus-mediated short-hairpin RNA (shRNA) in the BLA (Fig. 7A). The rats were decapitated for the Western blot assays 7 or 14 d after being treated with LV-shATF4 or LV-Scramble (n = 6 per group; Fig. 7B). Compared with the LV-Scramble groups, the ATF4 protein level significantly decreased 7 d (1-way ANOVA, F(1,11) = 56.02, p < 0.01) and 14 d (1-way ANOVA, F(1,11) = 80.64, p < 0.01) after LV-shRNA microinjection.
Figure 7.

Preventing eIF2α dephosphorylation by Sal003 did not impair the reconsolidation of morphine reward memory in ATF4 knockdown rats. The data are expressed as mean ± SEM. A, Lentiviral transfer vector-mediated knockdown of ATF4 in the BLA. The figure shows representative micrographs of enhanced green fluorescent protein (eGFP; green) after BLA microinjection. Lentiviral-vector-mediated eGFP expression within a defined boundary of the BLA (a: 4× magnification, scale bar, 200 μm; b: 40× magnification, scale bar, 20 μm) shows injection sites (black dots) within the BLA (c). B, Western blot data for ATF4 in BLA tissues collected from rats 7 or 14 d after bilateral intra-BLA infusion of 0.5 μl of LV-shATF4 or LV-Scramble (n = 6 per group). *p < 0.05 compared with LV-Scramble group. C, Timeline of the experiment. D, CPP scores in LV-shATF4- and LV-Scramble-injected rats during tests for the expression of morphine-induced CPP when injected with Sal003 (20 μm/side) or vehicle (0.5 μl/side) in the BLA immediately after exposure to the morphine-paired context on day 11. In the BLA, LV-shATF4 blocked the decrease in CPP scores induced by Sal003 injection after morphine memory retrieval (n = 7–8 per group). *p < 0.05 compared with LV-Scramble + Vehicle in CPP Test 2. E, Phosphorylated eIF2α, total eIF2α, ATF4, Zif268, and Fos protein levels and representative Western blots in the BLA after exposure to the morphine-paired context. The data are expressed as a percentage of molecular alterations in naive control rats (n = 6 per group). In the BLA, Sal003 (20 μm/side) prevented the decrease in phosphorylated eIF2α and ATF4 and prevented the increase in Zif268 and Fos protein levels after exposure to the morphine-paired context in rats injected with LV-Scramble. When ATF4 expression was knocked down in the BLA, Sal003 (20 μm/side) did not affect the increase in Zif268 or Fos after exposure to the morphine-paired context. *p < 0.05 compared with LV-Scramble + Vehicle group.
We then investigated whether the impairment of morphine reward memory by Sal003 can be blocked by prior knockdown of ATF4 in the BLA (Fig. 7C). We used a 2 (LV-shATF4 or LV-Scramble) × 2 (Sal003 or vehicle) factorial design. LV-shATF4 or LV-Scramble was injected in the BLA 24 h after the baseline preference test (n = 6–8 per group). The rats were trained for morphine-induced CPP for 8 d and tested for the expression of CPP on day 10 (CPP Test 1). On day 11, the rats received either vehicle (0.5 μl/side) or Sal003 (20 μm/side) in the BLA immediately after morphine-paired context exposure. Twenty-four hours later, the rats underwent CPP Test 2 (Fig. 7C). The two-way repeated-measures ANOVA of CPP scores with the within-subjects factor test phase (CPP Test 1 and CPP Test 2) and between-subjects factors lentivirus (scramble and ATF4 shRNA) and Sal003 dose (0 and 20 μm/side) revealed a significant test phase × lentivirus × Sal003 dose interaction (F(2,50) = 3.52, p < 0.05; Fig. 7D). The post hoc tests showed that CPP scores were significantly different between the groups in which Sal003 was injected into BLA in LV-scramble-injected or LV-shATF4-injected rats (p < 0.05). Finally, all of the rats were decapitated 10 min after CPP Test 2 for the subsequent determination of eIF2α phosphorylation, total eIF2α, ATF4, Zif268, and Fos in the BLA (Fig. 7E). We found that Sal003 prevented the retrieval-induced dephosphorylation of eIF2α in the BLA compared with the vehicle-treated groups (one-way ANOVA, F(3,23) = 13.77, p < 0.01), and prior LV-shATF4 microinjection blocked the increased expression of ATF4 in the Sal003-treated group compared with Sal003 injection in LV-scramble-injected group (one-way ANOVA, F(3,23) = 21.32, p < 0.01). Furthermore, given that increased ATF4 translation decreases cyclic adenosine monophosphate response element binding protein (CREB)-dependent transcription (Jiang et al., 2010), we determined the expression of the ATF4-regulated immediate early genes zif268 and c-fos, which have been implicated in the process of memory reconsolidation (Strekalova et al., 2003; Lee et al., 2004; Lee et al., 2006), while ATF4 was reduced by LV-shATF4 in the BLA. Sal003 increased ATF4 translation and prevented the increase in Zif268 (one-way ANOVA, F(3,23) = 11.39, p < 0.01) and Fos (one-way ANOVA, F(3,23) = 33.85, p < 0.01) expression in LV-Scramble-treated rats. Altogether, these findings suggest that ATF4 knockdown in the BLA blocked morphine cue memory impairments induced by the inhibition of eIF2α dephosphorylation.
Effect of preventing eIF2α dephosphorylation by Sal003 in the BLA on cue-maintained heroin-seeking behavior
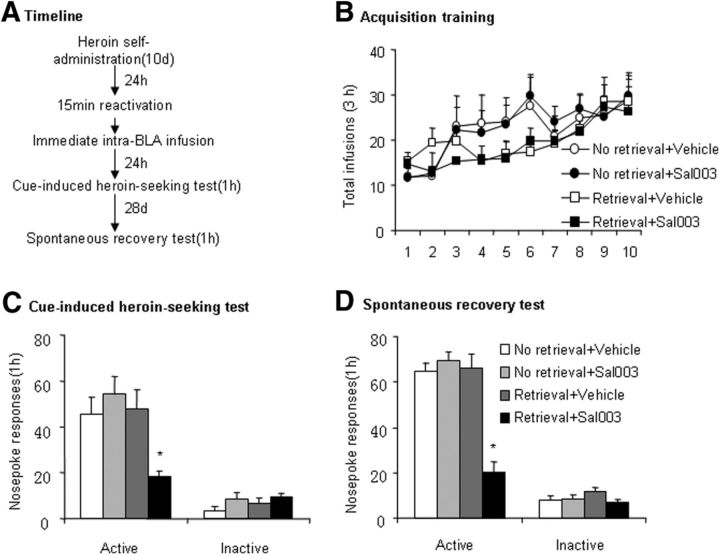
Finally, we assessed the effect of preventing eIF2α dephosphorylation by Sal003 in the BLA on the reconsolidation of heroin-associated cue memories in a self-administration model (Fig. 8A). We trained the rats to self-administer intravenous heroin over 10 d (Fig. 8B). The between-subjects factors were retrieval condition (retrieval and no retrieval) and Sal003 dose (0 and 20 μm/side). On day 11, we injected Sal003 or vehicle in the BLA immediately after the 15 min retrieval session or no retrieval. We tested cue-induced heroin-seeking behavior 24 h and 28 d after a microinjection of Sal003 or vehicle in the BLA (n = 7 per group). The two-way ANOVA revealed a significant retrieval condition × Sal003 dose interaction in the cue-induced heroin-seeking test (F(1,24) = 7.70, p < 0.05; Fig. 8C) and spontaneous recovery test (F(1,24) = 31.38, p < 0.01; Fig. 8D). The post hoc tests showed that active nose poke responses in the group of rats that received an injection of Sal003 in the BLA immediately after the retrieval of heroin-associated memories were significantly different from the other groups in both tests (p < 0.05), indicating that an intra-BLA injection of Sal003 immediately after memory retrieval disrupted the reconsolidation of heroin-associated cue memories and this effect lasted at least 28 d.
Figure 8.
Preventing eIF2α dephosphorylation by Sal003 in the BLA decreased cue-induced heroin craving and spontaneous recovery. The data are expressed as mean ± SEM. A, Timeline of the experiment. B, Total number of infusions across the acquisition of heroin self-administration. C, Number of responses at the active nose poke operandum in the cue-induced heroin-seeking test that was conducted 24 h after Sal003 (20 μm/side) or vehicle (0.5 μl/side) injection in the BLA after the retrieval session. D, Number of responses at the active nose poke operandum during the spontaneous recovery test that was conducted 28 d later. (n = 7 per group), *p < 0.05 compared with No retrieval + Vehicle group.
Discussion
We studied the role of eIF2α dephosphorylation in the amygdala in the reconsolidation of drug-associated cue memory assessed by the CPP and self-administration procedures. We found that the retrieval of either morphine or cocaine memory was associated with the dephosphorylation of eIF2α and ATF4 expression in the BLA. Intra-BLA infusion of Sal003 immediately after exposure to drug-paired cues impaired the reconsolidation of morphine and cocaine cue memories, which was associated with increases in eIF2α phosphorylation and ATF4 expression. This inhibitory effect on expression of morphine or cocaine CPP lasted at least 2 weeks and was not reversed by a morphine or cocaine priming injection. Furthermore, the knockdown of ATF4 expression before the retrieval blocked the disruption of the reconsolidation of morphine cue memory by Sal003. Finally, we investigated the role of eIF2α dephosphorylation in the reconsolidation of heroin cue memory in a self-administration procedure and found that intra-BLA infusion of Sal003 immediately after memory retrieval decreased cue-induced heroin-seeking behavior.
The effect of preventing eIF2α dephosphorylation by Sal003 on reconsolidation was anatomically and temporally specific. The retrieval of either morphine or cocaine cue memory was not associated with changes in eIF2α dephosphorylation in the CeA. Intra-CeA infusion of Sal003 immediately after retrieval had no effect on the reconsolidation of morphine cue memory. These results were consistent with previous studies indicating the BLA is involved in the reconsolidation of drug cue memories and fear memory. (Nader et al., 2000; Lee et al., 2005; Sanchez et al., 2010; Wu et al., 2011; Ding et al., 2013; Luo et al., 2013; Wells et al., 2013). Briefly, the BLA receives information about the motivational value or sensory-specific properties of drug-associated conditioned stimuli and reinforcers (Fuchs and See, 2002; Di Ciano and Everitt, 2004). The CeA is an output nucleus of the information from the BLA and connects to the brainstem and hypothalamus, which control conditioned responses (Johansen et al., 2011), and may be more important for stress-induced reinstatement (McFarland et al., 2004; Ma et al., 2008). Furthermore, we found intra-BLA infusion of Sal003 without the retrieval of morphine cue memory had no effect on the expression of morphine-induced CPP, indicating that the effect of inhibiting eIF2α dephosphorylation on the reconsolidation of drug cue memories depends on retrieval. Intra-BLA infusion of Sal003 6 h after retrieval had no effect on morphine-induced CPP. Reconsolidation refers to a process in which reactivated memory returns to a stable state to be persistently stored. The memory reconsolidation process was shown to be disrupted when postretrieval manipulations within a specific time window inhibited the expression of the learned conditioned response (Nader et al., 2000; Alberini, 2005; Milton and Everitt, 2010). Altogether, the present results indicate that eIF2α dephosphorylation in the BLA is critical for the reconsolidation of drug (morphine and cocaine) cue memories in both the CPP and self-administration procedures.
Role of eIF2α dephosphorylation in learning and memory
Our data extend previous work on the role of eIF2α dephosphorylation in learning and memory. Utilizing mouse genetics and pharmacology, a large body of evidence suggests that eIF2α dephosphorylation is essential for synaptic plasticity and LTM consolidation (Kelleher et al., 2004; Klann and Dever, 2004; Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007; Jiang et al., 2010; Stern et al., 2013). Genetic reductions of eIF2α phosphorylation in GCN2−/− and eIF2α+/S51A mice decreased the threshold of L-LTP and enhanced learning and memory in behavioral tasks (Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007). However, these data make it difficult to rule out the possibility that compensatory developmental changes in the mutant mice led to the enhancement of learning and memory. Jiang et al. (2010) demonstrated that CA1-restricted genetic manipulation of particular mRNA translations, using a new pharmacogenetic mouse model in which eIF2α phosphorylation was elicited specifically in the CA1 area, is sufficient to impair memory consolidation without affecting general translation. Recently, systemic or local microinjection of a protein kinase activated by double-stranded RNA (PKR) inhibitor that reduces eIF2α phosphorylation in the gustatory cortex enhanced taste memory in rats and mice (Stern et al., 2013). Moreover, mice treated with ISRIB, a small molecule that decreases eIF2α phosphorylation, exhibited enhanced spatial and fear-associated learning (Sidrauski et al., 2013).
However, the mechanisms that underlie the effect of eIF2α dephosphorylation on synaptic plasticity and LTM consolidation have not been elucidated. Substantial evidence indicates that ATF4, regulated by eIF2α phosphorylation, is crucial for L-LTP and LTM storage. ATF4, also known as CREB2, is a bZip transcription factor that interacts with the CCAAT/enhancer binding protein (C/EBP) family of transcription factors (Ameri and Harris, 2008). In Aplysia, ApCREB-2 that is partially homologous to murine ATF4 was shown to be a repressor of CREB (Bartsch et al., 1995). Specifically, reduced ATF4 expression in transgenic mice facilitates LTP and memory (Chen et al., 2003). In addition, the reduction of eIF2α phosphorylation in GCN2−/− and eIF2α+/S51A mice also decreased ATF4 expression and L-LTP induction was not prevented by Sal003 in slices from ATF4−/− mice (Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007). Moreover, the increased expression of ATF4 in the CA1 by PKR activation reduced the expression of the BDNF, c-fos, zif268, and c/EBPβ genes (Jiang et al., 2010), which were shown to be involved in the reconsolidation process of drug memory (Inda et al., 2005; Lee et al., 2006). In the present study, we found that eIF2α dephosphorylation in the BLA after memory retrieval was associated with a decrease in ATF4 levels. The infusion of Sal003 after memory retrieval, which inhibited CPP expression, also prevented eIF2α dephosphorylation and the decrease in ATF4 levels after CPP Test 2. These results indicate that the alterations of eIF2α dephosphorylation and ATF4 are correlated with the retrieval of drug reward memory. Moreover, we knocked down ATF4 expression by LV-shATF4 in the BLA before memory retrieval, which blocked the impairment of morphine cue memory induced by Sal003. Notably, the ATF4 levels did not significantly decline in the vehicle groups after LV-shATF4 or LV-scramble injection in the CPP experiment, whereas LV-shATF4 induced a significant reduction of ATF4 protein levels 7 and 14 d after virus injection, possibly due to a “floor effect” of ATF4 levels after treatments of CPP training and the retrieval test. Therefore, our data provide evidence that eIF2α dephosphorylation in the BLA mediates the reconsolidation of morphine cue memories and is dependent on ATF4.
Role of eIF2α dephosphorylation in drug memory
Inspired by studies that demonstrated a role for eIF2α dephosphorylation in synaptic plasticity and the consolidation of LTM (Costa-Mattioli and Sonenberg, 2006; Costa-Mattioli et al., 2007), we studied the role of eIF2α dephosphorylation in the reconsolidation of drug cue memories. It has been demonstrated that the reconsolidation of drug cue memories depends on protein synthesis (Lee et al., 2005; Milekic et al., 2006). Disruption of the reconsolidation of cocaine-associated cue memories blocked the expression of cocaine-induced CPP and decreased the activation of Zif268 and Fos (Miller and Marshall, 2005). In addition, Zif268 knockdown using an antisense oligonucleotide in the BLA after memory retrieval disrupted the reconsolidation of cocaine-associated cue memories and inhibited cue-induced cocaine-seeking behavior up to at least 27 d (Lee et al., 2005). Furthermore, under a second-order schedule of reinforcement, Zif268 antisense oligodeoxynucleotides in the BLA impaired the cue-induced maintenance of cocaine seeking (Lee et al., 2006). The phosphorylation of eIF2α activates ATF4 to regulate the transcription of genes, including c-fos and Zif268, during the initiation of translation, which controlled the synaptic plasticity required for the reconsolidation of drug cue memories in the present study. Notably, we found that the inhibition of eIF2α dephosphorylation by Sal003 immediately after the retrieval impaired the expression of morphine- and cocaine-induced CPP and subsequent expression of Zif268 and Fos in the BLA, and this effect was blocked by prior knockdown of ATF4 using LV-shATF4 in the BLA. These findings indicate that eIF2α-ATF4 signaling controls the reconsolidation of drug cue memories by regulating the expression of Zif268 and Fos. We further tested the effect of inhibiting eIF2α dephosphorylation on cue-induced heroin-seeking behavior in the self-administration procedure. Intra-BLA infusion of Sal003 after the retrieval of heroin-associated memory persistently inhibited cue-induced heroin seeking up to at least 28 d.
However, a general conclusion regarding the role of eIF2α dephosphorylation in the BLA in reward memory should be made with caution because eIF2α phosphorylation is known to impair both positive and negative forms of taste memory (Stern et al., 2013) and eIF2α dephosphorylation is necessary for the consolidation of normal memory (Costa-Mattioli et al., 2007; Jiang et al., 2010). Further studies are needed to determine whether eIF2α dephosphorylation is also involved in natural reward memory.
Concluding remarks
In conclusion, we found that eIF2α dephosphorylation in the BLA is involved in the retrieval of drug cue memories and is critical for the reconsolidation of drug cue memories in an ATF4-dependent manner. These findings extend the knowledge of eIF2α-ATF4 signaling in the BLA with regard to drug cue memories and may provide insights into the therapeutic potential of inhibiting eIF2α dephosphorylation in the treatment of drug addiction.
Footnotes
This work was supported by the Natural Science Foundation of China (Grants 31230033, 81221002, and 81225009, 31300930) and the National Basic Research Program of China (Grant 2011CB707805). We thank Jia-Jia Feng and Zeng-bo Ding for editorial assistance.
The authors declare no competing financial interests.
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/S0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sonenberg N. Translational control of long-term synaptic plasticity and memory storage by eIF2alpha. Crit Rev Neurobiol. 2006;18:187–195. doi: 10.1615/CritRevNeurobiol.v18.i1-2.190. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sonenberg N. Translational control of gene expression: a molecular switch for memory storage. Prog Brain Res. 2008;169:81–95. doi: 10.1016/S0079-6123(07)00005-2. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjević K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sonenberg N, Richter JD. Translational regulatory mechanisms in synaptic plasticity and memory storage. Prog Mol Biol Transl Sci. 2009a;90:293–311. doi: 10.1016/S1877-1173(09)90008-4. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009b;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M. Deletion of the eIF2alpha Kinase GCN2 fails to rescue the memory decline associated with Alzheimer's disease. PLoS One. 2013;8:e77335. doi: 10.1371/journal.pone.0077335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Ding ZB, Wu P, Luo YX, Shi HS, Shen HW, Wang SJ, Lu L. Region-specific role of Rac in nucleus accumbens core and basolateral amygdala in consolidation and reconsolidation of cocaine-associated cue memory in rats. Psychopharmacology (Berl) 2013;228:427–437. doi: 10.1007/s00213-013-3050-8. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ehrman R, Ternes J, O'Brien CP, McLellan AT. Conditioned tolerance in human opiate addicts. Psychopharmacology (Berl) 1992;108:218–224. doi: 10.1007/BF02245311. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gkogkas C, Sonenberg N, Costa-Mattioli M. Translational control mechanisms in long-lasting synaptic plasticity and memory. J Biol Chem. 2010;285:31913–31917. doi: 10.1074/jbc.R110.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Xue YX, Wang JS, Fang Q, Liu JF, Xue LF, Lu L. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology. 2011;36:1972–1981. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, Pierre P, Wagner G, LeDoux JE, Klann E. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci U S A. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Delgado-García JM, Carrión AM. Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J Neurosci. 2005;25:2070–2080. doi: 10.1523/JNEUROSCI.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Belforte JE, Lu Y, Yabe Y, Pickel J, Smith CB, Je HS, Lu B, Nakazawa K. eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci. 2010;30:2582–2594. doi: 10.1523/JNEUROSCI.3971-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/S0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue LF, Shaham Y, Lu L. Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Luo YX, Xue YX, Shen HW, Lu L. Role of amygdala in drug memory. Neurobiol Learn Mem. 2013;105:159–173. doi: 10.1016/j.nlm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Ma DY, Xu MY, Yang HC, Yang LZ. Effect of inhibition of the central nucleus of the amygdala and drug experience on the regions underlying footshock-induced reinstatement of morphine seeking. J Int Med Res. 2008;36:992–1000. doi: 10.1177/147323000803600516. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 5. Amsterdam: Elsevier Academic; 2005. [Google Scholar]
- Ren ZY, Liu MM, Xue YX, Ding ZB, Xue LF, Zhai SD, Lu L. A critical role for protein degradation in the nucleus accumbens core in cocaine reward memory. Neuropsychopharmacology. 2013;38:778–790. doi: 10.1038/npp.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siouda M, Yue J, Shukla R, Guillermier S, Herceg Z, Creveaux M, Accardi R, Tommasino M, Sylla BS. Transcriptional regulation of the human tumor suppressor DOK1 by E2F1. Mol Cell Biol. 2012;32:4877–4890. doi: 10.1128/MCB.01050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E, Chinnakkaruppan A, David O, Sonenberg N, Rosenblum K. Blocking the eIF2alpha kinase (PKR) enhances positive and negative forms of cortex-dependent taste memory. J Neurosci. 2013;33:2517–2525. doi: 10.1523/JNEUROSCI.2322-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. doi: 10.1037/0033-295X.91.2.251. [DOI] [PubMed] [Google Scholar]
- Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Zörner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes Brain Behav. 2003;2:3–10. doi: 10.1034/j.1601-183X.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Trinh MA, Klann E. Translational control by eIF2alpha kinases in long-lasting synaptic plasticity and long-term memory. Neurobiol Learn Mem. 2013;105:93–99. doi: 10.1016/j.nlm.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2013;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Xue YX, Ding ZB, Xue LF, Xu CM, Lu L. Glycogen synthase kinase 3beta in the basolateral amygdala is critical for the reconsolidation of cocaine reward memory. J Neurochem. 2011;118:113–125. doi: 10.1111/j.1471-4159.2011.07277.x. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34:6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]