Abstract
The hypothalamic NPY system plays an important role in regulating food intake and energy expenditure. Different biological actions of NPY are assigned to NPY receptor subtypes. Recent studies demonstrated a close relationship between food intake and growth hormone (GH) secretion; however, the mechanism through which endogenous NPY modulates GH release remains unknown. Moreover, conclusive evidence demonstrating a role for NPY and Y-receptors in regulating the endogenous pulsatile release of GH does not exist. We used genetically modified mice (germline Npy, Y1, and Y2 receptor knock-out mice) to assess pulsatile GH secretion under both fed and fasting conditions. Deletion of NPY did not impact fed GH release; however, it reversed the fasting-induced suppression of pulsatile GH secretion. The recovery of GH secretion was associated with a reduction in hypothalamic somatotropin release inhibiting factor (Srif; somatostatin) mRNA expression. Moreover, observations revealed a differential role for Y1 and Y2 receptors, wherein the postsynaptic Y1 receptor suppresses GH secretion in fasting. In contrast, the presynaptic Y2 receptor maintains normal GH output under long-term ad libitum-fed conditions. These data demonstrate an integrated neural circuit that modulates GH release relative to food intake, and provide essential information to address the differential roles of Y1 and Y2 receptors in regulating the release of GH under fed and fasting states.
Keywords: fasting, feeding, growth hormone, NPY, NPY-receptors, somatostatin
Introduction
Growth hormone (GH) secretion is under the reciprocal control of hypothalamic stimulatory growth hormone releasing hormone (GHRH) and inhibitory somatotropin release inhibiting factor (SRIF; somatostatin) neurons (Frohman et al., 1992). A close relationship exists between GH secretion and food intake. In humans, consumption of a high-caloric diet results in the early suppression of pulsatile GH secretion (Cornford et al., 2011). Similarly, consumption of a high-fat diet suppresses pulsatile GH secretion in rodents (Huang et al., 2012). In addition, food withdrawal immediately suppresses pulsatile GH secretion in both rats (Glad et al., 2011) and mice (Steyn et al., 2012). Altered GH secretion relative to food intake may, in part, be mediated through changes in GHRH and/or SRIF secretion (Bruno et al., 1993; Tannenbaum et al., 1996; Luque and Kineman, 2006; Steyn et al., 2012) that occur in response to hypothalamic orexigenic and anorexigenic systems.
Hypothalamic NPY neurons mediate their orexigenic effect through the activation of Y receptors (Y1, Y2, Y4, and Y5, and in mice and rabbits, the Y6 receptor; Herzog, 2003; Fetissov et al., 2004). Of these receptors, the Y1 receptor (Y1R) is the dominant postsynaptic receptor, whereas the Y2 receptor (Y2R) is mainly presynaptically expressed on NPY neurons (Chen and van den Pol, 1996; Balasubramaniam et al., 2007; Keen-Rhinehart and Bartness, 2007). Activation of the Y1R stimulates feeding whereas activation of Y2R inhibits NPY production and release (Keen-Rhinehart and Bartness, 2007; Ortiz et al., 2007). While the role of NPY and its receptors on food intake is well characterized (Herzog, 2003), their effects in regulating GH secretion remains largely unknown. Anatomical and neuropharmacological evidence provides insights into the possible interconnectivity between NPY and GH regulating systems. Early studies in rodents demonstrate an inhibitory role of NPY on the GH axis (Pierroz et al., 1996; Raposinho et al., 2001), while morphological studies in rats showed that NPY neurons in the arcuate nucleus (ARC) project to SRIF neurons located within the periventricular nucleus (PeVN; Hisano et al., 1990). Furthermore, observations in mice demonstrate an increase in hypothalamic levels of Npy mRNA, alongside an increase in Srif mRNA expression following 9 h of fasting (Steyn et al., 2012). Given that SRIF inhibits the release of GH secretion, these observations suggest that NPY neurons mediate the fasting-induced suppression of GH secretion, possibly through increasing SRIF neuronal activity. Accordingly, we investigated the actions of NPY neurons, and the Y1R and Y2R in mediating pulsatile GH secretion under ad libitum-fed and fasting conditions.
Materials and Methods
Mice.
All experiments were conducted in adult male mice (8–12 weeks of age) on either a wild-type or transgenic background. Heterozygous Npy+/−, Y1R+/−, and Y2R+/− mice, bred on a mixed C57BL/6129SvJ background, were used to generate homozygous Npy−/−, Y1R−/−, and Y2R−/− mice and age-matched wild-type littermates (Sainsbury et al., 2002; Howell et al., 2003; Karl et al., 2008). Mice were maintained under controlled light (12 h light/dark cycle, lights off at 18:00 h) and temperature (22 ± 2°C) conditions and had free access to food and water unless otherwise specified. Mice were individually housed at least 1 week before experiments. All experimental procedures were approved by the University of Queensland Animal Ethics Committee.
Confirmation of anatomical relationship between NPY and SRIF neurons in mice.
Brain tissues from transgenic NPY-humanized Renilla reniformis-GFP mice (B6.FVB-Tg(Npy-hrGFP)1Lowl/J; stock number 006417; The Jackson Laboratory; 9–10 weeks old; Wu et al., 2014) and C57BL/6 wild-type mice were collected for immunohistochemistry and in situ hybridization, respectively. Briefly, Npy-hrGFP mice were anesthetized with isoflurane and perfused with 0.1 m phosphate buffer, followed by 4% paraformaldehyde (Sigma-Aldrich). The brain was excised and post fixed in 4% paraformaldehyde overnight at 4°C and then replaced in 30% sucrose. For immunohistochemistry, coronal brain sections from Npy-hrGFP mice were cut at a thickness of 30 μm on a cryostat (Leica CM 1850) and every fourth section through the hypothalamus was collected and stored in cryoprotectant (30% sucrose, 1% polyvinyl pyrrolidone and 30% ethylene glycol in 0.1 m phosphate buffer) at −20°C. Sections were incubated with primary antibodies [rabbit anti-somatostatin-14, 1:5000; Bachem; mouse monoclonal antibody against the synaptic vesicle protein 2 (SV2), a marker for synaptic sites in neurons and endocrine cells; Buckley and Kelly, 1985; 1:100; Invitrogen] diluted in blocking serum for 72 h at 4°C on an orbital shaker. Following incubation in primary antibodies, sections were washed and incubated with secondary antibodies (donkey anti-rabbit 555, 1:500; Invitrogen and donkey anti-mouse IgG 647, 1:500; Life Technologies). To visualize immunoreactivity, sections were air dried, mounted with Golden anti-fade reagent with DAPI (Invitrogen), and examined using a confocal laser-scanning microscope (Olympus, FV 1000). Images were processed using ImageJ software (version 1.46r). For In situ hybridization, two sequential coronal brain sections (14 μm) were thaw mounted as mirrored images onto SuperFrost slides (Menzel-Gläser). Matched sections from the adjacent coronal brain level were processed for DIG-labeled Srif and Npy mRNA expression, following established in situ hybridization methodology (Tan et al., 2013). DNA oligonucleotides complementary to mouse Srif and Npy are 5′-TAATACGACTCACTATAGGGgggccaggagttaaggaaga-3′ and 5′-TAATACGACTCACTATAGGGgcagactggtttcaggggat-3′, respectively. Sections were imaged on an Aperio ScanScope XT slide scanner (Aperio Technologies). Extracted images were opened in ImageJ and matched sections were adjusted to mirror symmetry.
Determination of pulsatile GH secretion in Npy−/− mice under fasting and fed conditions.
At 8 weeks of age, pulsatile secretion of GH was assessed in Npy−/− mice and the corresponding age-matched wild-type littermates following the first 6 h of food withdrawal. At the start of dark cycle, animals were deprived of food (between 1800 and 1815 h). Starting at 1830 h, 36 sequential tail-tip blood samples were collected from individual mice at 10 min intervals under dim red light. Blood samples were collected and processed as described previously (Steyn et al., 2011). Following collection of blood samples, mice were returned to their home cage and allowed to settle for at least 2 weeks before repeat serial blood collection under ad libitum-fed conditions (10 weeks of age, sampling commenced at 0700 h). Samples were processed by an in-house GH ELISA to determine whole blood GH content (Steyn et al., 2011). One week following the second blood collection, mice were killed following 6 h of fasting (as detailed above). Body length was measured from nose to rump at the time of killing. Brains were collected, snap frozen, and kept at −80°C for further analysis.
Analysis of hypothalamic Srif and Ghrh gene expression.
We previously showed that the rise in hypothalamic Srif mRNA at 9 h of fasting in the mouse occurs alongside a rise in hypothalamic Npy mRNA expression. Moreover, treatment of NPY results in an increase in SRIF release from hypothalamic explants (Rettori et al., 1990). Accordingly, we anticipated that the recovery of GH release in Npy−/− mice occurred alongside a loss in NPY-mediated SRIF release. To address this, we determined the expression of Srif and Ghrh mRNA from hypothalamic tissue biopsies from Npy−/− mice and corresponding age-matched wild-type littermates under fasting and fed conditions. To confirm that loss of Npy−/− does not impact hypothalamic Srif and Ghrh mRNA expression under basal ad libitum-fed condition, Npy−/− mice and wild-type littermates were killed and brain tissues were collected between 0800 and 0900 h. These animals had free access to food and water the night before tissue collection. To determine whether loss of NPY will result in altered Srif expression, Npy−/− mice and wild-type littermates were killed following 6 h of food withdrawal in the dark cycle (tissue collected between 0000 and 0100 h; food was removed immediately following the onset of the dark cycle). For tissue collection, mice were anesthetized with a single intraperitoneal injection of sodium pentobarbitone (32.5 mg/ml, 1PO643-1; Virbac Animal Health). Once anesthetized, the brain from each mouse was immediately removed, snap frozen on dry ice, and stored at −80°C until microdissection of specific nuclei (ARC and PeVN), following established methodology (Steyn et al., 2012). In brief, frozen brain tissue was cut (300 μm) on a cryostat (Leica), and transferred to RNase free SuperFrost plus slides. Tissue biopsies representative of the ARC and PeVN located between −0.94 and −2.18 mm bregma (as shown in Fig. 3A) were processed for qRT-PCR. Total cellular RNA was extracted from brain tissues collected in TRIzol using PureLink RNA Micro Kit (Invitrogen). The final concentration and quality of RNA was determined via a spectrophotometer (NanoDrop, ND-1000; Thermo Scientific). First-strand cDNA was synthesized from 100 ng total RNA using the Vilo cDNA Synthesis kit (Invitrogen). qRT-PCR was performed using Taq polymerase (TaqMan) probes and the following primers: Ghrh, assay ID: Mm00439100_m1; Srif, assay ID: Mm00436671_ml; and Gapdh, assay ID: Mm9999915_gl. All primers were purchased from Applied Biosystems. The experiment was set up according to the protocol and reagents provided. Amplification in a 10 μl reaction volume was assessed using the QuantStudio 7 system (Applied Biosystems). Data were displayed as an amplification plot and analysis was done by ViiA7 Software v1.2 (Applied Biosystems). Changes in cycle threshold of the gene of interest were corrected to the housekeeping gene (Gapdh). Final measurements of the gene expression from Npy−/− mice were presented as relative levels corresponding to ad libitum-fed or fasting wild-type mice.
Figure 3.
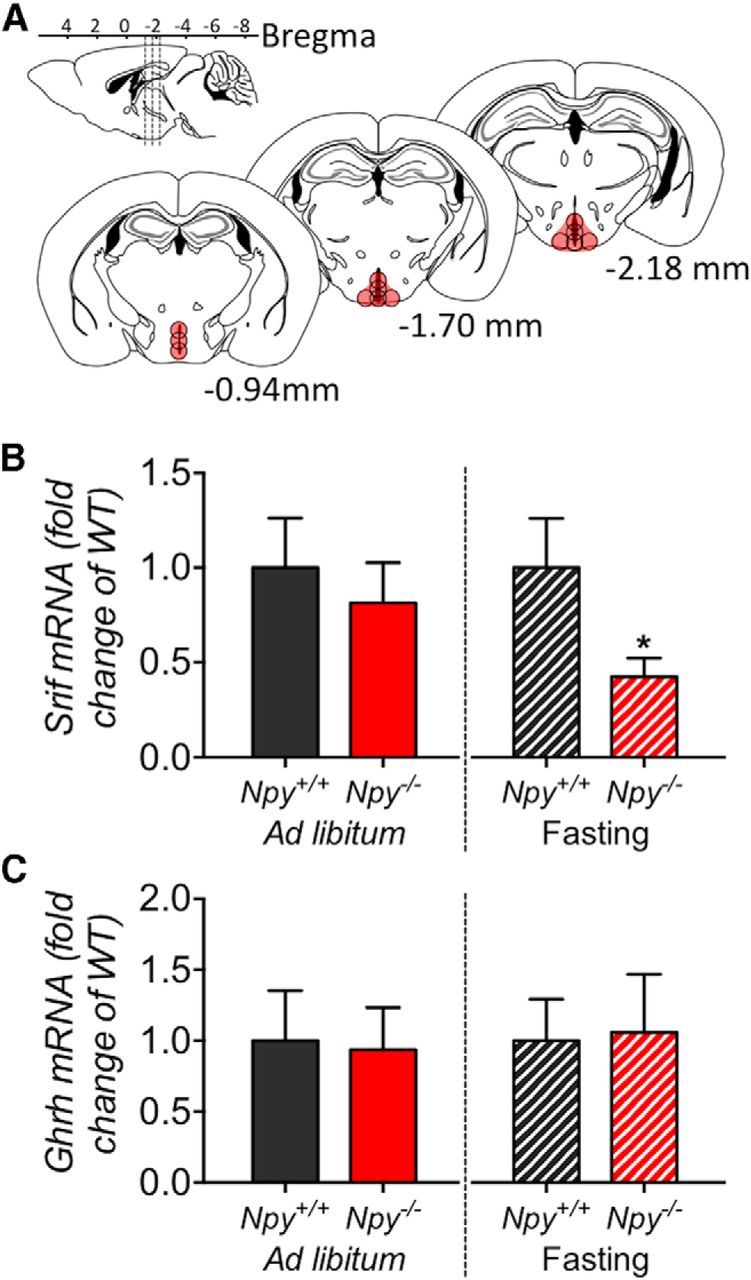
Comparison of Ghrh and Srif mRNA expression within the ARC/PeVN of Npy−/− mice and corresponding WT mice under ad libitum-fed conditions and following 6 h of fasting. Micropunch biopsies of tissue representing the ARC/PeVN (between −0.94 and −2.18 bregma) were collected from frozen brain sections (A, location of punch biopsies illustrated in red). Compared with wild-type mice, deletion of NPY did not result in a significant difference in Srif (B, left) or Ghrh (C, left) mRNA expression under ad libitum-fed conditions. In contrast, deletion of NPY resulted in a reduction of Srif mRNA expression (B, right) following 6 h of fasting. Ghrh mRNA expression remained unchanged (C, right). Data are presented as mean ± SEM, N = 5–6, *p < 0.05.
Determination of circulating blood glucose, NPY, and peptide YY (PYY) in response to acute food deprivation.
NPY neurons sense glucose (Murphy et al., 2009), and activation of hypothalamic NPY neurons occurs within 60 min of central glucoprivation (Minami et al., 1995). Accordingly, alterations in blood glucose levels might underlie altered NPY activity. To determine the time-dependent response of circulating levels of glucose relative to food withdrawal, blood glucose was assessed from tail blood using the Accu-Chek Performa blood glucose meter (Roche). Blood glucose measures were collected at 15 min intervals for a period of 2.5 h, starting at 1800 h (food was removed immediately following the onset of the dark cycle). Circulating gut hormone PYY is secreted in response to food ingestion and is a natural ligand of Y receptors (Batterham et al., 2002). Changes in circulating PYY may underlie Y-receptor-mediated GH secretion. Accordingly, circulating levels of PYY in response to fasting were determined by commercial ELISA kit (mouse metabolic magnetic bead panel, #MMHMAG-44K; Millipore) using terminal blood samples collected from C57BL/6 mice at 0, 45, and 90 min following the onset of food withdrawal. Samples were collected via cardiac puncture from anesthetized mice, and sampling intervals were selected based on observations from glucose measurements. Although peripheral NPY is largely a marker of sympathetic nervous system activity (Zukowska-Grojec et al., 1998), a number of studies in both humans and rats have demonstrated effects of NPY on GH secretion in pituitary cells (Adams et al., 1987; Chabot et al., 1988). Accordingly, we determined circulating levels of NPY in fasting mice to determine whether alterations in circulating levels of NPY may contribute to the suppression of GH release at this time. Circulating NPY was assessed by commercial ELSIA kit (rat/mouse NPY, #EZRMNPY-27K; Millipore) from terminal samples collected as described above.
Assessment of pulsatile GH secretion in Y1R−/− and Y2R−/− mice under fasting and ad libitum-fed conditions.
To determine whether the NPY-mediated GH secretion occurs via postsynaptic Y1R or presynaptic Y2R-mediated mechanisms, pulsatile GH secretion was further determined in germline Y1 or Y2 receptor knock-out mice and respective age-matched wild-type littermates under ad libitum-fed and fasting conditions. Experimental procedures were similar to that conducted in Npy−/− mice (as described above).
Statistical analysis.
The quantitative features underlying GH secretion and clearance associated with the observed GH concentration profiles were determined at a fixed half-life of 7.95 min by deconvolution analysis, as described previously (Huang et al., 2012). Resulting output measures are indicative of the secretion of GH from the pituitary gland, and thus a measure of hypothalamic-mediated pituitary activity that accounts for the observed circulating measures of GH. All data are presented as mean ± SEM unless otherwise stated. Differences between groups were identified by two-tailed unpaired t test unless otherwise stated. All measures (excluding deconvolution analysis) were performed using GraphPad Prism 6.0e. The threshold level for statistical significance was set at p < 0.05.
Results
Hypothalamic NPY neurons are structurally integrated with hypothalamic SRIF neurons
Immunofluorescence assessment of hypothalamic NPY and SRIF expression demonstrates close apposition of NPY-immunopositive fibers with SRIF-expressing neurons, specifically within the PeVN (Fig. 1C–E). Insets illustrate close apposition of NPY-GFP fibers with SRIF-immunoreactive cell bodies, while the 60× magnified view (Fig. 1F–H) demonstrates SV2 immunoreactivity in both SRIF (Fig. 1F) and NPY-immunopositive fibers (Fig. 1 G), and colocalization of SV2, SRIF, and NPY immunoreactivity (Fig. 1H). Thus, synaptic interactions occur between SRIF and NPY neurons. Immunofluorescence assessment of NPY-GFP and SRIF-positive immunoreactivity within the ARC demonstrate punctate SRIF expression, distributed among NPY-positive GFP neuronal cell bodies (Fig. 1I–K). Given that SRIF within the ARC was not clearly localized to cell bodies, we completed in situ hybridization assessment of Npy (Fig. 1L) and Srif (Fig. 1M) mRNA within this region. In situ hybridization confirmed the close proximity of Npy and Srif mRNA-expressing neurons (Fig. 1N). Collectively, observations provide a structural basis for potential physiological interactions between hypothalamic NPY and SRIF-expressing neurons.
Figure 1.
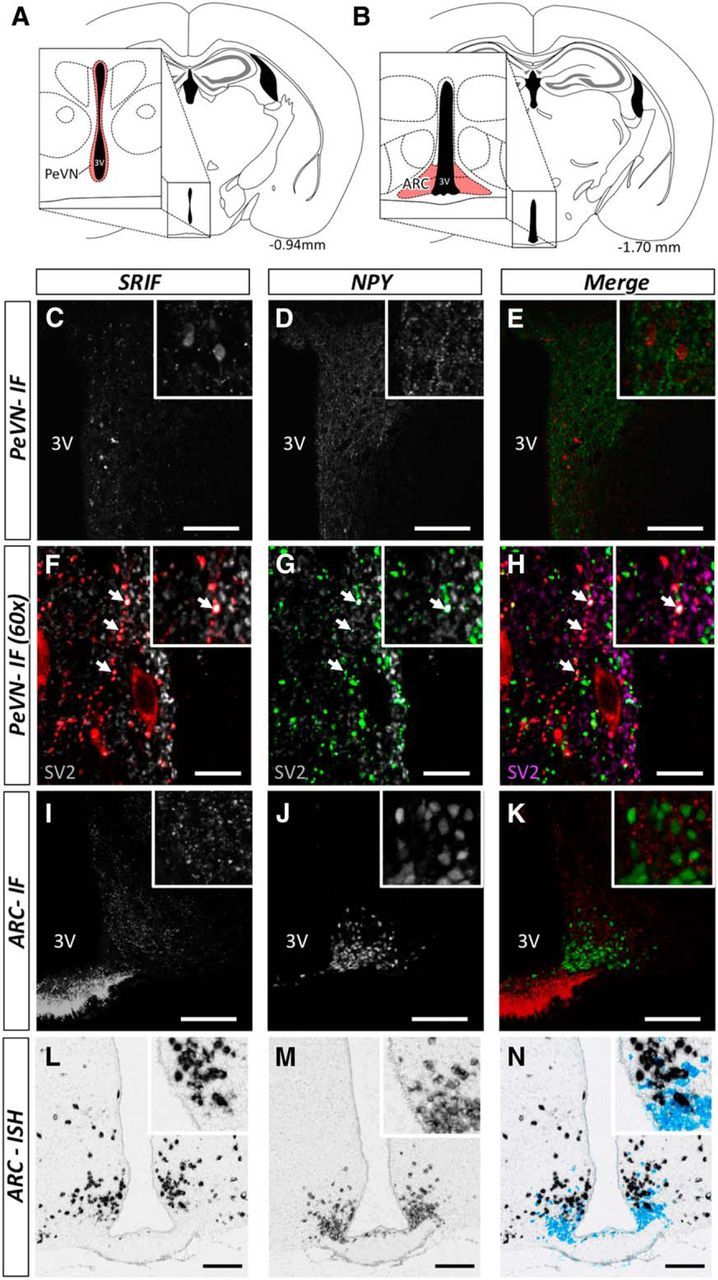
Illustration of anatomical interactions between SRIF and NPY-expressing neurons within the hypothalamus (A, B) by immunofluorescence (C–K) and in situ hybridization (L–N). Brain slices were collected between −0.94 and −1.70 bregma. Schematic representation of specific areas within the hypothalamus, representative of the PeVN (A, enlarged inset, shaded areas in red) and ARC (B, enlarged inset, shaded area in red). Within the PeVN (C–H) we observed a number of SRIF-immunopositive neurons (red) in close apposition with NPY-GFP-immunopositive fibers (green; C–E). Further assessment using SV2 (gray; F and G; purple; H) revealed the synaptic interactions between SRIF and NPY-positive fibers (F–H; colocalization is indicated by white arrows). SRIF-positive immunoreactivity and NPY-GFP within the ARC (I–K) demonstrate punctuate SRIF expression, distributed among NPY-positive GFP neuronal cell bodies. In situ hybridization illustrates the proportion of Srif (black) and Npy (light blue) mRNA-expressing neurons within the ARC (L–N). These two populations of neurons were in close proximity. Scale bars: C–E, I–N, 100 μm; F–H, 10 μm. Inserts illustrate a magnified view of the figures. Representative images illustrate interactions verified across four animals.
Deletion of NPY prevents fasting-induced suppression in GH secretion in mice
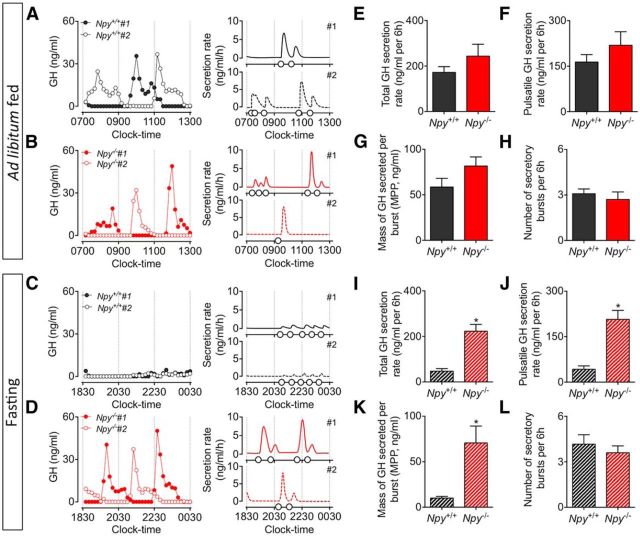
Representative secretory profiles and output figures illustrate the onset of pulsatile GH secretion in ad libitum-fed Npy−/− and corresponding wild-type mice (Fig. 2A,B), and the same mice following 6 h of fasting (Fig. 2C,D). Peaks in GH secretion occurred at regular intervals, and were dispersed by periods of low basal levels of secretion. There was no significant difference in GH release (including total; Fig. 2E; t(20) = 1.29, p = 0.21) and pulsatile (Fig. 2F; t(20) = 1.30, p = 0.27) GH secretion, the mass of GH secreted per burst (Fig. 2G; t(20) = 1.67, p = 0.11), and the number of secretory events (Fig. 2H; t(20) = 0.67, p = 0.51) between Npy−/− mice and corresponding wild-type mice under ad libitum-fed conditions, as determined by deconvolution analysis. During fasting, pulsatile GH secretion was significantly suppressed in wild-type mice (Fig. 2C) whereas GH secretion was sustained in Npy−/− mice (Fig. 2D). Deconvolution analysis of pulsatile measures of GH confirmed that deletion of NPY recovered GH secretion. This is characterized by a recovery in total (Fig. 2I; t(20) = 5.88, p < 0.01), pulsatile (Fig. 2J; t(20) = 5.54, p < 0.01), and mass of GH secretion per pulse (Fig. 2K; t(20) = 3.57, p < 0.01), whereas the number of secretory bursts remained unchanged (Fig. 2L; t(20) = 0.72, p = 0.48). Of interest, Npy mRNA exhibits circadian rhythm in gene expression with highest levels observed early in the dark cycle (Kohsaka et al., 2007). This suggests NPY neurons are primed at this time, and is in agreement with the immediate cessation of GH secretion as observed in wild-type mice following food withdrawal (Fig. 2C).
Figure 2.
Representative examples of pulsatile GH secretion profiles (2 animals; #1, closed circles; #2, open circles; A–D, left) and corresponding output figures (A–D, right) in male adult Npy−/− mice (red) and age-matched wild-type littermates (black). Assessment of pulsatile GH secretion was done in ad libitum-fed mice (A, B) and during the first 6 h of fasting (C, D). Samples were collected for 6 h at 10 min intervals, starting at 0700 h (fed) and 1830 h (fasting). Corresponding output figures illustrate the onset of GH pulses (pulse onset indicated by open circles along the x-axis). GH secretory parameters following deconvolution analysis are illustrated in E–H (ad libitum fed) and I–L (fasting). Total (E) and pulsatile GH secretion rate (F), the mass of GH secreted per burst (G), and number of secretory bursts (H) in Npy−/− mice were comparable to ad libitum-fed wild-type mice. In contrast, the fasting-induced suppression of GH secretion observed in wild-type mice was prevented in Npy−/− mice. This was characterized by a significant increase in total (I) and pulsatile GH secretion rate (J), and the mass of GH secreted per burst (K) in Npy−/− mice. The number of secretory bursts (L) remained unchanged. Data are presented as mean ± SEM, N = 10–12, *p < 0.05.
The recovery of GH secretion in Npy−/− mice following 6 h of fasting is associated with a reduction in hypothalamic Srif mRNA expression
Our previous results demonstrate that reduced GH secretion in fasting wild-type mice coincides with elevated Srif mRNA expression (Steyn et al., 2012). To further determine whether the recovery of pulsatile GH secretion in Npy−/− mice in response to fasting is associated with changes in primary hypothalamic GH regulators, Ghrh and Srif gene expression was assessed. Loss of NPY did not impact ARC/PeVN Srif (Fig. 3B, left; t(8) = 1.03, p = 0.55) or Ghrh (Fig. 3C, left; t(8) = 0.21, p = 0.84) mRNA expression under ad libitum-fed conditions. In contrast, Srif mRNA expression was significantly reduced in Npy−/− mice following the first 6 h of food withdrawal (Fig. 3B, right; t(9) = 2.74, p < 0.05), indicating that fasting may induce NPY-activated SRIF expression. No change in Ghrh mRNA expression within the ARC/PeVN area was observed (Fig. 3C, right; t(9) = 0.15, p = 0.88).
Fasting rapidly reduces circulating glucose levels, whereas circulating NPY and PYY levels remain unchanged
Increased NPY activity occurs in response to glucoprivation (Minami et al., 1995), and thus may underlie the trigger for the suppression of GH release in fasting mice. Circulating levels of glucose declined by 45 min of food withdrawal (Fig. 4A; two-way ANOVA; p < 0.01). In ad libitum-fed animals, blood glucose levels were maintained relative to starting measures, and increased slightly relative to the onset of nighttime feeding. Plasma NPY and PYY concentrations remained unchanged following 45 min (Fig. 4B; t(22) = 0.05, p = 0.96 and C; t(22) = 0.29, p = 0.77, respectively) and 90 min of fasting (Fig. 4B; t(22) = 1.79, p = 0.09 and C; t(22) = 0.11, p = 0.91, respectively). These observations suggest that circulating NPY and PYY in early fasting does not contribute to the NPY-mediated suppression of GH secretion and supports the central actions of NPY in suppressing GH release, presumably following alterations in blood glucose concentration.
Figure 4.
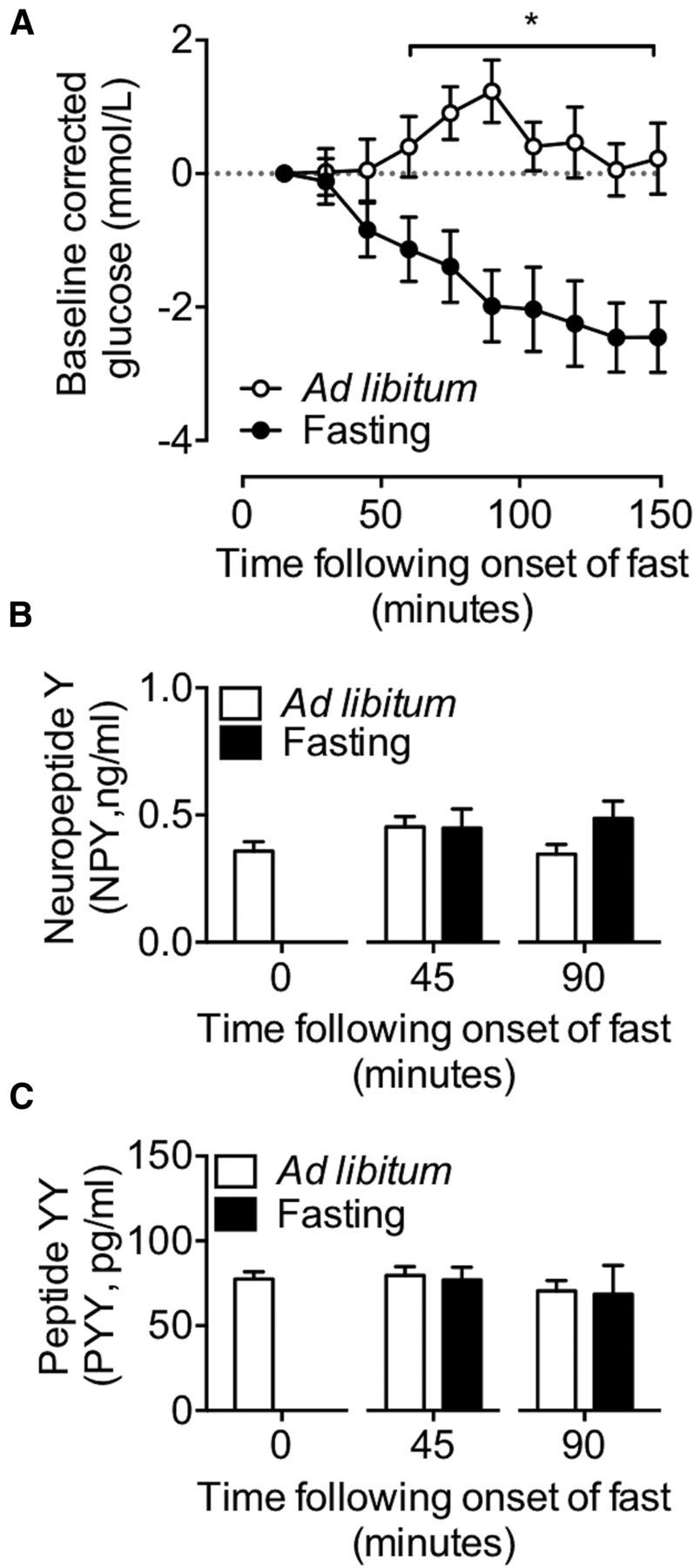
Assessment of circulating levels of blood glucose, NPY, and PYY in response to fasting. Glucose was measured every 15 min for a duration of 150 min, and corrected to starting levels (A). Circulating levels of NPY (B) and PYY (C) were determined following 45 and 90 min of fasting. Fasting resulted in a decline in circulating levels of glucose by 45 min of food withdrawal (A). Unlike glucose, circulating levels of NPY and PYY remained unchanged for the duration of the fasting period (B, C). Data are presented as mean ± SEM, N = 12, *p < 0.05.
The Y1R mediates GH secretion following acute fasting, whereas the Y2R modulates GH secretion under basal ad libitum-fed conditions
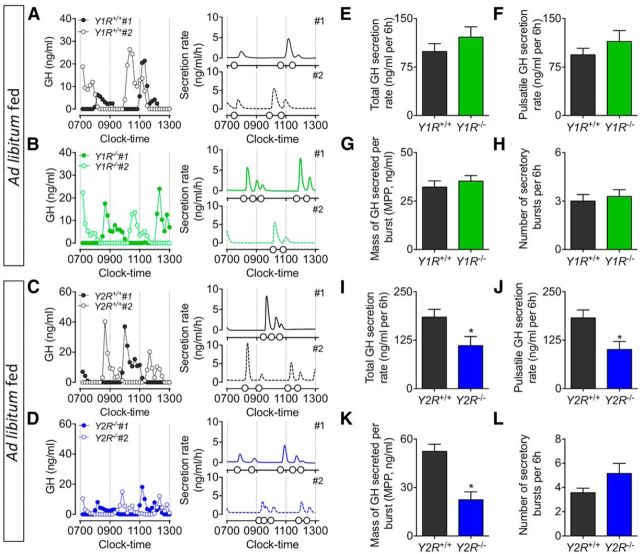
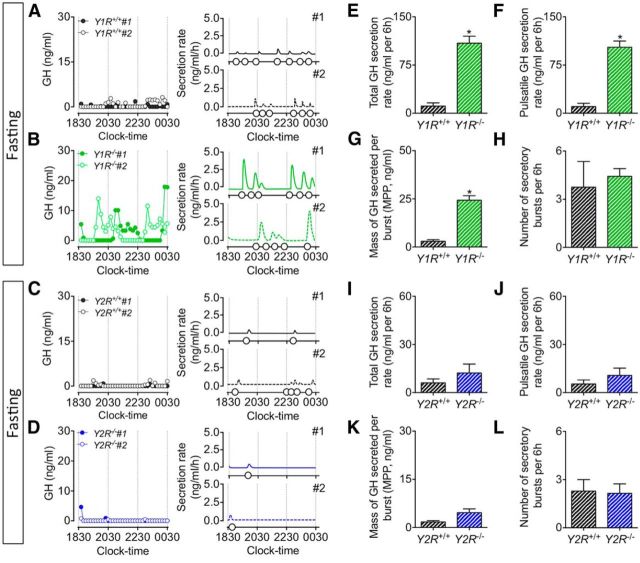
To determine whether the recovery of pulsatile GH secretion observed in Npy−/− mice following 6 h of fasting is assigned to specific receptor subtypes, pulsatile GH secretion was further assessed in postsynaptic Y1R and presynaptic Y2R germline knock-out mice. GH secretory profiles from ad libitum-fed Y1R−/− and Y2R−/− mice display regular pulsatile secretory patterns, characterized by spontaneous GH secretory events (Fig. 5A–D). The deletion of Y1R did not affect GH secretion in ad libitum-fed mice (including total; Fig. 5E; t(9) = 0.95, p = 0.37) and pulsatile (Fig. 5F; t(9) = 0.85, p = 0.42) GH secretion, the mass of GH secreted per burst (Fig. 5G; t(9) = 0.67, p = 0.52), and the number of secretory events (Fig. 5H; t(9) = 0.45, p = 0.67), whereas the loss of Y2R resulted in a significant decline in peak GH secretion, as demonstrated by a reduction in total (Fig. 5I; t(12) = 2.37, p < 0.05) and pulsatile (Fig. 5J; t(12) = 2.79, p < 0.05) GH secretion, and the mass of GH secreted per burst (Fig. 5K; t = (12) = 4.55, p < 0.01). Of interest, germline Y2R deletion increased pulse number (although this did not reach significance; Fig. 5L; t(12) = 1.25, p = 0.24) and pulse irregularity (as measured by approximate entropy; ApEn, a measure of pulse irregularity; Veldhuis et al., 2001; 0.45 ± 0.05 vs 0.64 ± 0.03, t(12) = 3.37, p < 0.01). GH secretory profiles from fasting Y1R−/− and Y2R−/− mice demonstrate a differential role for these receptors in fasting-mediated GH release (Fig. 6A–D). Deletion of Y1R prevented the fasting-induced suppression of pulsatile GH release (including total; Fig. 6E; t(9) = 6.43, p < 0.01) and pulsatile (Fig. 6F; t(0) = 6.80, p < 0.01) GH secretion, the mass of GH secreted per burst (Fig. 6G; t(9) = 6.71, p < 0.01), whereas fasting still suppressed pulsatile GH release in Y2R−/− mice (as total; Fig. 6I; t(12) = 1.00, p = 0.33) and pulsatile (Fig. 6J; t(12) = 1.02, p = 0.33) GH secretion, the mass of GH secreted per burst (Fig. 6K; t(12) = 2.17, p = 0.05). The number of secretory events in fasting Y1R−/− (Fig. 6H; t(9) = 0.51, p = 0.62) and Y2R−/− (Fig. 6L; t(12) = 0.15, p = 0.88) mice did not change relative to wild-type littermates. Collectively, our observations suggest that Y1 and Y2 receptors differentially regulate pulsatile GH release, wherein the Y1R modulates fasting control of GH release while the Y2R regulates pulse dynamics under ad libitum-fed conditions. We observed a significant reduction in body length specific to Y2R−/− mice (Fig. 7C; t(12) = 6.10 p < 0.01), whereas body length between wild-type littermates and NPY−/− (Fig. 7A; t(20) = 1.68, p = 0.11) and Y1R−/− (Fig. 7B; t(9) = 0.49, p = 0.64) mice remained unchanged. Given the role of GH in promoting linear growth (Attanasio and Shalet, 2007), we suggest reduced peak GH release contributes to the observed reduction in body length in Y2R−/− mice.
Figure 5.
Representative examples of pulsatile GH secretion profiles (2 animals; #1, closed circles; #2, open circles; A–D, left) and corresponding output figures (A–D, right) from ad libitum-fed male adult mice with germline deletion of Y1 (Y1R−/−; green, B) or Y2 (Y2R−/−; blue, D) receptors and age-matched littermates (black, A and C) under ad libitum-fed conditions. Samples were collected for 6 h at 10 min intervals, starting at 0700 h. Corresponding output figures illustrate the onset of GH pulses (pulse onset indicated by open circles along the x-axis). GH secretory parameters following deconvolution analysis are illustrated in E–H (Y1R−/−) and I–L (Y2R−/−). Total (E) and pulsatile GH secretion rate (F), the mass of GH secreted per burst (G), and number of secretory bursts (H) in Y1R−/− mice were comparable to ad libitum-fed wild-type mice. GH secretion was significantly reduced in Y2R−/− mice as characterized by a significant reduction in total (I), pulsatile (J), and the mass of GH secreted per burst (K). While not significant, the number of secretory bursts (L) was elevated in Y2R−/− mice (p = 0.06). Data are presented as mean ± SEM, N = 4–7, *p < 0.05.
Figure 6.
Representative examples of pulsatile GH secretion profiles (2 animals; #1, closed circles; #2, open circles; A–D, left) and corresponding output figures (A–D, right) from male adult mice with germline deletion of the Y1 (Y1R−/−; green, B) and Y2 (Y2R−/−; blue, D) receptor and age-matched littermates (black, A and C) following 6 h of food withdrawal. Samples were collected for 6 h at 10 min intervals, starting at 1830 h. Corresponding output figures illustrate the onset of GH pulses (pulse onset indicated by open circles along the x-axis). GH secretory parameters following deconvolution analysis are illustrated in E–H (Y1R−/−) and I–L (Y2R−/−). Deletion of Y1R resulted in a prevention of the fasting-induced suppression of pulsatile GH release, and was characterized by an increase in total (E) and pulsatile GH secretion rate (F), and the mass of GH secreted per burst (G). In contrast, pulsatile GH release was suppressed in fasting Y2R−/− mice. This was characterized by a comparable total (I) and pulsatile GH secretion (J), and the mass of GH secreted per burst (K) when compared with age-matched fasting wild-type littermates. Data are presented as mean ± SEM, N = 4–7, *p < 0.05.
Figure 7.
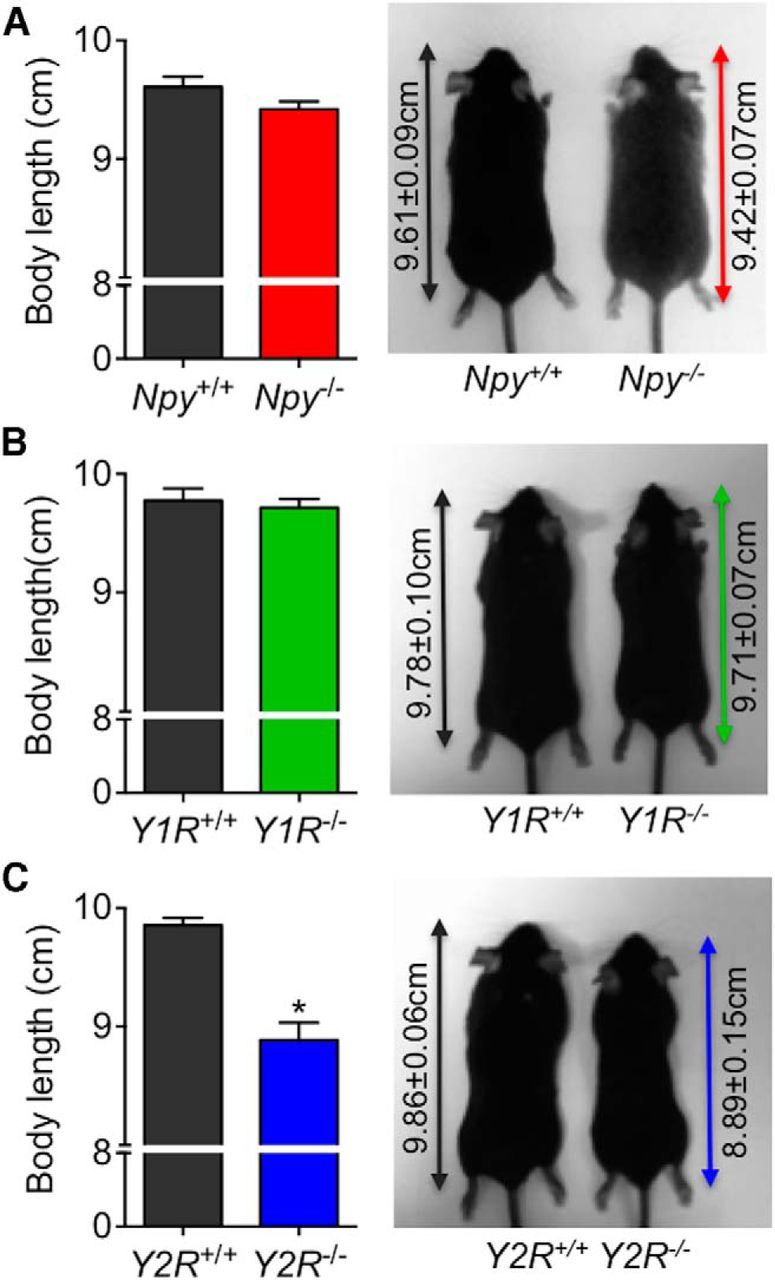
Body length and representative images of adult mice following germline deletion of NPY (Npy−/−; A), Y1R (Y1R−/−; B), and Y2R (Y2R−/−; C). Body length in Npy−/− and Y1R−/− mice was comparable to the age-matched wild-type mice, whereas Y2R−/− mice were significantly shorter compared with age-matched wild-type littermates. Body length was measured from nose to rump as indicated by the double arrow line. Data are presented as mean ± SEM, N = 4–12, *p < 0.05.
Discussion
Central injection of NPY inhibits GH secretion (Rettori et al., 1990), although the endogenous role of NPY in modulating GH release remains unknown. We provide evidence supporting functional interactions between hypothalamic NPY neurons, Y-receptors, and key components of the GH axis.
Fasting in the mouse immediately decreases pulsatile GH release (Steyn et al., 2012) and this coincides with an early rise in Npy and Srif mRNA expression within the hypothalamic ARC/PeVN complex. In comparison, Ghrh mRNA expression remains unchanged (Steyn et al., 2012). Thus, we anticipate that NPY neurons selectively activate SRIF neurons to suppress GH release. Using immunofluorescence and in situ hybridization, we confirmed synaptic interactions between hypothalamic NPY and SRIF-expressing neurons. This agrees with prior observations in rats (Kawano and Daikoku, 1988; Hisano et al., 1990).
Germline deletion of NPY does not alter GH pulsatility nor does it impact hypothalamic Ghrh and Srif mRNA expression in ad libitum-fed mice. Accordingly, NPY neurons do not modulate GH release under fed conditions. The suppression of GH secretion normally observed in fasting mice (Steyn et al., 2011, 2012) was completely reversed in Npy−/− mice. This occurred alongside reduced hypothalamic Srif mRNA expression. Our data suggest NPY neurons modulate SRIF neuronal activity and couple the suppression of GH release relative to food withdrawal. This functional interaction agrees with observations in which NPY stimulated the release of SRIF from cultured rat median eminence fragments (Rettori et al., 1990). Observations provide a physiological context for interactions between the SRIF neurons in the PeVN and NPY neurons in the ARC, wherein NPY-induced suppression of GH release can be prevented following structural disruption in NPY/SRIF connectivity (Minami et al., 1998).
Plasma glucose concentrations were significantly reduced within 45 min of food withdrawal. ARC NPY neurons are glucose sensitive (Claret et al., 2007; Murphy et al., 2009), and are activated by a decrease in extracellular glucose (Minami et al., 1995). We suggest diminishing glucose levels in mice following food withdrawal activates NPY neurons, which subsequently stimulate SRIF neurons to inhibit GH release. As NPY neurons remain inactive when food is sufficient (Becskei et al., 2009), deletion of NPY does not impact the release of GH in ad libitum-fed mice. Recent in vitro observations argue that a decrease in extracellular glucose concentrations activate glucose-sensing GHRH neurons (Stanley et al., 2013). If this occurs in vivo, a fall in blood glucose levels should increase GH release; however, hypoglycemia associated with fasting reduces GH release. In light of this, we anticipate that NPY neurons act as gatekeepers to control GHRH activity, activating SRIF neurons to suppress GHRH-induced GH release, regardless of potential GHRH glucose-sensing effects (Stanley et al., 2013).
The actions of NPY are mediated via a family of high-affinity receptors (Pedragosa-Badia et al., 2013). The Y1R is the dominant postsynaptic receptor regulating food intake (Keen-Rhinehart and Bartness, 2007), whereas the Y2R is an auto-receptor that inhibits the synthesis and release of NPY (Broberger et al., 1997). As seen in Npy−/− mice, pulsatile GH secretion in Y1R−/− mice did not change in ad libitum-fed mice, whereas the fasting-induced suppression of GH release was completely reversed. This corroborates observations in rats, where central administration of a Y1R agonist suppressed circulating levels of GH (Suzuki et al., 1996). While the Y1R is expressed on SRIF neurons within the dorsal horn of the spinal cord (Zhang et al., 1999), coexpression of Y1R on SRIF neurons within the ARC or PeVN is yet to be confirmed. The Y1R is widely expressed within the PeVN and PVN (Kishi et al., 2005), areas characterized by the abundant expression of Srif mRNA (Tan et al., 2013), and thus Y1Rs may directly act within SRIF neurons to modulate GH release.
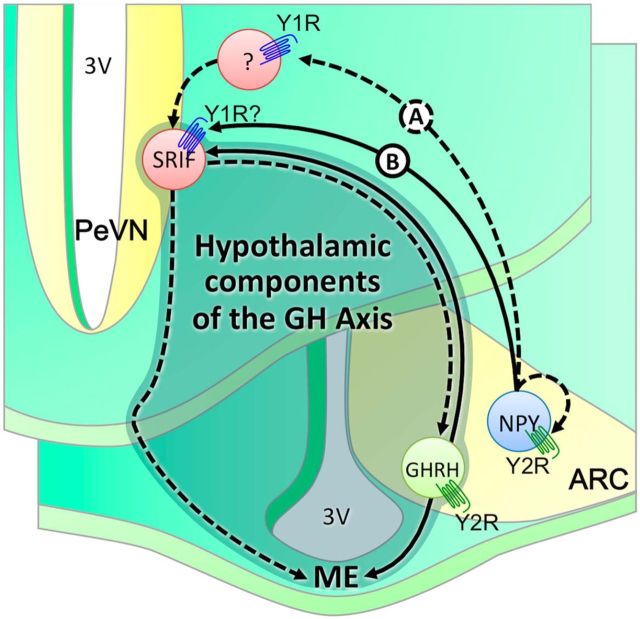
The Y1R commonly signals via inhibitory G (Gi)-protein-signaling pathways. For example, NPY (acting through the Y1R) inhibits GnRH activity via the Gi signaling cascade (Klenke et al., 2010). This inhibitory role contradicts the anticipated stimulatory role of the Y1R in modulating SRIF-mediated changes in GH release. It is possible that the Y1R functions through a yet undefined intermediate neuron to modulate SRIF activity. The Y1R may silence this intermediate neuron that would normally suppress the activity of SRIF neurons, resulting in the inhibition of GH release (Fig. 8, Scenario A). Importantly, acting through the Y1R, NPY increased calcium currents in a proportion of vagal sensory neurons, acting via Gi/Go proteins (Colmers and Bleakman, 1994). Thus potential direct stimulatory actions of Y1R on SRIF neurons cannot be excluded. Alternatively, the Y1R may alter SRIF activity, acting through coupling of Gβ and Gγ subunits. The proliferative effects of NPY on dentate β-tubulin-positive neuroblasts are mediated via the Y1R, and require ERK1/2 activation (Howell et al., 2005). Similarly, the observed proliferative effects of neuronal precursor cells within the adult olfactory epithelium occur via stimulation of the Y1R, and are mediated downstream through a kinase cascade involving PKC and ERK1/2 (Hansel et al., 2001). Given these effects, NPY may mediate SRIF activity via the Y1R, acting independent of classical Gi-mediated pathways (Fig. 8, Scenario B). Data confirming the coexpression of the Y1R on SRIF neurons does not exist, and the direct role of Y1R on SRIF activity remains unconfirmed.
Figure 8.
Schematic representation defining interactions between hypothalamic components of the GH axis (shaded), NPY neurons, and Y-receptors. Fasting induces the activation of NPY neurons in the ARC. Current and published observations suggest that altered NPY activity promotes SRIF activity, presumably through activation of the postsynaptic Y1 receptors. Acting through intermediate inhibitory neurons, the Y1R may stimulate SRIF-ergic tone through withdrawal in inhibition (Scenario A). Alternatively, Y1R may directly modulate SRIF-egic tone via nonclassical G-protein-mediated pathways (Scenario B). In both scenarios, increased SRIF-ergic tone will inhibit GHRH-induced GH release, or directly inhibit somatotroph activity and thus GH release. Accordingly, GH release is not suppressed in fasting mice following germline deletion of NPY or Y1R. Under ad libitum-fed conditions, activation and/or inactivation of NPY neurons in the ARC are under tight control of Y2 receptors. Deletion of Y2 receptors results in impaired peak GH release, presumably through a potential increase in NPY feedback to SRIF neurons resulting in an increase in SRIF-ergic tone. Of interest, GHRH neurons express the Y2R (Lin et al., 2007) and promote GHRH activity (Osterstock et al., 2010). Thus, germline loss of Y2R may directly impact GHRH activity in ad libitum-fed mice, and thus GHRH-mediated GH release. PVN, paraventricular nucleus; 3V, third ventricle; ME, medium eminence. Dashed lines represent an inhibitory effect whereas solid lines represent a stimulatory effect.
Fasting reduces pituitary GH immunoreactivity in wild-type but not Y2R−/− mice (Lin et al., 2007), providing evidence for Y2R-mediated GH synthesis. These observations do not directly test whether the Y2R modulates GH release. We now demonstrate that the Y2R does not contribute to suppressed GH release in fasting mice. Rather, the Y2R regulates peak GH release in ad libitum-fed mice. Moreover, extended analysis of ApEn demonstrates a rise in pulse irregularity in Y2R−/− mice, signifying an overall derangement of GH pulse profile. Thus, the Y2R likely regulates interactions between hypothalamic GH pulse generators, the SRIF and GHRH neurons. This is consistent with the reduced body length seen in Y2R−/− mice. Therefore, while the Y2R directly regulates bone formation (Baldock et al., 2002), the Y2R may also facilitate pubertal growth by sustaining optimal GH release.
The Y2R inhibits NPY release (Chen and van den Pol, 1996; Broberger et al., 1997) and deletion of Y2R increases hypothalamic Npy mRNA expression (Sainsbury et al., 2002). Consequently, the suppression of GH secretion in ad libitum-fed Y2R−/− mice may occur in response to increased hypothalamic NPY activity. Given observed interactions between NPY and SRIF neurons, we predict increased inhibitory SRIF tone and thus GH release. The Y2R is expressed on GHRH neurons (Lin et al., 2007), and Y2R agonist increases the discharge rate of GHRH neurons (Osterstock et al., 2010). Thus the loss of Y2R may contribute to altered GHRH tone, resulting in reduced-fed GH release. This requires further validation.
The differential effects of Y2R and NPY deletion on GH release suggest that factors other than NPY may modulate Y2R-mediated GH release, such as PYY, which activates the Y2R (Batterham et al., 2002). We anticipate that postprandial fluctuations in PYY secretion may underlie the selective role of Y2R in GH release in ad libitum-fed mice. This was somewhat corroborated by PYY data in fasting mice, as circulating PYY levels did not change during the fasting period. It is thus unlikely that PYY contributes to the loss of GH release at this time. In pigs, PYY3–36 (a potent agonist for Y2R; Dumont et al., 1994) treatment stimulates GH release following an overnight fast (Ito et al., 2006). As PYY expression rises in response to food intake (Batterham et al., 2002), PYY may signal the hypothalamus to sustain optimal GH release under fed conditions. Whether PYY modulates GH release, and whether these actions are mediated selectively via Y2R expression on GHRH, needs further assessment. Regardless, observations highlight a critical role for the Y2R (and potentially other Y-receptors) in growth and development. These complex interactions require extensive investigation, including the detailed assessment of peak pulsatile GH release in fed and fasting states.
Our observations demonstrate that NPY neurons represent a key hypothalamic node that integrates the production and release of GH relative to food intake. We propose that NPY neurons modify SRIF activity to suppress GH release in mice during fasting (Fig. 8). While the exact mechanism is not defined, these actions are likely mediated via postsynaptic Y1Rs acting via an intermediate neuron, or directly via SRIF neurons. We confirmed that the Y2R does not contribute to altered GH release in fasting mice. Rather, the Y2R sustains peak pulsatile GH output specifically in ad libitum-fed mice, potentially in response to peripheral factors released in response to feeding. Current observations are limited to germline knock-out animals, and thus cannot account for the existence of compensatory pathways that develop in the absence of NPY, Y1R, or Y2R signaling. Confirmation of detailed circuitry requires further assessment of GH release following selective alteration of hypothalamic NPY neuron activity, or Y1R and Y2R expression. Our data provide exciting evidence to confirm that the release of GH is tightly coupled to hypothalamic food intake mechanisms that mediate orexigenic responses.
Footnotes
This work was supported by the National Health and Medical Research Council and The University of Queensland. L.H. receives a postgraduate scholarship from the China Scholarship Council and The University of Queensland. We thank Dr. Yan Zhao, Dr. Shyuan Ngo, and Ying Wan for assisting with experiments.
The authors declare no competing financial interests.
References
- Adams EF, Venetikou MS, Woods CA, Lacoumenta S, Burrin JM. Neuropeptide Y directly inhibits growth hormone secretion by human pituitary somatotropic tumours. Acta Endocrinol. 1987;115:149–154. doi: 10.1530/acta.0.1150149. [DOI] [PubMed] [Google Scholar]
- Attanasio AF, Shalet SM. Growth hormone and the transition from puberty into adulthood. Endocrinol Metab Clin North Am. 2007;36:187–201. doi: 10.1016/j.ecl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A, Joshi R, Su C, Friend LA, James JH. Neuropeptide Y (NPY) Y2 receptor-selective agonist inhibits food intake and promotes fat metabolism in mice: combined anorectic effects of Y2 and Y4 receptor-selective agonists. Peptides. 2007;28:235–240. doi: 10.1016/j.peptides.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Becskei C, Lutz TA, Riediger T. Diet-derived nutrients mediate the inhibition of hypothalamic NPY neurons in the arcuate nucleus of mice during refeeding. Am J Physiol Regul Integr Comp Physiol. 2009;297:R100–R110. doi: 10.1152/ajpregu.91014.2008. [DOI] [PubMed] [Google Scholar]
- Broberger C, Landry M, Wong H, Walsh JN, Hökfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Song J, Xu Y, Berelowitz M. Regulation of hypothalamic preprogrowth hormone-releasing factor messenger ribonucleic acid expression in food-deprived rats: a role for histaminergic neurotransmission. Endocrinology. 1993;133:1377–1381. doi: 10.1210/endo.133.3.8103451. [DOI] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot JG, Enjalbert A, Pelletier G, Dubois PM, Morel G. Evidence for a direct action of neuropeptide Y in the rat pituitary gland. Neuroendocrinology. 1988;47:511–517. doi: 10.1159/000124963. [DOI] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Multiple NPY receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Bleakman D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994;17:373–379. doi: 10.1016/0166-2236(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Cornford AS, Barkan AL, Horowitz JF. Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J Clin Endocrinol Metab. 2011;96:824–830. doi: 10.1210/jc.2010-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Pheng LH, Fournier A, St-Pierre S, Quirion R. Peptide YY derivatives as selective neuropeptide Y/peptide YY Y1 and Y2 agonists devoided of activity for the Y3 receptor sub-type. Brain Res Mol Brain Res. 1994;26:320–324. doi: 10.1016/0169-328X(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Kopp J, Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Frohman LA, Downs TR, Chomczynski P. Regulation of growth-hormone secretion. Front Neuroendocrinol. 1992;13:344–405. [PubMed] [Google Scholar]
- Glad CA, Kitchen EE, Russ GC, Harris SM, Davies JS, Gevers EF, Gabrielsson BG, Wells T. Reverse feeding suppresses the activity of the GH axis in rats and induces a preobesogenic state. Endocrinology. 2011;152:869–882. doi: 10.1210/en.2010-0713. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knock-out models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Hisano S, Tsuruo Y, Kagotani Y, Daikoku S, Chihara K. Immunohistochemical evidence for synaptic connections between neuropeptide Y-containing axons and periventricular somatostatin neurons in the anterior hypothalamus in rats. Brain Res. 1990;520:170–177. doi: 10.1016/0006-8993(90)91703-J. [DOI] [PubMed] [Google Scholar]
- Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Steyn FJ, Tan HY, Xie TY, Veldhuis JD, Ngo ST, Chen C. The decline in pulsatile GH secretion throughout early adulthood in mice is exacerbated by dietary-induced weight gain. Endocrinology. 2012;153:4380–4388. doi: 10.1210/en.2012-1178. [DOI] [PubMed] [Google Scholar]
- Ito T, Thidarmyint H, Murata T, Inoue H, Neyra RM, Kuwayama H. Effects of peripheral administration of PYY3–36 on feed intake and plasma acyl-ghrelin levels in pigs. J Endocrinol. 2006;191:113–119. doi: 10.1677/joe.1.06855. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Kawano H, Daikoku S. Somatostatin-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies. J Comp Neurol. 1988;271:293–299. doi: 10.1002/cne.902710209. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1728–R1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lin S, Lin EJ, Boey D, Lee NJ, Slack K, During MJ, Sainsbury A, Herzog H. Fasting inhibits the growth and reproductive axes via distinct Y2 and Y4 receptor-mediated pathways. Endocrinology. 2007;148:2056–2065. doi: 10.1210/en.2006-1408. [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. doi: 10.1210/en.2005-1549. [DOI] [PubMed] [Google Scholar]
- Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by administration of 2-deoxy-D-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Brain Res Mol Brain Res. 1995;33:305–310. doi: 10.1016/0169-328X(95)00151-H. [DOI] [PubMed] [Google Scholar]
- Minami S, Kamegai J, Sugihara H, Suzuki N, Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr J. 1998;45:S19–S26. doi: 10.1507/endocrj.45.Suppl_S19. [DOI] [PubMed] [Google Scholar]
- Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L, Martin WJ, Routh VH. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol. 2009;296:C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz AA, Milardo LF, DeCarr LB, Buckholz TM, Mays MR, Claus TH, Livingston JN, Mahle CD, Lumb KJ. A novel long-acting selective neuropeptide Y2 receptor polyethylene glycol-conjugated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents. J Pharmacol Exp Ther. 2007;323:692–700. doi: 10.1124/jpet.107.125211. [DOI] [PubMed] [Google Scholar]
- Osterstock G, Escobar P, Mitutsova V, Gouty-Colomer LA, Fontanaud P, Molino F, Fehrentz JA, Carmignac D, Martinez J, Guerineau NC, Robinson IC, Mollard P, Méry PF. Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus. PLoS One. 2010;5:e9159. doi: 10.1371/journal.pone.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedragosa-Badia X, Stichel J, Beck-Sickinger AG. Neuropeptide Y receptors: how to get subtype selectivity. Front Endocrinol. 2013;4:5. doi: 10.3389/fendo.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology. 1996;137:3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Pierroz DD, Broqua P, White RB, Pedrazzini T, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol Cell Endocrinol. 2001;185:195–204. doi: 10.1016/S0303-7207(01)00620-7. [DOI] [PubMed] [Google Scholar]
- Rettori V, Milenkovic L, Aguila MC, McCann SM. Physiologically significant effect of neuropeptide-y to suppress growth-hormone release by stimulating somatostatin discharge. Endocrinology. 1990;126:2296–2301. doi: 10.1210/endo-126-5-2296. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci U S A. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S, Domingos AI, Kelly L, Garfield A, Damanpour S, Heisler L, Friedman J. Profiling of glucose-sensing neurons reveals that GHRH neurons are activated by hypoglycemia. Cell Metab. 2013;18:596–607. doi: 10.1016/j.cmet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152:3165–3171. doi: 10.1210/en.2011-0253. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Leong JW, Huang L, Tan HY, Xie TY, Nelson C, Waters MJ, Veldhuis JD, Epelbaum J, Chen C. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology. 2012;153:273–282. doi: 10.1210/en.2011-1681. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Okada K, Minami S, Wakabayashi I. Inhibitory effect of neuropeptide Y on growth hormone secretion in rats is mediated by both Y1- and Y2-receptor subtypes and abolished after anterolateral deafferentation of the medial basal hypothalamus. Regul Pept. 1996;65:145–151. doi: 10.1016/0167-0115(96)00085-7. [DOI] [PubMed] [Google Scholar]
- Tan HY, Huang L, Simmons D, Veldhuis JD, Steyn FJ, Chen C. Hypothalamic distribution of somatostatin mRNA expressing neurones relative to pubertal and adult changes in pulsatile growth hormone secretion in mice. J Neuroendocrinol. 2013;25:910–919. doi: 10.1111/jne.12078. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Epelbaum J, Videau C, Dubuis JM. Sex-related alterations in hypothalamic growth hormone-releasing hormone mRNA-but not somatostatin mRNA-expressing cells in genetically obese Zucker rats. Neuroendocrinology. 1996;64:186–193. doi: 10.1159/000127117. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus SM. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, Andrews ZB. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology. 2014;155:840–853. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tong YG, Bao L, Hökfelt T. The neuropeptide Y Y1 receptor is a somatic receptor on dorsal root ganglion neurons and a postsynaptic receptor on somatostatin dorsal horn neurons. Eur J Neurosci. 1999;11:2211–2225. doi: 10.1046/j.1460-9568.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.RES.83.2.187. [DOI] [PubMed] [Google Scholar]