Abstract
The role of the p75 neurotrophin receptor (p75NTR) in adult cholinergic basal forebrain (cBF) neurons is unclear due to conflicting results from previous studies and to limitations of existing p75NTR-knock-out mouse models. In the present study we used a novel conditional knock-out line (ChAT-cre p75in/in) to assess the role of p75NTR in the cBF by eliminating p75NTR in choline acetyl-transferase-expressing cells. We show that the absence of p75NTR results in a lasting increase in cBF cell number, cell size, and cholinergic innervation to the cortex. Analysis of adult ChAT-cre p75in/in mice revealed that mutant animals show a similar loss of cBF neurons with age to that observed in wild-type animals, indicating that p75NTR does not play a significant role in mediating this age-related decline in cBF neuronal number. However, the increased cholinergic axonal innervation of the cortex, but not the hippocampus, corresponded to alterations in idiothetic but not allothetic navigation. These findings support a role for p75NTR-mediated regulation of cholinergic-dependent cognitive function, and suggest that the variability in previous reports of cBF neuron number may stem from limited spatial and temporal control of p75NTR expression in existing knock-out models.
Keywords: cholinergic basal forebrain, hippocampus, knock-out, Morris water maze, navigation, p75 neurotrophin receptor
Introduction
Cholinergic basal forebrain (cBF) neurons regulate a wide array of brain functions, including learning, memory and attention (Baxter and Chiba, 1999). Throughout life, cBF neurons require a supply of neurotrophins, particularly nerve growth factor (NGF), from target areas of the cortex and hippocampus for their function and survival (Conner et al., 2009). The p75 neurotrophin receptor (p75NTR), together with Trk receptors, mediates the effects of the neurotrophins, and cBF neurons comprise one of the few neuronal populations in which expression of p75NTR is maintained throughout life (Yeo et al., 1997). However, the normal function of p75NTR within these neurons in the adult brain is unclear.
Analyses of mice in which exons of the nerve growth factor receptor (Ngfr) gene that encodes p75NTR have been genetically deleted have variously reported an increase (Van der Zee et al., 1996; Yeo et al., 1997; Naumann et al., 2002), decrease (von Schack et al., 2001), or no change in the number of cBF neurons, an increase in the size of these neurons, changes to hippocampal synaptic activity (Greferath et al., 2000) and neuron structure (Yeo et al., 1997), and either improved (Barrett et al., 2010) or impaired spatial learning (Catts et al., 2008; Dokter et al., 2014); the latter coupled to reduced hippocampal neurogenesis. One of the reasons for this variability lays in the shortcomings of existing complete p75NTR knock-out strains. First, adult phenotypes may manifest as altered development and/or non-cell-autonomous functions of p75NTR. Indeed, we have previously proposed that changes to hippocampal function in p75NTR knock-out animals could be driven by changes to septohippocampal cholinergic innervation (Catts et al., 2008). Second, both the widely used exon 3 strain and the subsequently generated exon 4 strains maintain expression of truncated versions of p75NTR protein (von Schack et al., 2001; Paul et al., 2004). Although these animals do not express a p75NTR protein capable of ligand binding, the truncated proteins mimic naturally occurring proteolytic fragments of p75NTR and are capable of proapoptotic signaling (Murray et al., 2003; Paul et al., 2004). Together, these factors limit the interpretation of results obtained with existing knock-out models. In this study, we generated a novel conditional p75NTR-deficient mouse strain, which we determined does not express a p75NTR transcript, and analyzed the effect of removing p75NTR from postmitotic cBF neurons.
Materials and Methods
Animals.
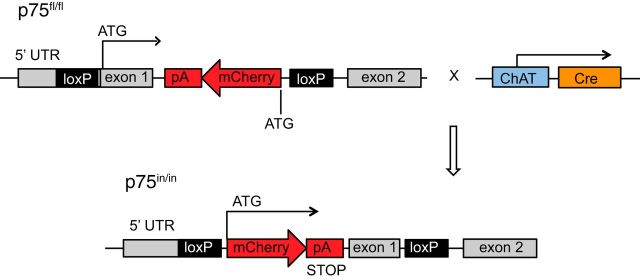
The p75NTR conditional knock out mouse was generated by Ozgene Pty (Australia) using C57BL6 embryonic stem cells. The genetic modification involved addition of one loxP site within the 5′ untranslated region of Ngfr and another loxP site within intron 1 (Fig. 1). A mutated loxP sequence pair meant that recombination leads to inversion rather than excision of the sequence flanked by the loxP sites in cre-expressing cells (Oberdoerffer et al., 2003). The sequence between the loxP sites contained the ATG start codon of Ngfr, as well as the mCherry coding sequence and a polyadenylation STOP signal, which were inserted in the antisense orientation within the intron 1 sequence. The mCherry and the STOP signal sequences were integrated within the first intron so that p75NTR expression remained unchanged in a wild-type context when cre recombinase was absent. However, when cre recombinase was present, the coding sequence for Ngfr exon 1 was replaced with the mCherry gene sequence.
Figure 1.
The Ngfr mutant gene (p75fl/fl) and inversion strategy used to generate p75in/in mutants. In cells expressing cre recombinase, the loxP-flanked region inverts to generate p75in/in mutants that express mCherry rather than p75NTR from the endogenous Ngfr promoter. UTR, Untranslated region.
The other mouse strains used in the study, p75exonIII (Lee et al., 1992) and choline-acetyltransferase(ChAT)-IRES-cre (Rossi et al., 2011), have been previously described. The ChAT-cre p75in/in animals showed variable recombination in the basal forebrain, falling predominantly into two groups: 65.59 ± 1.31% recombination, and 91.95 ± 1.66% recombination. Only mice from the latter group were used for analysis in the present study.
Male and female animals were used for cell counts (1-, 4-, and 8-month-old) and examination of cholinergic innervation (8-month-old). Only males (4-month-old) were used for ELISA assays, examination of neurogenesis and behavioral testing.
Histological tissue preparation.
Brains from 4% formaldehyde-perfused mice were postfixed overnight, preserved in 20% sucrose solution for 24 h, and embedded in OCT compound (Tissue-Tek). Coronal sections (40 μm) were cut in three serially adjacent sets through the basal forebrain and hippocampus using a sliding microtome (SM2000r, Leica). For in situ hybridization procedures, tissue was cut at 14 μm and stored in 0.1% sodium azide in 0.1 m PBS until immunohistochemistry was performed.
Immunohistochemistry.
For immunofluorescence labeling, free-floating sections were immunostained using goat anti-ChAT (1:200; Millipore), rabbit anti-DsRed (1:200; Clontech), goat anti-p75NTR (extracellular domain, 1:500; R&D Systems), rabbit anti-p75NTR (intracellular domain, 1:200; Promega), and goat anti-doublecortin (1:200; Santa Cruz Biotechnology) antibodies, followed by the appropriate secondary antibody (1:1000; Life Technologies). Sections were mounted onto slides and coverslipped using fluorescence mounting medium (Dako).
For visualization of cBF axons, ChAT was visualized using goat anti-ChAT antibody (1:1000; Millipore), biotinylated donkey anti-goat IgG (1:1000; Jackson Immunoresearch Laboratories) and ABC reagent (Vector Elite kit: Vector Laboratories). Black immunoreactive cytoplasm labeled for ChAT was revealed by a nickel-intensified diaminobenzidine reaction and slices were coverslipped with DePeX (Sigma-Aldrich).
In situ hybridization.
The p75NTR intracellular domain digoxigenin-labeled Ngfr antisense riboprobe spanned nucleotides 811–1226 (McQuillen et al., 2002). In situ hybridization was performed using previously described methods (Chambers et al., 2009). The stain was developed with the color substrate NBT/BCIP. Sections were subsequently washed in water and dehydrated in ethanol and xylene before being mounted in DePeX mounting medium (VWR International).
Histological quantifications.
For all histological quantification experiments, a minimum of three mice were used for each genotype. All cell counts for medial septum/diagonal band of Broca (MS/DBB) sections were performed from 1.3 mm anterior to bregma to 0.1 mm anterior to bregma. Every third section (8–10 sections per animal) was counted. Cells to be used for area measurements were selected at random from the MS/DBB region in a separate cohort. Neurogenesis was assessed by the number of doublecortin-labeled cells per 1 mm of dentate gyrus length. All measurements and analyses were performed using Imaris 7.2.3 software (Bitplane).
Quantification of ChAT intensity.
Levels of ChAT were assessed using ImageJ 1.45 s (NIH). For each animal, 5 hippocampal and 5 barrel cortex slices were selected at random and analyzed by measuring the gray value of pixels along 7 lines drawn through the CA1 and dentate gyrus regions of the hippocampus and one line per cortical layer of the barrel cortex (Lein et al., 2007). These values were then pooled, averaged, and inversed to obtain the average ChAT intensity for each animal.
Neurotrophin ELISAs.
Neurotrophin expression was analyzed in supernatant prepared from hippocampal homogenates. Mice were killed by cervical dislocation and the hippocampi dissected, weighed, and snap-frozen with liquid nitrogen. The neurotrophins were extracted by adding 20–30 v/w lysis buffer (0.05 m sodium acetate, 1 m sodium chloride, 1% bovine serum albumin, 1% Triton-X, Roche complete inhibitor cocktail tablet) to the hippocampi before homogenization using the Bullet Blender Storm 24 (Next Advance). NGF and brain-derived neurotrophic factor (BDNF) ELISAs were then performed as per the manufacturer's instructions (Biosensis). The resulting measurements were normalized for tissue weight.
Behavioral tasks.
Spatial memory was assessed using the Morris water maze as previously described (Catts et al., 2008), except without flag training, and data were analyzed using Ethovision XT (Noldus). Idiothetic navigation was assessed using the passive avoidance paradigm described previously (Hamlin et al., 2013).
Statistics.
Results are expressed as mean ± SEM. Statistical analyses were conducted using Student's t tests (Graphpad Prism 6) with the significance threshold set at p < 0.05.
Results
A novel ChAT-cre p75in/in mouse model eliminates expression of p75NTR at both the protein and mRNA levels
To investigate the role of p75NTR in cBF structure and function we generated a conditional knock-out mouse line that we termed p75fl/fl (Fig. 1; see Materials and Methods for details). In non-cre-expressing p75fl/fl cells, p75NTR expression proceeds normally, as no significant difference was seen in the number of p75NTR-positive cells in the adult MS/DBB (wild-type: 1966 ± 139.8 cells vs p75fl/fl: 1843 ± 38.55 cells; n = 3 animals per group); however, upon introduction of cre recombinase, the Ngfr genomic DNA flanked by loxP sites is inverted and, in p75in/in cells, expression of p75NTR is replaced with that of mCherry.
To remove p75NTR from postmitotic cBF neurons we crossed our p75fl/fl line with a mouse line expressing ChAT-IRES-cre recombinase, which removed p75NTR in fully differentiated cBF neurons from postnatal day (P)4 (Rossi et al., 2011). We termed the resulting offspring ChAT-cre p75in/in.
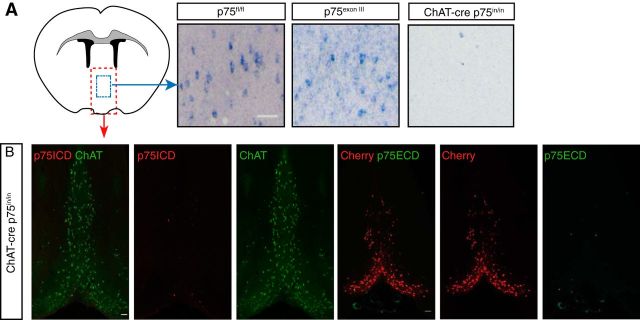
To confirm that the inversion strategy resulted in complete loss of p75NTR mRNA and protein expression we performed in situ hybridization for the p75NTR transcript using a probe for the 3′ sequence of Ngfr mRNA (encoding exon 6, the intracellular domain). mRNA corresponding to the p75NTR intracellular domain was present in the parental p75fl/fl animals lacking cre transgene coexpression but was absent from the majority of cBF neurons in the ChAT-cre p75in/in mutant mice (Fig. 2A). Although Ngfr mRNA was present in p75exonIII mutant animals (Fig. 2A), p75NTR protein expression was not detected (data not shown). In the basal forebrain of ChAT-cre p75in/in mutant mice, mCherry rather than p75NTR was expressed by the majority of ChAT-positive neurons. Antibodies to the p75NTR extracellular domain or intracellular domain did not label mCherry-positive or most ChAT-positive neurons (Fig. 2B). These data indicate that the cre-mediated conditional removal of p75NTR fully eliminates expression of p75NTR in the vast majority of cBF neurons, and we are therefore confident that the present mouse does not express alternative/truncated versions of p75NTR.
Figure 2.
Loss of p75NTR expression in p75in/in mutants. A, In situ hybridization for p75NTR intracellular domain (p75ICD) mRNA in coronal sections of the medial septum/diagonal band of Broca (MS/DBB) of mutant animals. p75fl/fl and p75exonIII animals contain many p75ICD mRNA-expressing cells, whereas expression of p75NTR mRNA in ChAT-cre p75in/in mice is almost completely absent. B, Immunostaining of the MS/DBB in coronal sections from ChAT-cre p75in/in animals. Expression of the extracellular (p75ECD) or intracellular (p75ICD) domain of p75NTR is not found in ChAT- or Cherry-positive neurons; n = 3 animals per group. Scale bars: A, 20 μm; B, 100 μm.
Postmitotic loss of p75NTR increases cell number, cell size and cholinergic innervation but does not regulate age-related death of cBF neurons
We next determined the number of cBF neurons in histological sections of the MS/DBB from ChAT-cre p75in/in mice (Fig. 3A). Analysis of ChAT-cre p75in/in animals revealed a significant increase in cBF neuron number (25% more, relative to p75fl/fl animals), indicating a role of p75NTR in facilitating the programmed cell death of postmitotic cholinergic neurons that occurs between P4 and P15 (Ward and Hagg, 1999).
Figure 3.
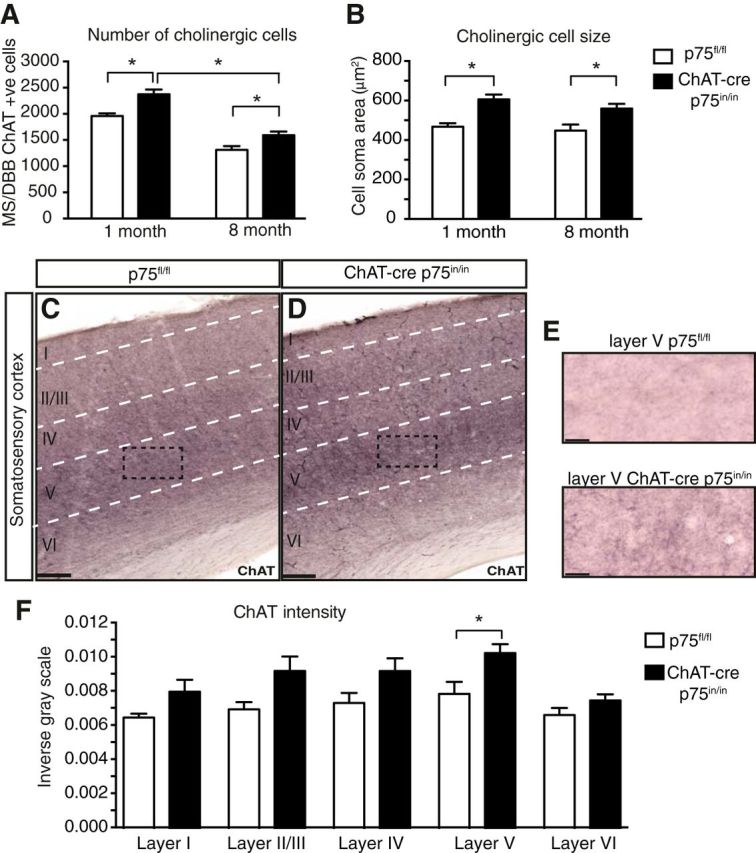
Absence of p75NTR from the adult cBF induces a lasting increase in cell numbers and cell surface area and an increase in cholinergic innervation to layer V somatosensory cortex. Quantification of ChAT-positive cell number (A) and surface area (B) of cBF neurons in ChAT-cre p75in/in mice at 1 and 8 months of age (n = 3 animals per group, 90 cells per genotype). ChAT-immunostained coronal sections of the cortex (C, D, and expanded in E) of p75fl/fl (C) and ChAT-cre p75in/in (D) animals. F, Quantification of the intensity of immunostaining of ChAT-positive fibers reveals a significant increase in the intensity of ChAT in layer V of the cortex; n = 3 animals, 15 sections per group. Scale bars: C, D, 100 μm; E, 20 μm.
We then examined whether the age-induced decrease in cBF neurons observed in wild-type animals was ameliorated in ChAT-cre p75in/in animals. Although the number of cBF neurons in 8-month-old ChAT-cre p75in/in animals was still significantly higher than that of p75fl/fl controls, there was nevertheless a significant decrease in the number of ChAT-cre p75in/in cBF neurons in the older animals compared with 1-month-old ChAT-cre p75in/in animals (Fig. 3A). As observed previously (Yeo et al., 1997; Greferath et al., 2000), the size of cBF neurons in ChAT-cre p75in/in animals was significantly larger in both young and older animals (Fig. 3B). Together, these data indicate that the presence of p75NTR does not affect the age-related loss in cBF neuron number, but that there is a genotype effect on cell size that persists from 1 to 8 months of age.
To determine whether the increase in cell size and number corresponded to altered innervation to, and/or expression of neurotrophins in target tissues, the levels of NGF and BDNF in freshly dissected hippocampi of mutant and control animals were quantified by ELISA. No differences were found, indicating that loss of p75NTR expression in efferent cBF axons has no indirect effects on neurotrophin levels in the hippocampus (NGF, p75fl/fl: 9.502 ± 1.706 vs ChAT-cre p75in/in: 10.34 ± 1.020 pg/ml per milligram of tissue; BDNF, p75fl/fl: 40.79 ± 6.485 vs ChAT-cre p75in/in: 43.72 ± 11.69 pg/ml per milligram of tissue; n = 8 controls, 5 mutants). No significant difference in the extent of cholinergic fiber innervation to the hippocampus was found between ChAT-cre p75in/in and p75fl/fl animals (p75fl/fl 0.008 ± 0.0008 vs ChAT-cre p75in/in 0.008 ± 0.0003 measured by inverse gray value; n = 3 animals, 15 sections per group). However, quantification of cholinergic nerve terminals in the barrel cortex of the same animals revealed a significant increase in cholinergic innervation of layer V neurons (Fig. 3C–F). In contrast to previous analyses of p75NTR mutant mice (Catts et al., 2008; Zuccaro et al., 2014), adult neurogenesis, as assessed by the number of doublecortin-positive immature neurons along the length of the dentate gyrus, was unchanged in mutant mice compared with controls (p75fl/fl 48.02 ± 7.66 cells/mm vs ChAT-cre p75in/in 44.58 ± 3.10 cells/mm; n = 3 animals, 9 slices per group). These results indicate that p75NTR in the cBF does not exhibit non-cell-autonomous effects on hippocampal neurogenesis and neurotrophin expression but does play a role in cBF innervation to the cortex.
p75NTR plays a role in idiothetic but not allothetic navigation
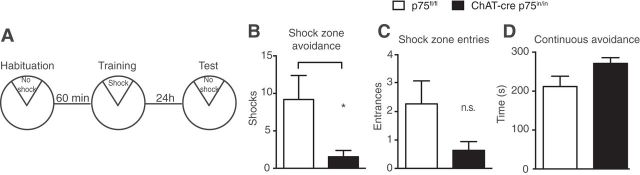
We next investigated spatial learning, memory and flexibility by comparing the performance of ChAT-cre p75in/in and p75fl/fl animals in both idiothetic (self-centered) and allothetic (world-centered) navigation tasks (Fig. 4). Because p75NTR expression is observed during embryogenesis from embryonic day 11.5 in the cholinergic motor neurons of the spinal cord, we first subjected the animals to a 30 min open-field test of general locomotor activity and anxiety. No differences were observed in locomotor or exploratory activity between mutant and control animals (distance traveled, p75fl/fl 2746 ± 447.1 vs ChAT-cre p75in/in 2532 ± 264.6 centimeters; thigmotaxic time, p75fl/fl 24.72 ± 0.6 vs ChAT-cre p75in/in 24.91 ± 0.5 min; n = 9 controls, 11 mutants).
Figure 4.
Mutant animals show an improvement in idiothetic navigation. A, Idiothetic navigation was tested using a modified version of the active place avoidance in an uncued nonrotating arena. On the test day, mutant animals received significantly fewer shocks compared with control animals (B; p = 0.0323), showed a tendency for fewer entries into the shock zone (C; p = 0.0738), and spent longer periods avoiding the shock zone (D; p = 0.0665); n = 11 animals per group. n.s., Not significant.
Next, the cued Morris water maze was used to assess allothetic navigation, previously reported to be altered in p75exonIII animals (Greferath et al., 2000; Catts et al., 2008; Dokter et al., 2014). No significant differences were observed between the two groups in learning (both groups average <20 s by day 6 of training), memory (probe trial: p75fl/fl 11 ± 0.7 vs ChAT-cre p75in/in 7.3 ± 6.7 s to platform), or memory flexibility (probe trial 2: p75fl/fl 5 ± 0.8 vs ChAT-cre p75in/in 9.2 ± 2.5 s to new platform), indicating that these animals do not have any major alterations in allothetic spatial navigation (n = 6 controls, 7 mutants). By contrast, in the uncued place avoidance test of egocentric navigation, in which animals with cBF lesions perform poorly (Hamlin et al., 2013), ChAT-cre p75in/in animals received significantly fewer shocks during the test and showed marked trends for fewer number of entries into the shock zone and more time avoiding this region compared with p75fl/fl controls (Fig. 4A–D). Together, these data indicate that p75NTR plays an important role in idiothetic navigation.
Discussion
Our analysis of a novel conditional p75in/in mutant animal illustrates that loss of p75NTR in mature postmitotic neurons induces a lasting increase in cBF cell size and number but does not play a role in age-related neuronal cell death. Furthermore, we show that loss of basal forebrain p75NTR has no overt effect on hippocampal function, but does result in increased cholinergic innervation to layer V cortical neurons, corresponding to alterations in idiothetic but not hippocampally dependent allothetic spatial navigation.
p75NTR regulates cBF neuron structure and function throughout life
p75NTR is well known for mediating the developmental programmed cell death of a range of peripheral neuronal populations, and previous reports of the phenotype of both p75exonIII and p75exonIV mice have suggested that a failure of programmed cell death between P6 and P15 results in increased cBF neuronal numbers in adult animals (Van der Zee et al., 1996; Yeo et al., 1997; Ward and Hagg, 1999; Naumann et al., 2002). Our finding of an increase in ChAT-positive basal forebrain neurons at 1 month of age is consistent with programmed cell death in cBF neurons being regulated by p75NTR-apoptotic signaling.
By contrast, the age-mediated loss of basal forebrain neurons in the ChAT-cre p75in/in animals between 1 and 8 months of age was not significantly regulated by p75NTR, indicating that p75NTR is not engaged in proapoptotic signaling under basal conditions. Although there is significant evidence for p75NTR being involved in mediating cell death in conditions of trauma and neurodegeneration where age is a factor (Ibáñez and Simi, 2012), expression of p75NTR per se is not apoptotic. Only in adverse conditions where proapoptotic ligands are significantly increased might p75NTR be triggered to promote cell death, whereas age-mediated cBF neuronal loss may occur due to reduced TrkA signaling and/or downregulation of NGF (Parikh et al., 2013), which primarily maintains cholinergic function.
Despite this, cBF neuron size was significantly increased in ChAT-cre p75in/in animals, and this increased cell size was maintained throughout life. It is possible, however, that the difference in cell size observed at 8 months of age reflects the phenotype present at 1 month. Nonetheless, NGF signaling regulates cell size (Lloyd, 2013), and as it is not influenced by TrkA expression (Sanchez-Ortiz et al., 2012), cell size may be a direct effect of p75NTR signaling. Although it is unclear exactly what increased cell size represents, it could reflect an increased axonal arbor. Together our findings indicate that p75NTR is mediating atrophic actions in postmitotic cBF neurons in the adult that are independent of neuronal survival.
Re-evaluation of the role for cBF p75NTR in the hippocampus
A number of hippocampal changes have previously been reported in p75exonIII animals that could have manifested though indirect effects, such as changes in activity of the septo-hippocampal cBF projections or alterations in hippocampal development. However, we found no evidence of hippocampal changes in animals in which loss of p75NTR in the brain was restricted to cBF neurons. Despite observing increased numbers of septal cBF neurons in ChAT-p75in/in adult animals, we found no evidence of altered innervation to the hippocampus, no change in neurotrophin levels that could induce septal reorganization and affect behavior (Conner et al., 2009), and no effect on hippocampal adult neurogenesis. This suggests that the previously observed increases in hippocampal cholinergic innervation are more likely due to the loss of full-length p75NTR expression in all cells, including those in the hippocampus, throughout developmental and adult life, and may reflect a non-cell-autonomous effect that is not observed when p75NTR is selectively deleted from the cBF. Although herein we demonstrate that loss of p75NTR from basal forebrain terminals does not directly mediate the rate of adult neurogenesis, we cannot rule out the possibility that increased innervation to the hippocampus (Yeo et al., 1997) may still play a role in this phenotype. Regardless, we now consider a direct effect of p75NTR on developmental or adult hippocampal neurogenesis (Catts et al., 2008; Bernabeu and Longo, 2010), or non-cell-autonomous effects as the most likely explanation for the previous results, while also considering that expression of p75NTR mRNA alternative transcripts or functional truncated proteins could have contributed to these previously reported phenotypes.
cBF p75NTR regulates idiothetic but not allocentric navigation
We and others have previously reported that the p75exonIII animals have subtle memory alterations (Greferath et al., 2000; Catts et al., 2008; Barrett et al., 2010; Dokter et al., 2014). However, our ChAT-cre p75in/in animals did not display any disruption in allothetic navigation learning, memory, or flexibility as assessed using the cued Morris water maze paradigm, albeit consistent with the lack of any obvious change in hippocampal structure. We consider that the previously reported changes to performance in this task in p75exonIII mutants is more likely due to elimination of p75NTR from the developing and/or adult hippocampus and subsequent effects on adult neurogenesis (Catts et al., 2008; Bernabeu and Longo, 2010), given the important role of neurogenesis in regulating allothetic spatial performance (Vukovic et al., 2013).
By contrast, the ChAT-cre p75in/in mutant animals performed better in the idiothetic place avoidance task than littermate control animals. We have previously found that lesions of the cBF cause an impairment in the same idiothetic task, with lesioned animals displaying disorganized behavior and reduced shock zone avoidance (Hamlin et al., 2013), and that basal forebrain lesions have, at best, subtle effects on Morris water maze performance (Moreau et al., 2008). Conversely, in the present study we saw improved idiothetic performance, corresponding to an increase in cholinergic innervation in the barrel cortex. Together these results suggest that loss of p75NTR within cBF neurons positively regulates cognitive processes via the cBF corticopetal neurons that underpin idiothetic navigation. This is congruent with our observation of increased cortical cholinergic innervation to layer V neurons in ChAT-cre p75in/in animals. The mechanism by which increased cholinergic innervation of layer V cortical neurons results in improved idiothetic navigation is a topic for future study. However, it has previously been demonstrated that the somatosensory cortex is a key area for the integration of kinesthetic information in rodents (Petreanu et al., 2012), as well as humans (for review, see Lopez, 2013). This is supported by reports that vestibular disorders underpinned by dysfunction of the somatosensory cortex result in deficits in egocentric spatial navigation due to sensory and perceptual conflict or incoherence (Weniger et al., 2012; Rousseaux et al., 2014).
In summary, our work demonstrates that p75NTR plays a predominantly atrophic role in postmitotic cBF structure and function, as its loss results in an adult phenotype that includes increased cBF neuron number and size, increased cholinergic innervation to the cortex and altered idiothetic navigation performance. In contrast to previous models of p75NTR deficiency, there was no obvious effect on hippocampal function resulting from the loss of cBF p75NTR. Our work therefore highlights that conditional p75NTR knock-out strains with spatial and temporal control over gene disruption, including the novel strain described here, are required to tease out the many roles that p75NTR plays in cellular neurogenesis, specification, maturation, and maintenance.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia (Project Grant 1049236 and Career Development Fellowship 569601 to E.J.C.) and the Australian Research Council and NuNerve Pty (Linkage Project Grant LP10012610). Z.B. is the recipient of an Australian Postgraduate Award and University of Queensland Advantage Scholarship. We thank the staff of the University of Queensland Biological Resources Facility for breeding and maintaining the animals used in this study, members of the Coulson laboratory past and present for helpful discussions, and Rowan Tweedale for critical reading of the paper and editorial assistance.
The authors declare no competing financial interests.
References
- Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, Trieu J, Murphy M. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20:145–152. doi: 10.1002/hipo.20598. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/S0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bernabeu RO, Longo FM. The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 2010;11:136. doi: 10.1186/1471-2202-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur J Neurosci. 2008;28:883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- Chambers D, Wilson LJ, Alfonsi F, Hunter E, Saxena U, Blanc E, Lumsden A. Rhombomere-specific analysis reveals the repertoire of genetic cues expressed across the developing hindbrain. Neural Dev. 2009;4:6. doi: 10.1186/1749-8104-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, Sejnowski TJ, Tuszynski MH. NGF is essential for hippocampal plasticity and learning. J Neurosci. 2009;29:10883–10889. doi: 10.1523/JNEUROSCI.2594-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokter M, Busch R, Poser R, Vogt MA, von Bohlen Und Halbach V, Gass P, Unsicker K, von Bohlen Und Halbach O. Implications of p75NTR for dentate gyrus morphology and hippocampus-related behavior revisited. Brain Struct Funct. 2014 doi: 10.1007/S00429-014-0737-5. doi: 10.1007/S00429-014-0737-5. Advance online publication. Retrieved March 6, 2014. [DOI] [PubMed] [Google Scholar]
- Greferath U, Bennie A, Kourakis A, Bartlett PF, Murphy M, Barrett GL. Enlarged cholinergic forebrain neurons and improved spatial learning in p75 knockout mice. Eur J Neurosci. 2000;12:885–893. doi: 10.1046/j.1460-9568.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Windels F, Boskovic Z, Sah P, Coulson EJ. Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation. PloS One. 2013;8:e53472. doi: 10.1371/journal.pone.0053472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35:431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-L. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lloyd AC. The regulation of cell size. Cell. 2013;154:1194–1205. doi: 10.1016/j.cell.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Lopez C. A neuroscientific account of how vestibular disorders impair bodily self-consciousness. Front Integr Neurosci. 2013;7:91. doi: 10.3389/fnint.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, DeFreitas MF, Zada G, Shatz CJ. A novel role for p75NTR in subplate growth cone complexity and visual thalamocortical innervation. J Neurosci. 2002;22:3580–3593. doi: 10.1523/JNEUROSCI.22-09-03580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau PH, Cosquer B, Jeltsch H, Cassel JC, Mathis C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus. 2008;18:610–622. doi: 10.1002/hipo.20422. [DOI] [PubMed] [Google Scholar]
- Murray SS, Bartlett PF, Lopes EC, Coulson EJ, Greferath U, Cheema SS. Low-affinity neurotrophin receptor with targeted mutation of exon 3 is capable of mediating the death of axotomized neurons. Clin Exp Pharmacol Physiol. 2003;30:217–222. doi: 10.1046/j.1440-1681.2003.03827.x. [DOI] [PubMed] [Google Scholar]
- Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P, Otipoby KL, Maruyama M, Rajewsky K. Unidirectional Cre-mediated genetic inversion in mice using the mutant loxP pair lox66/lox71. Nucleic Acids Res. 2003;31:e140. doi: 10.1093/nar/gng140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Howe WM, Welchko RM, Naughton SX, D'Amore DE, Han DH, Deo M, Turner DL, Sarter M. Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur J Neurosci. 2013;37:278–293. doi: 10.1111/ejn.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CE, Vereker E, Dickson KM, Barker PA. A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci. 2004;24:1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu NL, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature. 2012;489:299–303. doi: 10.1038/nature11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux M, Honoré J, Saj A. Body representations and brain damage. Neurophysiol Clin. 2014;44:59–67. doi: 10.1016/j.neucli.2013.10.130. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortiz E, Yui D, Song D, Li Y, Rubenstein JL, Reichardt LF, Parada LF. TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J Neurosci. 2012;32:4065–4079. doi: 10.1523/JNEUROSCI.6314-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee CE, Ross GM, Riopelle RJ, Hagg T. Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science. 1996;274:1729–1732. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Vukovic J, Borlikova GG, Ruitenberg MJ, Robinson GJ, Sullivan RK, Walker TL, Bartlett PF. Immature doublecortin-positive hippocampal neurons are important for learning but not for remembering. J Neurosci. 2013;33:6603–6613. doi: 10.1523/JNEUROSCI.3064-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, Hagg T. p75NGFR and cholinergic neurons in the developing forebrain: a re-examination. Brain Res Dev Brain Res. 1999;118:79–91. doi: 10.1016/S0165-3806(99)00133-9. [DOI] [PubMed] [Google Scholar]
- Weniger G, Ruhleder M, Lange C, Irle E. Impaired egocentric memory and reduced somatosensory cortex size in temporal lobe epilepsy with hippocampal sclerosis. Behav Brain Res. 2012;227:116–124. doi: 10.1016/j.bbr.2011.10.043. [DOI] [PubMed] [Google Scholar]
- Yeo TT, Chua-Couzens J, Butcher LL, Bredesen DE, Cooper JD, Valletta JS, Mobley WC, Longo FM. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J Neurosci. 1997;17:7594–7605. doi: 10.1523/JNEUROSCI.17-20-07594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccaro E, Bergami M, Vignoli B, Bony G, Pierchala BA, Santi S, Cancedda L, Canossa M. Polarized expression of p75NTR specifies axons during development and adult neurogenesis. Cell Rep. 2014;7:138–152. doi: 10.1016/j.celrep.2014.02.039. [DOI] [PubMed] [Google Scholar]