Abstract
The roles of the motor cortex in the acquisition and performance of skilled finger movements have been extensively investigated over decades. Yet it is still not known whether these roles of motor cortex are expertise-dependent. The present study addresses this issue by comparing the effects of noninvasive transcranial direction current stimulation (tDCS) on the fine control of sequential finger movements in highly trained pianists and musically untrained individuals. Thirteen pianists and 13 untrained controls performed timed-sequence finger movements with each of the right and left hands before and after receiving bilateral tDCS over the primary motor cortices. The results demonstrate an improvement of fine motor control in both hands in musically untrained controls, but deterioration in pianists following anodal tDCS over the contralateral cortex and cathodal tDCS over the ipsilateral cortex compared with the sham stimulation. However, this change in motor performance was not evident after stimulating with the opposite montage. These findings support the notion that changes in dexterous finger movements induced by bihemispheric tDCS are expertise-dependent.
Keywords: dexterity, expertise, motor cortex, motor skill acquisition, tDCS
Introduction
Skilled finger movements represent a highly sophisticated human activity. The primary motor cortex plays the most important role in dexterous use of the hand (Gentner et al., 2010). Previous studies have demonstrated an increase in neural activity of the motor cortex during the acquisition of hand motor skills (Grafton et al., 1995; Karni et al., 1995; Honda et al., 1998; Steele and Penhune, 2010). Furthermore, extensive training of sequential finger movements enlarges neural representations of finger muscles in the primary motor cortex (Pascual-Leone et al., 1995). Enhancement of motor cortical excitability by transcranial direct current stimulation (tDCS) delivered over the primary motor cortex has also been found to improve fine motor control of the contralateral hand (Nitsche et al., 2003; Vines et al., 2008b; Reis et al., 2009). In addition, suppression of the ipsilateral motor cortex by tDCS enhanced hand motor skills (Vines et al., 2008a), possibly due to reduced intercortical inhibition of the contralateral cortex (Williams et al., 2010; Sehm et al., 2013). However, previous studies have focused primarily on neuroplasticity subserving motor skill acquisition in untrained healthy individuals (Dayan and Cohen, 2011) and in patients with neurological disorders (Fregni and Pascual-Leone, 2007; Bradnam et al., 2012; Schulz et al., 2013). Our understanding of the function of the motor cortex in skilled finger movements in highly trained individuals, such as musicians, is limited.
Evidence does exist for neuroplastic adaptations of the motor cortex through extensive musical training (Münte et al., 2002). Several studies have demonstrated reduced activation of the primary motor cortex contralateral to the hand that underwent complex finger movements in musicians, compared with untrained individuals (Jäncke et al., 2000; Krings et al., 2000). Musicians with focal dystonia are characterized by abnormal overactivation of the motor cortex (Pujol et al., 2000; Haslinger et al., 2010) and reduction of surround inhibition (Rosenkranz et al., 2009), which may underlie loss of fine motor control (Furuya and Altenmüller, 2013a, b). It is therefore possible that facilitation of motor cortical excitability degrades skilled finger movements of the contralateral hand in trained musicians as opposed to untrained individuals. Indeed, a long-term manual training study reported a nonlinear relation between motor cortical excitability and training duration (Koeneke et al., 2006). In contrast, training of complex finger movements elicited a larger improvement in motor skills and larger increase in neuronal activity at the contralateral primary motor cortex for pianists than nonmusicians (Hund-Georgiadis and von Cramon, 1999). This finding raises the alternative possibility that fine motor control of the hand may be improved by facilitating the contralateral motor cortex, as has been observed in untrained individuals.
To address the question of whether functional changes in the motor cortex during fine motor control depend on expertise, we assessed differences in the effects of noninvasive transcranial stimulation on skilled finger movements of highly trained pianists compared with untrained individuals. Probing the effects of tDCS possibly overcomes a potential limitation of the above-mentioned previous neuroimaging studies, namely, the difficulty in deciding whether distinct motor cortical activity in musicians is the cause or an epiphenomenon of their superior motor performance. We also assessed polarity-dependent changes in fine motor control of the hand following tDCS to determine whether facilitation and suppression yield symmetric behavioral outcomes.
Materials and Methods
Participants.
Thirteen right-handed expert pianists with no history of neurological disorders (8 females, 18–31 years old) and 13 right-handed nonmusicians with no experience of studying piano playing (6 females, 20–31 years old) participated in the experiment. All expert pianists had majored in piano at music conservatories and had a history of at least 13 years of extensive piano training (range 13–27 years; 18.0 ± 3.7 years). The Edinburgh handedness test showed that all participants were right-handed (range from 40 to 80 for all participants) (Oldfield, 1971). In accordance with the Declaration of Helsinki, the experimental procedures were explained to all participants. Informed consent was obtained from all participants before participation in the experiment, and the experimental protocol was approved by the ethics committee of the Hanover Medical School.
Experimental design.
Each individual was asked to participate in three experimental sessions with different stimulation protocols, each of which was separated by >2 weeks to minimize any carryover effect of the stimulation. The order of the stimulation protocols was balanced across participants, and the experimental design was double-blinded.
Each experimental session consisted of a pretest, stimulation, and post-test. tDCS was applied during a state of rest. Participants sat on a chair and did not perform any movements. We used a bihemispheric tDCS that may elicit more pronounced improvement of hand motor skills (Vines et al., 2008b) and facilitation of motor cortical excitability (Lindenberg et al., 2013) than conventional unihemispheric stimulation. Two active water-soaked tDCS electrodes were put on locations C3 and C4 (primary motor cortex), which were identified using the international 10–20 electroencephalogram system. We stimulated the motor cortex because this region represents the motor program responsible for acquisition of hand motor skills (Gentner et al., 2010). The current montage was adopted to modulate cortical excitability of both hemispheres simultaneously. To minimize current shunt between the electrodes over the scalp, the location of the electrodes was carefully selected so that the distance between the edges of the electrodes was at least 6 cm (Rush and Driscoll, 1968). tDCS was applied throughout the entire stimulation session, which lasted for 15 min. The three stimulation protocols were referred to as “RaLc,” “RcLa,” and “sham.” For the “RaLc” and “sham” conditions, the cathodal electrode (excitability diminution) was placed on the left hemisphere and the anodal (excitability enhancement) on the right side of the cortex, and vice versa for the “RcLa” condition. The intensity of stimulation was 2 mA; tDCS lasted for 15 min for the “RaLc” and “RcLa” protocols, but for only the initial 30 s for the “sham” protocol. tDCS was induced through sponge electrodes (surface = 35 cm2) and delivered by a battery-driven constant-current stimulator (eldith). This method has already been used in numerous studies and is regarded as safe (Nitsche and Paulus, 2000). The ramping time of the stimulation was 8 s.
During the pretest and post-test sessions, each participant played a sequence of keystrokes for 8 s with the right and left hands in synchrony with a metronome (interkeystroke interval = 333 ms) as accurately as possible. A sequence consists of successive strikes of four adjacent piano keys with four fingers serially and repeatedly (i.e., L → R → M → I → M → R → L →…; I, M, R, and L indicates the index, middle, ring, and little finger, respectively). The keys to be struck were G, F, E, and D for the right hand, and C–F for the left hand. We chose a short test sequence to reduce performance improvements due to repetition.
Before the first day of experiment, each participant was asked to familiarize themselves with the task. In particular, nonmusicians were instructed during a preliminary session as to which keys had to be struck with which fingers. The familiarization session continued until he/she could perform the task consistently without erroneous keystrokes. This session took maximally 1 min for the expert pianists and maximally 10 min for the nonmusicians. At the end of the session, all participants could perform the sequential keystrokes readily.
Data acquisition and analysis.
We recorded the time of each keystroke in pre- and post-test conditions with a custom-made script running on LabView (Furuya and Soechting, 2010). The SD of the interkeystroke intervals (IKI-SD) across strokes, and both mean and SD of the finger-key contact duration (CD-mean, CD-SD) across strokes were computed. A small value of the IKI-SD and CD-SD indicates high evenness of striking and lifting keys with very low rhythmic variability. A small value of the CD-mean indicates quick transition from the striking to lifting motion. In particular, a quick transition of movement direction of individual fingers is difficult in the current movement task because the performed sequential movements make independent movement of the fingers difficult. The amount of change in each of the variables by the stimulation was evaluated by computing a ratio value between the pretest and post-test. We computed a post/pre ratio value to normalize potential interindividual differences in fine motor control across participants.
Statistical analysis.
Based on previous findings regarding the tDCS effects on fine motor control (Lefebvre et al., 2014) and independent control of finger movements (Waters-Metenier et al., 2014), we hypothesized changes in movement variability of key-presses (IKI-SD) and key-releases (CD-SD) and movement quickness (CD-mean) following the stimulation and their difference between the trained and untrained players. To test whether the effect of the stimulation depended on electrode montage, performing hand, and/or expertise, a three-way ANOVA with mixed design using “stimulation protocol” (“RaLc,” “RcLa,” sham) and “hand” (right and left) as the within-factors and “group” (expert pianists and nonmusicians) as the between-factor was performed (p < 0.05). Each of the dependent variables was standardized by dividing by the baseline performance (i.e., a ratio value between the pretest and post-test). We did not expect effects of repetition of the tests over 3 d on motor performance because: (1) the sequence used for the test was fairly short; and (2) the initial familiarization session was done until the performance reached a plateau. Indeed, none of the aforementioned dependent variables showed any significant intersession learning effects (p > 0.05). Post hoc tests were performed using t tests with multiple-comparison correction (Benjamini and Hochberg, 1995) in case of significant ANOVA results. The statistical analyses were performed using R (version 3.0.2). We used a partial η-squared (η2) measure as an index of effect size, which was computed using an R package called “ez.”
Results
Baseline performance at the pretest
To assess whether motor performance at pretest was invariant across stimulation protocols and different between the groups, a three-way ANOVA with mixed design was performed (Table 1). The results showed a significant main effect of group on each of the variables (IKI-SD: F(1,24) = 78.12, p = 5.17 × 10−9, η2 = 0.63; CD-mean: F(1,24) = 4.98, p = 0.04, η2 = 0.24; CD-SD: F(1,12) = 7.86, p = 0.01, η2 = 0.22), which confirms larger values for the nonmusicians than the pianists. No main effect of protocol or interaction effect of protocol and group was identified for any of the variables (p > 0.05, η2<0.01), which indicates no difference of motor performance at the pretest across the stimulation protocols.
Table 1.
Baseline motor performance at the pretesta
| Hand | Motor performance (ms) |
||||||
|---|---|---|---|---|---|---|---|
| Pianists |
Nonmusicians |
||||||
| RaLc | RcLa | Sham | RaLc | RcLa | Sham | ||
| IKI-SD | Right | 10.6 (2.6) | 12.1 (3.8) | 11.4 (2.1) | 29.4 (7.3) | 27.0 (7.7) | 27.4 (8.7) |
| Left | 10.8 (3.4) | 10.9 (2.6) | 11.4 (2.8) | 32.9 (15.2) | 30.0 (9.6) | 30.0 (7.9) | |
| CD-mean | Right | 321.1 (40.4) | 311.4 (35.1) | 326.1 (42.2) | 404.4 (40.5) | 406.6 (39.5) | 398.6 (41.6) |
| Left | 310.4 (31.3) | 318.1 (40.4) | 319.8 (41.5) | 404.1 (43.0) | 399.9 (38.0) | 389.9 (20.9) | |
| CD-SD | Right | 23.4 (10.0) | 22.0 (8.7) | 23.7 (11.2) | 51.0 (33.5) | 48.4 (27.5) | 48.3 (23.4) |
| Left | 20.8 (7.6) | 21.6 (9.1) | 19.6 (8.2) | 63.9 (46.8) | 56.5 (32.6) | 52.7 (25.0) | |
a Values are mean (SD).
Timing of key-striking and key-lifting motions at pretest and post-tests
Figure 1 displays group means of the key-striking and key-release timing of the first seven strokes at the pretest (top) and post-test (bottom) with the left hand in the RcLa condition by each of expert pianists (left) and nonmusicians (right). Time 0 indicates the moment of the initial stroke. At the pretest, the finger-key contact duration was ∼300 ms and constant across strokes for the pianists, and 400 ms and variable across strikes for the nonmusicians. At the post-test, this feature was maintained for the experts, whereas the nonmusicians displayed shorter and more consistent finger-key contact duration.
Figure 1.
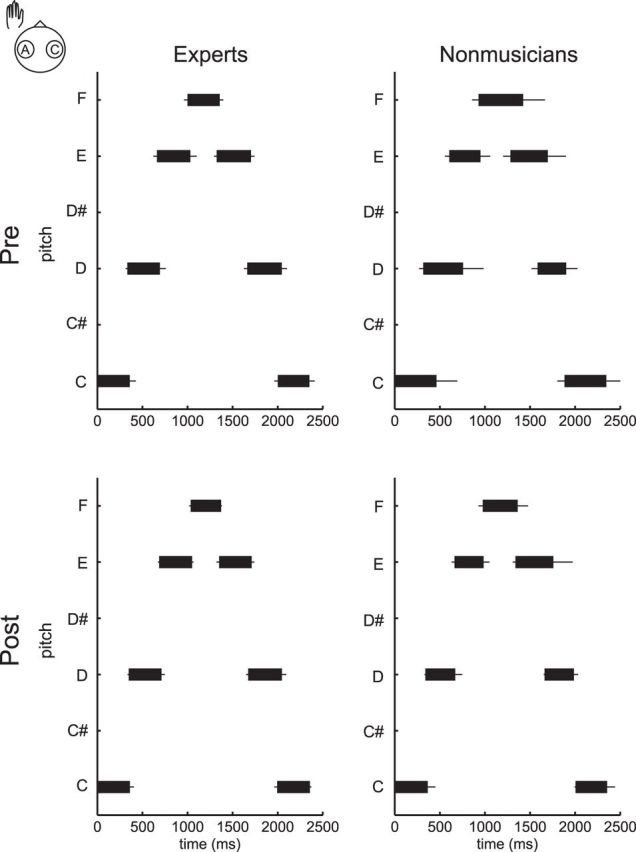
Group means of the key-press and key-release timings of the first seven strokes in the pretest (top) and post-test (bottom), with the left hand in the RcLa condition in the expert pianists (left) and nonmusicians (right). A left and right edge of a black horizontal bar corresponds to timing of the key-press and key-release of each stroke, respectively. x-axis and y-axis indicates time and pitch, respectively. Time 0 indicates the moment of the initial stroke.
Group differences in effects of bihemispheric tDCS
Figure 2 illustrates group means of ratio values for the IKI-SD, CD-mean, and CD-SD between pretest and post-test with the right and left hands in three stimulation conditions in the pianists and nonmusicians. For the IKI-SD (Fig. 2A,B), three-way mixed-design ANOVA identified both interaction effect between group and protocol (F(2,48) = 10.19, p = 2.06 × 10−4, η2 = 0.16) and main effect of group (F(1,24) = 17.46, p = 3.35 × 10−4, η2 = 0.15). Post hoc tests found a larger value for the experts than the nonmusicians in the RcLa condition at the right hand, and in the RaLc condition at the left hand. Only for the nonmusicians, there was a significant difference between the RaLc and sham conditions at the left hand. None of the remaining interaction and main effects was significant (p > 0.05).
Figure 2.
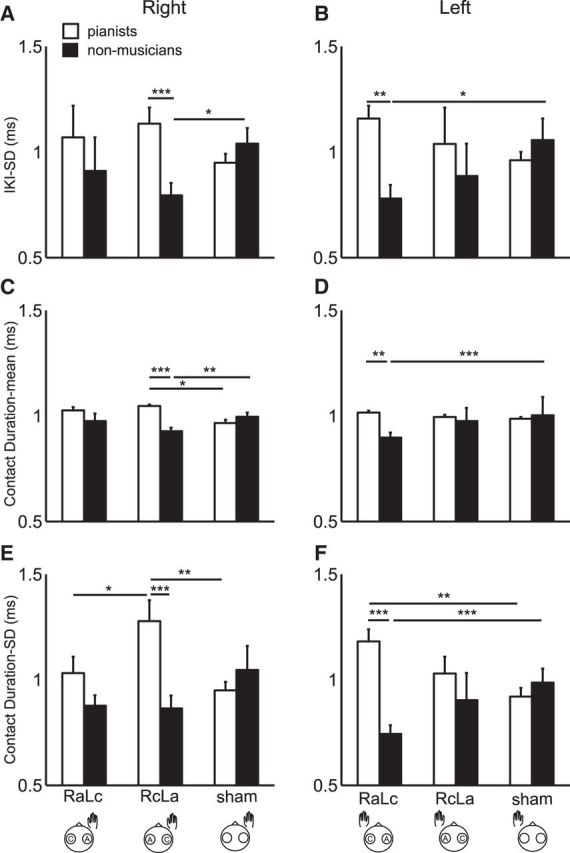
Group means of the change in the IKI-SD (A, B), CD-mean (C, D), and CD-SD (E, D) following the stimulation for the expert pianists (black) and nonmusicians (white) at the right (A, C, E) and left hand (B, D, F). x-axis indicates the stimulation protocols. *p < 0.05. **p < 0.01. ***p < 0.001. Error bar indicates SEM within each of the groups.
For the CD-mean (Fig. 2C,D), the three-way mixed-design ANOVA identified significant interaction effects of group and protocol (F(2,48) = 14.51, p = 1.17 × 10−5, η2 = 0.21). In addition, there was a main effect of group (F(1,24) = 5.25, p = 0.03, η2 = 0.03) and condition (F(2,48) = 3.96, p = 0.03, η2 = 0.07). None of the remaining interactions or main effects was significant (p > 0.05). For the right hand, post hoc tests identified a larger value in the RcLa condition compared with the sham condition in the pianists, and vice versa in the nonmusicians. In addition, only in the RcLa condition, the value was larger for the pianists than the nonmusicians. For the left hand, nonmusicians showed a smaller value in the RaLc condition compared with the sham condition. In addition, only in the RaLc condition, a group difference was evident, displaying a smaller value for the nonmusicians. There was no significant difference between the right and left hands for any of the groups or protocols.
For CD-SD (Fig. 2E,F), the three-way mixed-design, ANOVA identified significant interaction effects of group, protocol, and hand (F(2,48) = 7.59, p = 0.001, η2 = 0.06) and of group and protocol (F(2,48) = 16.50, p = 3.51 × 10−6, η2 = 0.23). In addition, there was a main effect of group (F(1,24) = 13.77, p = 0.001, η2 = 0.09). Again, none of the remaining interactions and main effects was significant (p > 0.05). For the right hand, post hoc tests identified a larger value in the RaLc condition compared with the sham condition in the pianists, and vice versa in the nonmusicians. In addition, only in the RaLc condition, the value was larger for the pianists than the nonmusicians. For the left hand, the RcLa condition showed a larger value compared with both the RaLc and sham conditions only for the experts. In addition, the value was significantly larger for the experts than the nonmusicians in the RcLa condition.
Discussion
The results of the present study demonstrate contrasting effects of noninvasive transcranial stimulation over the primary motor cortex on fine control of finger movements between pianists and untrained individuals. These findings thus provide evidence for expertise-dependent functional roles of motor cortices in fine motor control. In untrained individuals, both finger-key contact duration and its variability during keystrokes, as well as the variability of the interkeystroke interval, were decreased following anodal and cathodal stimulation over the contralateral and ipsilateral motor cortex. This observation of enhanced fine motor control corroborates previous findings of improved accuracy of finger movements caused by anodal tDCS over the contralateral motor cortex (Vines et al., 2006), cathodal tDCS over the ipsilateral motor cortex (Vines et al., 2008a), and both (Vines et al., 2008b; Waters-Metenier et al., 2014). These findings suggest an association between facilitation of motor cortical excitability and skilled finger movements. Skill acquisition of sequential finger movements through practice in untrained individuals involves enhancement of contralateral motor cortical excitability (Pascual-Leone et al., 1995). The present bihemispheric tDCS may further enhance facilitation of motor cortical excitability associated with acquisition of fine motor control.
By contrast, the highly trained pianists did not display such an improvement in motor skill following stimulation. Instead, the movement variability of the hand contralateral to the anodal stimulation was even increased after the stimulation, which indicates deterioration of skilled finger movements. Skillful finger movements during successive pressing of keys require independent control of individual fingers, such as flexing one finger for pressing down a key while extending the adjacent finger for lifting a key (Furuya et al., 2011). A fine-tuned spatiotemporal pattern of activation and deactivation of multiple finger muscles thus ensures accurate production of precisely timed sequential finger movements (Parlitz et al., 1998; Winges et al., 2013). Neuroimaging studies also report smaller motor cortical activations in pianists compared with untrained individuals during complex finger movements, which suggests selective recruitment of specific motor neurons (Jäncke et al., 2000; Krings et al., 2000). Facilitation of motor cortical excitability in trained pianists may therefore disrupt selective neuromuscular recruitment, such as unwanted facilitation of motor neurons innervating muscles to be suppressed. In line with this hypothesis, studies using transcranial magnetic stimulation have demonstrated loss of surround inhibition, which compromises fine motor control in the hand muscles of patients with focal hand dystonia (Rosenkranz et al., 2009).
The differential findings on motor cortical excitability between the skilled pianists and nonmusicians indicate a different functional architecture of the motor cortex. Playing the piano requires many varying patterns of movement coordination across joints and muscles (Gentner et al., 2010; Furuya et al., 2011), which may require a fine-tuned, elaborated motor cortex organization. Such a network might be disturbed more easily than a motor cortex containing relatively nonspecialized motor programs. It is also possible that the relation between training and motor cortical excitability forms an inverse U-shape, which may explain our observation of both improvement and deterioration of fine motor control in the pianists and nonmusicians after facilitating stimulation. Indeed, improved motor performance seems to be associated with reduced excitability enhancement after an initial phase of enhanced excitability of the respective area (Pascual-Leone et al., 1994).
Interestingly, motor skill was not altered following stimulation with the opposite montage (i.e., cathodal over the contralateral motor cortex and anodal over the ipsilateral one). This indicates polarity-dependent effects of bihemispheric tDCS over motor cortices on motor performance. However, importantly, there was no difference in the performance change both between the RaLc and RcLa montages for each hand, and between the hands for each montage. Therefore, the actual relevance of anodal versus cathodal stimulation still remains unclear. It seems unlikely that a lack of behavioral changes indicates no neurophysiological changes elicited by the stimulation because previous studies have demonstrated modulation of motor cortical excitability in an opposite direction between the contralateral and ipsilateral sides with bihemispheric tDCS (Paquette et al., 2011; Zheng et al., 2011; Mordillo-Mateos et al., 2012).
The present study cannot rule out the possibility that the current stimulation affected motor cortices adjacent to the stimulated regions, such as supplementary motor areas (SMA), because of the relatively large electrode size. An fMRI study showed that anodal tDCS over the primary motor cortex with a similar montage facilitated SMA activation (Kwon et al., 2008). A number of studies have demonstrated that SMA plays a role in the performance of complex sequential finger movements (Sadato et al., 1997). Thus, the behavioral changes obtained following tDCS may be attributed to modulation of neuronal activation, not only of the primary motor cortices but also of SMA and other adjacent regions.
A further limitation of this study is an apparent difference in the amount of difficulty in memorizing and performing the movement sequence between pianists and nonmusicians. Indeed, during the familiarization session, pianists performed the movement sequence only a few times, whereas nonmusicians had to perform it much more frequently to reach stable performance levels. One may therefore suspect that the expertise-dependent behavioral changes originated from a difference in offline learning during rest between expert pianists and nonmusicians. Although we cannot completely exclude this possibility, a familiarization session to practice the sequence of finger movements was performed only for the first experiment, and the order of three experiments was randomized across participants. Moreover, an expertise-dependent difference in offline learning does not provide any reasonable explanation for the performance-diminishing effect of anodal tDCS on fine motor control of the contralateral hand in expert pianists. We thus believe that the observed expertise-dependent behavioral changes resulted only from the stimulation. The present study may have implications for the area of neuroenhancement. In particular, “doping” by transcranial stimulation is receiving increasing attention among lay people and the press. As shown here in the area of highest possible motor performance, it seems that transcranial stimulation may not only be unable to improve but may even worsen performance under specific conditions, which would make uncontrolled use of the technique for enhancement of performance questionable.
Footnotes
This work was supported by the Alexander von Humboldt Foundation and the Japan Society for the Promotion of Science.
The authors declare no competing financial interests.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22:2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Furuya S, Altenmüller E. Flexibility of movement organization in piano performance. Front Hum Neurosci. 2013a;7:173. doi: 10.3389/fnhum.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Altenmüller E. Finger-specific loss of independent control of movements in musicians with focal dystonia. Neuroscience. 2013b;247:152–163. doi: 10.1016/j.neuroscience.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Furuya S, Soechting JF. Role of auditory feedback in the control of successive keystrokes during piano playing. Exp Brain Res. 2010;204:223–237. doi: 10.1007/s00221-010-2307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Flanders M, Soechting JF. Hand kinematics of piano playing. J Neurophysiol. 2011;106:2849–2864. doi: 10.1152/jn.00378.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010;20:1869–1874. doi: 10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Altenmüller E, Castrop F, Zimmer C, Dresel C. Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology. 2010;74:1790–1797. doi: 10.1212/WNL.0b013e3181e0f784. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning: a PET study. Brain. 1998;121:2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res. 1999;125:417–425. doi: 10.1007/s002210050698. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res. 2000;10:177–183. doi: 10.1016/S0926-6410(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Herwig U, Ziemann U, Jäncke L. Extensive training of elementary finger tapping movements changes the pattern of motor cortex excitability. Exp Brain Res. 2006;174:199–209. doi: 10.1007/s00221-006-0440-8. [DOI] [PubMed] [Google Scholar]
- Krings T, Töpper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A. Cortical activation patterns during complex motor tasks in piano players and control subjects: a functional magnetic resonance imaging study. Neurosci Lett. 2000;278:189–193. doi: 10.1016/S0304-3940(99)00930-1. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Chang MC, Jang SH. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435:56–59. doi: 10.1016/j.neulet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard JL, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single session of dual-tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabil Neural Repair. 2014;28:100–110. doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Flöel A. Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci. 2013;33:9176–9183. doi: 10.1523/JNEUROSCI.0055-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordillo-Mateos L, Turpin-Fenoll L, Millán-Pascual J, Núñez-Pérez N, Panyavin I, Gómez-Argüelles JM, Botia-Paniagua E, Foffani G, Lang N, Oliviero A. Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimul. 2012;5:214–222. doi: 10.1016/j.brs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L. The musician's brain as a model of neuroplasticity. Nat Rev Neurosci. 2002;3:473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paquette C, Sidel M, Radinska BA, Soucy JP, Thiel A. Bilateral transcranial direct current stimulation modulates activation-induced regional blood flow changes during voluntary movement. J Cereb Blood Flow Metab. 2011;31:2086–2095. doi: 10.1038/jcbfm.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlitz D, Peschel T, Altenmüller E. Assessment of dynamic finger forces in pianists: effects of training and expertise. J Biomech. 1998;31:1063–1067. doi: 10.1016/S0021-9290(98)00113-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset-Llobet J, Rosinés-Cubells D, Deus J, Narberhaus B, Valls-Solé J, Capdevila A, Pascual-Leone A. Brain cortical activation during guitar-induced hand dystonia studied by functional MRI. Neuroimage. 2000;12:257–267. doi: 10.1006/nimg.2000.0615. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Butler K, Williamon A, Rothwell JC. Regaining motor control in musician's dystonia by restoring sensorimotor organization. J Neurosci. 2009;29:14627–14636. doi: 10.1523/JNEUROSCI.2094-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesth Analg. 1968;47:717–723. [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Gerloff C, Hummel FC. Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579–587. doi: 10.1016/j.neuropharm.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Sehm B, Kipping J, Schäfer A, Villringer A, Ragert P. A comparison between uni- and bilateral tDCS effects on functional connectivity of the human motor cortex. Front Hum Neurosci. 2013;7:183. doi: 10.3389/fnhum.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010;30:8332–8341. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008a;28:1667–1673. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared with uni-hemisphere stimulation. BMC Neurosci. 2008b;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci. 2014;34:1037–1050. doi: 10.1523/JNEUROSCI.2282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398–410. doi: 10.2522/ptj.20090075. [DOI] [PubMed] [Google Scholar]
- Winges SA, Furuya S, Faber NJ, Flanders M. Patterns of muscle activity for digital coarticulation. J Neurophysiol. 2013;110:230–242. doi: 10.1152/jn.00973.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


