Abstract
For most organisms, sensitive recognition of even slight changes in environmental temperature is essential for adjusting their behavioral strategies to ensure homeostasis and survival. However, much remains to be understood about the molecular and cellular processes that regulate thermosensation and the corresponding behavioral responses. Planarians display clear thermotaxis, although they have a relatively simple brain. Here, we devised a quantitative thermotaxis assay and unraveled a neural pathway involved in planarian thermotaxis by combinatory behavioral assays and RNAi analysis. We found that thermosensory neurons that expressed a planarian Dugesia japonica homolog of the Transient Receptor Potential Melastatin family a (DjTRPMa) gene were required for the thermotaxis. Interestingly, although these thermosensory neurons are distributed throughout their body, planarians with a dysfunctional brain due to regeneration-dependent conditional gene knockdown (Readyknock) of the synaptotagmin gene completely lost their thermotactic behavior. These results suggest that brain function is required as a central processor for the thermosensory response. Therefore, we investigated the type(s) of brain neurons involved in processing the thermal signals by gene knockdown of limiting enzymes for neurotransmitter biosynthesis in the brain. We found that serotonergic neurons with dendrites that were elongated toward DjTRPMa-expressing thermosensory neurons might be required for the processing of signals from thermosensory neurons that results in thermotaxis. These results suggest that serotonergic neurons in the brain may interact with thermosensory neurons activated by TRPM ion channels to produce thermotaxis in planarians.
Keywords: planarian, serotonergic neurons, thermosensation, thermotaxis, TRP cation channels
Introduction
The brain functions as an information-processing center in which a variety of internal and external signals are decoded into defined molecular and cellular functions that often result in distinct behaviors appropriate for the environment. Temperature affects not only animal behaviors but also physiological functions such as gene expression, energy usage, and cell growth in organisms. Planarians are among the most responsive animals to changes of temperature throughout their lives. For example, planarians change their body size, fission rate, and conversion between sexual and asexual state and sustain their homeostasis according to the environmental temperature (Curtis, 1902; Cole, 1926; Best et al., 1969; Vowinckel, 1970; Grasso and Benazzi, 1973). Furthermore, planarians belong to an evolutionarily early group that acquired a CNS and show stereotypical behaviors in response to environmental stimuli (Pearl, 1903) perceived through sensory neurons projecting to an inverted U-shaped brain (Agata et al., 1998; Okamoto et al., 2005). Despite its relatively simple structure, the planarian brain is divided into several functional and structural domains composed of several types of neurons distinguished by their neurotransmitters and neural modulators, such as glutamate, dopamine, serotonin, GABA, acetylcholine, and neuropeptides, which are quite similar to those used by mammals (Umesono and Agata, 2009; Collins et al., 2010; Umesono et al., 2011).
Increasing knowledge has been gained recently about the morphogenesis of the planarian brain and its robust regenerative ability (Agata and Watanabe, 1999; Reddien and Sanchez Alvarado, 2004; Shibata et al., 2010; Umesono et al., 2011; Umesono et al., 2013). Moreover, tractable planarian behavioral assay systems that can be quantified for many variables, such as time spent in a target zone, speed, and distance, and that can be used in combination with RNAi of neuron-specific genes, have provided molecular and cellular knowledge about the information-processing machinery in the brain (Inoue et al., 2004; Takano et al., 2007; Nishimura et al., 2008). Therefore, the planarian brain, with its remarkable regenerative ability, may provide a unique opportunity to examine molecular mechanisms underlying the thermosensory system and its physiological and behavioral functions in vivo.
Crucial advances in understanding the molecular mechanisms of temperature sensing by the nervous system have been achieved by recent studies of transient receptor potential (TRP) ion channels that are conserved across the animal kingdom (Montell, 2005) and are involved in a wide variety of sensory systems, including thermosensation (Komatsu et al., 1996; Harteneck et al., 2000; Caterina and Montell, 2005; Rosenzweig et al., 2005; Kahn-Kirby and Bargmann, 2006; Tominaga, 2007; Talavera et al., 2008; Xiao and Xu, 2011). Although the signal transduction processes of the TRP family receptors in response to temperature stimulation have been studied extensively, much remains to be understood about the neural pathways in the brain that transform the activation of TRP channels by temperature stimulation into thermotactic behavioral responses. Our findings in this study suggest that serotonergic neurons in the brain and TRP Melastatin (TRPM) family channel activation in thermosensory neurons in the body periphery may be required for thermotaxis in planarians.
Materials and Methods
Animals.
A clonal strain of planarian (Dugesia japonica), SSP, was used in this study. Animals were cultured at 23°C in tap water. For amputation, they were starved for at least 1 week, anesthetized by chilling on ice, and then cut. Planarians that were 7 mm in length were used for all experiments. All planarians were maintained and manipulated according to a protocol approved by the Animal Care and Use Committee of Kyoto University.
Thermotaxis behavior assay.
All behavioral experiments were performed in a dark room with only a red light, a wavelength which cannot be sensed by planarians (Azuma et al., 1994; Azuma et al., 1999). Thermotaxis assays were performed for 180 s using a 6 cm Petri dish and a 2-cm-diameter vial containing frozen acetic acid or melting ice (11% of the area of the total assay field). The vial containing 10 ml of frozen acetic acid was removed from a refrigerator and kept at room temperature (25°C) until the acetic acid started to melt. Then, an assay plate and the vial were set as shown in Figure 2A and the surface of the plate was covered with 5 ml of autoclaved tap water at room temperature (25°C). The assay plate and the vial were left for 3 min in this state to allow formation of a radial thermal gradient within the assay plate. The temperature across the assay field was measured with a Thermo GEAR G100 infrared thermography camera (Nippon Avionics; see Fig. 2B). To assay planarian behavior at various temperatures, the behavior was analyzed using a temperature-controlled Thermo Plate (Tokai Hit) based on a previously reported phototactic behavior assay system (Inoue et al., 2004), the planarian thermotactic behavior was recorded using a video camera (Sony), and the tracked data were analyzed as described previously (Inoue et al., 2004).
Figure 2.
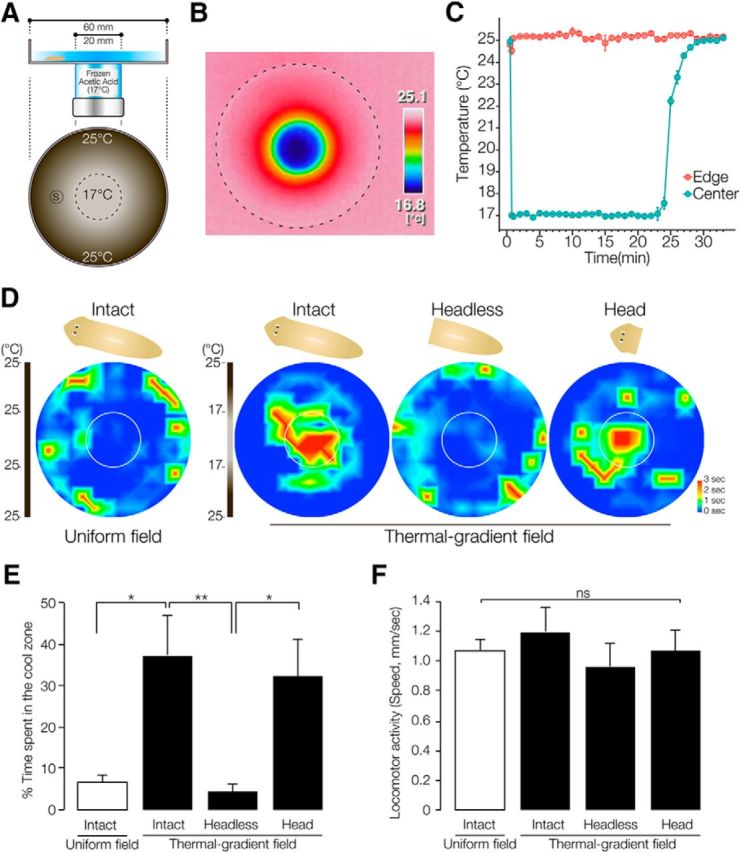
Planarian thermotaxis assay using thermal gradient from 17°C to 25°C. A, Schematic drawing of the assay system for thermotaxis. A vial containing frozen acetic acid was set under the center of an assay plate (60 mm diameter). The diameter of the bottom of the vial was 20 mm. A planarian was placed on the assay plate. S, Start area. B, Thermal image of a temperature gradient measured using an infrared thermography camera. C, Time course of the temperature change of the thermal gradient field. D, Heat map view of the average time spent at the indicated location by 10 each of intact planarians, headless body fragments, and amputated headpieces (n = 10, t = 180 s). Planarians showed a preference for moving along the edge of the uniform-field dish. In contrast, planarians gathered in the cool part of the thermal gradient dish. Headless planarians did not show thermotaxis, whereas amputated headpieces of planarians showed thermotaxis. E, Percentage of time spent in the cool target area is shown as mean ± SEM of 10 independent animals. *p < 0.05; **p < 0.01. F, Locomotor activity is shown as the speed of movement. ns, Not significant by one-way ANOVA followed by Tukey's multiple-comparisons test.
Treatment with a chemical antagonist.
The TRPM8 channel inhibitor N-(3-aminopropyl)-2-[(3-methylphenyl) methoxy]-N-(2-thienylmethyl) benzamide hydrochloride (AMTB; Tocris Bioscience) (Lashinger et al., 2008) was dissolved in dimethyl sulfoxide (DMSO) to 100 mm concentration and used at the indicated concentration(s) for each experiment. Planarian behaviors were analyzed in culture water supplemented with the antagonist or DMSO alone.
Statistical evaluation.
Data were analyzed by one-way ANOVA and the statistical significance of differences between test results was determined by Student's t test; p values >0.05 were taken as not significant (ns).
Phylogenetic analysis.
A phylogenetic tree was generated using ClustalW2 by aligning the transmembrane region of 81 TRP channel amino acid sequences from planarian (D. japonica), mouse (Mus musculus), human (Homo sapiens), rat (Rattus norvegicus), frog (Xenopus laevis), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), and nematode (Caenorhabditis elegans). The phylogenetic tree was drawn using FigTree software.
Whole-mount in situ hybridization.
The plasmids pBluescript SK(−) containing the planarian DjTRP family genes were linearized using BamHI. Using these linearized plasmids as templates, digoxigenin-labeled antisense RNA probes were synthesized using T7 RNA polymerase (Fermentas). Whole-mount in situ hybridization was performed as described previously (Tasaki et al., 2011). Alkaline phosphatase enzyme reactions for signal detection of in situ hybridization were performed identically at room temperature, 26°C, to allow direct comparison between experimental animals and controls. Images were obtained using an M205FA bright-field stereoscopic microscope (Leica). Images were processed with LAS-AF, ImageJ, and Adobe Photoshop software. For quantification of the number of expressing cells, images were processed with ImageJ and the number of expressing cells was calculated using the static ggplot2 R (Wickham, 2009).
Whole-mount immunohistochemistry.
Whole-mount immunostaining was performed as described previously (Inoue et al., 2007). Planarians were stained using the following dilutions of mouse antibodies: 1/2000 mouse anti-planarian synaptotagmin (anti-DjSYT; Tazaki et al., 1999), 1/2000 mouse anti-planarian tryptophan hydroxylase (DjTPH; Nishimura et al., 2007a), and 1/2000 rabbit anti-planarian glutamic acid decarboxylase (DjGAD; Nishimura et al., 2008) in 10% goat serum in 0.1% of Triton X-100-containing PBS (TPBS). After washing, the samples were incubated with fluorescence-labeled goat secondary antibodies (Alexa Fluor 488-labeled anti-mouse IgG(H+L) antibody or Alexa Fluor 594-labeled anti-rabbit IgG(H+L) antibody) and 10 μg/ml Hoechst 33342 (Life Technologies) in TPBS containing 10% goat serum overnight at 4°C. Fluorescence was detected with an FV10 confocal scanning microscope [Olympus; 10×/0.4 numerical aperture (NA) or a 60×/1.34 NA oil-immersion objective lens]. Images were processed with FV10-ASW (Olympus). All images were obtained using the same photography conditions to allow direct comparison between experimental animals and controls. Fluorescence was quantified using the ImageJ program and fluorescence intensity was expressed as the mean gray value.
Double fluorescent staining for immunohistochemistry and whole-mount in situ hybridization.
Planarians were examined by whole-mount in situ hybridization using the method described above with a Tyramide Signal Amplification (TSA) kit (Life Technologies). All peroxidase enzyme reactions for signal detection of the in situ hybridization using a TSA kit were performed identically at room temperature (25°C) to allow direct comparison between experimental animals and controls. The planarians were postfixed with 4% paraformaldehyde and then rinsed with TPBS. Immunohistochemical processing was performed as above.
RNAi Double-stranded RNA (dsRNA) was prepared as described previously (Shibata et al., 2012; Rouhana et al., 2013). Twenty-five microliters of chicken liver solution, 5 μl of 2% agarose, and 5 μl of 2 μg/μl dsRNA were mixed and presented to a group of 10 planarians. This food solution was prepared in small aliquots (∼30 μl) and stored frozen at −30°C. Two successive feedings were conducted at 3 d intervals after the first feeding. Control animals were fed dsRNA for GFP, a gene that is not found in planarians. For Readyknock (Takano et al., 2007), dsRNA was injected into the posterior intestinal duct of planarians using a Drummond Scientific Nanoject injector. Four hours after the injection, planarians were amputated posterior to the auricles and the resulting regenerants were used for the analysis at 7 d of regeneration.
Data deposition.
The sequences of DjTRPAa, DjTRPMa, DjTRPMb, DjTRPMLa, DjTRPPa, DjTRPVa, and DjTRPVb reported here have been deposited in the DNA Data Bank of Japan (DDBJ) and the National Center for Biotechnology Information (NCBI; Accession numbers AB845354, AB845353, AB981783, AB981784, AB981785, AB981786, and AB981787).
Results
Tractable assay for thermotactic behavior analysis in planarians
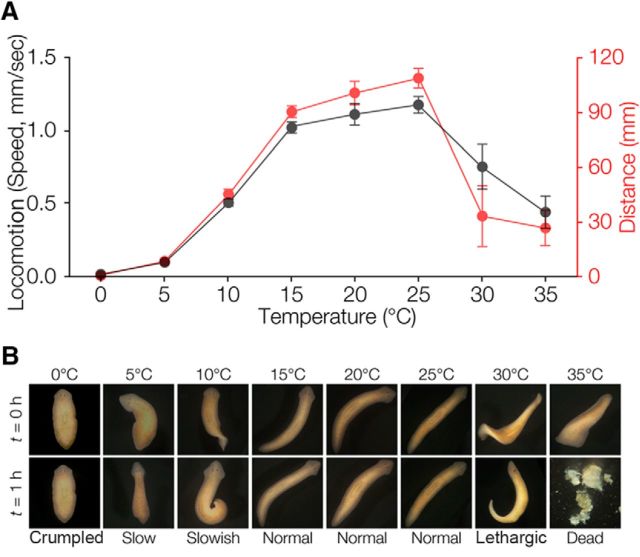
To determine the optimal temperature range for planarian, we analyzed behavior and physiological reactions at several different temperatures. The results showed that planarians showed normal behaviors from 15°C to 25°C and they could move freely for a distance of ∼100 mm during a 90 s duration (Fig. 1A), whereas planarian locomotor activity was drastically suppressed below 10°C and the total distance moved during 90 s was also shorter below 10°C compared with above 15°C. Above 30°C, planarians lost their normal locomotor activities and coordinated behavior. Planarians' body crumpled and the planarians lost their motility at low temperature (5–10°C). Above 30°C, they displayed hyperkinesia and thereafter became lethargic or died within 1 h (Fig. 1B). These observations indicated that the optimal temperature range for normal locomotion and survival of planarian was between ∼15°C and 25°C.
Figure 1.
Planarian behavior at various temperatures. A, Locomotor activity is shown as the speed of movement and total distance moved during 90 s. B, Planarians soon after the temperature of their environment was changed and 1 h after. Low temperature (below10°C) interfered with planarian motility. At high temperature (>30°C), planarians' survival was decreased.
Next, to observe and quantify planarian temperature-sensing behavior, a tractable thermotaxis assay method was developed based on a similar assay used to study C. elegans (Hedgecock and Russell, 1975; Mori and Ohshima, 1995). For this assay, a radial thermal gradient from 17°C to room temperature (25°C), which is suitable for easily creating a stable thermal gradient over the optimal temperature range for planarian, was made by placing a vial of frozen acetic acid (melting point, 17°C) at the center of a round, water-filled assay plate (Fig. 2A). Measurement of the temperature of the assay field with an infrared thermography camera showed a radial thermal gradient from 17°C to 25°C (Fig. 2B). Measurement of the time course of the change of the temperature of the center region and the edge region of the assay plate showed that the thermal gradient of this assay was stable for >20 min (Fig. 2C).
Planarians could move a distance of ∼100 mm during 90 s (Fig. 1A) and most stopped moving after 180 s during the assay; therefore, we decided to use a duration of 180 s to analyze planarians' thermotactic behavior. First, we tested the behavior of intact planarians in a normal field (uniform-field plate at 25°C). Figure 2D shows the averaged movements of 10 planarians together with a heat map (in which warmer colors indicate locations where much time was spent and cool colors indicate locations where little time was spent) and indicates that planarians showed a default behavior of tending to move near the edge of a dish in a uniform temperature field. In contrast, planarians placed in a plate with a thermal gradient showed a preference to move from the 25°C region to the 17°C region (Fig. 2D, central circle). Although the planarians had been cultured at 23°C, they showed a preference to move to the cooler (17°C) zone. In contrast, decapitated headless planarians did not move to the 17°C region and instead moved along the edge of the dish, like planarians on the uniform field. Surprisingly, even the amputated head piece moved to the 17°C region, indicating that the head region is necessary and sufficient for thermotaxis in planarian (Fig. 2D). Next, the average time spent by the animals (n = 10) in the central 17°C region during a 180 s test interval (the “thermotaxis score”) was measured to assess the ability of animals to recognize the temperature and move to the cooler region. The intact animals spent a large fraction of their time in the target zone after reaching it (percentage of time ± SEM, 37.4 ± 9.3%; Fig. 2E). In contrast, headless animals showed a much lower thermotaxis score (4.4 ± 2.0%; Fig. 1D). There was no difference in the speed of movement of intact, headless, or headpiece planarians on the thermal gradient (1.19 ± 0.16, 0.96 ± 0.10, and 1.06 ± 0.15 mm/s, respectively) or uniform field (1.07 ± 0.08 mm/s) plate, indicating that the difference of thermotaxis between animals on a thermal gradient and a uniform field was not the result of slowing of the locomotor activity by low temperature at 17°C (Fig. 2F). These results indicated that this assay method is useful for analyzing planarian thermotactic behavior and that planarians' thermotaxis is dependent on their head region.
Planarians prefer cooler temperature independent of their culture temperature
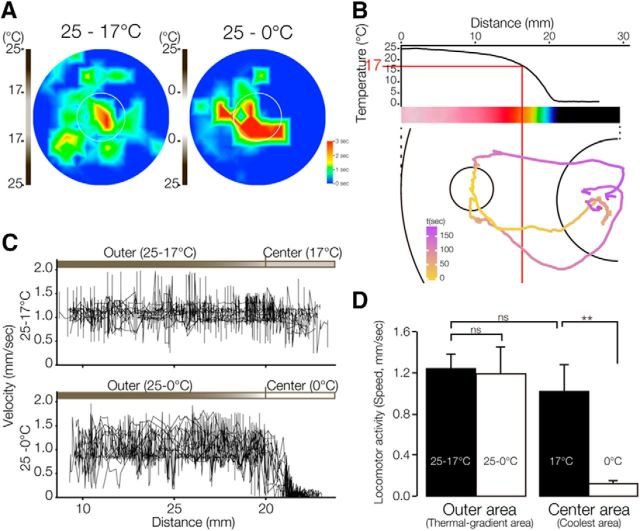
To investigate whether planarians prefer 17°C specifically, or even colder regions, frozen water was used instead of frozen acetic acid to create a thermal gradient from 25°C to 0°C in the thermotaxis assay. When planarians were placed in a thermal gradient plate with a vial of ice water under the center of the plate, they tended to go to the central region, the same as they did on a thermal gradient from 25°C to 17°C (Fig. 3A). The trajectories of the thermotactic movement of three individuals on the thermal gradient using ice revealed that planarians grown at 23°C passed through the 17°C zone without stopping and showed cryophilic movement down the temperature gradient (called “negative thermotaxis”), rather than heat-avoidance behavior (Fig. 3B).
Figure 3.
Thermal preference of planarians on a thermal gradient from 0°C to 25°C. A, Heat map of planarian thermotaxis from 17°C to 25°C and from 0°C to 25°C on a thermal gradient field that was created with a vial containing melting acetic acid or ice water, respectively, under the center of an assay plate (60 mm diameter) filled with water at 25°C. Planarians showed a preference for moving to cooler temperature. B, Top, Graph of the actual temperature on a thermal gradient field from 0°C to 25°C and a heat map from 0°C to 25°C measured using an infrared thermography camera. Bottom, Representative trajectories of 3 planarians starting from the circle on a thermal gradient plate using 0°C ice water at the center. Lines are shown in pseudocolor to represent the time of movement. C, Velocity plot of planarians moving toward the coolest area. Top, Planarian velocity plot on thermal gradient field from 0°C to 25°C. Bottom, Planarian velocity plot on thermal gradient field from 17°C to 25°C. Near the 0°C region, animals' motility was drastically inhibited. D, Statistical comparison of the planarian locomotor activity between a thermal gradient with coolest area at 17°C (black columns) and a thermal gradient with coolest area at 0°C (white columns). **p < 0.01. ns, Not significant.
When we examined the rate of movement of the animals as a function of temperature, we noted that their movement was decreased by the 0°C ice compared with their movement on the 17°C to 25°C thermal gradient field (Figs. 1, 3C). Statistical analysis clearly indicated that planarians moving into the center (0°C) region showed considerably slower movement (0.1 ± 0.1 mm/s) than those in outer (17°C to 25°C) regions (1.0 ± 0.2 mm/s; Fig. 3D). In contrast, planarians' locomotion in a 17°C center region was not slower than that in the outer regions. Note that, although coldness slowed planarians' movement, planarians moved into the region at ∼0°C, suggesting that they have a preference to move toward a temperature much colder than 17°C even though they lost their mobility at such a cold temperature. However, we used a thermal gradient using frozen acetic acid in the subsequent experiments because planarian movement was slowed and animals become immobilized by a field with a 0°C region.
Recovery of thermotaxis during head regeneration
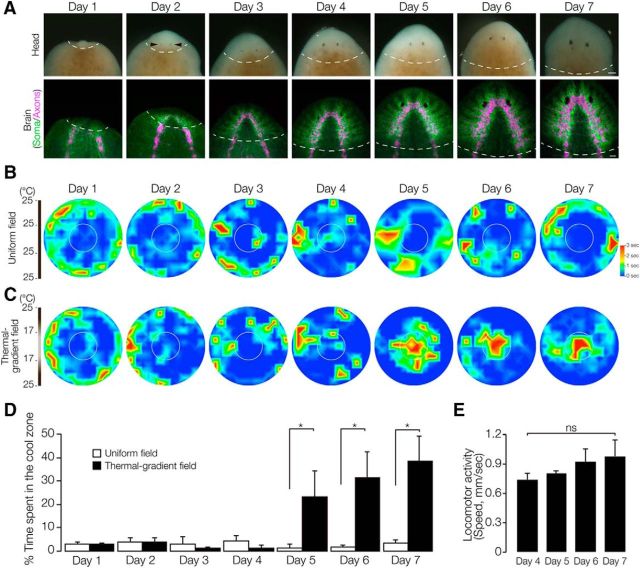
Next, animals at different stages of head regeneration were subjected to the thermotaxis assay to study the process of recovery of thermotactic behavior during head regeneration. Figure 4A shows the progressive development of head and brain neurons during head regeneration visualized by in situ hybridization of the pan-neural gene Djsyt and immunostaining of DjSYT to detect neural cells (green) and neural axons (magenta), respectively. In day 1 regenerants, DjSYT-positive nerves derived from the cephalic blastema could not be detected. From day 2, newly developed neural cells and axons were detected. At day 4, the head morphogenesis was complete, including regeneration of the eyes, consistent with a previous report (Cebrià et al., 2002), although the regenerated brain was small compared with the fully regenerated brain seen at day 7. After day 4 of regeneration, the newly formed brain gradually continued to grow.
Figure 4.
Process of recovery of thermotaxis during head regeneration. A, Time course of planarian head regeneration. Top, Head-amputated planarian regenerating a head. Bottom, Regenerating brain visualized by in situ hybridization for Djsyt mRNA in green (neural cell bodies) and visualized by immunostaining against DjSYT protein in magenta (axons). The dashed line indicates the border between the newly formed region and old stump region. Arrows indicate eyes. Scale bar, 300 μm. B, Heat map of the averaged thermotaxis of 10 planarians assayed individually using a uniform-field dish (n = 10, t = 180 s). C, Heat map of the average thermotaxis of 10 planarians assayed individually using a thermal gradient dish (n = 10, t = 180 s). D, The time spent in the cool target area is shown as mean ± SEM of 10 independent animals. White columns show the values on the uniform-field plate. Black columns show the values on the thermal gradient plates. *p < 0.05. E, Locomotor activity calculated as the average speed of planarians in the uniform-field plate during head regeneration. ns, Not significantly different by one-way ANOVA followed by Tukey's multiple-comparisons test.
In contrast to the gradual morphological regeneration of the neurons and brain, functional recovery of thermotaxis occurred abruptly during head regeneration. Figure 4, B and C, shows the planarian movements during head regeneration in a uniform field and thermal gradient field, respectively. The thermotactic response was still weak in 4 day regenerants, because most of these animals did not reach the target zone within the 180 s duration of the assay. Strong recovery of the thermotactic behavior, however, was observed at 5 d after amputation (Fig. 4C), although planarians on the uniform field plate preferred to move around the edge of the dish or to move randomly around their starting position (Fig. 4B). To further analyze the data, recovery was quantified and plotted graphically (Fig. 4D). The average time spent in the target zone during the 180 s test was measured to determine the recovery of thermotaxis during head regeneration. These analyses clearly indicated that, although the thermotaxis was very weak at day 4 (1.5 ± 1.0%, 3/10; percentage of time ± SEM, number that reached the target zone), which was similar to the thermotaxis score with a uniform field (4.3 ± 2.3%, 3/10), the thermotactic response was abruptly upregulated 5 d after amputation (26.4 ± 11.4%, 8/10). This result was consistent with the process of recovery of negative phototaxis in planarian (Inoue et al., 2004). Notably, we could not find any significant increase of the speed of movement between day 4 and day 5 (Fig. 4E), suggesting that the recovery of thermotaxis observed at day 5 was not due to increased locomotor or other physical activity, but rather was due to some other factor such as reestablishment of brain function.
Planarian TRP family genes
To investigate possible genes and sensory neurons involved in thermosensory behavior, we focused on the TRP superfamily because it is known that TRP family genes are required for various kinds of sensing, including thermosensation and mechanosensation, in many metazoan species ranging from flies to mammals (Severino et al., 2007; Talavera et al., 2008; Sokabe and Tominaga, 2009). We found at least seven candidate genes with high homology to known TRPs in our planarian EST database and named them DjTRPAa, DjTRPMa, DjTRPMb, DjTRPMLa, DjTRPPa, DjTRPVa, and DjTRPVb (Fig. 5A). We reasoned that, if these genes function primarily in planarian thermosensation, then they should be expressed predominantly in thermosensory neurons located in the body periphery. To test this, the expression patterns of the genes were analyzed by whole-mount in situ hybridization and the results revealed that DjTRPAa and DjTRPMa were expressed in a scattered pattern throughout the body, implying that the expressing cells might be sensory neurons (Fig. 5B,C), whereas DjTRPMb was expressed ubiquitously (Fig. 5D), DjTRPMLa was expressed in the intestinal duct (Fig. 5E), DjTRPPa was expressed in the dorsoventral boundary and dorsal midline (Fig. 5F), and DjTRPVa and DjTRPVb were expressed in mesenchymal cells (Fig. 5G,H). These various expression patterns of planarian TRP family genes suggested that planarians possess diverse functions of TRP family genes in multiple tissues. Further careful observation of the expression patterns of DjTRPAa and DjTRPMa revealed that both genes were expressed more strongly in the head compared with the trunk region (Fig. 5I,J). To verify this observation, the numbers of expressing cells were counted. The results clearly showed that DjTRPMa and DjTRPAa were expressed in cells located in the peripheral region of the entire body and were especially highly expressed in the head region (Fig. 5K,L). These results suggested that DjTRPAa and DjTRPMa could be candidates as thermosensory-neuron-specific genes.
Figure 5.
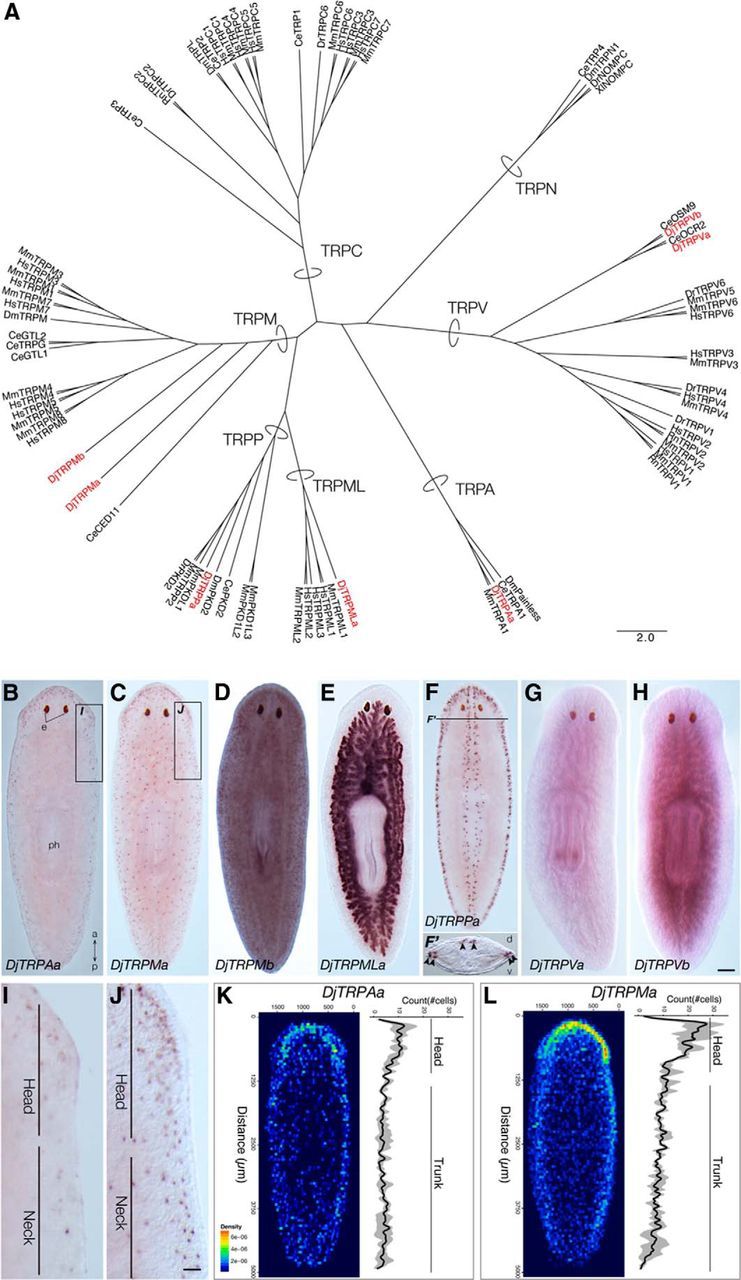
Planarian TRP family genes. A, Phylogenetic tree of representative TRP channels generated in ClustalX2 by aligning the transmembrane domains of 81 TRP channels from various species. Ce, C. elegans; Dm, D. melanogaster; Dr, D. rerio; Hs, H. sapiens; Mm, M. musculus; Rn, R. norvegicus; Xl, X. laevis. The scale bar indicates substitutions per site. B–H, Expression pattern of planarian TRP family genes: DjTRPAa (B), DjTRPMa (C), DjTRPMb (D), DjTRPMLa (E), DjTRPPa (F), DjTRPVa (G), and DjTRPVb (H). Scale bar, 300 μm. e, Eyes; ph, pharynx; a, anterior; p, posterior, d, dorsal; and v, ventral. F′, Transverse section at the F′ level in F. Arrowheads indicate the gene-expressing dorsoventral boundary cells and dorsomedial cells. I, J, Magnified views of the head regions of the planarians indicated by boxes in B and C, respectively. Scale bar, 100 μm. K, L, Averaged heat map view of 2D density plot (left) and histogram along anteroposterior axis of the number of cells expressing genes DjTRPAa (K) and DjTRPMa (L) (n = 4). Although both DjTRPAa and DjTRPMa were predominantly expressed in the head, both of them were also expressed throughout the body.
TRPM family gene might be required for the thermotactic behavior in planarian
To investigate the functions of DjTRPAa and DjTRPMa, we prepared gene knockdown planarians by RNAi treatment and analyzed their thermotactic behavior. The results revealed that DjTRPMa(RNAi) animals showed a defect in thermotaxis, whereas DjTRPAa(RNAi) animals showed normal thermotactic behavior (Fig. 6A). qRT-PCR analysis showed that RNAi caused specific gene knockdown (Fig. 6B), suggesting that DjTRPMa might be required for the thermotaxis. Quantitative analysis of time spent in the coolest (center) area of the assay plate performed (Fig. 6C) by measuring the time spent resting (moving at <0.1 mm/s) in the cool center zone showed that DjTRPMa(RNAi) planarians never rested in the coolest center area, whereas control and DjTRPAa(RNAi) planarians spent a large fraction of their time in the coolest center area (Fig. 6D). This suggested that DjTRPMa(RNAi) planarians could not recognize the temperature, resulting in their random movement in the assay field. There was no difference in locomotor activity among the control and two different RNAi animals (Fig. 6E), suggesting that loss of either of these two genes did not affect movement itself.
Figure 6.
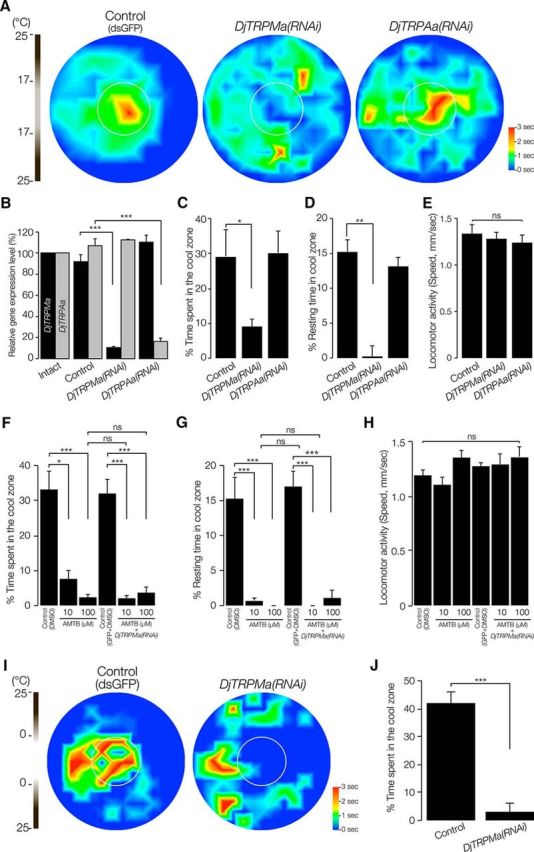
Thermotaxis assay of DjTRPAa(RNAi) and DjTRPMa(RNAi) planarians. A, Heat map of RNAi-treated (DjTRPMa or DjTRPAa) planarians using thermal gradient assay plate. Planarians fed dsRNA of GFP were used as a negative control (n = 10, t = 180 s). B, qRT-PCR analysis shows the specific reduction of genes of control, DjTRPMa(RNAi), and DjTRPAa(RNAi) planarians. C, Percentage of time spent in the cool target area is shown as mean ± SEM of 10 independent animals. *p < 0.05. D, Percentage resting (speed of movement ∼0.1 mm/s) time in the cool zone (center area) is shown as mean ± SEM **p < 0.01. E, Locomotor activity was determined by measuring the speed. ns, Not significantly different by one-way ANOVA followed by Tukey's multiple-comparisons test. F, The percentage time spent in the cool target area is shown as mean ± SEM of 10 independent animals using a thermal gradient assay plate with 10 or 100 μm AMTB with or without DjTRPMa(RNAi) treatment. 0.1% DMSO plus GFP-dsRNA injection was used as a negative control (n = 10, t = 180 s). *p < 0.05; ***p < 0.005. ns, Not significantly different. G, Percentage resting (speed of movement ∼0.1 mm/s) time in the cool zone (center area) is shown as mean ± SEM. ***p < 0.005. ns, Not significantly different. H, Locomotor activity was not affected by AMTB treatment or by both DjTRPMa(RNAi) and AMTB treatment. ns, Not significantly different by one-way ANOVA followed by Tukey's multiple-comparisons test. I, DjTRPMa(RNAi) planarians did not move toward 0°C. J, The percentage time spent in the 0°C target area (n = 10, t = 180 s). ***p < 0.005.
To further investigate whether DjTRPMa functions in thermotaxis, we performed the thermotaxis assay in the presence of AMTB, which specifically antagonizes the TRPM8 channel, which is known to be a thermosensitive TRPM family protein (Lashinger et al., 2008). AMTB treatment drastically interfered with the thermotactic behavior (Fig. 6F). The decrease of time spent in the coolest center area clearly demonstrated that AMTB treatment inhibited planarian thermotaxis in a dose-dependent manner (Fig. 6F). In addition, when we performed thermotactic behavior analysis of DjTRPMa(RNAi) planarians in the presence and absence of AMTB, we could not find additive effects of AMTB and DjTRPMa(RNAi) treatment, suggesting that AMTB might specifically inhibit DjTRPMa, although we could not directly investigate the specific target of AMTB (Fig. 6C,F). Measurement of the time spent resting in the cool center zone showed that planarians in the thermal gradient field containing AMTB never rested in the coolest center area (Fig. 6G), although AMTB did not affect the locomotor activity of planarians (Fig. 6H). DjTRPMa(RNAi) and TRPM8 channel antagonist treatment caused quite similar behavioral patterns, although we could not find any orthologous relationship between mammalian TRPM8 and DjTRPMa. Together, these results suggested that DjTRPMa might be required for thermotaxis, and DjTRPMa-expressing cells might act as thermosensory neurons.
Next, we performed the thermotaxis assay on a 0°C to 25°C thermal gradient field to investigate whether DjTRPMa functions in a particular temperature range, such as from 15°C to 25°C (in which planarian showed normal locomotor activity). Intriguingly, DjTRPMa(RNAi) animals did not enter the 0°C region and they tended to move around the edge of the dish (the same as the behaviors on a 17°C to 25°C thermal gradient field; Fig. 6I,J), suggesting that DjTRPMa might function in thermosensing over a relatively wide range of (at least) low temperatures.
Analysis of the brain neurons involved in thermotaxis using Readyknock of synaptotagmin
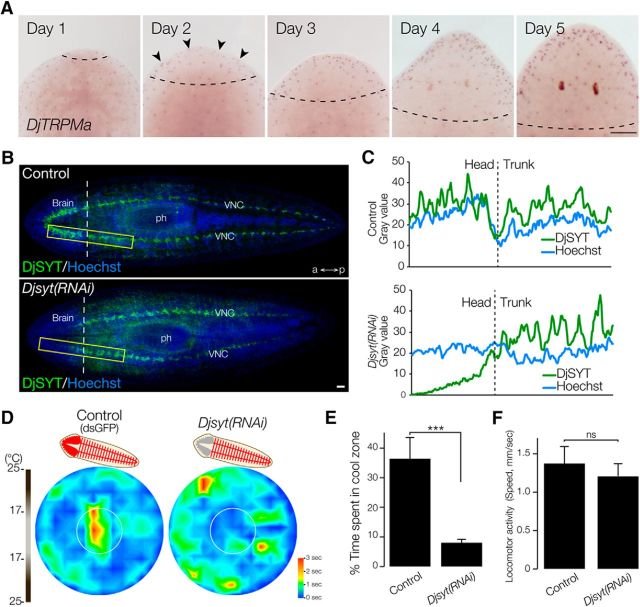
To determine whether the expression of DjTRPMa during head regeneration might be the cause of the reestablishment of thermotactic behavior at day 5 (Fig. 4C,D), we analyzed the expression of DjTRPMa during head regeneration. Unexpectedly, the expression of DjTRPMa and differentiation of DjTRPMa-expressing thermosensory neurons were detected in day 2 regenerants (Fig. 7A), suggesting that the recovery of thermotaxis might not depend solely on the regeneration of thermosensory neurons.
Figure 7.
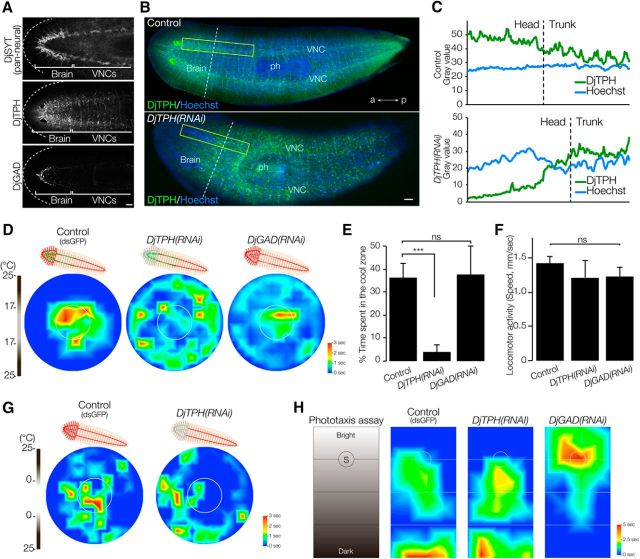
Brain neural activity is required for thermotaxis. A, Expression pattern of DjTRPMa during head regeneration. The dashed line indicates the border between the newly formed region and old stump region. Arrowheads indicate newly regenerated DjTRPMa-expressing cells. Scale bar, 300 μm. B, Readyknock of Djsyt. Left, Immunohistochemical detection of DjSYT protein shown in green in control (top) and in Readyknock (bottom) animals 7 d after decapitation. Samples were stained with Hoechst 33342 (for nuclei, shown in blue) to visualize planarian tissues, including brain. The dashed line indicates the border between the newly formed region and old stump region. ph, pharynx; a, anterior; p, posterior. Scale bar, 200 μm. C, Graphs show fluorescence intensity of DjSYT and Hoechst staining corresponding to yellow-boxed areas in B. D, Heat map of control and Djsyt(RNAi) planarians at 7 d of head regeneration using a thermal gradient dish (n = 10, t = 180 s). E, The time spent in the cool target area is shown as mean ± SEM of 10 independent animals. Synaptic transmission in brain-defective planarians produced by Djsyt(RNAi) did not result in normal thermotaxis. ***p < 0.005. F, Locomotor activity was not affected by Djsyt(RNAi). ns, Not significantly different.
A previous study demonstrated that dsRNA-induced knockdown affects protein expression more severely in the differentiating cells in the regeneration blastema than in the preexisting terminally differentiated cells. Such knockdown is called Regeneration-dependent conditional gene knockdown (Readyknock; Takano et al., 2007). Immunohistochemical analysis revealed that Readyknock using Djsyt RNAi treatment caused severe reduction of the level of DjSYT protein, which is involved in synaptic transmission (Tazaki et al., 1999), only within the newly formed brain in the head at day 7 of regeneration, although strong signals of the DjSYT protein were still detected in the preexisting ventral nerve cords (VNCs) in the trunk region (Fig. 7B). Measurement of the fluorescence intensity clearly indicated that the amount of DjSYT protein in the newly regenerated head region of the Djsyt(RNAi) planarians was drastically decreased (Fig. 7C). When Djsyt(RNAi) planarians were exposed to light or chemical stimuli, they could not distinguish the direction of the light or chemicals and moved randomly (Inoue et al., 2004; Takano et al., 2007). To investigate the brain functions involved in thermotaxis, the thermotactic behavioral assay was performed after Readyknock of Djsyt and revealed that Djsyt(RNAi) planarians did not preferentially move toward the cool temperature, but instead showed random movements in the assay plate (Fig. 7D). Quantitative analysis of time spent in the coolest center area clearly demonstrated that loss of the DjSYT in the brain inhibited planarian thermotaxis (Fig. 7E) without causing any defect of locomotor activity (Fig. 7F). These results strongly suggest that neural activity in the brain is required for planarian thermotactic behavior and that the thermotaxis assay system is useful for analyzing the function of the planarian brain and nervous system–related genes.
Readyknock of neural genes to identify the type(s) of neurons involved in processing of temperature signals in the brain
Next, to unravel the information-processing neural pathway and mechanism, we performed RNAi of various genes specifically expressed in the CNS. Previous RNAi studies revealed that the genes encoding rate-limiting enzymes for the synthesis of neurotransmitters are involved in various behaviors in planarian (Nishimura et al., 2007b, 2010). DjTPH is expressed in the brain and in the VNCs, including in sensory cells (Nishimura et al., 2007a; Fig. 8A), whereas GABAergic neurons are distributed only in the brain (Nishimura et al., 2008; Fig. 8A). In addition, planarian serotonergic neurons are involved in several kinds of behavioral functions, including locomotion using motile cilia distributed throughout the body (Currie and Pearson, 2013; März et al., 2013). Utilizing Readyknock, we attempted to demonstrate that knockdown of serotonergic neurons in the brain inhibits activities in the brain specifically without causing locomotor defects due to a defect of cilia motility. Immunofluorescence analysis using antibody against DjTPH in DjTPH(RNAi) planarians at 7 d of head regeneration revealed a drastic reduction of DjTPH protein specifically in the newly regenerated head region despite otherwise normal brain regeneration (Fig. 8B,C). The thermotaxis assay using DjTPH(RNAi) planarians revealed that they did not show normal thermotaxis (Fig. 8D). In contrast, Readyknock of the gene encoding planarian glutamic acid decarboxylase (DjGAD), a rate-limiting enzyme for GABA synthesis, did not cause a defect in thermotaxis (Fig. 8D). Figure 8E shows the time spent in the coolest center region of the thermotaxis assay and indicates that DjTPH(RNAi) planarians had a severe defect of thermotaxis, in contrast to the normal thermotaxis of DjGAD(RNAi) planarians. We did not find any defect of locomotor activity in either of these RNAi planarians (Fig. 8F). In addition, we could not find any differences in DjTPH(RNAi) planarian behaviors between 17°C and 25°C and in the 0°C to 25°C thermal gradient fields (Fig. 8G). In contrast, DjTPH(RNAi) planarians showed normal negative phototactic behavior as a control, whereas DjGAD(RNAi) planarians lost their photosensing ability, consistent with a previous report (Fig. 8H; Nishimura et al., 2008).
Figure 8.
Serotonergic neurons may be required for thermosensing in the brain. A, Planarian brain visualized by DjSYT (top), serotonergic neurons visualized by DjTPH (middle), and GABAergic neurons visualized by DjGAD (bottom) immunostaining. The dashed line indicates planarian head. Scale bar, 150 μm. B, Readyknock of DjTPH. Left, Immunohistochemical detection of DjTPH protein (green) in control (top) and in RNAi (bottom) animals 7 d after decapitation. Samples were stained with Hoechst 33342 (for nuclei, shown in blue) to visualize planarian tissues, including brain. The dashed line indicates border between the newly formed region and old stump region. ph, pharynx; a, anterior; p, posterior. Scale bar, 200 μm. C, Graphs show fluorescence intensity of DjTPH and Hoechst staining corresponding to yellow-boxed areas in B. D, Heat map of the Readyknock (DjTPH and DjGAD)–treated planarians at day 7 of head regeneration using thermal gradient dish (n = 10, t = 180 s). E, Time spent in the cool target area is shown as mean ± SEM of 10 independent animals. ***p < 0.005. ns, Not significantly different. Serotonergic neuron-defective planarians did not show normal thermotaxis, whereas DjGAD(RNAi) planarians showed normal thermotaxis. F, Locomotor activity was not affected by DjTPH RNAi or DjGAD RNAi. ns, Not significantly different by one-way ANOVA followed by Tukey's multiple-comparisons test. G, DjTPH(RNAi) planarians did not move toward 0°C. H, Negative phototaxis indicated by heat map of the Readyknock (DjTPH and DjGAD)–treated planarians at day 7 of head regeneration (n = 10, t = 60 s).
Double staining of DjTRPMa and DjTPH
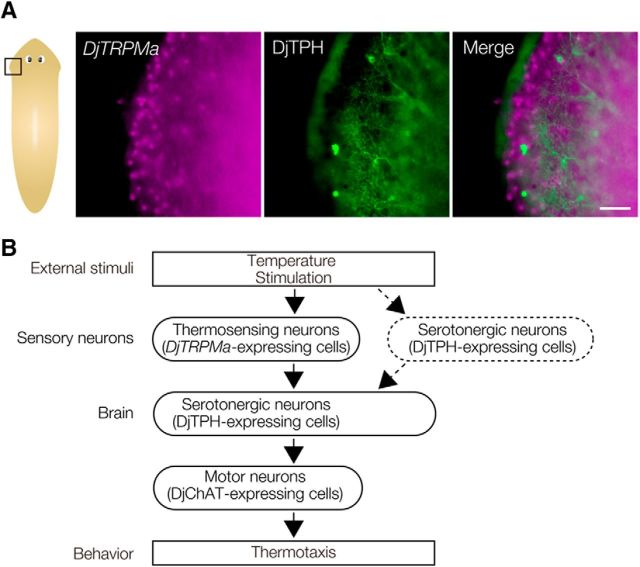
The above data showed that DjTRPMa-expressing sensory neurons and DjTPH-expressing serotonergic neurons in the brain are involved in thermotaxis in planarian, suggesting that the thermosensory neurons might interact with serotonergic neurons in the brain to produce thermotactic behavior. To determine the localization of the temperature-sensing neurons (presumably DjTRPMa-expressing neurons) and serotonergic neurons (DjTPH-expressing neurons), double staining for DjTRPMa-expression and DjTPH was performed. A previous study demonstrated that our anti-DjTPH antibody could recognize the cell bodies as well as axons and dendrites of neurons (Nishimura et al., 2007a), so this antibody was a good tool for analyzing the colocalization of DjTPH protein and expression of the DjTRPMa gene. Such analysis did not reveal any DjTPH-positive neurons that expressed DjTRPMa (Fig. 9A). However, dendritic projections of DjTPH-positive serotonergic neurons were observed to elongate toward neurons expressing DjTRPMa (Fig. 9A). These results suggest that temperature signals are received by DjTRPMa-expressing thermosensory neurons and then these signals are transduced to serotonergic neurons in the brain as part of the neural pathway to produce thermotactic behavior.
Figure 9.
Neural pathway involved in thermotaxis in planarian. A, Pattern of distribution of DjTRPMa-expressing cells visualized by in situ hybridization and serotonergic neurons visualized by immunohistochemistry using anti-DjTPH antibody. Dendrites of serotonergic neurons elongated toward DjTRPMa-expressing sensory neurons. The region of higher-magnification views is indicated by the square box in the schematic view. Scale bar, 100 μm. B, Neural network controlling planarian behavior. The DjTRPMa-expressing neurons act as thermosensory neurons and serotonergic neurons in the brain are required for the thermotactic behavior observed in this study.
Discussion
Thermotaxis in planarian
Thermosensing and appropriate responses to environmental temperature are among the most important primary functions of organisms (Ramot et al., 2008; Garrity et al., 2010; Luo et al., 2010; Wetsel, 2011). The relatively simple planarian brain is nevertheless highly organized, and improved molecular tools and methods for analyzing gene and cellular functions make it possible now to unravel the mechanisms of planarian brain functions and the physiology linking neural differentiation and animal behavior (Inoue et al., 2004; Takano et al., 2007). Here, we established a new behavioral analysis system for thermotaxis and used it to quantify the movements of planarians on a thermal gradient field, including time spent in the target zone and speed of locomotion (Fig. 2). Interestingly, planarians showed a preference for cooler temperatures and tended to stay in the coolest area in the thermal gradient assay dish (which was either 17°C or 0°C in the present experiments) independent of their culture temperature (Figs. 2, 3), although cold interfered with their movement (Figs. 1, 3). In contrast, after nematodes have been cultured in the presence of food at a certain temperature, they actively move toward that temperature on a thermal gradient (Mori, 1999). In addition, C. elegans move along isothermal lines when they are near their experience-dependent preferred temperature on a thermal gradient (Hedgecock and Russell, 1975), but planarians do not show such isothermal behavior. Larval and adult flies show a strong temperature preference, peaking at 24°C, independent of prior adaptation (Sayeed and Benzer, 1996; Lee et al., 2005). These results indicate that planarian thermotaxis (lacking preference for a specific temperature) has a unique character that is different from that of the thermotaxis in other animals studied previously, and that we speculate might be adaptive in the wild. It is generally said that poikilotherms must obtain moderate heat from their environment to sustain physiological processes such as metabolism by instinctively moving to places with warm temperature. The cryophilic behavior of planarians suggests that this behavior may also have some biological function(s), such as changing of the reproduction system from the asexual to sexual state, changing the size at which they undergo fission, or reducing their energy usage by lowering their metabolic rate by cooling their body (Cole, 1926; Vowinckel, 1970; Gillooly et al., 2001).
The process of the recovery of planarian thermosensing and thermotaxis during head regeneration was also analyzed. Robust recovery of thermotaxis was only observed from day 5 of head regeneration (Fig. 4). We found that both DjTRPMa-expressing thermosensory neurons and serotonergic neurons regenerated at 2 d after amputation (Fig. 7A) and projections of dendrites of serotonergic neurons toward DjTRPMa-expressing neurons were observed during head regeneration at 3 d after amputation (data not shown), suggesting that the recovery of thermotaxis might not depend solely on the regeneration of thermosensory neurons or signal-processing serotonergic neurons. Consistent with this, recovery of phototaxis in planarians also occurs at day 5 during head regeneration, after the axons of visual neurons have completely projected to the visual center in the brain, and this recovery is dependent on secreted neuropeptides specifically expressed starting at day 5 in the visual center neurons to which the visual neurons project (Inoue et al., 2004). These results suggest that functional restoration of the regulatory systems of various signal-sensing systems in the brain might occur in a coordinated manner.
Thermosensory neurons express DjTRPMa
Recently, several members of the TRP family of ion channels have been found to act as temperature-responsive ion channels (Baez-Nieto et al., 2011; Brauchi and Orio, 2011; Harteneck et al., 2011; Planells-Cases et al., 2011; Vay et al., 2012). Based on amino acid sequence comparisons, these channels are subdivided into five groups, TRPA, TRPC, TRPM, TRPN, and TRPV, and into two other subgroups, TRPML and TRPP, and are conserved across the animal kingdom (Montell, 2005). Therefore, we searched for planarian TRP family genes as tools to identify the thermosensory neurons and thereby obtained partial sequences of seven DjTRP family genes, the deduced amino acid sequences of which contain transmembrane regions highly homologous with those of other animals' TRPs (Fig. 5A).
Combinatory RNAi or chemical inhibitor treatment and thermotactic behavior analysis revealed that DjTRPMa might be required for thermotaxis in planarian (Fig. 6). Gene expression analysis suggested that DjTRPMa-expressing cells located throughout the body may be thermosensory neurons (Fig. 5C,J,L) and Readyknock analysis of DjTRPMa (data not shown) indicated that temperature signals may be sensed throughout the body and may be sent to the brain to produce appropriate behavioral responses. DjTRPAa did not appear to contribute to thermosensation of cool temperatures (Fig. 5B). Almost all animal sensing machineries, not only those for thermosensation, depend on TRPs (Christensen and Corey, 2007; Talavera et al., 2008), so it is possible that DjTRPAa may be important for some other sensory system(s) such as taste, smell, or various forms of mechanosensation. The establishment of assay methods for various behaviors will be essential for discovering the mechanisms and functions of genes involved in these sensory systems.
Temperature sensitivity is remarkably diverse depending on the TRP molecule(s) involved (Patapoutian et al., 2003). For example, TRPV1–4 are activated at a relatively warm temperature range, whereas TRPM8 and TRPA1 are activated by cold stimuli (Patapoutian et al., 2003; Tominaga, 2007; Vay et al., 2012). Because planarians commonly live at temperatures <25°C in nature and in the laboratory, the function of planarian DjTRPMa may thus be consistent with the functions of TRPMs in other organisms. This would indicate that the evolutionarily early group Lophotrochozoa might have already acquired thermoregulatory mechanisms used in common by Ecdysozoa and Vertebrata, including humans. Interestingly, DjTRPMa(RNAi) disturbed thermotaxis toward 0°C as well as 17°C (Fig. 6), suggesting that DjTRPMa proteins might possibly function in thermotaxis to cool temperature over a wide range of temperature (from 0°C to 25°C), although mammals sense cool temperature with two TRP channels, TRPA1 and TRPM8 (Patapoutian et al., 2003; Tominaga, 2007; Vay et al., 2012).
Serotonergic neurons in the brain process temperature signals from thermosensory neurons
The extraordinary regenerative potential of planarians makes them very useful for analyzing phenotypes during the processes of brain formation and function in vivo. Removal of the head or loss of synaptotagmin function in the brain caused complete loss of thermotaxis even though planarians have thermosensory neurons along their entire anteroposterior body axis (Figs. 2, 5, 7). This clearly indicated that temperature stimuli received by sensory neurons are processed in the brain. To identify the neural circuit(s) regulating thermotaxis, we investigated the types of neurons in such circuits in the brain using the thermotaxis behavioral assay and RNAi. Importantly, previous reports indicated that individual neurotransmitters were used by discrete cells in planarian, suggesting that inhibition of the gene encoding the rate-limiting enzyme for synthesis of one neurotransmitter(s) might lead to inhibition of the corresponding neural function (Nishimura et al., 2007b; Nishimura et al., 2008). Before trying to identify such neurons, we first examined the neurotransmitters used by thermosensory neurons. Although we tried to identify the neural type(s) by immunohistochemical staining to determine whether the thermosensory neurons expressing the DjTRPMa gene were GABAergic, dopaminergic, serotonergic, or octopaminergic neurons, we have not yet been able to identify the neurotransmitter used by the DjTRPMa-expressing thermosensory neurons. However, we did succeed in showing that DjTPH-positive serotonergic neurons are required for thermotaxis (Fig. 8D,E).
As shown in Figure 9A, the thermosensory neurons were not serotonergic neurons, but projections from DjTPH-positive neurons with many branched dendrites were elongated toward DjTRPMa-positive neurons. This suggests that serotonergic neurons in the brain might be involved in processing the information from thermosensory neurons activated by DjTRPMa channels to produce thermotactic behavior (Fig. 9B). Unfortunately, we could not visualize the axons of the sensory neurons to analyze whether DjTRPMa-positive thermosensory neurons connect to serotonergic neurons directly, so we could not exclude the possibility that in addition to DjTRPMa-expressing neurons, serotonergic neurons, and/or neurons expressing other TRP family proteins in the peripheral nerves in the head play a role as sensory neurons.
Planarians receive many kinds of external signals via independent sensory neurons such as visual neurons of the eyes, chemosensory neurons of the auricles, and thermosensory neurons in the body periphery, and these signals are then processed by neurons in the brain. We previously demonstrated that loss of DjGAD gene expression caused a defect in planarians' negative phototaxis, and further analysis revealed that GABAergic neurons are necessary for processing the signals from visual neurons (Fig. 8H; Nishimura et al., 2008). Furthermore, studies on the gene encoding planarian tyrosine hydroxylase demonstrated that dopaminergic neurons, together with motor neurons regulated by cholinergic neurons, coordinate the muscular locomotion in planarian (Fig. 9B; Nishimura et al., 2007b; Nishimura et al., 2010). In this study, we have identified some elements of the neural pathway for thermosensory responses in planarians, including thermosensory neurons in the periphery of the body and information-processing neurons in the brain that presumably receive the signals from those thermosensory neurons. Further investigation of the mechanisms underlying the coordination of neural pathways and neural physiology in planarians should provide insights into how this neural-information-processing machinery produces the behavioral responses to complex environmental cues.
Footnotes
This work was supported by a Grant-in-Aid for Young Scientists (B) to T.I., The Kyoto University Research Funds for Young Scientists to T.I., The Brain Science Foundation funds to T.I., a Grant-in-Aid for Scientific Research on Innovative Areas to K.A., a Grant-in-Aid for Creative Scientific Research to K.A., and the Grants to Excellent Graduate Schools (MEXT) program of Kyoto University. We thank Elizabeth Nakajima for critical reading of the manuscript.
The authors declare no competing financial interests.
References
- Agata K, Watanabe K. Molecular and cellular aspects of planarian regeneration. Semin Cell Dev Biol. 1999;10:377–383. doi: 10.1006/scdb.1999.0324. [DOI] [PubMed] [Google Scholar]
- Agata K, Soejima Y, Kato K, Kobayashi C, Umesono Y, Watanabe K. Structure of the planarian central nervous system (CNS) revealed by neuronal cell markers. Zool Sci. 1998;15:433–440. doi: 10.2108/zsj.15.433. [DOI] [PubMed] [Google Scholar]
- Azuma K, Okazaki Y, Asai K, Iwasaki N. Electrical responses and K+ activity changes to light in the ocellus of the planarian Dugesia japonica. Comp Biochem Physiol A Physiol. 1994;109:593–599. doi: 10.1016/0300-9629(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Azuma K, Iwasaki N, Ohtsu K. Absorption spectra of planarian visual pigments and two states of the metarhodopsin intermediates. Photochem Photobiol. 1999;69:99–104. doi: 10.1111/j.1751-1097.1999.tb05312.x. [DOI] [Google Scholar]
- Baez-Nieto D, Castillo JP, Dragicevic C, Alvarez O, Latorre R. Thermo-TRP channels: biophysics of polymodal receptors. Adv Exp Med Biol. 2011;704:469–490. doi: 10.1007/978-94-007-0265-3_26. [DOI] [PubMed] [Google Scholar]
- Best JB, Goodman AB, Pigon A. Fissioning in planarians: control by the brain. Science. 1969;164:565–566. doi: 10.1126/science.164.3879.565. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orio P. Voltage sensing in thermo-TRP channels. Adv Exp Med Biol. 2011;704:517–530. doi: 10.1007/978-94-007-0265-3_28. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Montell C. Take a TRP to beat the heat. Genes Dev. 2005;19:415–418. doi: 10.1101/gad.1294905. [DOI] [PubMed] [Google Scholar]
- Cebrià F, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. Dissecting planarian central nervous system regeneration by the expression of neural-specific genes. Dev Growth Differ. 2002;44:135–146. doi: 10.1046/j.1440-169x.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- Cole WH. Temperature and locomotion in Planaria. J Gen Physiol. 1926;9:503–511. doi: 10.1085/jgp.9.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, 3rd, Hou X, Romanova EV, Lambrus BG, Miller CM, Saberi A, Sweedler JV, Newmark PA. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 2010;8:e1000509. doi: 10.1371/journal.pbio.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KW, Pearson BJ. Transcription factors lhx1/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development. 2013;140:3577–3588. doi: 10.1242/dev.098590. [DOI] [PubMed] [Google Scholar]
- Curtis WC. The life history, the normal fission and the reproductive organs of Planaria maculata. Proc Boston Soc Nat Hist. 1902;30:515–559. [Google Scholar]
- Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Grasso M, Benazzi M. Genetic and physiologic control of fissioning and sexuality in planarians. J Embryol Exp Morphol. 1973;30:317–328. [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/S0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Klose C, Krautwurst D. Synthetic modulators of TRP channel activity. Adv Exp Med Biol. 2011;704:87–106. doi: 10.1007/978-94-007-0265-3_4. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kumamoto H, Okamoto K, Umesono Y, Sakai M, Sánchez Alvarado A, Agata K. Morphological and functional recovery of the planarian photosensing system during head regeneration. Zool Sci. 2004;21:275–283. doi: 10.2108/zsj.21.275. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hayashi T, Takechi K, Agata K. Clathrin-mediated endocytic signals are required for the regeneration of, as well as homeostasis in, the planarian CNS. Development. 2007;134:1679–1689. doi: 10.1242/dev.02835. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/S0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, Davenport EA, Hoffman BE, Laping NJ, Su X. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008;295:F803–F810. doi: 10.1152/ajprenal.90269.2008. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Luo L, Gershow M, Rosenzweig M, Kang K, Fang-Yen C, Garrity PA, Samuel AD. Navigational decision making in Drosophila thermotaxis. J Neurosci. 2010;30:4261–4272. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März M, Seebeck F, Bartscherer K. A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development. 2013;140:4499–4509. doi: 10.1242/dev.100081. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet. 1999;33:399–422. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Yoshimoto K, Takeuchi K, Taniguchi T, Agata K. Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesia japonica. Neurosci Res. 2007a;59:101–106. doi: 10.1016/j.neures.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Sano S, Yoshimoto K, Inden M, Takata K, Taniguchi T, Shimohama S, Agata K. Reconstruction of dopaminergic neural network and locomotion function in planarian regenerates. Dev Neurobiol. 2007b;67:1059–1078. doi: 10.1002/dneu.20377. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Umesono Y, Takeuchi K, Takata K, Taniguchi T, Agata K. Identification of glutamic acid decarboxylase gene and distribution of GABAergic nervous system in the planarian Dugesia japonica. Neuroscience. 2008;153:1103–1114. doi: 10.1016/j.neuroscience.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zool Sci. 2005;22:535–546. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Pearl R. The movements and reactions of fresh-water planarians: A study in animal behaviour. Q J Microsc Sci. 1903;46:509–714. [Google Scholar]
- Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv Exp Med Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- Ramot D, MacInnis BL, Lee HC, Goodman MB. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J Neurosci. 2008;28:12546–12557. doi: 10.1523/JNEUROSCI.2857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Weiss JA, Forsthoefel DJ, Lee H, King RS, Inoue T, Shibata N, Agata K, Newmark PA. RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: methodology and dynamics. Dev Dyn. 2013;242:718–730. doi: 10.1002/dvdy.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci U S A. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino M, Pedersen AF, Trajkovska V, Christensen E, Lohals R, Veng LM, Knudsen GM, Aznar S. Selective immunolesion of cholinergic neurons leads to long-term changes in 5-HT2A receptor levels in hippocampus and frontal cortex. Neurosci Lett. 2007;428:47–51. doi: 10.1016/j.neulet.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Shibata N, Rouhana L, Agata K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Dev Growth Differ. 2010;52:27–41. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- Shibata N, Hayashi T, Fukumura R, Fujii J, Kudome-Takamatsu T, Nishimura O, Sano S, Son F, Suzuki N, Araki R, Abe M, Agata K. Comprehensive gene expression analyses in pluripotent stem cells of a planarian, Dugesia japonica. Int J Dev Biol. 2012;56:93–102. doi: 10.1387/ijdb.113434ns. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Tominaga M. A temperature-sensitive TRP ion channel, Painless, functions as a noxious heat sensor in fruit flies. Commun Integr Biol. 2009;2:170–173. doi: 10.4161/cib.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Pulvers JN, Inoue T, Tarui H, Sakamoto H, Agata K, Umesono Y. Regeneration-dependent conditional gene knockdown (Readyknock) in planarian: demonstration of requirement for Djsnap-25 expression in the brain for negative phototactic behavior. Dev Growth Differ. 2007;49:383–394. doi: 10.1111/j.1440-169X.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- Talavera K, Nilius B, Voets T. Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31:287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tasaki J, Shibata N, Nishimura O, Itomi K, Tabata Y, Son F, Suzuki N, Araki R, Abe M, Agata K, Umesono Y. ERK signaling controls blastema cell differentiation during planarian regeneration. Development. 2011;138:2417–2427. doi: 10.1242/dev.060764. [DOI] [PubMed] [Google Scholar]
- Tazaki A, Gaudieri S, Ikeo K, Gojobori T, Watanabe K, Agata K. Neural network in planarian revealed by an antibody against planarian synaptotagmin homologue. Biochem Biophys Res Commun. 1999;260:426–432. doi: 10.1006/bbrc.1999.0933. [DOI] [PubMed] [Google Scholar]
- Tominaga M. The role of TRP channels in thermosensation. In: Liedtke WB, Heller S, editors. TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton, FL: CRC; 2007. [PubMed] [Google Scholar]
- Umesono Y, Agata K. Evolution and regeneration of the planarian central nervous system. Dev Growth Differ. 2009;51:185–195. doi: 10.1111/j.1440-169X.2009.01099.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Tasaki J, Nishimura K, Inoue T, Agata K. Regeneration in an evolutionarily primitive brain–the planarian Dugesia japonica model. Eur J Neurosci. 2011;34:863–869. doi: 10.1111/j.1460-9568.2011.07819.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Tasaki J, Nishimura Y, Hrouda M, Kawaguchi E, Yazawa S, Nishimura O, Hosoda K, Inoue T, Agata K. The molecular logic for planarian regeneration along the anterior-posterior axis. Nature. 2013;500:73–76. doi: 10.1038/nature12359. [DOI] [PubMed] [Google Scholar]
- Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowinckel C. The role of illumination and temperature in the control of sexual reproduction in the planarian Dugesia tigrina (Girard) Biol Bull. 1970;138:77–87. doi: 10.2307/1540293. [DOI] [Google Scholar]
- Wetsel WC. Sensing hot and cold with TRP channels. Int J Hyperthermia. 2011;27:388–398. doi: 10.3109/02656736.2011.554337. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis: Springer. 2009 [Google Scholar]
- Xiao R, Xu XZ. C. elegans TRP channels. Adv Exp Med Biol. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]