Abstract
Involvement of the type 1 cannabinoid receptor (CB1R) in the effects of alcohol on the brain is supported by animal experiments, but how in vivo CB1R levels are altered in alcoholic patients is still unclear. To assess the short-time effects of a binge drinking episode on CB1R availability, 20 healthy social drinkers underwent [18F]MK-9470-positron emission tomography (PET) at baseline and after intravenous ethanol administration (ALC ACU). Moreover, 26 alcoholic patients underwent sequential CB1R PET after chronic heavy drinking (ALC CHR) and after 1 month of abstinence (ALC ABST). Seventeen healthy subjects served as controls.
Compared with baseline, ALC ACU resulted in a global increase of CB1R availability (+15.8%). In contrast, a global decreased CB1R availability was found in ALC CHR patients (−16.1%) compared with controls, which remained unaltered after abstinence (−17.0%). Voxel-based analysis showed that ALC CHR patients had reduced CB1R availability, especially in the cerebellum and parieto-occipital cortex. After abstinence, reduced CB1R availability extended also to other areas such as the ventral striatum and mesotemporal lobe.
In conclusion, whereas the acute alcohol effect is an increase in CB1R availability, chronic heavy drinking leads to reduced CB1R availability that is not reversible after 1 month of abstinence. Longer follow-up is required to differentiate whether this is a compensatory effect of repeated endocannabinoid overstimulation or an enduring trait-like feature. An enhanced CB1R signaling may offer a new therapeutic direction for treatment of the negative affective state produced by alcohol withdrawal and abstinence, which is critical for the maintenance of alcohol addiction.
Keywords: abstinence, acute alcohol effect, alcoholism, cannabinoid CB1 receptor, chronic alcohol effect, PET
Introduction
Alcoholism is among the most common complex psychiatric disorders affecting society, and represents a leading cause of morbidity, mortality and socioeconomic problems (Grant, 1997). Treatment is complex and multidisciplinary, and although a number of pharmacotherapeutic interventions have been suggested for alcohol dependence, these remain unsatisfactory. This underlines the need to further understand the pathogenesis of alcohol dependence and effects of ethanol on the brain, to improve outcome. The effect of chronic alcohol consumption on the CNS has been related to its action on several monoamine neuroreceptor systems causing neuroadaptive changes (Vengeliene et al., 2008). However, the exact signaling pathways and mechanisms underlying the development of alcohol dependence and the propensity to relapse are still unclear.
Recently, the endocannabinoid system (ECS) has been recognized as an important target in alcohol drinking behavior and dependence (Pava and Woodward, 2012). The type 1 cannabinoid receptor (CB1R) is the most abundant G-protein-coupled receptor in the CNS. Its presynaptic activation by (endo)cannabinoids results in mainly inhibition of GABA-ergic and glutamatergic neurotransmission (Katona and Freund, 2008). Deficiencies or adaptations of the endocannabinoid signaling pathways in the brain may play an important role in the vulnerability to or development of alcohol dependence.
To date, involvement of the CB1R in the neural circuitry mediating the positive reinforcing properties of alcohol is mainly supported by animal experiments. In rats acute administration of CB1R agonists (CP55,940 and WIN 55,212–2) induces a dose-dependent increase in alcohol intake (Colombo et al., 2002), while the CB1R inverse agonist SR-141716A results in a reduction of voluntary alcohol intake, preference, and craving (Economidou et al., 2006). Similarly, CB1R knock-out mice show a reduced preference for and intake of alcohol (Hungund et al., 2003; Wang et al., 2003; Thanos et al., 2005). Preclinical studies have demonstrated that chronic ethanol exposure results in reduced CB1R mRNA expression (Ortiz et al., 2004; Mitrirattanakul et al., 2007), CB1R density, and functionality (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999b; Vinod et al., 2006). Although this was also confirmed by our microPET study where 7 d of forced chronic ethanol exposure resulted in a decreased CB1R binding (Ceccarini et al., 2013a), conflicting results have been found. No alterations in CB1R binding or limited region-dependent CB1R alterations were detected in rodents treated chronically with ethanol (Gonzalez et al., 2002; Pava et al., 2012). Moreover, the first two human positron emission tomography (PET) studies have also found conflicting results. In alcoholic patients after 4 weeks of abstinence, Neumeister et al. (2012) found elevated CB1R binding, whereas the opposite was observed by Hirvonen et al. (2013).
Because of known interspecies differences and given the above conflicting data, we used CB1R PET to investigate (1) the acute, short-term effect of ethanol in a cohort of young male healthy social drinkers using an ethanol activation paradigm for the first time and (2) CB1R availability in a prospectively recruited cohort of male alcoholic patients at admission for a binge drinking period and after a subsequent voluntary period of monitored rehabilitation.
Materials and Methods
Subjects.
For the acute ethanol administration study, 20 male healthy volunteers were included (age 31.6 ± 10.3 years). Participants were excluded if they met one of the following criteria: history of neurological dysfunction, other major psychiatric disorder, and current use of psychoactive medication or drug abuse. Volunteers were recruited in response to advertisements in the departmental homepage. Before entering the study, subjects were screened by a trained psychiatrist using the Structured Clinical Interview for DSM-IV Axis I psychiatric disorders (SCID; First et al., 2001). Volunteers were excluded if they consumed >10 alcoholic drinks per week.
Patients were recruited during hospitalization for an acute binge drinking episode in an inpatient university center for psychiatric disorders. They all met criteria for alcohol dependence according to the Diagnostic and Statistical Manual of Mental Disorders, fourth version (DSM-IV) criteria (APA, 1994). The SCID questionnaire was taken within a week before the PET scan. During the psychiatric interview a medical and psychiatric history was taken, including detailed alcohol history and consumption within the last year (Table 1). According to the indices calculated from the Lifetime Drinking History questionnaire (Skinner and Sheu, 1982), heavy drinking was defined as >6 drinks per day, where a standard alcoholic drink contains ∼10 g ethanol. According to the SCID, patients did not have other psychiatric or substance-dependence history (other than tobacco smoking). A total of 18 patients (69%) smoked cigarettes. Moreover, regarding coffee consumption, a total of 14 controls (82%) and 22 patients (85%) were regular coffee drinkers.
Table 1.
Demographic and clinical characteristics of healthy volunteers (A) and alcoholic patients (B)
| A ALC ACU Healthy volunteersa (n = 20) | B ALC CHR-ABST |
|||
|---|---|---|---|---|
| Alcoholic patients (CHR) (n = 26) | Alcoholic patients (ABST) (n = 19) | Healthy controlsb (n = 17) | ||
| Age (years) | 31.6 ± 10.3 | 49.5 ± 7.6 | 49.4 ± 7.5 | 41.6 ± 15.5 |
| Weight (kg) | 80.6 ± 7.4 | 78.1 ± 14.0 | 81.5 ± 14.2 | 84.1 ± 9.4 |
| In past year, number alcoholic drinks per day | 0.7 ± 0.4 | 19.1 ± 6.8 | 19.1 ± 6.5 | 0.6 ± 0.5 |
| Beverage type (%) | ||||
| Beer | 57.8 ± 42.0 | 52.9 ± 43.3 | ||
| Wine | 31.9 ± 37.2 | 32.6 ± 37.5 | ||
| Liquor | 22.8 ± 38.9 | 32.2 ± 43.1 | ||
| Age at first heavy drinking (years) | — | 30.5 ± 13.4 | 28.9 ± 13.6 | — |
| Estimated heavy drinking duration from the beginning alcohol use (years) | — | 19.1 ± 12.1 | 20.3 ± 13.0 | — |
| Days of alcohol abstinence before PET (days) | — | 5.7 ± 1.8 | 34.6 ± 4.9 | — |
Data are expressed as mean ± SD; (A) ALC ACU, alcohol acute condition; (B) ALC CHR, chronic alcohol condition; ALC ABST, alcohol abstinence condition.
aGroup of healthy volunteers who received acute alcohol challenge (ALC ACU condition).
bData from 12 healthy volunteers who had undergone the baseline condition of the ALC ACU study were also included in this healthy control group.
On the day of PET scanning, all subjects underwent blood and urine testing including general screening and toxicology for benzodiazepines, neuroleptics, opiates, cocaine and metabolites, amphetamines, and cannabinoids. After the first PET scan, the patients stayed at a specialized withdrawal center to abstain from alcohol consumption in a supervised treatment program. Alcohol and drug abstention was verified by multiple daily alcohol breath screening and urine drug tests.
For the alcoholic patients study, 26 male alcoholic patients (ALC) after a chronic (CHR) alcoholism period (ALC CHR) were recruited. Nineteen of the ALC patients abstained from alcohol for an average of 35 ± 5 d (abstinence (ABST) condition, ALC ABST). A control group consisting of 17 healthy nonalcoholic age-matched male subjects was included (CON). Twelve of the control subjects were also included in the acute alcohol study and their baseline data were used for this second study. Demographic and drinking characteristics are given in Table 1.
The study was approved by the local ethics committee and performed according to the World Medical Association Declaration of Helsinki. After complete description of the study to the subjects, written informed consent was obtained.
Alcohol administration paradigm and alcohol effects.
For the acute administration study, all alcohol infusions were performed at the same time of day (between 14:00 and 14:30 h) to minimize influences by alcohol sensitivity. Alcohol was intravenously administered as a 10% w/v solution in 5% w/v glucose through an indwelling catheter in a forearm vein for 120 min. The alcohol infusion speed was based on an alcohol breath clamping paradigm allowing volunteers to achieve a target level of breath alcohol concentration (BrAC) of 0.7 g/L and maintain this for an extended period of time (Zoethout et al., 2009; Gomez et al., 2012). The BrAC clamping method is based upon the theory that for substances with marked saturable elimination in the relevant concentration range (like alcohol), an approximately linear relationship exists between the applied infusion rate and the resulting change in alcohol concentration. When alcohol elimination is fully saturated, it is excreted at a constant (g/min) rate, independent of concentration. Therefore, when the input (i.e., infusion rate) is changed (g/min), this will result in a proportional change in alcohol concentration. The change in alcohol level required to achieve the target concentration can then be used to back-extrapolate the infusion rate that corresponds to, and should hence lead to this necessary change. Following this approach, the pseudo-steady-state alcohol level of 0.7 g/L was maintained by continuous assessment of the BrAC to provide on-line adjustments in the intravenous infusion speed of the ethanol solution. This alcohol infusion paradigm was originally introduced and described by O'Connor et al. (1998) and recently adapted by Zoethout et al. (2008, 2009). Following this approach, the pseudo-steady-state alcohol level of 0.7 g/L was maintained by continuous assessment of the BrAC by serial breathalyzer measurements using an electronic hand-held BrAC measuring instrument (Dräger Breathalyzer Alcotest 7410Plus) to provide on-line adjustments in the intravenous infusion speed of the ethanol solution. BrAC sample measurements were obtained at regular time points (each 5 min from the start of the infusion until the PET), and the measured BrAC was then entered into a spreadsheet, which calculated the corresponding infusion rate to maintain the BrAC at 0.7 g/L. The alcohol infusions were given at a constant rate fixed at 720 ml/h, during the first 5 min, and at 600 ml/h, during the second 5 min, to achieve the target BrAC. After this fixed loading phase, rates were necessarily modified at 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, and 120 min, according to the obtained BrAC samples, which were measured at the same time points. To verify the alcohol target level achievement, a final BrAC sample was measured when the PET ended (at 120 min). The controlled alcohol administration was initiated simultaneously on radiotracer injection.
Volunteers were required to comment on the momentary subjective effects of alcohol using the Visual Analog Scale (VAS) self-report questionnaire (Bond and Lader, 1974), which assesses subjective effects similarly to those obtained using the Drug Effects Questionnaire or the Biphasic Alcohol Effects Scale before (Rupp et al., 2007; Webster et al., 2011), starting (t = 0 min), during (t = 45 min), and at the end of the alcohol administration (t = 120 min). We used VAS for rating the following 15 subjective alcohol effects: “bad drug effect,” “good drug effect,” “uncomfortable,” “clumsy,” “bored,” “slurred speech,” “dizziness,” “sense of nausea,” “confused,” “sleepiness,” “distorted sense of time,” “floating sensation,” “tired,” “talkative,” and “anxious.” The VAS scales consisted of a series of 15 horizontal 100 mm lines, each labeled with the corresponding adjective. Zero millimeter on the line corresponded to “not at all,” and 100 mm corresponded to “extremely.” Subjects were asked to place a mark on each line indicating how they felt at that precise moment. The outcome measure was the distance in millimeters to the marker on each scale.
[18F]MK-9470 characteristics and preparation.
CB1R imaging was done using the radioligand [18F]MK-9470 (N-[2-(3-cyanophenyl)-3-(4-(2-[18F]fluoroethoxy) phenyl)-1-methylpropyl]-2-(5-methyl-2-pyridyloxy)-2-methyl propanamide), which is an inverse agonist with a high affinity and specificity for the CB1R (Burns et al., 2007; Casteels et al., 2012). The [18F]MK-9470 precursor was obtained from Merck Research Laboratories and labeling was performed on-site using 2-[18F]fluoroethylbromide. Tracer preparation and characteristics have been described previously (Burns et al., 2007). The radioligand had a radiochemical purity > 95% and a mean specific radioactivity of 197.0 ± 85.3 GBq/μmol. The tracer was administered in a sterile solution of 5 mm sodium acetate buffer (pH 5.5) containing 6% ethanol.
PET data acquisition.
For the acute administration study, each volunteer obtained two CB1R PET scans on separate days: during a baseline (BL) condition, and during an acute alcohol administration (ALC ACU) paradigm 10 ± 5 d later. Due to the nature of the study, the design was not blinded. For the second substudy, patients were scanned at two time points: (1) within the first week (6 ± 2 d) following CHR alcohol consumption with binge drinking exacerbation (ALC CHR) and (2) after a month (35 ± 5 d) of alcohol ABST (ALC ABST).
All subjects fasted for at least 4 h before the PET session. Before each [18F]MK-9470 administration (injected activity 148.3 ± 44.2 MBq), subjects were placed with the head placed in a vacuum cushion and the body fixed to minimize head movement. PET data were acquired on an ECAT EXACT HR+ (Siemens) camera in 3D mode. A dynamic PET scan was started 90 min post injection with 30 min scanning session (six frames of 300 s). PET images were reconstructed with a standard 3D filtered backprojection algorithm including scatter and attenuation correction (68Ge source). Reconstructed images were post smoothed with a Gaussian filter with full-width at half-maximum (FWHM) of 4.8 mm. Additionally, all subjects underwent magnetic resonance imaging (MRI), both T1-weighted 3D-MPRAGE and T2-weighted, to exclude structural brain abnormalities and to anatomically coregister with the PET images. MRI was acquired on a 1.5 T Vision Scanner (Siemens).
Image processing.
Since there is no region in the human brain devoid of CB1R, the tracer plasma concentration is typically required to quantify the [18F]MK-9470 binding. With comparable peripheral tracer metabolization in subjects, the index of CB1R availability can be quantified on the basis of previously validated quantification method using modified standardized uptake value (mSUV; Thie et al., 2007; Sanabria-Bohórquez et al., 2010; Casteels et al., 2012), thereby not requiring invasive blood sampling (Sanabria-Bohórquez et al., 2010; Casteels et al., 2012). Parametric mSUV images were generated by summation of the 90–120 min image data, corrected for injected dose and subject's body weight: mSUV = [activity concentration (KBq/cc) × (subject's body weight (Kg) + 70/2)]/injected dose (MBq). Possible group differences in peripheral metabolism were excluded by comparing mSUV to the fractional uptake ratio (FUR) values, an index proportional to the CB1R total volume of distribution (Sanabria-Bohórquez et al., 2010). For the subjects with full dynamic measurements with venous blood sampling, the parametric FUR images were calculated as the ratio of total radioactivity concentration in tissue at the end of the scan and the integral of metabolite corrected plasma radioactivity from the time of injection to the end of the scan (Sanabria-Bohórquez et al., 2010). To evaluate whether this simplified quantification method was also applicable in this patient group as shown in neurodegenerative disorders, eating disorders (Van Laere et al., 2010, 2012; Gérard et al., 2011), cannabis addiction (Ceccarini et al., 2013c), and schizophrenia (Ceccarini et al., 2013b), [18F]MK-9470 plasma concentration and blood metabolite measurements were performed for a subset (n = 5) of subjects both in the first study at BL and ALC ACU conditions, as well as for patients and controls in the second study between 0–120 min post injection. Venous sampling for input curve determination and blood metabolites was done as described previously (Sanabria-Bohórquez et al., 2010). There were no group differences in peripheral metabolism and metabolite corrected venous input function that could lead to bias in CB1R availability determination by mSUV. We found a strong linear relationship between mSUV and FUR values (R2 = 0.99) for all subgroups, indicating that tracer peripheral clearance and/or metabolization was not different thus allowing the use of the mSUV measure (data not shown). Moreover, changes in CB1R availability were the same as obtained with both FUR and mSUV method for all regions of interest, indicating the robustness of the findings independent of tracer influx (data not shown).
Parametric maps of CB1R availability were generated in PMOD version 2.95 (PMOD Technologies). For each subject, all PET frames were realigned in SPM2 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK). [18F]MK-9470 mSUV images were then coregistered to the subject′s MRI and spatially normalized to a specific CB1R template (Van Laere et al., 2008) constructed in Montreal Neurological Institute (MNI) space (2 × 2 × 2 mm), as described previously (Ceccarini et al., 2013b). Before further statistical analyses, normalized PET images were masked within the brain 80% isocontour of the CB1R template and smoothed with a FWHM of 10 mm.
PET data analyses.
A voxel-based statistical parametric analysis (SPM2) was conducted. For assessment of absolute CB1R availability differences, no proportional scaling was used and a relative gray matter analysis threshold of 80% was used to exclude extracerebral activity. To prevent false-positive findings, data were explored at a voxel-level pFWE < 0.05 corrected for multiple comparisons, with a cluster-level pcluster < 0.05 and extent threshold Kext > 200 voxels (unless indicated otherwise). Next to the voxel-based analysis, also a predefined volume of interest (VOI) analysis was performed using an in-house previously created set of VOIs (Van Laere et al., 2006; Ceccarini et al., 2013b). Average mSUV values within the VOIs were determined and compared using ANOVA and paired t tests, for ALC conditions in patients and social drinkers, respectively, followed by Bonferroni test (Statistica version 9.0; StatSoft).
Additionally PET data have been corrected for atrophy by post hoc partial volume correction (PVC) on the reconstructed data, using the Alfano method in the software package PVEOut software (Quarantelli et al., 2004; n = 22 ALC CHR, n = 13 CON). Segmentation of T1 3D-MPRAGE images were performed using SPM. For each subject the segmented MRI dataset were coregistered to PET data and spatially normalized to the standard MNI space using the same CB1R template.
We performed also voxel-based morphometry (VBM) analysis to assess morphological changes in both gray and white matter differences between the ALC and CON groups. Normalized MRIs were segmented into gray and white matter maps and a voxelwise comparison of the local gray matter and white matter concentration was performed.
Moreover, correlation analyses were performed between CB1R availability and drinking parameters and subjective response changes. Correlation analysis was performed using the percentage change in mSUV between BL and ALC ACU (ΔmSUV), calculated as follows:
Data were first inspected for normality distribution by Kolmogorov–Smirnov testing. Pearson and (r) Spearman rank (rs) coefficients were computed for normally and non-normally distributed data, respectively. All results were considered statistically significant at p < 0.05.
Results
Alcohol administration and subjective effects of alcohol
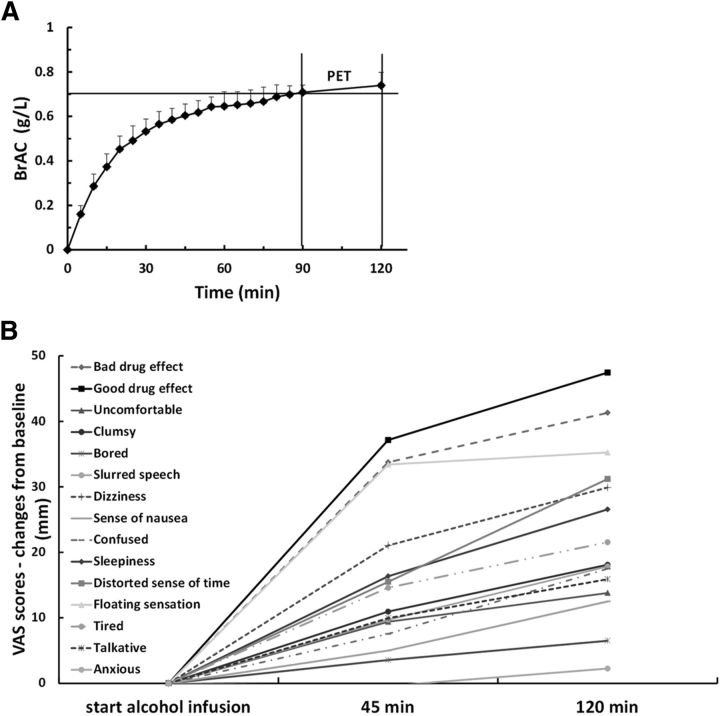
All volunteers completed the study without adverse events. Alcohol clamping succeeded for all subjects. The average BrAC profile over time is presented in Figure 1A. On average, the volunteers reached the pseudo-steady-state alcohol concentration target level 0.7 ± 0.03 g/L within ∼66 ± 13.4 min after the start of the alcohol infusion. During the pseudo-steady-state phase, they maintained an average BrAC of 0.73 ± 0.04 g/L, which was quite well maintained between the start and the end of PET acquisition. On average, it could be observed that, although the alcohol clamping procedure slightly exceeds its BrAC target level by 5.7% during the plateau phase, the intravenous loading regimen did not produce an overshoot. The mean infusion rate needed to maintain the stable BrAC during the plateau phase was 200 ± 55 ml/h. The subjects received, on average, a total amount of alcohol of 75.4 ± 7.2 g, comparable to six beers or five glasses of wine or four liquors (Skinner and Sheu, 1982). Subjective VAS results are summarized in Figure 1B. The group showed a significant increase of the subjective effect for each VAS item after the alcohol administration (at 120 min), except for the VAS feelings bored and anxious. The two VAS items that scored the highest significant alcohol-induced increase were the feelings of good drug effect (average increase of 47.5 mm, p = 0.3 · 10−7), and the feelings of bad drug effect (average increase of 41.3 mm, p = 9.8 · 10−7; Fig. 1B). The feelings of floating sensation and distorted sense of time were also significantly increased by alcohol (average increase of 35.3 mm, p = 1.1 · 10−5 and of 31.2 mm, p = 7.0 · 10−5, respectively). Additionally, some of the VAS items that scored the highest significant increase from baseline were negatively associated with the weekly alcohol consumption (good drug effect: rs = −0.53, p = 0.017; floating sensation: rs = −0.73, p < 0.001; dizziness: rs = −0.60, p = 0.005; talkative: rs = −0.64, p = 0.002; tired: rs = −0.46, p = 0.041).
Figure 1.
BrAC profile and subjective responses to alcohol. A, Average BrAC profile with its SDs error bars. Alcohol was infused between t = 0 and 120 min, and PET was performed between t = 90 and 120 min, as indicated by the two vertical bars. The target BrAC (0.7 g/L) is marked by the horizontal line. B, Bar mean scores of the VAS changes (mm) used to measure self-reported subjective responses to ALC from the start of the ALC treatment (0 min) to the end of ALC administration (120 min).
CB1R availability after acute alcohol administration
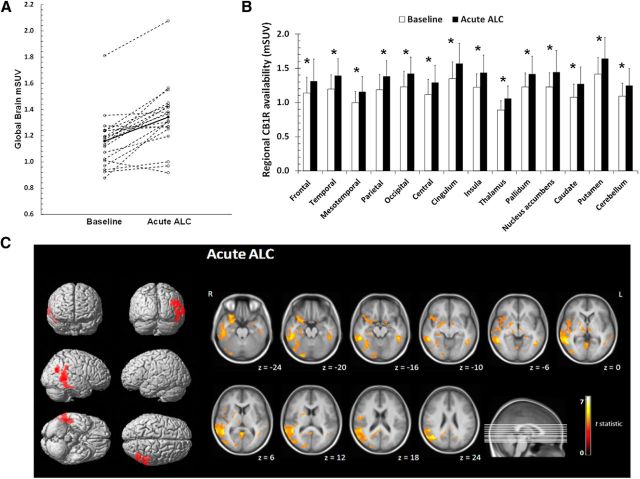
Acute alcohol administration resulted in a significant increase in absolute CB1R availability compared with BL, throughout all gray matter regions (pheight < 0.001; Fig. 2A–C). The most significant clusters (pFWE < 0.05) of increased CB1R availability were located in the inferior temporal gyrus and the inferior parietal lobule (Fig. 2C, Table 2, ALC ACU > baseline). On a gray matter global VOI basis, the level of increase was on average +15.8% (range: 14.1–18.9%; p = 0.014; Fig. 2A).
Figure 2.
CB1R increases in healthy volunteers after acute alcohol administration. A. Scatter plot of the mean global gray matter mSUV (modified standardized uptake value) in healthy volunteers for the baseline condition and after the acute alcohol (Acute ALC) administration. Full circles and full lines represent the mean mSUV in each condition. B, Bar chart of mean regional differences in CB1R availability between baseline and Acute ALC (significant differences vs controls are indicated with * p < 0.05, Bonferroni post hoc correction). Error bars indicate 1 SD. C, SPM results showing increases in CB1R availability after Acute ALC in social drinkers compared with the baseline condition overlaid on a single-subject rendered representation. Clusters are shown at the level of pFWE < 0.05 (corrected) with a cluster extent threshold of 200 voxels (L, left; R, right). From top to bottom: anterior–posterior; right–left; inferior– superior (left). Clusters of interest are overlaid on a T1 MRI template. Images are in radiological orientation (right).
Table 2.
Voxel-based analysis of CB1R changes in healthy subjects after acute alcohol administration and in alcoholic patients after chronic heavy drinking and abstinence
| Cluster level |
Voxel level |
Peak voxel MNI coordinates |
Intensity difference (%) | Cluster location | ||||
|---|---|---|---|---|---|---|---|---|
| pcor | kEXT | pFWE | T | x | y | z | ||
| ALC ACU > baselinea | ||||||||
| 0.004 | 901 | 0.001 | 7.37 | 50 | −52 | 2 | +12.1 | Inferior temporal gyrus (fusiform gyrus), BA 37 |
| 0.002 | 6.53 | 48 | −46 | 6 | +6.6 | Middle temporal gyrus, BA 21 | ||
| 0.004 | 6.28 | 52 | −44 | −12 | +10.4 | Inferior temporal gyrus, BA 20 | ||
| 0.005 | 6.09 | 50 | −40 | 32 | +12.9 | Inferior parietal lobule (supramarginal gyrus), BA 40 | ||
| 0.007 | 5.87 | 50 | −46 | 20 | +11.6 | Transverse temporal gyrus, BA 41 | ||
| 0.015 | 5.45 | 46 | −38 | 10 | +9.5 | Transverse temporal gyrus, BA 41 | ||
| 0.019 | 5.33 | 70 | −44 | 2 | +13.3 | Middle temporal gyrus, BA 21 | ||
| 0.023 | 5.22 | 68 | −48 | 12 | +12.9 | Middle temporal gyrus, BA 21 | ||
| 0.030 | 5.05 | 48 | −26 | −22 | +13.9 | Inferior temporal gyrus, BA 20 | ||
| 0.049 | 4.78 | 64 | −54 | 20 | +10.6 | Middle temporal gyrus, BA 21 | ||
| 0.016 | 272 | 0.002 | 6.54 | 40 | −62 | 26 | +9.1 | Inferior parietal lobule (angular gyrus), BA 39 |
| CHR patients < healthy controlsb | ||||||||
| 0.033 | 4282 | 0.010 | 4.38 | 50 | −52 | −34 | −25.9 | Cerebellum crus I |
| 0.015 | 4.24 | 52 | −56 | −32 | −25.1 | Cerebellum crus I | ||
| 0.020 | 4.13 | 48 | −64 | −30 | −24.6 | Cerebellum crus I | ||
| 0.020 | 4.12 | 44 | −50 | −44 | −24.1 | Cerebellum crus II | ||
| 0.032 | 3.94 | 4 | −80 | −20 | −23.2 | Lateral occipital gyrus, BA 18 | ||
| 0.047 | 2795 | 0.045 | 3.80 | 42 | −44 | 56 | −25.9 | Inferior parietal lobule, BA 40 |
| 0.048 | 3.54 | 38 | −42 | 58 | −25.3 | Somatosensory cortex, BA 2 | ||
| 0.050 | 3.75 | −12 | −82 | 38 | −24.0 | Lateral occipital gyrus, BA 19 | ||
| < 0.001 | 3.67 | 28 | −82 | 32 | −23.4 | Lateral occipital gyrus, BA 19 | ||
| < 0.001 | 3.66 | 28 | −66 | 56 | −22.4 | Superior parietal lobule, BA 7 | ||
| < 0.001 | 3.58 | −36 | −52 | 54 | −24.3 | Inferior parietal lobule, BA 40 | ||
| ABST patients < healthy controlsc | ||||||||
| <0.001 | 22958 | 0.012 | 4.65 | −10 | 10 | 10 | −21.6 | Caudate nucleus |
| 0.014 | 4.61 | 48 | −64 | −30 | −23.4 | Cerebellum crus I | ||
| 0.015 | 4.56 | −2 | −82 | 36 | −24.0 | Lateral occipital gyrus, BA 19 | ||
| 0.016 | 4.55 | 22 | −82 | −36 | −20.9 | Cerebellum crus II | ||
| 0.017 | 4.51 | 50 | −58 | −32 | −24.0 | Cerebellum crus I | ||
| 0.021 | 4.43 | 4 | −78 | −22 | −23.0 | Cerebellar vermis | ||
| 0.021 | 4.42 | −8 | 10 | 6 | −21.7 | Caudate nucleus | ||
| 0.022 | 4.42 | −12 | 2 | 12 | −20.3 | Caudate nucleus | ||
| 0.025 | 4.36 | 10 | 12 | 2 | −22.6 | Nucleus accumbens | ||
| 0.034 | 4.23 | 18 | −36 | −4 | −20.6 | Parahippocampal gyrus | ||
| 0.035 | 4.22 | 32 | 20 | −26 | −17.8 | Superior temporal gyrus, BA 38 | ||
| 0.036 | 4.20 | 6 | 12 | −2 | −21.5 | Nucleus accumbens | ||
| 0.038 | 4.18 | 30 | 2 | −24 | −18.5 | Amygdala extending to parahippocampal gyrus | ||
| 0.038 | 4.18 | 14 | 8 | 8 | −20.4 | Caudate nucleus | ||
| 0.039 | 4.17 | 2 | 16 | −4 | −20.4 | Subgenual anterior cingulate cortex, BA 25 | ||
| 0.042 | 4.14 | 50 | −4 | 26 | −19.3 | Precentral gyrus, BA 4 | ||
| 0.043 | 4.12 | 54 | 4 | 30 | −19.9 | Precentral gyrus, BA 4 | ||
| 0.044 | 4.11 | 8 | 16 | −2 | −21.3 | Nucleus accumbens | ||
| 0.045 | 4.11 | 38 | −22 | −16 | −19.2 | Hippocampus extending to parahippocampal gyrus | ||
| 0.045 | 4.11 | 40 | −48 | 58 | −23.7 | Inferior parietal lobule, BA 40 | ||
| 0.047 | 4.09 | 50 | −26 | 26 | −18.8 | Insula | ||
| 0.047 | 4.09 | −46 | −16 | −18 | −19.0 | Inferior temporal gyrus, BA 20 | ||
| 0.048 | 4.08 | −2 | 14 | −2 | −20.6 | Caudate nucleus | ||
CHR, alcoholics, chronic condition; ABST, alcoholics, abstinence condition; ALC ACU, acute alcohol condition; pcor, corrected p value; kEXT, cluster size extent (number of voxels); pheight, significant p threshold; FWE, familywise error (=peak height corrected for multiple voxel comparisons); T, peak voxel t statistic.
aSignificant clusters obtained at a voxel-level pheight < 0.001 corrected with a cluster-level pcluster < 0.05 corrected and a cluster threshold of 200 voxels.
bSignificant clusters obtained at a voxel-level pheight < 0.001 uncorrected with a cluster-level pcluster < 0.05 corrected and a cluster threshold of 200 voxel.
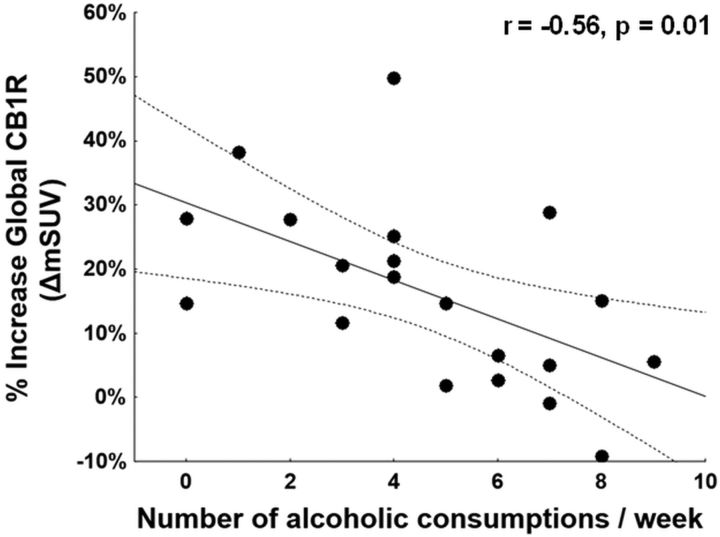
Correlating alcohol-induced changes in CB1R availability (ΔmSUV) with the number of documented alcoholic consumptions per week, we found that lower increase of ΔmSUV was significantly associated with greater alcoholic consumption per week mainly in all the cerebral gray matter areas (r = −0.56, p = 0.01 for the whole-brain VOI), indicating a lower effect in subjects with more previous drinking (Fig. 3). Furthermore, the alcohol-induced increase of CB1R availability (ΔmSUV) was significantly correlated with specific subjective alcohol's effects. Indeed, although we did not find any correlations between the total VAS score and ΔmSUV in any region, when the VAS items were considered separately, there was a positive relationship between ΔmSUV and VAS dizziness in the parietal lobe, secondary somatosensory cortex (mann area (BA), 5) and precuneus (BA 7), and with floating sensation in the superior frontal gyrus (BA 6, BA 8) and somatosensory cortex (BA 123, BA 5; rs ≥ 0.46, p ≤ 0.04). No correlations were found between baseline CB1R availability and subjective reports of feelings from alcohol exposure.
Figure 3.
Negative correlation between the percentage change of the global CB1R availability between ALC and BL condition (ΔmSUV) in relation to the number of alcoholic consumptions per week (r = −0.56, p = 0.01).
CB1R availability after chronic heavy drinking and abstinence in patients
VBM analysis on all datasets did not show significant changes in gray and white matter concentration between patient and control groups. A widespread decrease in CB1R availability was found in both ALC CHR and ALC ABST compared with CON (Fig. 4A–C). Analysis of partial volume corrected data confirmed these results. PVC mSUVALC CHR was significantly lower than PVC mSUVCON in all brain regions (frontal lobe: PVC mSUVALC CHR = 0.97 ± 0.19, PVC mSUVCON = 1.31 ± 0.26, p = 0.00005; temporal lobe: PVC mSUVALC CHR = 1.04 ± 0.15, PVC mSUVCON = 1.31 ± 0.18, p = 0.00003; mesotemporal lobe: PVC mSUVALC CHR = 0.90 ± 0.12, PVC mSUVCON = 1.07 ± 0.14, p = 0.00025; parietal lobe: PVC mSUVALC CHR = 0.98 ± 0.17, PVC mSUVCON = 1.28 ± 0.18, p = 0.00002; occipital lobe: PVC mSUVALC CHR = 1.06 ± 0.16, PVC mSUVCON = 1.35 ± 0.20, p = 0.00003; striatum: PVC mSUVALC CHR = 1.17 ± 0.15, PVC mSUVCON = 1.40 ± 0.17, p = 0.00009).
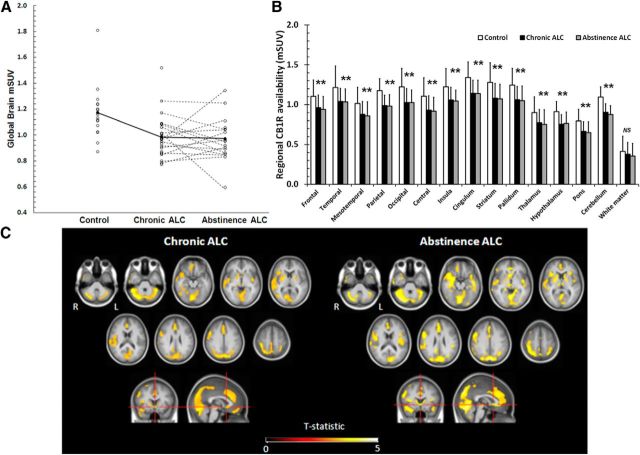
Figure 4.
CB1R decreases in alcoholic patients after chronic heavy drinking and abstinence. A, Scatter plot of the mean global gray matter mSUV (modified standardized uptake value) for controls, alcoholic patients after the chronic exposure to alcohol (Chronic ALC), and during the alcohol abstinence condition (Abstinence ALC). Full circles and full lines represent the mean mSUV in each condition. B, Bar chart of mean regional differences in CB1R availability between chronic ALC, abstinence ALC, and controls. Significant differences versus controls are indicated with *p < 0.05, Bonferroni post hoc correction. Error bars indicate 1 SD. C, SPM results showing decreases in CB1R availability in Chronic ALC (left) and in Abstinence ALC (right) conditions compared with controls. SPM t-maps are overlaid on a T1 MRI template at pheight < 0.001 (uncorrected) with a cluster level pcluster < 0.05 corrected and extent and a cluster threshold of 200 voxels (L, left, R, right).
SPM analysis found a relatively homogeneous gray matter reduction of CB1R availability for both the CHR alcohol exposure and the ABST period compared with controls. The most significant decreases at the CHR phase were found in the cerebellum and in the parieto-occipital cortex (Fig. 4C, Table 2, CHR patients < healthy controls). In the ALC ABST condition the same clusters were observed, with extension to the bilateral caudate nucleus, nucleus accumbens, lateral and mesotemporal cortex, and precentral frontal cortex (Fig. 4C, Table 2, ABST patients < healthy controls). The secondary VOI analysis showed that, the average gray matter mSUV of ALC CHR was 16.1% lower compared with CON (p = 0.003) and for the ALC ABST with 17.0% compared with CON (p = 0.003).
On both SPM and VOI analysis, patients did not show any significant CB1R changes after the period of ABST compared with CHR (Fig. 4). Figure 4B shows that the regional variations of CB1R decrease across cortical and subcortical regions are limited as the changes are relatively uniform across cortical and subcortical regions (ALC CHR: range −12.9 to 17.5%; ALC ABST: range −14.8 to 20.1%). There were no statistically significant differences in decrease between regions (p > 0.3). Yet, although 69% of the alcoholic patients were also tobacco smokers, the tobacco use did not significantly confound the main finding on the CB1R differences, since mSUV was not different between smoking and nonsmoking patients and including smoking status as a covariate variable in the statistical model did not influence the outcome. Moreover, regular coffee consumption could not contribute to the observed effects considering that no significant difference in daily coffee drinking was present between the two groups (14 controls and 22 patients were regular coffee drinkers, 82 and 85%, respectively).
There were no significant correlations between drinking history variables (number of alcoholic drinks per day, heavy drinking duration from the beginning alcohol use, period between the first heavy drinking, the worst alcoholic period, and age of the day of scanning) and CB1R availability.
Discussion
To our knowledge, this PET study provides the first in vivo evidence that acute exposure to alcohol is related to a (presumably) transient increase in CB1R availability. Moreover, we found that chronic long-term alcohol exposure leads to significantly decreased CB1R availability and that this downregulation is not reversible in the short term, as observed after ∼1 month of abstinence.
Acute effects of alcohol on CB1R availability
In healthy social drinkers, we found for the first time that acute alcohol administration resulted in a rapid increase of CB1R availability, which was modulated by routine alcohol consumption and related to subjective alcohol effects. The synthesis of endocannabinoids has been shown to be enhanced by acute ethanol administration (Caillé et al., 2007; Basavarajappa et al., 2008). To be able to interpret these findings further, we have also recently investigated alterations in CB1R availability and endocannabinoid anandamide levels in the same rat subjects to several ethanol conditions using [18F]MK-9470 microPET and microdialysis (Ceccarini et al., 2013a). We confirmed that acute ethanol exposure is related to an increased CB1R availability that is correlated to the ethanol-induced increased anandamide levels of that region (Ceccarini et al., 2013a). This is also in accordance with the observation that an acute anandamide administration resulted in an increased CB1R density in the hippocampus (Romero et al., 1995). Therefore, our results are in line with increased endocannabinoid content that could drive an increased CB1R expression and a rapid upregulation, providing evidence that (moderate) activation of CB1R activity can result in receptor regulation.
Additionally, we found a negative correlation between alcohol-induced increase in CB1R availability and the average number of drinks per week, indicating that the acute increase in CB1R availability is blunted in more regularly consuming subjects. This might be a sensitization phenomenon where the “response capacity” of the CB1R to alcohol insult is reduced. Alternatively, this may be related to the interaction between the ECS and the mesocorticolimbic dopaminergic pathway. Microdialysis studies demonstrated that CB1R is involved in the reward pathway, confirming that dopamine release induced by some drugs of abuse including alcohol is CB1R dependent (Hungund et al., 2003).
CB1R availability changes upon chronic alcohol administration and abstinence
In alcohol-dependent patients, we found that chronic long-term heavy drinking led to a profound decreased CB1R availability, and this reduction remained unaltered after 1 month of abstinence. Our current findings are perfectly consistent with the CB1R PET results of Hirvonen et al. (2013), where they observed an irreversible CB1R reduction after 2–4 weeks of abstinence. This lack of reversibility is in contrast with the reversible CB1Rs downregulation found in cannabis users (Hirvonen et al., 2012; Ceccarini et al., 2013c), suggesting a more persistent neuroadaptive change in CB1Rs in alcohol dependence. Also, several preclinical studies found a decreased CB1R density and CB1R gene expression after chronic alcohol administration in several subcortical structures (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999b; Ortiz et al., 2004; Vinod et al., 2006; Mitrirattanakul et al., 2007). Accordingly, we have also provided preclinical evidence that 1 week of chronic ethanol exposure leads to decreased CB1R availability (Ceccarini et al., 2013a). A plausible mechanism could be the persistent overstimulation of the CB1R by its endogenous ligands, as the synthesis of both endocannabinoids is increased in reward-related areas after chronic alcohol exposure and withdrawal (Basavarajappa and Hungund, 1999a; Basavarajappa et al., 2000; Gonzalez et al., 2002; Mitrirattanakul et al., 2007; Vinod et al., 2012). The observed long-term CB1Rs decrease may thus reflect a compensatory downregulation due to chronic stimulation, as for other neurotransmitter systems associated with addiction after 2–4 weeks of abstinence (Volkow et al., 2012), or may be alternatively the consequence of other molecular mechanisms such as cross-desensitization, allosteric modulation, disruption in CB1R dimerization and trafficking, or altered receptor/binding protein interactions.
Changes in ECS functioning in different stages of alcohol dependence and potential therapeutic implications
Overall, our findings are supported by a model recently proposed on ECS changes across the different stages of alcohol dependence (Pava and Woodward, 2012). To begin, acute alcohol consumption enhances endocannabinoid signaling to produce its acute and positive reinforcing properties through secondary effects on the mesocorticolimbic dopaminergic pathway (Hungund et al., 2003; Riegel and Lupica, 2004; Everitt and Robbins, 2005; Perra et al., 2005; Cheer et al., 2007). On the other hand, consistent with increased endocannabinoid activity resulting from alcohol consumption, a downregulation of CB1Rs expression either immediately after chronic ethanol treatment or during acute withdrawal is found (Basavarajappa et al., 1998; Vinod et al., 2006; Mitrirattanakul et al., 2007; Pava and Woodward, 2012; Ceccarini et al., 2013a). Because of the established role of CB1R in positive reinforcement from alcohol, the persistent and irreversible profound CB1R downregulation we have found after chronic alcohol consumption might suggest a possible candidate mechanism that might contribute to the neuroadaptation process that gradually reduces the acute and positive reinforcing properties of alcohol. Additionally, the fact that CB1R downregulation persists several weeks into abstinence might suggest that, in alcoholic patients, decreased CB1R availability could represent an enduring trait-like feature. Longer follow-up is necessary to differentiate this. Moreover, during a several week long withdrawal and abstinence period, endocannabinoid tone is persistently increased well into abstinence and CB1R expression is reduced during the initial phase of withdrawal. Over time endocannabinoid levels should remain elevated and CB1R expression is consequently upregulated over time to allow the endocannabinoid tone to respond to phasic changes. In this phase, brain stress systems become overactive and alcohol use becomes pursued in large part to ameliorate negative emotionality and promote stress coping (i.e., phase of reinstatement to alcohol-seeking following abstinence; Koob, 2008). The hyperexcitability associated with the initial phase of abstinence results in a large increase in endocannabinoid release and concomitant reductions in CB1R expression. Given the interaction between the ECS and stress and emotionality (Rademacher and Hillard, 2007; McLaughlin et al., 2013), a large body of evidence supports a role for the ECS in the negative affect and stress phase associated with withdrawal (Hill et al., 2008; Little et al., 2008). Other studies have implicated the ECS in dampening the stress response to an acute ethanol injection (Cippitelli et al., 2008), which is attenuated by acute injection of the CB1R inverse agonist rimonabant (Rubio et al., 2008). Additionally, CB1Rs also play a critical role in modulating stress responses through actions in the hippocampus, basolateral amygdala, and prefrontal cortex (Hill et al., 2010). Therefore, the downregulation of CB1Rs in these stress-related areas we have found during early abstinence can be expected to contribute to enhanced negative emotionality and impaired stress coping providing a powerful incentive for resumption of alcohol intake to restore endocannabinoid signaling and to promote stress coping to basal levels.
Therefore, to prevent relapse, an enhancement of the endocannabinoid function might offer a new cannabinoid-based therapeutic direction for treatment of alcohol dependence. First, increased endocannabinoid tone might restore affective homeostasis during abstinence, hence reducing the motivation to consume alcohol for its negatively reinforcing properties, and is protective against the hyperexcitability developed during alcohol withdrawal (i.e., hyperglutamatergic state; Rubio et al., 2011), due to the CB1R-related inhibitory effects on glutamate release (i.e., neuroprotection). In line with our hypothesized mechanism, an increased anandamide level by selective FAAH inhibitor URB597 did reverse alcohol-induced anxiety associated to alcohol withdrawal (Cippitelli et al., 2008), whereas treatment with rimonabant failed to prevent foot shock stress-induced alcohol relapse (Economidou et al., 2006) and alcohol relapse (Soyka et al., 2008; George et al., 2010). Accordingly, pharmacological strategies aimed at enhancing CB1R signaling may offer therapeutic advantages to treat the negative affective state produced by alcohol withdrawal in late stage, which is critical for the maintenance of alcohol addiction.
Study limitations
To have the subjects evaluable on the PET scanner, patients were scanned within the first week of sobriety following a period of chronic alcohol consumption. This means that the evaluated condition may correspond to an early abstinence rather than a pure stable chronic condition. However, we could not find an influence on the number of delay days and reduction of CB1R. Methodologically, the use of mSUV as quantitation measure has been shown to be a good index of tracer availability in the absence of metabolization or input differences between groups (Burns et al., 2007; Sanabria-Bohórquez et al., 2010; Casteels et al., 2012). This was also explicitly demonstrated in this study. Possible increases in cerebral blood flow induced by acute ethanol administration could theoretically alter tracer kinetics by enhanced influx. However, this was explicitly demonstrated not to be the case for [18F]MK-9470 (Casteels et al., 2012; Ceccarini et al., 2013a). Third, this study only included male subjects. Gender-dependent differences in CB1R binding and affinity have been demonstrated in humans and in rats (Rodríguez de Fonseca et al., 1994; Van Laere et al., 2008). Further studies should attempt to investigate whether acute and chronic alcohol effects might lead to gender-dependent changes in CB1R. Finally, it would be interesting to correlate CB1R availability to measures of alcohol dependence severity, craving, and withdrawal intensity across different stages of alcohol dependence.
Conclusions
In conclusion this study indicates that acute alcohol consumption is associated with an increase in endocannabinoid signaling, indicated by an increased CB1R availability, which is related to behavioral effects of alcohol. Second, chronic alcohol consumption in alcohol-dependent subjects is characterized by reduction in CB1R availability that is not reversible after several weeks of imposed alcohol abstinence. Together, these findings strongly show that acute and chronic alcohol consumption produce relevant in vivo alterations of CB1R availability that deserve further study in the light of rational therapeutic strategies envisaging the ECS.
Footnotes
Financial support of the Research Council KU Leuven (OT/05/58) is gratefully acknowledged. K.V.L. and S.C are Senior Clinical Investigators of the Flemish Foundation of Scientific Research (FWO). K.V.L. and G.B. did not receive financial support related to this work, they received financial support for clinical trial studies in collaboration with Merck, Janssen Pharmaceutics, GE Healthcare, and research grants from the FWO. We are grateful to all study participants. We acknowledge the Leuven PET radiopharmacy and nuclear medicine team for their collaboration, as well as Merck & Co for the availability of the [18F]MK-9470 precursor.
The authors declare no competing financial interests.
References
- APA. Diagnostic and statistical manual of mental disorders (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999a;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999b;815:89–97. doi: 10.1016/S0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/S0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/S0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem. 2008;107:1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. doi: 10.1111/j.2044-8341.1974.tb02285.x. [DOI] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels C, Koole M, Celen S, Bormans G, Van Laere K. Preclinical evaluation and quantification of [18F]MK-9470 as a radioligand for PET imaging of the type 1 cannabinoid receptor in rat brain. Eur J Nucl Med Mol Imaging. 2012;39:1467–1477. doi: 10.1007/s00259-012-2163-3. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Casteels C, Koole M, Bormans G, Van Laere K. Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: a combined PET and microdialysis study. Eur J Nucl Med Mol Imaging. 2013a;40:1582–1594. doi: 10.1007/s00259-013-2456-1. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, Enning F, Koethe D, Leweke FM, Van Laere K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage. 2013b;79:304–312. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Dieter K, van Os J, Henquet C, Van Laere K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2013c doi: 10.1111/adb.12116. doi: 10.1111/adb.12116. Advance online publications. Retrieved Dec. 27, 2013. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. New York: Biometrics Research; 2001. User Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P W/PSY SCREEN) [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, Singley ED, O'Malley SS, Bishai R, Rawlings RR, Kunos G. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology. 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard N, Pieters G, Goffin K, Bormans G, Van Laere K. Brain type 1 cannabinoid receptor availability in patients with anorexia and bulimia nervosa. Biol Psychiatry. 2011;70:777–784. doi: 10.1016/j.biopsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend. 2002;66:77–84. doi: 10.1016/S0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB(1) receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knock-out mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Dang SS, Wainwright SR, Galea LA, Hillard CJ, Gorzalka BB. Upregulation of CB(1) receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav Brain Res. 2013;237:333–337. doi: 10.1016/j.bbr.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Murrough JW, Henry S, Bailey CR, Luckenbaugh DA, Tuit K, Zheng MQ, Galatzer-Levy IR, Sinha R, Carson RE, Potenza MN, Huang Y. Positron emission tomography shows elevated cannabinoid CB (1) receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–2109. doi: 10.1111/j.1530-0277.2012.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. doi: 10.1111/j.1530-0277.1998.tb03639.x. [DOI] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Pérez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Blake EM, Green ST, Mizroch BJ, Mulholland PJ, Woodward JJ. Tolerance to cannabinoid-induced behaviors in mice treated chronically with ethanol. Psychopharmacology. 2012;219:137–147. doi: 10.1007/s00213-011-2387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology. 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, Alfano B, Brunetti A, Baron JC, Salvatore M. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med. 2004;45:192–201. [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Romero J, García L, Fernández-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1995;51:731–737. doi: 10.1016/0091-3057(95)00023-P. [DOI] [PubMed] [Google Scholar]
- Rubio M, Fernández-Ruiz J, de Miguel R, Maestro B, Michael Walker J, Ramos JA. CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology. 2008;54:976–988. doi: 10.1016/j.neuropharm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rubio M, Villain H, Docagne F, Roussel BD, Ramos JA, Vivien D, Fernandez-Ruiz J, Ali C. Pharmacological activation/inhibition of the cannabinoid system affects alcohol withdrawal-induced neuronal hypersensitivity to excitotoxic insults. PLoS One. 2011;6:e23690. doi: 10.1371/journal.pone.0023690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp TL, Acebo C, Van Reen E, Carskadon MA. Effects of a moderate evening alcohol dose. I: sleepiness. Alcohol Clin Exp Res. 2007;31:1358–1364. doi: 10.1111/j.1530-0277.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- Sanabria-Bohórquez SM, Hamill TG, Goffin K, De Lepeleire I, Bormans G, Burns HD, Van Laere K. Kinetic analysis of the cannabinoid-1 receptor PET tracer [(18)F]MK-9470 in human brain. Eur J Nucl Med Mol Imaging. 2010;37:920–933. doi: 10.1007/s00259-009-1340-5. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Thie JA, Hubner KF, Isidoro FP, Smith GT. A weight index for the standardized uptake value in 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography. Mol Imaging Biol. 2007;9:91–98. doi: 10.1007/s11307-006-0068-x. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47:740–747. [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage. 2008;39:1533–1541. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Casteels C, Dhollander I, Goffin K, Grachev I, Bormans G, Vandenberghe W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease in vivo. J Nucl Med. 2010;51:1413–1417. doi: 10.2967/jnumed.110.077156. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Casteels C, Lunskens S, Goffin K, Grachev ID, Bormans G, Vandenberghe W. Regional changes in type 1 cannabinoid receptor availability in Parkinson's disease in vivo. Neurobiol Aging. 2012;33:620.e1–8. doi: 10.1016/j.neurobiolaging.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, Manzanares J, Cooper TB, Hungund BL, Colombo G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol. 2012;17:62–75. doi: 10.1111/j.1369-1600.2010.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Johnson FK, Stauffer J, Setnik B, Ciric S. Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users. Drugs R D. 2011;11:259–275. doi: 10.2165/11593390-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoethout RW, van Gerven JM, Dumont GJ, Paltansing S, van Burgel ND, van der Linden M, Dahan A, Cohen AF, Schoemaker RC. A comparative study of two methods for attaining constant alcohol levels. Br J Clin Pharmacol. 2008;66:674–681. doi: 10.1111/j.1365-2125.2008.03268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoethout RW, Schoemaker RC, Zuurman L, van Pelt H, Dahan A, Cohen AF, van Gerven JM. Central nervous system effects of alcohol at a pseudo-steady-state concentration using alcohol clamping in healthy volunteers. Br J Clin Pharmacol. 2009;68:524–534. doi: 10.1111/j.1365-2125.2009.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]