Abstract
Synaptic plasticity is a cellular mechanism putatively underlying learning and memory. However, it is unclear whether learning induces synaptic modification globally or only in a subset of neurons in associated brain regions. In this study, we genetically identified neurons activated during contextual fear learning and separately recorded synaptic efficacy from recruited and nonrecruited neurons in the mouse basolateral amygdala (BLA). We found that the fear learning induces presynaptic potentiation, which was reflected by an increase in the miniature EPSC frequency and by a decrease in the paired-pulse ratio. Changes occurred only in the cortical synapses targeting the BLA neurons that were recruited into the fear memory trace. Furthermore, we found that fear learning reorganizes the neuronal ensemble responsive to the conditioning context in conjunction with the synaptic plasticity. In particular, the neuronal activity during learning was associated with the neuronal recruitment into the context-responsive ensemble. These findings suggest that synaptic plasticity in a subset of BLA neurons contributes to fear memory expression through ensemble reorganization.
Introduction
One cellular mechanism underlying fear conditioning is likely to be synaptic plasticity in the amygdala. Fear conditioning potentiates synaptic strength in the amygdala (McKernan and Shinnick-Gallagher, 1997; Tsvetkov et al., 2002) and is impaired when synaptic plasticity in the amygdala is inhibited (Fanselow and Kim, 1994; Rumpel et al., 2005). However, it is unclear whether fear learning induces synaptic modification in the amygdala globally or only in a subset of neurons. Because studies using activity-dependent gene expression showed that a subset of amygdala neurons are involved in fear conditioning (Han et al., 2007, 2009; Reijmers et al., 2007), we hypothesized that fear learning induces synaptic modification only in neurons that are recruited into the memory trace. Furthermore, if this is the case, synaptic modification in a subset of neurons would likely lead to a reorganization of amygdala neuronal ensemble activity.
Here, we tested these ideas by combining whole-cell recordings with imaging of neuronal activity histories. We examined synaptic plasticity and ensemble reorganization associated with contextual fear conditioning. Further, we focused our analysis on the basolateral amygdala (BLA) because of its importance in contextual fear conditioning (Maren, 2001).
Materials and Methods
Animals.
All experiments were approved by the animal experiment ethics committee at the University of Tokyo (approval number 24-8, 24-10), and were in accordance with the University of Tokyo guidelines for the care and use of laboratory animals. For catFISH, we used male C57BL/6J mice (8–15 weeks old; SLC) on a 12 h light/dark cycle with lights on from 7:00 A.M. to 7:00 P.M., with access to food and water ad libitum. For electrophysiological experiments, we used male or female Arc-dVenus transgenic mice (3–5 weeks old; Eguchi and Yamaguchi, 2009).
Behavioral procedures.
In the electrophysiological experiments with fear memory retrieval (Fig. 1), we examined synaptic efficacy in the neurons that were activated during memory retrieval. On day 1, mice in the fear conditioning (FC) group were conditioned in a conditioning chamber with three footshocks (1 mA, 2 s, 150 s intervals). Mice in the immediate shock (IS) group received a footshock (1 mA, 6 s) immediately after they were placed in the chamber. On day 2, both groups were re-exposed to the chamber for 5 min and killed 5 h later, when dVenus fluorescence reaches a plateau (Eguchi and Yamaguchi, 2009). In the electrophysiological experiments without fear memory retrieval (Fig. 2), we examined synaptic efficacy in the neurons that were activated during fear conditioning. Mice were conditioned in the conditioning chamber with three footshocks and killed 5 h later.
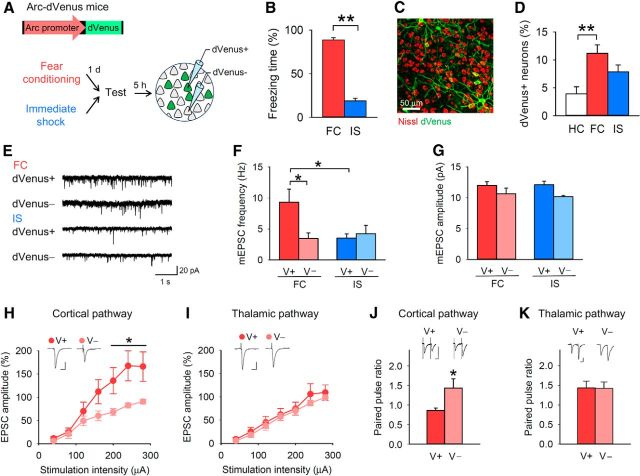
Figure 1.
Contextual fear conditioning induces presynaptic potentiation in BLA neurons recruited into a fear memory trace. A, Experimental procedure. B, The FC group showed longer freezing time during the test than the IS group. C, A representative fluorescence image of BLA neurons with dVenus. D, Re-exposure to the conditioning context increased the proportion of dVenus+ neurons in the BLA (one-way ANOVA, F(2,18) = 6.7, p = 0.0068; Tukey's test, home cage (HC) vs FC, p = 0.0050). E, F, dVenus+ (V+) neurons of the FC group had a higher mEPSC frequency than dVenus− (V−) neurons of the FC group and dVenus+ neurons of the IS group (n = 10 dVenus+ and 10 dVenus− neurons per behavioral group from 14 mice in the FC group and 12 mice in the IS group; two-way ANOVA, F(1,36) = 4.9, p = 0.033; dVenus+ (FC) vs dVenus− (FC), p = 0.042; dVenus+ (FC) vs dVenus+ (IS), p = 0.024). G, There was no significant group × dVenus interaction of mEPSC amplitude (F(1,36) = 0.33, p = 0.57). H, I, The EPSC amplitude of the dVenus+ neurons in the FC group was higher than that of the dVenus− neurons in the FC group in the cortical but not thalamic pathway (n = 7–10 neurons from 5 mice; cortical pathway, repeated-measures ANOVA, F(6,108) = 4.5, p = 4.1 × 10−4). Calibration: 20 ms, 100 pA. J, K, The PPR in dVenus+ neurons in the FC group was lower than that of dVenus− neurons in the FC group in the cortical but not thalamic pathway (n = 7–11 neurons from 7 mice; cortical pathway, Student's t test, t(11) = 2.3, p = 0.041). Calibration: 20 ms, 50 pA. **p < 0.01, *p < 0.05.
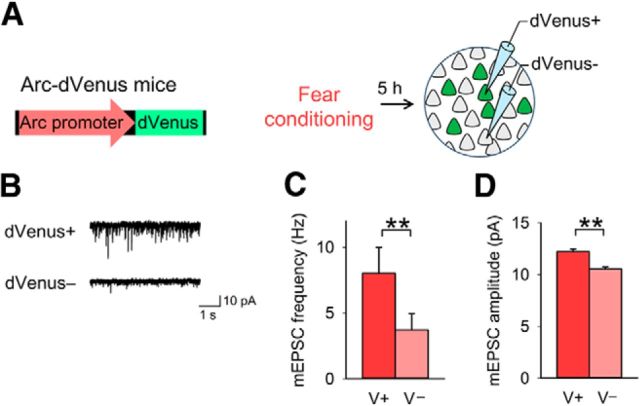
Figure 2.
Enhanced synaptic efficacy in the BLA neurons recruited into a fear memory trace is due to fear conditioning but not fear memory retrieval. A, Experimental procedure. B, Representative mEPSC traces recorded from dVenus+ and dVenus− neurons. C, D, dVenus+ neurons had a higher mEPSC frequency (C) and amplitude (D) than dVenus− neurons (n = 6 dVenus+ and 6 dVenus− neurons from 5 mice; frequency, paired t test, t(5) = 5.4, p = 0.0029; amplitude, t(5) = 7.8, p = 0.00056); **p < 0.01.
In the catFISH experiments that examined fear conditioning-induced ensemble reorganization, the behavioral test comprised three sessions. In session 1, mice in the FC group were placed in the conditioning chamber for 5 min. The mice were returned to their home cage and 36 min later, in session 2, they were conditioned with three footshocks (1 mA, 2 s, 150 s intervals) in the chamber. They were returned to their home cage again, and 20 min later (session 3) they were re-exposed to the chamber for 5 min to induce recall of the fear memory. The IS group, a control for associative learning, was given footshocks 0, 10, and 20 s after being placed in the chamber in session 2, instead of the fear conditioning protocol. In the catFISH experiments that examined the time course of the cytoplasmic Homer 1a signals, mice were exposed to the conditioning chamber for 5 min without shock. They were killed immediately, 25 min or 70 min after the context exposure.
All sessions were video recorded to enable the automatic scoring of freezing behavior according to the previously described method (Nomura and Matsuki, 2008).
Electrophysiology.
Mice were deeply anesthetized with diethyl ether and decapitated 5 h after re-exposure to the conditioning context. Brains were removed quickly, and coronal slices (300 μm thick) containing the BLA were prepared with a vibratome (VT 1200S; Leica) in ice-cold, oxygenated (95% O2/5% CO2) modified artificial CSF (mACSF) containing 222.1 mm sucrose, 27 mm NaHCO3, 1.4 mm NaH2PO4, 2.5 mm KCl, 0.5 mm ascorbic acid, 1 mm CaCl2, and 7 mm MgSO4.
Picrotoxin (100 μm) was added to ACSF (127 mm NaCl, 1.6 mm KCl, 1.24 mm KH2PO4, 1.3 mm MgSO4, 2.4 mm CaCl2, 26 mm NaHCO3, and 10 mm glucose). Whole-cell patch-clamp recordings were performed with glass microelectrodes (3–8 MΩ) filled with internal solution (135 mm K-gluconate, 4 mm KCl, 10 mm phosphocreatine-Na2, 10 mm HEPES, 4 mm MgATP, and 0.3 mm Na2GTP, pH 7.2–7.3, 280–295 mOsm). Because the BLA receives projections from diverse cortices and thalamus including the entorhinal and perirhinal cortices and mediodorsal thalamic nucleus, which are involved in contextual fear conditioning (Maren and Fanselow, 1997; Sacchetti et al., 1999; Li et al., 2004), we examined the effect of contextual fear conditioning on the cortico-BLA and thalamo-BLA synapses. Electrical stimulation was applied to the internal capsule to evoke EPSCs in BLA neurons from thalamic afferents, or to the external capsule to evoke EPSCs from cortical afferents, using bipolar tungsten electrodes (0.1–1 MΩ). Paired stimuli were given with an interstimulus interval of 50 ms, and the ratio between the amplitude of the second and first EPSCs was calculated. Miniature EPSCs (mEPSCs) were recorded at a holding potential of −70 mV in the presence of tetrodotoxin (1 μm). mEPSCs were detected using an in-house MATLAB program and were defined as inward currents with amplitudes >7 pA unless stated (Miura et al., 2012). Data were sampled at 20 kHz and filtered at 2 kHz using an Axopatch 200B, 700B amplifier (Molecular Devices), DIGIDATA1320A, 1440A (Molecular Devices), and pClamp 10.2 (Molecular Devices). All data were acquired, stored, and analyzed using Clampex 10, Clampfit, and MATLAB.
Fluorescence in situ hybridization.
Mice were killed immediately after session 3, and their brains were removed and frozen quickly. In situ hybridization was performed according to previously published protocols (Nomura et al., 2012). The coronal brain sections (20 μm) were hybridized with the riboprobes (DIG-Homer 1a antisense riboprobe, 2 μg/ml; Fluorescein-Arc antisense riboprobe, 1 μg/ml). The signals were detected with an anti-Fluorescein HRP-conjugated antibody (1:200; PerkinElmer); a TSA Plus DNP System (1:10; PerkinElmer); an anti-DIG peroxidase-conjugated antibody (1:500; Roche); Tyramide-biotin (1:5000), an Alexa488-conjugated anti-DNP antibody (Invitrogen); and Alexa594-conjugated streptavidin (Invitrogen). The nuclei were counterstained with Hoechst.
Z-stacks of 1-μm-thick optical sections were acquired with LSM-510 (Zeiss) and CV1000 (Yokogawa Denki) confocal microscopes using 40× objective lenses. Only cells that were presumptive neurons with whole, large nuclei stained diffusely with the Hoechst dye were included in the analysis. The designation “intranuclear positive” was assigned to neurons that exhibited one or two of the characteristic intense intranuclear areas of fluorescence. The designation “cytoplasmic positive” was assigned to neurons that contained perinuclear/cytoplasmic labeling over multiple optical sections.
Immunohistochemistry.
For dVenus detection, mice were transcardially perfused with 4% PFA in PBS 5 h after re-exposure to the conditioning context. The sections were incubated with rabbit anti-GFP antibody (1:1000; Invitrogen), Alexa488 anti-rabbit IgG (1:500; Invitrogen), and NeuroTrace Blue (1:50; Invitrogen).
Data analysis.
All values are given as the mean ± SEM. Two-way factorial ANOVA, repeated-measure ANOVA, Student's t test, the Tukey–Kramer test, and the paired t test were used for appropriate comparisons.
An overlap score between neuronal populations that were active during both sessions 1 and 3 was obtained as follows: S1 = percentage of total cytoplasmic Homer 1a+ neurons, S3 = percentage of total nuclear Arc+ neurons, S13 = percentage of neurons in which both cytoplasmic Homer 1a and nuclear Arc were observed, chance level (C13) = S1 × S3/100, overlap score between sessions 1 and 3 = (S13 − C13)/(S3 − C13) × 100. The recruiting score was calculated as the percentage of neurons that changed their activity from inactive in session 1 to active in session 3.
Results
Contextual fear conditioning induces presynaptic potentiation in BLA neurons recruited into a fear memory trace
To identify the neurons that were recruited into a memory trace, we used Arc-dVenus transgenic mice. These mice express a destabilized version of the fluorescent protein Venus under the control of the Arc promoter (Eguchi and Yamaguchi, 2009). The Arc-dVenus mice allowed us to identify the neurons that were recruited into the memory trace by dVenus fluorescence produced by Arc activation during fear memory expression. The mice received contextual fear conditioning, and 24 h later they were re-exposed to the conditioning context (Fig. 1A). They showed robust freezing during the re-exposure to the context (Fig. 1B), suggesting that they recalled the contextual fear memory. They were killed 5 h after the context re-exposure for the subsequent electrophysiological studies. An IS group, which received a footshock immediately after they were placed in the chamber, was prepared as a control for associative fear learning (Fig. 1B). The percentage of dVenus+ BLA neurons in FC mice was higher than that of home cage (HC) controls (Fig. 1C,D).
Brain slices were prepared for whole-cell recordings from BLA neurons. We measured mEPSCs from dVenus+ (V+) and dVenus− (V−) neurons of the mice in the FC and IS groups. The mEPSC frequency of the dVenus+ neurons in the FC group was higher than that of the dVenus− neurons in the FC group and the dVenus+ neurons in the IS group (Fig. 1E,F). There was no significant group × dVenus interaction for mEPSC amplitude (Fig. 1G).
To probe the synaptic efficacy of dVenus+ neurons in the FC group, we measured the evoked EPSCs and paired-pulse ratio (PPR). The EPSC amplitude of the dVenus+ neurons in the FC group was higher than that of the dVenus− neurons in the FC group in the cortical, but not the thalamic, pathway (Fig. 1H,I). PPR in the dVenus+ neurons of the FC group was lower than that in dVenus− neurons of the FC group in the cortical, but not the thalamic, pathway (Fig. 1J,K). These results indicate that the synaptic efficacy of cortico-amygdala synapses is presynaptically enhanced in the FC group, and that this enhancement is restricted to neurons that were recruited into the memory trace (i.e., dVenus+ neurons).
We tested whether fear memory retrieval is required for the high mEPSC frequency in the recruited neurons. Arc-dVenus mice were killed 5 h after they received fear conditioning without re-exposure to the conditioning context (Fig. 2A). BLA dVenus+ neurons showed higher mEPSC frequency and amplitude than dVenus− neurons (Fig. 2B–D). These results suggest that the high mEPSC frequency in the recruited neurons is attributable to fear conditioning.
In all the analyses described above, we detected mEPSCs with a 7 pA threshold. This analysis strictly removed noise (false positive EPSCs) but could miss small mEPSCs. To avoid missing small mEPSCs, we also performed an additional analysis detecting mEPSCs with a 5 pA threshold. Similarly with the 7 pA threshold analyses, we found that BLA dVenus+ neurons showed a higher mEPSC frequency (dVenus+: 12.1 ± 2.8 Hz; dVenus−: 6.9 ± 2.1 Hz; paired t test, t(5) = 4.7, p = 0.0051) and amplitude (dVenus+: 10.0 ± 0.19 pA; dVenus−: 8.3 ± 0.14; t(5) = 9.9, p = 0.0002) than dVenus− neurons.
Fear conditioning reorganizes a context-responsive BLA neuronal ensemble based on the activity of individual BLA neurons during fear conditioning
Because synaptic potentiation in a subset of neurons is likely to lead to a reorganization of neuronal ensembles, we tested whether a BLA neuronal ensemble responsive to context is altered by fear conditioning. Mice in the FC group were subjected to context exposure in session 1 (S1), contextual fear conditioning in session 2 (S2), and context re-exposure in session 3 (S3) with 36 and 20 min intervals (Fig. 3A). They spent a much greater time freezing in session 3 versus session 1 (Fig. 3B). The IS group demonstrated significantly less freezing in session 3 than the FC group.
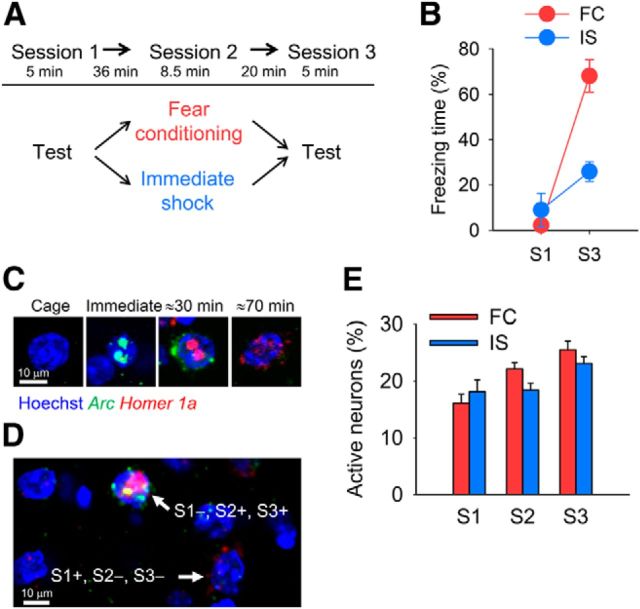
Figure 3.
Large-scale activity imaging before, during, and after fear conditioning in the BLA. A, Experimental procedure (n = 8 mice per group). B, The FC but not the IS group exhibited contextual conditioned fear in session 3 (repeated-measures ANOVA, F(1,14) = 23.8, p = 0.00025; FC vs IS in session 3, p = 0.00017). C, Representative images of individual BLA neurons from fluorescent in situ hybridization of Arc and Homer 1a. D, A representative image of Arc and Homer 1a RNA expression from the FC group. A neuron with nuclear and cytoplasmic Arc and nuclear Homer 1a was activated in sessions 2 and 3 (S1−, S2+, S3+). A neuron with only cytoplasmic Homer 1a was activated in session 1 (S1+, S2−, S3−). E, Percentage of BLA neurons active in each session was comparable between the FC and IS groups (n = 319.0 ± 18.1 neurons in FC group and 313.3 ± 17.6 neurons in IS group per mouse).
To identify neurons that were active during each session, we used temporal activity mapping with cellular resolution by observing Arc and Homer 1a RNA (i.e., catFISH; Guzowski et al., 1999; Marrone et al., 2008). Transcribed Arc RNA first appears in neuronal nuclei, after which processed Arc mRNA accumulates in the cytoplasm. Homer 1a RNA is transcribed later than Arc RNA. Previously, we reported that nuclear Arc is observed immediately after neuronal activity and that cytoplasmic Arc and nuclear Homer 1a are observed 25–30 min after neuronal activity in the amygdala (Hashikawa et al., 2011; Nomura et al., 2012). Because the characteristics of the cytoplasmic Homer 1a signal have been described for hippocampal neurons (Marrone et al., 2008) but not amygdala neurons, we first examined the time course of the cytoplasmic Homer 1a signal after neuronal activity. We found that in the basolateral amygdala, as well as the hippocampal CA1, more cytoplasmic Homer 1a+ neurons were observed when mice were killed 70 min after context exposure compared with 0 and 25 min (p = 0.00052 and 0.0013, respectively). Therefore, in the following analysis, we identified neurons that were activated during session 1 (∼70 min before being killed), session 2 (∼30 min before being killed), and session 3 (immediately before being killed) based on the “cytoplasmic Homer 1a,” “nuclear Homer 1a and cytoplasmic Arc,” and “nuclear Arc,” respectively (Fig. 3C,D). We measured the proportions of active neurons in the BLA during sessions 1, 2, and 3 (Fig. 3D). There was no group effect or group × session interaction (Fig. 3E).
To determine whether fear conditioning alters the context-responsive neuronal ensemble, the overlap between the neuronal populations that were active during sessions 1 and 3 was examined. The overlap score of the FC group was lower than that of the IS group (Fig. 4A). This result indicates that fear conditioning alters the context-responsive neuronal ensemble.
Figure 4.
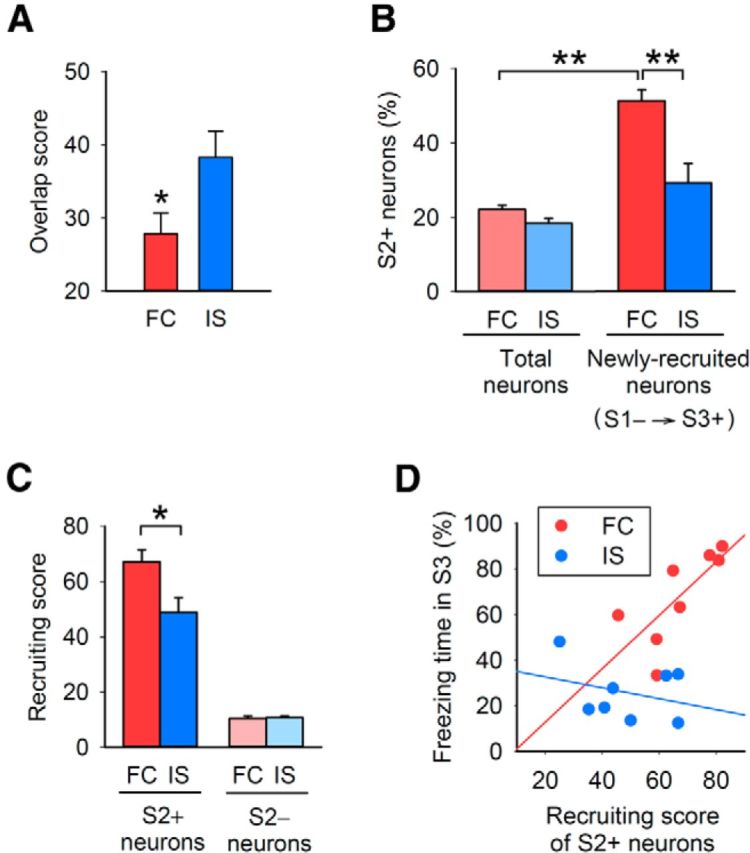
Fear conditioning alters a context-responsive BLA neuronal ensemble based on the neuronal activity during fear conditioning. A, BLA neurons that were active in session 3 overlapped less with those that were active in session 1 in the FC group compared with the IS group (Student's t test, t(14) = 2.3, p = 0.039). B, BLA neurons newly recruited into the context-responsive ensemble displayed preferential activity in session 2 depending on fear learning (repeated-measures ANOVA, F(1,14) = 11.0, p = 0.0051; total vs newly recruited neurons in the FC group, p = 1.5 × 10−5). C, The activities of individual BLA neurons during fear conditioning predicted their recruitment into the context-responsive ensemble (F(1,14) = 7.08, p = 0.019; S2+ (FC) vs S2− (FC), p = 0.00062; S2+ (FC) vs S2+ (IS), p = 0.021). D, The recruiting effect of activity during fear conditioning in the BLA correlated with the strength of fear memory expression (Pearson's correlation coefficient, r = 0.74, t(7) = 2.7, p = 0.036; Z-test, FC vs IS, z = 2.0, p = 0.023); **p < 0.01, *p < 0.05.
We further hypothesized that the newly recruited neurons were preferentially activated during conditioning, because synaptic potentiation was observed in neurons that were activated during conditioning. To test this, we measured the percentage of neurons that were active during session 2 (S2+) among the total BLA neurons and among the newly recruited neurons. In the FC group, the S2+ percentage in the newly recruited neurons was higher than that in the total BLA neurons (Fig. 4B). In the IS group, on the other hand, there was no significant difference.
To determine whether neurons activated during fear conditioning are newly recruited into the context-responsive ensemble, we calculated a recruiting score consisting of the percentage of S2+ or S2− neurons that changed their activity from inactive in session 1 to active in session 3. In the FC group, the recruiting score of the S2+ neurons was higher than that of the S2− neurons (Fig. 4C). Further analysis also indicated that the recruiting effect of session 2 activity was associated with fear conditioning: First, the recruiting score of S2+ neurons of the FC group was higher than that of the IS group (Fig. 4C); second, the recruiting score in the FC group correlated positively with the freezing time in session 3 (Fig. 4D). Together, these results indicate that fear conditioning alters the context-responsive neuronal ensemble depending on the neuronal activity during fear conditioning.
Discussion
We demonstrated that contextual fear conditioning induces presynaptic potentiation in cortical, but not thalamic, synapses on BLA neurons that were recruited into the fear memory trace. In accordance with the hypothesis that synaptic modification in a subset of neurons is likely to lead to a reorganization of neuronal ensembles, we also found that fear conditioning alters a context-responsive neuronal ensemble depending on the neuronal activity during fear conditioning. The recruiting effect of activity during fear conditioning correlated with the strength of fear memory expression. Overall, these data suggest that synaptic plasticity in a subset of BLA neurons is likely to contribute to fear memory expression through ensemble reorganization.
A novel finding of this study is that synaptic potentiation associated with learning occurs specifically in neurons that are recruited into the memory trace. Although previous studies showed that fear conditioning induces synaptic potentiation in the BLA synapses (McKernan and Shinnick-Gallagher, 1997; Tsvetkov et al., 2002), it is unclear whether fear conditioning induces synaptic modification of the amygdala globally or only in a subset of neurons. In this study, we challenged this issue by measuring synaptic efficacy from neurons recruited into a memory trace, which are labeled with Arc promoter-driven dVenus. We found that presynaptic potentiation is specifically induced in the recruited neurons (dVenus+ neurons). The observed increase in mEPSC frequency in dVenus+ neurons from conditioned mice reflects modifications in whole neurons, whereas the decrease in the PPR was limited to the cortical pathway alone. This might be due to the greater influence of cortical inputs on BLA neurons relative to other inputs. Our findings do not rule out postsynaptic plasticity (Rumpel et al., 2005; Zhou et al., 2009) as another mechanism for contextual fear conditioning. The amplitude of mEPSCs in the dVenus+ neurons was higher compared with dVenus− neurons, but there was no group × dVenus interaction. The postsynaptic enhancement effect of fear conditioning might be masked by footshock stress, which was also given to the IS group.
The activity of individual neurons during learning is likely to determine their recruitment into the memory trace. In this study, we used large-scale activity mapping with the immediate-early genes Arc and Homer 1a to determine how contextual fear conditioning alters BLA neuronal ensembles. Remarkably, we measured the activities of >300 neurons per mouse separately over three sessions by analyzing subcellular distributions of Arc and Homer 1a RNA. In doing so, we found that the neurons activated during memory retrieval were preferentially activated during preceding fear conditioning, and the neurons that were activated during fear conditioning were also preferentially activated during memory retrieval. The importance of neuronal activity during learning in recruitment is supported by previous studies using CREB transfection. CREB transfection upregulates excitability of transfected neurons (Zhou et al., 2009) and increases the recruitment ratio of those neurons into a fear memory trace (Han et al., 2007).Together, these findings suggest that the recruitment of neurons into a memory trace is defined by the activity of individual neurons during learning.
In conclusion, we have demonstrated that presynaptic potentiation occurs specifically in neurons that are recruited into a fear memory trace. This synaptic plasticity likely contributes to the ensemble reorganization, an event that positively correlated with the strength of fear memory expression. Together, our findings suggest that synaptic plasticity in a subset of BLA neurons contributes to fear memory expression through the reorganization of a context-responsive neuronal ensemble.
Footnotes
This work was supported by a Grant-in-Aid for Young Scientists (B) (25830002 to H.N.); Grant-in-Aid for Scientific Research on Innovative Areas, “Mesoscopic Neurocircuitry” (23115101 to H.N.); “The Science of Mental Time” (26119507 to H.N. and 25119004 to Y.I.); and “Memory Dynamism” (26115509 to H.N.). We thank Dr. Hiroyuki Hioki (Kyoto University) for technical advice in in situ hybridization and immunohistochemistry, Dr. Paul F Worley (Johns Hopkins University) for the Arc and Homer 1a cDNA used in this study, and the University of Tokyo/Leica microsystems imaging center for their assistance in obtaining the imaging data.
The authors declare no competing financial interests.
References
- Eguchi M, Yamaguchi S. In vivo and in vitro visualization of gene expression dynamics over extensive areas of the brain. Neuroimage. 2009;44:1274–1283. doi: 10.1016/j.neuroimage.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D, L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037/0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Matsuki N, Nomura H. Preferential arc transcription at rest in the active ensemble during associative learning. Neurobiol Learn Mem. 2011;95:498–504. doi: 10.1016/j.nlm.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Li XB, Inoue T, Nakagawa S, Koyama T. Effect of mediodorsal thalamic nucleus lesion on contextual fear conditioning in rats. Brain Res. 2004;1008:261–272. doi: 10.1016/j.brainres.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Schaner MJ, McNaughton BL, Worley PF, Barnes CA. Immediate-early gene expression at rest recapitulates recent experience. J Neurosci. 2008;28:1030–1033. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Miura Y, Naka M, Matsuki N, Nomura H. Differential calcium dependence in basal and forskolin-potentiated spontaneous transmitter release in basolateral amygdala neurons. Neurosci Lett. 2012;529:1–6. doi: 10.1016/j.neulet.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Nomura H, Matsuki N. Ethanol enhances reactivated fear memories. Neuropsychopharmacology. 2008;33:2912–2921. doi: 10.1038/npp.2008.13. [DOI] [PubMed] [Google Scholar]
- Nomura H, Nonaka A, Imamura N, Hashikawa K, Matsuki N. Memory coding in plastic neuronal subpopulations within the amygdala. Neuroimage. 2012;60:153–161. doi: 10.1016/j.neuroimage.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/S0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]