Abstract
Context
Asians have a high prevalence of insulin resistance, even in the nonobese state. Whereas both visceral fat accumulation (VFA) and fatty liver (FL) have been shown to be associated with insulin resistance, it is still unclear which is a better marker to predict insulin resistance in nonobese Asians.
Objective
The aim of this study was to investigate the relation between VFA or FL and insulin resistance in nondiabetic nonobese Japanese men who do not have diabetes.
Design and Participants
We studied 87 nonobese (body mass index <25 kg/m2) Japanese men without diabetes. Using a two-step hyperinsulinemic euglycemic clamp, we evaluated insulin sensitivity in adipose tissue, muscle, and liver. Intrahepatic lipid and abdominal visceral fat area were measured by 1H-magnetic resonance spectroscopy and MRI, respectively. Subjects were divided into four groups based on the presence or absence of VFA (visceral fat area ≥100 cm2) and FL (intrahepatic lipid ≥ 5%): control (non-VFA, non-FL; n = 54), VFA only (n = 18), FL only (n = 7), and VFA plus FL (n = 8).
Results
Subjects in the FL only and VFA plus FL groups had insulin resistance in adipose tissue and muscle, as well as relatively lower hepatic insulin sensitivity. The specific insulin sensitivities in these organs were comparable in the VFA only and control groups.
Conclusions
In nonobese Japanese men without diabetes, subjects with FL only or VFA plus FL but not VFA only had insulin resistance, suggesting that FL may be a more useful clinical marker than VFA to predict insulin resistance in nonobese Japanese men without diabetes.
Keywords: fatty liver, visceral fat, ectopic fat, nonobese
The number of individuals with type 2 diabetes mellitus has been increasing worldwide, particularly in Asia [1]. Insulin resistance is recognized as an important contributor to the development of type 2 diabetes and obesity exacerbates its onset [2], however, several studies suggested that insulin resistance in nonobese Asians is also an important contributor to metabolic abnormalities [3–6]. For example, we performed a two-step hyperinsulinemic euglycemic clamp study in 90 nonobese [body mass index (BMI) <25 kg/m2] Japanese men who did not have diabetes and found that even those with only one cardiometabolic risk factor had insulin resistance in muscle but not in liver [4], and the metabolic abnormalities in these subjects were closely associated with insulin resistance [4]. However, the mechanisms of insulin resistance in nonobese Asians have not yet been fully elucidated.
Insulin resistance in nonobese Asians may be caused by at least in part by dysregulated lipid storage [7]. Several studies have revealed that Asians, especially East Asians [8], have a low fat-storage capacity in their subcutaneous adipose tissue [8–10]. Even moderate weight gain in East Asians may easily exceed the lipid storage capacity of subcutaneous adipose tissue [8–10], and thus lipid may accumulate in visceral adipose tissue, muscle, and liver, the so-called lipid spillover hypothesis. Accumulation of visceral and ectopic fat may therefore somehow elicit insulin resistance in muscle and liver [7, 11]. Indeed, visceral fat accumulation (VFA) is closely associated with insulin resistance in nonobese East Asians [3–7]. Also, Asians were found to easily develop nonalcoholic fatty liver disease (NAFLD) [10, 12, 13], and intrahepatic lipid (IHL) accumulation measured by 1H-magnetic resonance spectroscopy (MRS) has been closely associated with insulin resistance in nonobese Asians [6] and nonobese Japanese men with [14] and without diabetes [4].
Against this background, it is unsurprising that VFA has been shown to correlate with fatty liver (FL) [15], and that both VFA and FL predict insulin resistance in nonobese Asians [4, 14]. However, although FL is reported to be a better marker of obesity-related metabolic derangements than VFA in obese patients [16], it is still unclear which parameter better predicts insulin resistance in nonobese Asians. To address this issue, we compared the degree of insulin sensitivity among four groups defined by the presence and absence of VFA and FL, based on our previous data on tissue-specific insulin sensitivity derived using a two-step euglycemic hyperinsulinemic clamp in 87 nonobese (BMI < 25 kg/m2), middle-aged Japanese men without diabetes [4].
1. Research Design and Methods
A. Subjects
The Sportology Center Core Study was a prospective observational study involving hypothesis-driven, hypothesis-generating research on the mechanisms underlying metabolic abnormalities in nonobese Japanese subjects without diabetes [4]. In that study, we recruited Japanese men without diabetes with a BMI of 21 to 27.5 kg/m2 (≥21.0 to <27.5 kg/m2) and between 30 and 50 years of age. In the current study, we selected those with a BMI of 21 to 25 kg/m2 (≥21.0 to <25.0 kg/m2).
All participants gave written informed consent to participate in the study. This study was approved by the ethics committee of Juntendo University and carried out in accordance with the principles outlined in the Declaration of Helsinki.
B. Study Design and Measurements
All subjects were recruited at a screening session and were examined at three subsequent visits for baseline evaluation. We performed a two-step euglycemic hyperinsulinemic clamp combined with stable glucose tracer methodology, each for a duration of 180 minutes, with a constant insulin infusion of 10 and 20 mU/m2 per minute (0 to 180 and 180 to 360 minutes, respectively). The metabolic clearance rate of serum insulin (MCRI) during glucose clamp at the second step was calculated as previously described [17]. The IHL and intramyocellular lipid (IMCL) were measured with 1H-MRS and percent fat was measured bioimpedance method; biochemical tests were measured by standard methods and hormonal levels were measure by radioimmunoassay or enzyme-linked immunosorbent assay. These and other methods have been previously reported in detail [4].
C. Statistical Analysis
Data are presented as mean ± SD. The relation between IHL and VFA was assessed by Spearman rank correlation coefficient. Data were compared by one-way ANOVA or Kruskal-Wallis analysis for continuous variables, and groups were compared using the Tukey-Kramer or Games-Howell post hoc tests. All statistical tests were two-sided with a 5% level of significance.
2. Results
A. Comparison of Body Fat Distribution and Fat Derived Factors Among Groups
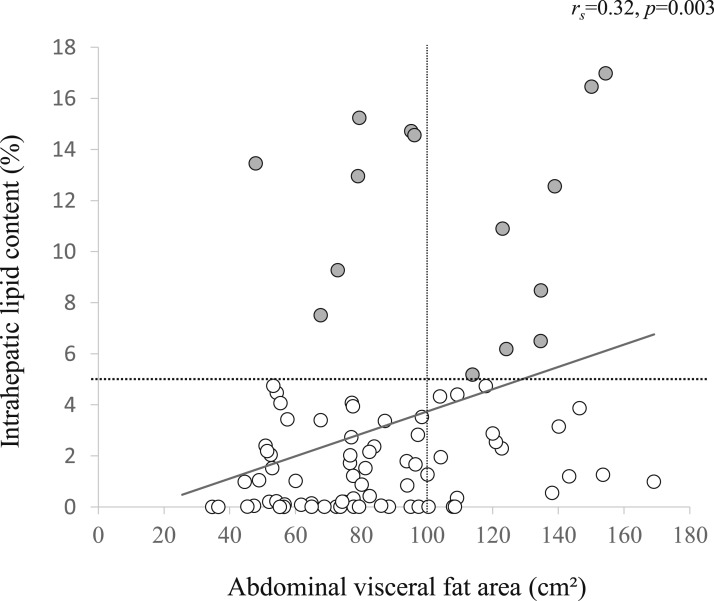
The anthropometric data of the study subjects are shown in Table 1. All subjects were middle-aged men, and the mean values of BMI, IHL, abdominal visceral fat area, and cardiometabolic risk factors were within the normal ranges. The VFA was correlated with IHL, but correlation coefficient was low (rs = 0.32, P = 0.003) (Fig. 1). According to the generally accepted definition, subjects were divided into the following four groups based on the presence or absence of VFA (visceral fat area ≥100 cm2) [18] and FL (IHL 5%) [15]: control group (non-VFA, non-FL; n = 54), VFA only group (n = 18), FL only group (n = 7), and VFA plus FL group (n = 8) (Fig. 1). The anthropometric data in each group are shown in Table 1. Trends of BMI, waist circumference, total body fat content, and abdominal subcutaneous fat area in all four groups resembled the trends of VFA rather than FL. On the other hand, IMCL levels were comparable among the groups (data not shown). In terms of adipose tissue-derived factors, free fatty acid (FFA) was elevated and total and high molecular weight adiponectin were reduced in the VFA only, FL only and VFA plus FL groups relative to the control group.
Table 1.
Clinical Characteristics of the Study Subjects
| Total | Control | VFA Only | FL Only | VFA Plus FL | P a | |
|---|---|---|---|---|---|---|
| Number of subjects | 87 | 54 | 18 | 7 | 8 | |
| IHL, % | 3.2 ± 4.3 | 1.3 ± 1.5 | 2.0 ± 1.6 | 12.5 ± 3.0b,c | 10.4 ± 4.6b,c | <0.001 |
| Abdominal visceral fat area, cm2 | 87.3 ± 31.2 | 69.7 ± 16.9 | 123.2 ± 20.5b | 76.9 ± 16.6c | 134.3 ± 13.7b,d | <0.001 |
| Age, y | 42.1 ± 5.1 | 41.4 ± 5.1 | 43.2 ± 4.4 | 40.6 ± 5.4 | 46.3 ± 4.5 | 0.046 |
| BMI, kg/m2 | 23.5 ± 1.0 | 23.3 ± 1.1 | 23.8 ± 0.7 | 23.6 ± 0.7 | 24.1 ± 0.6b | 0.033 |
| Waist circumference, cm | 83.6 ± 5.2 | 81.6 ± 4.6 | 87.0 ± 3.5b | 84.8 ± 6.2 | 88.2 ± 4.7b | <0.001 |
| Total body fat content, % | 21.5 ± 4.1 | 20.7 ± 4.4 | 22.1 ± 2.7 | 20.9 ± 3.4 | 26.1 ± 2.2b,c,d | <0.001 |
| Abdominal subcutaneous fat area, cm2 | 120.9 ± 37.3 | 113.2 ± 38.8 | 130.5 ± 32.5 | 123.7 ± 32.8 | 148.6 ± 25.7 | 0.044 |
| Fasting plasma glucose, mg/dL | 96.1 ± 7.5 | 95.0 ± 7.7 | 96.1 ± 5.7 | 101.9 ± 7.1 | 98.9 ± 8.1 | 0.090 |
| Fasting serum insulin, μU/mL | 5.8 ± 2.5 | 5.0 ± 1.7 | 6.2 ± 2.4 | 8.9 ± 2.9b | 8.2 ± 3.2 | <0.001 |
| Triglyceride, mg/dL | 141.0 ± 76.9 | 120.6 ± 67.0 | 170.9 ± 93.4 | 193.1 ± 82.4 | 166.0 ± 53.2 | 0.005 |
| Free fatty acid, μEq/L | 372.6 ± 107.9 | 342.8 ± 108.1 | 412.2 ± 87.6 | 435.7 ± 78.6 | 437.6 ± 103.9 | 0.007 |
| Aspartate aminotransferase, IU/L | 22.2 ± 6.8 | 20.6 ± 5.9 | 23.3 ± 6.4 | 29.1 ± 10.0b | 24.6 ± 6.4 | 0.007 |
| Alanine aminotransferase, IU/L | 25.7 ± 15.3 | 22.3 ± 9.7 | 23.1 ± 6.9 | 45.1 ± 36.4 | 37.3 ± 16.1 | 0.005 |
| γ-glutamyl transferase, IU/L | 45.1 ± 40.9 | 40.1 ± 43.7 | 51.5 ± 42.1 | 57.0 ± 22.0 | 54.6 ± 27.8 | 0.021 |
| High molecular weight adiponectin, μg/mL | 1.5 ± 1.1 | 1.8 ± 1.2 | 1.0 ± 0.7b | 0.6 ± 0.3b | 1.0 ± 0.8 | 0.001 |
| VO2peak, mL/kg/min | 32.3 ± 6.6 | 33.8 ± 6.4 | 31.6 ± 6.0 | 29.6 ± 3.5 | 25.7 ± 7.4b | 0.006 |
Data are expressed as mean ± SD.
Abbreviation: VO2peak, peak oxygen consumption.
P value for one-way ANOVA or Kruskal-Wallis analysis.
P < 0.05 for Tukey-Kramer or Games-Howel; vs control.
P < 0.05 for Tukey-Kramer or Games-Howel; vs VFA Only.
P < 0.05 for Tukey-Kramer or Games-Howel; vs FL Only.
Figure 1.
Relation between abdominal visceral fat area and IHL content in all subject. Dashed lines indicate the cutoffs for the grouping of VFA ( cm2) and FL (IHL 5%) in the current study. Open circles, subjects without FL; gray circles, subjects with FL.
B. Insulin Sensitivity in Adipose Tissue, Muscle and Liver
Table 2 shows the data from the two-step hyperinsulinemic euglycemic clamp study. Hepatic insulin sensitivity, defined as percent reduction of endogenous glucose production/steady-state serum insulin at the first step [19], was relatively lower in the FL only and VFA plus FL groups than in the other two groups. On the other hand, muscle insulin sensitivity, defined as rate of disappearance/steady-state serum insulin at the second step [20], were lower in the FL only and VFA plus FL groups compared with the control group. Also, adipose tissue insulin sensitivity, defined as FFA suppression/insulin at the first step [19], was substantially lower in both the FL only and VFA plus FL groups compared with the control group. Consistently, our preliminary analysis showed that compared with one to one matched control subjects by age and BMI [21], the FL only group and VFA plus FL group had substantially or relatively impaired insulin sensitivity in muscle, liver, and adipose tissue, respectively; however, insulin sensitivity in those were comparable between age and BMI matched control and VFA only group (data not shown).
Table 2.
Euglycemic Hyperinsulinemic Clamp Data
| Control | VFA Only | FL Only | VFA Plus FL | P a | |
|---|---|---|---|---|---|
| %Reduction of EGP/SSSI at first step, %/μU·mL−1 | 3.6 ± 1.0 | 3.7 ± 0.8 | 2.6 ± 0.7 | 2.7 ± 1.2 | 0.006 |
| Rd/SSSI at second step, mg/kg FFM·min−1/μU·mL−1 | 0.23 ± 0.07 | 0.21 ± 0.09 | 0.12 ± 0.04b | 0.13 ± 0.04b | <0.001 |
| %FFA suppression/insulin at first step, %/μU·mL−1 | 4.4 ± 1.0 | 3.9 ± 0.9 | 2.7 ± 0.7b,c | 2.8 ± 1.0b | <0.001 |
| MCRI at second step, mL/min per m2 | 551.7 ± 78.7 | 536.7 ± 125.4 | 478.9 ± 52.9 | 488.1 ± 77.4 | 0.080 |
Data are expressed as mean ± SD.
Abbreviations: SSSI, steady-state serum insulin; EGP, endogenous glucose production; Rd, rate of disappearance.
P value for one-way ANOVA or Kruskal-Wallis analysis.
P < 0.05 for Tukey-Kramer or Games-Howel; vs control.
P < 0.05 for Tukey-Kramer or Games-Howel; vs VFA only.
3. Discussion
In nonobese Asians, as in other ethnic groups, VFA and FL were reported to be associated with insulin resistance and metabolic abnormalities [3–7, 14], however, it is still unclear which parameter better predicts insulin resistance. In the current study, we found that subjects with FL but without VFA had insulin resistance in adipose tissue and muscle, as well as relatively lower hepatic insulin sensitivity. In contrast, subjects with VFA but without FL showed similar insulin sensitivity of muscle, liver, and adipose tissue as control subjects. Subjects with both VFA and FL had more body fat than other groups, but similar insulin resistance as subjects with FL alone. Although VFA has been recognized as a clinical marker to predict insulin resistance [3–7, 14], the current study showed that nonobese Japanese men with VFA alone (without FL) did not demonstrate insulin resistance and their insulin sensitivity was similar to men without VFA or FL. In contrast, the presence of FL alone was well associated with insulin resistance in adipose tissue and muscle.
The FL only group showed insulin resistance in adipose tissue as well as muscle, despite the low fat content in adipose tissue. Previously, it was demonstrated that compared with individuals of other ethnicities, including white, black, Hispanic, and Southeast Asian, East Asians have a lower fat-storage capacity in their subcutaneous adipose tissue and the highest accumulation of visceral fat with increasing adiposity [8]. However, in this study, subjects in the FL only group did not seem to store lipids sufficiently in either visceral or subcutaneous fat, which may have caused insulin resistance. Indeed, another indicator of adipose tissue dysfunction, namely decreased adiponectin levels, was observed in the FL only group. Adiponectin is one of the adipokines that promotes lipid oxidation in muscle and liver and maintains insulin sensitivity [22]. Indeed, decreased adiponectin levels were previously linked to FL and insulin resistance in humans [16, 23], and also associated with increased IMCL and impaired peripheral insulin sensitivity after a high-fat diet in nonobese men [24]. Although adiponectin levels were also decreased in the VFA only and VFA plus FL groups, they were lowest in the FL only group despite this group’s lower adiposity. Thus, despite lower adiposity, the FL only group demonstrated obvious adipose tissue dysfunction.
Although VFA has been established as a marker of insulin resistance in nonobese East Asians [3–7], subjects with VFA but without FL did not have insulin resistance in the present study. In contrast, as discussed above, subjects with FL but without VFA had insulin resistance in adipose tissue and muscle. However, this is not surprising if adipose tissue insulin resistance is considered as upstream of insulin resistance in muscle and liver as well as IHL accumulation. For example, circulating FFAs are a major source of liver fat in NAFLD patients [25]. Adipose tissue insulin resistance is associated with insulin resistance in muscle and liver in obese subject [26]. The FFA elevation by lipid infusion induces insulin resistance in muscle and liver in healthy individuals [27, 28]. Our recent study also revealed that in nonobese apparently healthy Japanese men, reduced adipose tissue insulin sensitivity was associated with moderate IHL accumulation and muscle insulin resistance [19]. Taken together, it is speculated that FL but not VFA alone is a good marker to predict adipose tissue insulin resistance, thus FL is a better predictor for insulin resistance in peripheral tissues than VFA.
Unlike our results, Ding et al. [29] demonstrated that VFA is a better marker for insulin resistance than IHL accumulation in nonobese Asian. We do not know exact reason why this discrepancy occurred, however, we suppose that the differences in study subjects and protocol may contribute to the opposite results. For example, one large difference was observed in the degree of IHL accumulation. In the study, the IHL level in the high IHL group was 4.4% (median), whereas it was 12.5% (mean) in the current study (Table 1). In addition, ∼40% of study subjects were female and BMI and VO2max were matched among the groups [29].
The current study has several limitations. First, we included only men. Fat distribution differs between women and men and therefore it is unclear if our data can be applied to women. Second, both the FL and VFA plus FL groups contained a relatively small number of subjects. However, because two-step hyperinsulinemic euglycemic clamp is a complicated technique, the number of subjects is considered reasonable. Third, this study showed clinical relevance of FL and VFA as a marker of insulin resistance, thus the causal relations involved have not been identified. There may be several potential confounders of the association between FL or VFA and insulin resistance [29–31].
In conclusion, in nonobese Japanese men without diabetes, subjects with FL alone or VFA plus FL demonstrated insulin resistance in adipose tissue and muscle, whereas subjects with VFA alone did not. These data suggest that FL (IHL ≥ 5%) may be a useful marker than VFA (visceral fat area ≥100 cm2) for predicting insulin resistance in nonobese Japanese men without diabetes.
Acknowledgments
We thank Mutsuko Yoshikawa, Miyuki Iwagami, Naoko Daimaru, Eriko Magoshi, and Emi Miyazawa for their excellent technical assistance. We also thank Hikari Taka and Tsutomu Fujimura (Juntendo University) for performing the LC-MS analysis.
Financial Support: High Technology Research Center Grant, Strategic Research Foundation at Private Universities and KAKENHI (23680069, 26282197, 17K19929) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Japan Diabetes Foundation, Suzuken Memorial Foundation, Mitsukoshi Welfare Foundation, and Diabetes Masters Conference.
Author Contributions: S.K., Y.S., H.K., K.T., and Y.T. researched the data and contributed to study design, data collection, interpretation of results, wrote, and edited the manuscript. S.K., T.F., Y.F., D.S., R.S., M.N-Y., and K.S. participated in data collection, data analysis, and contributed to the discussion. H.D., S.A., A.K., and R.K. contributed to the discussion. H.W. contributed to study design, reviewed, and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- FFA
free fatty acid
- FL
fatty liver
- IHL
intrahepatic liquid
- IMCL
intramyocellular lipid
- MCRI
metabolic clearance rate of serum insulin
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- VFA
visceral fat accumulation
References and Notes
- 1. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. [DOI] [PubMed] [Google Scholar]
- 2. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. [DOI] [PubMed] [Google Scholar]
- 3. Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Maruyama N, Morioka K, Nakatani K, Yano Y, Adachi Y. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26(8):2341–2344. [DOI] [PubMed] [Google Scholar]
- 4. Takeno K, Tamura Y, Kawaguchi M, Kakehi S, Watanabe T, Funayama T, Furukawa Y, Kaga H, Yamamoto R, Kim M, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Taka H, Fujimura T, Sawada SS, Giacca A, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Relation between insulin sensitivity and metabolic abnormalities in Japanese men with BMI of 23-25 kg/m2. J Clin Endocrinol Metab. 2016;101(10):3676–3684. [DOI] [PubMed] [Google Scholar]
- 5. Chooi YC, Ding C, Chan Z, Choo J, Sadananthan SA, Michael N, Lee Y, Velan SS, Magkos F. Moderate weight loss improves body composition and metabolic function in metabolically unhealthy lean subjects. Obesity (Silver Spring). 2018;26(6):1000–1007. [DOI] [PubMed] [Google Scholar]
- 6. Ding C, Chan Z, Chooi YC, Choo J, Sadananthan SA, Chang A, Sasikala S, Michael N, Velan SS, Magkos F. Regulation of glucose metabolism in nondiabetic, metabolically obese normal-weight Asians. Am J Physiol Endocrinol Metab. 2018;314(5):E494–E502. [DOI] [PubMed] [Google Scholar]
- 7. Rattarasarn C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte. 2018;7(2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96(4):714–726. [DOI] [PubMed] [Google Scholar]
- 9. Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas. 2010;65(4):315–319. [DOI] [PubMed] [Google Scholar]
- 10. Azuma K, Kadowaki T, Cetinel C, Kadota A, El-Saed A, Kadowaki S, Edmundowicz D, Nishio Y, Sutton-Tyrrell K, Okamura T, Evans RW, Takamiya T, Ueshima H, Curb JD, Abbott RD, Kuller LH, Kelley DE, Sekikawa A; ERA JUMP study group. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 2009;58(8):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes. 2017;41(6):853–865. [DOI] [PubMed] [Google Scholar]
- 12. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia—as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10(5):307–318. [DOI] [PubMed] [Google Scholar]
- 13. Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103(48):18273–18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furukawa Y, Tamura Y, Takeno K, Funayama T, Kaga H, Suzuki R, Watanabe T, Kakehi S, Kanazawa A, Kawamori R, Watada H. Impaired peripheral insulin sensitivity in non-obese Japanese patients with type 2 diabetes mellitus and fatty liver. J Diabetes Investig. 2017;9(3):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu AH, Duan-Mu YY, Zhang Y, Wang L, Guo Z, Yu YQ, Wang YS, Cheng XG. Correlation between non-alcoholic fatty liver disease and visceral adipose tissue in non-obese Chinese adults: a CT evaluation. Korean J Radiol. 2018;19(5):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elahi D, Nagulesparan M, Hershcopf RJ, Muller DC, Tobin JD, Blix PM, Rubenstein AH, Unger RH, Andres R. Feedback inhibition of insulin secretion by insulin: relation to the hyperinsulinemia of obesity. N Engl J Med. 1982;306(20):1196–1202. [DOI] [PubMed] [Google Scholar]
- 18. Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66(11):987–992. [DOI] [PubMed] [Google Scholar]
- 19. Sugimoto D, Tamura Y, Takeno K, Kaga H, Someya Y, Kakehi S, Funayama T, Furukawa Y, Suzuki R, Kadowaki S, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Kanazawa A, Kawamori R, Watada H. Clinical features of nonobese, apparently healthy, Japanese men with reduced adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2019;104(6):2325–2333. [DOI] [PubMed] [Google Scholar]
- 20. Abdul-Ghani M, DeFronzo RA. Fasting hyperglycemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J Clin Endocrinol Metab. 2007;92(5):1778–1784. [DOI] [PubMed] [Google Scholar]
- 21. Haukoos JS, Lewis RJ. The Propensity Score. JAMA. 2015;314(15):1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17(2):185–196. [DOI] [PubMed] [Google Scholar]
- 23. Yatsuzuka S, Shimomura Y, Akuzawa M, Ando Y, Kobayashi I, Nakano T, Tokita Y, Nagamine T, Ono H, Tanaka A, Schaefer E, Nakajima K. Plasma adiponectin is a more specific marker of fatty liver than a marker of metabolic syndrome in Japanese men. Ann Clin Biochem. 2014;51(Pt 1):68–79. [DOI] [PubMed] [Google Scholar]
- 24. Sakurai Y, Tamura Y, Takeno K, Kumashiro N, Sato F, Kakehi S, Ikeda S, Ogura Y, Saga N, Naito H, Katamoto S, Fujitani Y, Hirose T, Kawamori R, Watada H. Determinants of intramyocellular lipid accumulation after dietary fat loading in non-obese men. J Diabetes Investig. 2011;2(4):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012; 55(5):1389–1397. [DOI] [PubMed] [Google Scholar]
- 27. Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72(5):1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Häring HU, Jacob S. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50(11):2579–2584. [DOI] [PubMed] [Google Scholar]
- 29. Ding C, Chan Z, Chooi YC, Choo J, Sadananthan SA, Michael N, Velan SS, Leow MKS, Magkos F. Visceral adipose tissue tracks more closely with metabolic dysfunction than intrahepatic triglyceride in lean Asians without diabetes. J Appl Physiol (1985). 2018;125(3):909–915. [DOI] [PubMed] [Google Scholar]
- 30. Haufe S, Engeli S, Budziarek P, Utz W, Schulz-Menger J, Hermsdorf M, Wiesner S, Otto C, Haas V, de Greiff A, Luft FC, Boschmann M, Jordan J. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes. 2010;59(7):1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McMillan KP, Kuk JL, Church TS, Blair SN, Ross R. Independent associations between liver fat, visceral adipose tissue, and metabolic risk factors in men. Appl Physiol Nutr Metab. 2007;32(2):265–272. [DOI] [PubMed] [Google Scholar]