Abstract
A cortical-basal ganglia network involving, particularly, the posterior region of dorsomedial striatum (DMS) has been implicated in the acquisition of goal-directed actions; however, no direct evidence of learning-related plasticity in this striatal region has been reported, nor is it known whether, or which, specific cell types are involved in this learning process. The striatum is primarily composed of two classes of spiny projection neurons (SPNs): the striatonigral and striatopallidal SPNs, which express dopamine D1 and D2 receptors, respectively. Here we establish that, in mice, the acquisition of goal-directed actions induced plasticity in both D1- and D2-SPNs specifically in the DMS and, importantly, that these changes were in opposing directions; after learning, AMPA/NMDA ratios were increased in D1-SPNs and reduced in the D2-SPNs in the DMS. Such opposing plasticity could provide the basis for rapidly rebiasing the control of task-specific actions, and its dysregulation could underlie disorders associated with striatal function.
Keywords: dorsal striatum, electrophysiology, goal-directed action, plasticity
Introduction
Disorders of the basal ganglia, although commonly related to problems with motor control, have long been known to produce cognitive symptoms, including a deficit in the control of volitional action (Gotham et al., 1988; Albin et al., 1989). These deficits suggest a role for aspects of basal ganglia function in complex reward-related behavior; indeed, recent evidence has implicated the striatum in the learning process that underlies the acquisition of goal-directed actions (Lauwereyns et al., 2002; Balleine et al., 2007; Hikosaka, 2007). Such actions are mediated both by their causal relationship to and the value of their consequences, information that is encoded through the formation of specific action–outcome associations (Rescorla, 1991; Dickinson, 1994). This capacity for goal-directed action is fundamental to our ability to control the environment in the service of our basic needs and desires. As an executive function, it is usually regarded as involving the frontal lobe (Stuss and Alexander, 2000); however, recent evidence suggests that cortical processes play only a transitory role in goal-directed learning (Ostlund and Balleine, 2005; Tran-Tu-Yen et al., 2009) and that their acquisition depends primarily on plasticity in its striatal targets particularly, in rodents, a region of posterior dorsomedial striatum (pDMS) (Balleine et al., 2007; Balleine and O'Doherty, 2010). Previous studies found that lesion, inactivation, or blockade of plasticity in the pDMS abolished the acquisition of the goal-directed action (Yin et al., 2005; Shiflett et al., 2010); however, to date, no direct evidence of plasticity in the pDMS related to this learning has been reported.
As with other regions of striatum, the projection neurons in the pDMS are medium spiny projection neurons (SPNs), which constitute 95% of the striatal neuronal population and fall into two classes based on gene expression and their efferent projections: one expressing dopamine D1 receptors and projecting to the substantia nigra pars reticulata and a second expressing D2 receptors and projecting to the external globus pallidus (Gerfen and Surmeier, 2011). Both D1R- and D2R-expressing SPNs receive excitatory glutamatergic inputs from cortex and modulatory dopaminergic inputs from the midbrain, but as D1R and D2R are coupled with the excitatory Gs/olf and inhibitory Gi/o proteins, respectively, dopamine exerts opposing effects on their activity (Tritsch and Sabatini, 2012); and these cell types have been linked to the activation and inhibition of the behavioral functions of striatum, respectively (Kravitz et al., 2010).
Here we assessed goal-directed, learning-related plasticity in these two distinct populations of neurons by measuring phosphorylation of extracellular signal-regulated kinase (pERK) using immunohistochemical staining and the AMPA/NMDA EPSC ratio using ex-vivo patch-clamp electrophysiological recording. Two transgenic mouse lines that express GFP either in drd1-expressing SPNs (D1-GFP mice) or in the drd2-expressing SPNs (D2-GFP mice) (Gong et al., 2003) were used to distinguish these two neuronal populations.
Materials and Methods
Animals.
Male, 7–8 weeks old, C57BL/6J–Swiss Webster hybrid transgenic mice expressing the enhanced green fluorescent protein under the control of the promoter for either the D1 or the D2 dopamine receptor (D1-GFP or D2-GFP mice) (Gong et al., 2003) were used. The D1-GFP and D2-GFP transgenes are hemizygous. The mice were housed in a 12 h light/12 h dark cycle in a temperature-controlled (21°C) and humidity-controlled (50%) environment. All experiments were conducted according to the ethical guidelines approved by the University of Sydney Animal Care and Ethics Committee.
Instrumental conditioning.
Med Associates operant chambers were used for instrumental conditioning. Food supply was controlled to maintain the weight of each mouse at 80%–90% of its ad libitum level for the duration of instrumental conditioning. Two sessions of magazine-entry training were given, during each of which 30 grain pellets (20 mg each) were delivered on a random time 60 s schedule. There followed four instrumental training sessions in which mice had to press a lever to receive a grain pellet. Each session lasted until either 30 grain pellets were delivered or 1 h of time had passed. In the first sessions, the pellets were delivered on a continuous reinforcement schedule (CRF), whereas in sessions 2, 3, and 4 they were delivered on a random interval (RI) 15, 30, and 30 s, respectively. Each trained mouse had a yoked control mouse treated in the same way except that, during instrumental conditioning, the delivery of the pellet was determined by the mouse to which it was yoked rather than the lever.
Previous work in our laboratory has established that this minimal training paradigm generates goal-directed, instrumental conditioning. To confirm this, a mixed group of D1- and D2-GFP mice (n = 16) were trained and given a specific satiety devaluation test the day after the fourth training session. Half of the mice (pellet condition) were given 1 h free access to grain pellets (devalued condition), which they normally earned during the training sessions, whereas the remainder (chow condition) were given free access to the chow that they received in their home cages, for the same duration as a control for the effects of general satiety (nondevalued condition). The devaluation effect was tested in a 5 min extinction test conducted immediately after the satiety treatment. No pellets were delivered during the test. A second devaluation test was conducted the day after the first devaluation test for which the satiety (pellet or chow) conditions were reversed. As there was no significant difference between D1- and D2-GFP mice in their behavioral performance (p > 0.05), data from both days for both mouse lines were pooled for analysis.
Electrophysiological recording.
One day after the last instrumental conditioning session, mice were killed for ex vivo striatal slice recording. Mice were anesthetized using a ketamine and xylazine mixture (210 and 14 mg/kg body weight, respectively, i.p.) and decapitated. Parahorizontal striatal slices, including surrounding cortical regions (380 μm thickness), were cut on a vibratome in an ice-cold dissection solution containing the following (in mm): 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, 10 glucose, and 200 sucrose (saturated with 95% O2/5% CO2, osmolarity 295–305 mOsm). Slices were recovered for at least 1 h in an ACSF solution containing the following (in mm): 126 NaCl, 2.5 KCl, 2 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, 10 glucose (saturated with 95% O2/5% CO2, osmolarity 310–320 mOsm).
During recording, slices were perfused continuously with ACSF (1–2 ml/min) at 31°C-32°C. Picrotoxin (100 μm) was added to the ACSF solution to suppress any GABAergic inhibitory response. Whole-cell voltage-clamp recordings were performed on the D1R- and D2R-expressing SPNs. SPNs were identified by their medium size and lack of spontaneous firing. D1R- and D2R- expressing SPNs were distinguished from each other by the absence or presence of GFP fluorescence, respectively. Recording pipettes (2.5–4 mΩ) were filled with an internal solution containing the following (in mm): 120 CsMeSO3, 15 CsCl, 8 NaCl, 10 HEPES, 0.4 EGTA, 3 QX-314, 2 Mg2ATP, and 0.33 Na3GTP (pH 7.3 and osmolarity 280–290 mOsm). For both DMS and dorsolateral striatum (DLS) recordings, a bipolar stimulation electrode was placed in the white matter between the dorsal striatum and the cortex (see Fig. 2D) and used to evoke EPSC at a frequency of 0.05 Hz. Stimulation intensity was set to induce EPSCs with amplitude 50–200 pA. Neurons were voltage-clamped either at −70 mV or at 40 mV. The ratio, AMPA/NMDA, was calculated by dividing the peak amplitude of the EPSC recorded at −70 mV (AMPA) by the averaged magnitude, 50–60 ms after the stimulation artifact, of the EPSC recorded at 40 mV (NMDA).
Figure 2.
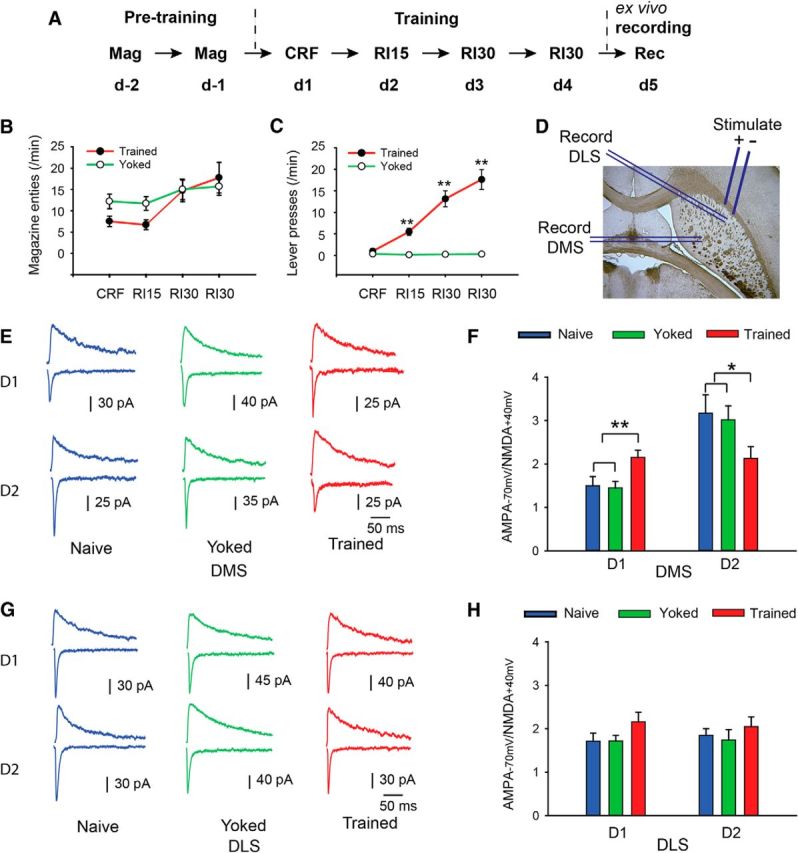
Ex vivo electrophysiological assessment of plasticity in D1R- and D2R-expressing SPNs in the pDMS after action–outcome learning. A–C, D2-GFP mice were given training sessions in which reward delivery was either paired with lever pressing (trained) or unpaired with lever pressing (yoked). Rate of magazine entries (two-way repeated-measures ANOVA, F(1,117) = 0.5, p > 0.05; n = 22 and 19 for trained and yoked mice, respectively) and rate of lever presses (two-way repeated-measures ANOVA, F(1,117) = 50.5, p < 0.01; post hoc test, **p < 0.01) were recorded and compared between the yoked and the trained mice. D, Parahorizontal striatal slices were prepared, and the stimulation electrode was placed at the white matter between the cortex and the dorsal striatum, and either GFP-positive or GFP-negative neurons in the pDMS or the DLS were recorded. E–H, In the trained and yoked groups, the induction of action–outcome learning had a clear effect on plasticity with the D1R-expressing SPNs in the pDMS demonstrating a higher AMPA/NMDA ratio in the trained group than in the yoked or naive groups. Conversely, the D2R-expressing SPNs in the pDMS demonstrated a lower AMPA/NMDA ratio in the trained group than in the yoked or naive groups (E, F), indicating opposing changes in plasticity in the D1- and D2-expressing neurons. For D1 neurons in the pDMS, one-way ANOVA revealed no difference between naive versus yoked groups (F < 1), but a significant difference between the trained group and both the naive and yoked groups (F(1,46) = 10.9, p < 0.01; post hoc test, **p < 0.01). n = 13–22. For the D2 neurons in the pDMS, the naive and yoked groups again did not differ (F < 1). However, the trained group differed from both the naive and yoked groups (F(1,41) = 5.70). *p < 0.05. n = 13–15. Furthermore, these effects were only found in the pDMS and did not extend to the DLS: the AMPA/NMDA ratio of the D1R- and D2R-expressing SPNs in the DLS was not significantly different between the trained and yoked or naive groups (G, H: ANOVA, p > 0.05, n = 12–19).
Immunofluorescence imaging.
Immediately after the last training session, the mice were anesthetized with lethabarb (300 mg/kg; i.p. injection) and transcardially perfused with 4% PFA in 0.1 m sodium phosphate buffer, pH 7.4. The brains were removed and postfixed overnight in the same solution at 4°C. The brains were then cut into 30-μm-thick coronal sections using a vibratome (VT1000, Leica Microsystems) and stored at −30°C in a cryoprotectant solution (30% ethylene glycol, 30% glycerol, and 0.1 m sodium phosphate buffer) until they were further processed for immunofluorescence detection. Three sections were selected from each mouse in each of two ranges of bregma coordinates (0 to −0.3 mm, and 0.4 to 0.7 mm) and used for immunofluorescence detection of the pDMS and DLS, respectively.
Free-floating sections were rinsed in TBS containing NaF (0.25 m Tris, 0.5 m NaCl, 0.1 mm NaF, pH 7.6) three times for 10 min each and then treated in 3% H2O2 for 5 min. For the D1-GFP mice, sections were permeabilized and blocked using 0.5% Triton X-100 and 10% normal goat serum in TBS for 30 min, probed with rabbit anti-phospho-p44/42 MAPK (ERK1/2) (1:1000; Cell Signaling Technology) and chicken anti-GFP (1:1000; Aves Laboratories) for 48 h at 4°C, and labeled with Cy3-conjugated donkey anti-rabbit secondary antibody (1:1000, Invitrogen) and FITC-conjugated goat anti-chicken secondary antibody (1:1000, Jackson ImmunoResearch Laboratories) for 4 h at room temperature.
Because the GFP fluorescence signal in D2-GFP mice can be readily detected by microscope, immunostaining of the GFP was skipped in the D2-GFP mice. Sections from the D2-GFP mice were permeabilized and blocked using 0.5% Triton X-100 and 10% normal horse serum in TBS for 30 min, probed with rabbit anti-phospho-p44/42 MAPK (ERK1/2) (1:1000; Cell Signaling Technology) for 48 h at 4°C, and labeled with AlexaFluor-546-conjugated goat anti-rabbit IgG secondary antibody (1:1000, Invitrogen) for 4 h at room temperature.
Sections were finally mounted in ProLong Gold (Invitrogen) medium and incubated at 4°C for at least 12 h before imaging. Images of the pDMS and the DLS were obtained using confocal laser scanning microscopy (Olympus Fluoview FV300, BX61WI microscope) using a 20× objective (numerical aperture 0.75). Sequential laser scanning (step size: 1.16 μm, 15–19 layers) was applied to obtain a stack of 635.90 μm2 optical sections in each hemisphere of each brain section. All stacks were processed and quantified using ImageJ. Each stack was transformed into a Z-projection with maximum projection, and the numbers of FITC- and Cy3-fluorescent cells in the D1-GFP group, and GFP- and AlexaFluor-546-fluorescent cells in the D2-GFP group, were counted by an experimenter blind to training condition.
Results
Goal-directed learning increases activity in D1R-expressing SPNs in dorsomedial striatum
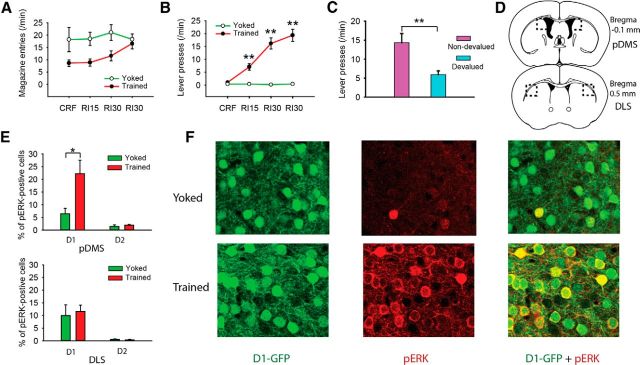
For each mouse line, we established two groups: one trained with action and outcome delivery paired and a second serving as a control group given yoked exposure to the reward outcome and for which lever pressing and reward delivery were unpaired (Fig. 1B). As expected, lever pressing in the trained group increased over the 4 d relative to the yoked group (Fig. 1B). Both the D1- and D2-GFP mice behaved similarly to their wild-type littermates or C57B6 mice. Indeed, a separate group composed of both D1- and D2-GFP mice were given paired training and then tested in extinction after devaluation of the outcome by specific satiety or after satiety on their maintenance diet. These mice demonstrated sensitivity to outcome devaluation, indicating that their performance was goal-directed (Fig. 1C). Analysis of the immunohistochemistry in the initial two groups found that pERK was significantly elevated in the D1R-expressing SPNs (GFP-positive cells in D1-GFP mice) in the pDMS (Fig. 1D–F) of the trained mice. In contrast, the percentage of pERK-positive neurons in D2R-expressing SPNs (GFP-positive cells in D2-GFP mice) in the pDMS did not differ between the yoked and the trained mice (Fig. 1D,E). These effects were specific to the pDMS; in the DLS, the percentage of pERK-positive D1R-expressing and D2R-expressing SPNs did not differ between groups (Fig. 1D,E). These results confirm the involvement of a D1 receptor-related process in goal-directed learning in the pDMS and suggest that D1 and D2 SPNs play distinct roles in this process.
Figure 1.
D1R- and D2R-expressing SPN involvement in pERK-related activity associated with action–outcome encoding. A, B, Groups of D1- and D2-GFP mice (n = 4–6) were either trained to lever press for food pellets (trained) or received the food pellet delivery unpaired with lever pressing (yoked). Rate of magazine entries (two-way repeated-measures ANOVA, F(1,48) = 6.8, p < 0.05; post hoc test, p > 0.05) and rate of lever presses (two-way repeated-measures ANOVA, F(1,48) = 101, p < 0.01; post hoc test, **p < 0.01) were recorded and compared between the yoked and the trained mice. C, This training schedule induced a goal-directed outcome association, as a separate group of D1- and D2-GFP mice (n = 9 and 7, respectively) that were trained using the same schedule, demonstrated sensitivity toward outcome devaluation (one-way ANOVA, F(1,30) = 10.4). *p < 0.01. D, The pERK-positive neurons in the pDMS (top) and DLS (bottom) were counted in the indicated regions of coronal sections (dashed line). E, The pERK-positive neurons in the pDMS (top) and DLS (bottom) were expressed as the percentage of D1R-expressing SPNs (GFP-positive cells in D1-GFP mice) and the D2R-expressing SPNs (GFP-positive cells in D2-GFP mice). The percentage of pERK-positive neurons among the D1R-expressing SPNs in the pDMS was significantly increased (F(1,9) = 6.32). *p < 0.05, trained versus yoked in D1 group of the pDMS. Other comparisons in the top and bottom were not significant (all F values <1). F, Representative samples from D1-GFP mice illustrating the proportion of pERK-positive neurons in the pDMS in the yoked and trained groups.
One concern over the interpretation of the data in this experiment is that the changes in pERK might reflect higher activity in the trained mice. However, this was not the case. We used magazine entry as a means of comparing activity in the two groups, which, indeed, was actually numerically higher early in training in the yoked group than in the trained group, although this difference diminished with training (Fig. 1A); indeed, there was a main effect of magazine entry during training (Fig. 1).
Opposing synaptic plasticity in the D1R- and D2R-expressing SPNs in dorsal striatum is induced by goal-directed learning
Although the results of the immunohistochemistry were clear, it is possible that levels of pERK detected shortly after training reflected a temporary change in neuronal activity associated with performance rather than learning (Obrietan et al., 1998; Kelleher et al., 2004; Thomas and Huganir, 2004; Eckel-Mahan et al., 2008). To overcome this limitation, we used ex vivo electrophysiology to record long-term changes in the plasticity of D1R- and D2R-expressing SPNs in the pDMS and DLS in striatal slices prepared the day after the final training session (Fig. 2). As GFP fluorescence in the D2-GFP mice can be detected directly without any signal amplification, we performed electrophysiological recording on this mouse line. As the large majority of the GFP-negative cells are D1R-expressing SPNs, we recorded from both the GFP-negative and GFP-positive neurons to sample both the D1R- and D2R-expressing SPNs, respectively. We again used two groups: a trained group and a yoked control group (Fig. 2A–C). Synaptic plasticity is manifest in the trafficking and modulation of glutamate receptors (Kerchner and Nicoll, 2008), which produces a change in the ratio of AMPA- to NMDA-induced currents (AMPA/NMDA ratio). To test whether any synaptic plasticity occurred in the striatum on formation of the action–outcome association, we measured the AMPA/NMDA ratio of D1R- and D2R-expressing SPNs in the pDMS and DLS, on the day after the fourth training session (Fig. 2D).
We found that the D1R-expressing SPNs in the pDMS demonstrated a higher AMPA/NMDA ratio in the trained group than in both the yoked or naive groups (Fig. 2E,F). In contrast, the D2R-expressing SPNs demonstrated a lower AMPA/NMDA ratio in the trained group than in the yoked or naive group (Fig. 2E,F). We also measured the AMPA/NMDA ratios in the trained and yoked groups in the D1R- and D2R-expressing SPNs in the DLS, a region of striatum known not to be necessary for goal direct learning (Yin et al., 2008; Balleine and O'Doherty, 2010). In sharp contrast to the pDMS, no significant change was found between the trained and the yoked or naive groups for both types of SPNs in this area (Fig. 2G,H). Finally, to eliminate the possibility that the changes in synaptic strength reflected the higher activity of the trained mice, we again recorded the rate of magazine entries. Again, numerically more magazine entries were observed in the yoked than in the trained group early in training; and again, this difference reduced as training progressed (Fig. 2B). Statistical analysis found, however, no significant differences between groups in this experiment (Fig. 2).
Changes in synaptic plasticity in SPNs are often accompanied by selective trafficking of AMPA and NMDA receptors (Conrad et al., 2008) that may be reflected in synaptic current decay times. For example, a reduced decay time constant (τ) may indicate increased contribution of Ca2+-permeable GluR2 subunits to the synaptic AMPA current (Thiagarajan et al., 2005). With training, however, we found no significant differences in τ for either AMPA or NMDA receptor currents in pDMs or DLS. There was a small trend for a reduced τ of AMPAR currents in pDMS D1 neurons (4.86 ± 0.26 ms, n = 22) versus naive (5.52 ± 0.35 ms, n = 13), but the yoked group was also reduced (4.83 ± 0.34 ms, n = 13). Conversely, a small increase in τ of AMPAR currents in pDMS D2 neurons (4.70 ± 0.32 ms, n = 15) versus naive (3.87 ± 0.36 ms, n = 14) and yoked (4.10 ± 0.32 ms, n = 15) groups was not significant. These results suggest that the subunit composition of AMPARs and NMDARs was not substantially altered during training. Because decay of macroscopic synaptic currents might not be sensitive enough to detect altered subunit composition, it will be important to confirm this in future by examining τ of quantal synaptic currents and rectification index.
Discussion
The current results provide evidence of a functionally important, localized, bidirectional change in plasticity in both direct and indirect pathway neurons associated with encoding the action–outcome associations that enable goal-directed action. Our data suggest that, during the course of acquiring goal-directed actions, activation, which is indicated by calcium rise, in both D1- and D2-expressing SPNs (Cui et al., 2013), induces opposing synaptic plasticity at both populations of neurons, and the fact these changes persisted at least 24 h after training suggests that they likely reflect LTP- and LTD-like plasticity in D1R- and D2R-expressing SPNs, respectively. Mechanically, this is possible: the formation of both LTP and LTD requires synaptic activation and an increase in intracellular calcium (Akopian et al., 2000; Kim et al., 2013).
These results also suggest that direct and indirect pathways of the striatum are not only structurally but also functionally heterogeneous. Models of the basal ganglia emphasize the importance of the interaction between direct and indirect pathways for specific functional effects (Alexander and Crutcher, 1990; Gerfen and Surmeier, 2011). It is important from this perspective, therefore, that we found differences in the AMPA/NMDA ratio in the D1 and D2 SPNs of naive animals in the pDMS, suggesting that the balance of activity in these circuits may be weighted toward the indirect pathway and so toward the inhibition of functional output from this region in untrained animals. As this region has been most directly linked with the acquisition and deployment of goal-directed actions (i.e., actions that are highly flexible, rapidly acquired, and under cognitive control), this finding implies that the basal ganglia is biased against allowing cognitive states rapidly to elicit action. Indeed, the current results suggest that, in order for cognitive processes reliably to elicit actions, plasticity affecting both direct and indirect pathway neurons may be required; the acquisition and deployment of goal-directed action depend on an increase in the synaptic plasticity of direct, and a reduction in the activity of indirect, pathway neurons.
It is also interesting to note that this is not true for other regions of the striatum, most notably, as others have found and we report here (see also Yin et al., 2009), the DLS, which has been related to skilled or reflexive motor movements that depend on the occurrence of specific eliciting stimuli for their deployment (Graybiel, 2008). Indeed, evidence suggests that the striatum plays a pivotal role in executing responses of many types dependent on rewarding, reinforcing, and punishing feedback as well as skilled movements and conditioned reflexes (Yin et al., 2009; Kravitz et al., 2012; Tai et al., 2012). As these functions are known to depend on distinct learning rules and distinct regions of the striatum, these data suggest that they may also depend on different relative changes in the plasticity in direct and indirect pathway neurons (Reynolds et al., 2001; Lovinger, 2010). Hence, establishing the necessary plasticity in the striatum subserving specific behavioral functions will require comparisons of striatal subregions as well as the different cell types within those regions.
Such differences also have clear implications for disorders associated with striatal function; these disorders often induce region-specific changes in activity in the striatum (Dichter et al., 2012), and the possibility that distinct functions and plasticity processes are mediated by those regions suggests that both behavioral and plasticity-related measures may be usefully applied to enhance diagnostic acuity and tailor treatment options.
Footnotes
This work was supported by the Australian Research Council Grant DP110105636, and a Laureate Fellowship from the Australian Research Council to B.W.B. and an National Health and Medical Research Council Senior Principal Research Fellowship to M.J.C.
The authors declare no competing financial interests.
References
- Akopian G, Musleh W, Smith R, Walsh JP. Functional state of corticostriatal synapses determines their expression of short- and long-term plasticity. Synapse. 2000;38:271–280. doi: 10.1002/1098-2396(20001201)38:3<271::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Instrumental conditioning. In: Mackintosh NJ, editor. Animal cognition and learning. London: Academic; 1994. pp. 4–79. [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson's disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Hawes SL, Gillani F, Wallace LJ, Blackwell KT. Signaling pathways involved in striatal synaptic plasticity are sensitive to temporal pattern and exhibit spatial specificity. PLoS Comput Biol. 2013;9:e1002953. doi: 10.1371/journal.pcbi.1002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Associative relations in instrumental learning: the Eighteenth Bartlett Memorial Lecture. Q J Exp Psychol. 1991;43:1–23. doi: 10.1080/14640749108400996. [DOI] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tran-Tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur J Neurosci. 2009;30:464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]