Abstract
Context
In patients with primary aldosteronism (PA), it remains unclear whether aldosterone-producing adenomas are likely to develop in the left or right adrenal gland.
Objective
To investigate left-right differences of PA laterality diagnoses via CT imaging and adrenal vein sampling (AVS).
Design
Retrospective, observational study.
Patients
From the Japan Primary Aldosteronism Study, 1493 patients with PA were enrolled who underwent CT and ACTH-stimulated AVS.
Measurements
Left or right adrenal nodular lesion distribution and laterality observed on CT scans and from AVS were noted.
Results
Both on CT scans and AVS, unilateral results were observed more frequently on the left side than on the right side (25.1% vs 15.4% and 17.3% vs 13.5%, respectively; P < 0.01for both diagnostic techniques). There was no significant difference in the concordance rate for CT and AVS between patients with left and right unilateral nodular lesions observed on CT scans (44.1% and 50.9%, respectively; P = 0.15). In patients with nodules <20 mm, the concordance rate was significantly greater on the right side than the left side (45.8% vs 56.4%; P = 0.03). In patients with bilateral results of AVS, unilateral nodular lesions were detected more frequently on the left side than the right side (17.8% vs 9.4%; P < 0.01).
Conclusion
These results suggest aldosterone-producing adenomas and nonfunctioning tumors are more likely to develop on the left side in patients with PA and that misdiagnosis of CT-based lateralization may occur more frequently on the left side.
Keywords: hypertension, primary aldosteronism, computed tomography, adrenal vein 87 sampling, left-right difference
Primary aldosteronism (PA) is characterized by autonomous hypersecretion of aldosterone and is the most common cause of secondary hypertension, accounting for either 6% or 8% of all hypertensive patients [1]. Patients with PA are at a higher risk of cardiovascular morbidity than those with essential hypertension [2]. The two common PA subtypes, aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism, must be diagnosed correctly, because the former condition requires an adrenalectomy, whereas the latter is treated by medication [2].
Two modalities of lateralization diagnosis—adrenal imaging, including CT and MRI, and adrenal vein sampling (AVS)—are commonly used to determine the localization of unilateral aldosterone production in patients with PA. It is well known that there are often discrepancies between the imaging diagnosis including CT and MRI and the lateralization diagnosis of AVS [3–5]. Reports [3–5] have discussed unilateral or bilateral diagnoses, although they did not consider whether the unilateral lesions were located on the left or right side. Recently, a few reports discussed left-right differences in laterality diagnosed using both imaging and AVS [6, 7]. However, those studies either had a small sample size [6] or did not use a unified diagnostic imaging modality [7].
In theory, adrenal tumors are considered to occur equally on the left or right side. However, several studies focusing on other objectives have suggested a potential lateralizing asymmetry with a greater prevalence of left-sided adrenal tumors [8–10]. Recently, Hao et al. [11], using the data from a large cohort study, investigated left-right differences in incidentally discovered adrenal adenomas, excluding functioning tumors. They found adrenal adenomas were more prevalent on the left side than the right side. In the current study, we investigated left-right differences determined from CT imaging and AVS, using the data from a large cohort of a multicentric collaborative study for PA, the Japan Primary Aldosterone Study (JPAS).
1. Patients and Methods
A. Patients
Twenty-seven centers participated in the JPAS. Patients with confirmed PA who underwent AVS from January 2006 to October 2016 were enrolled in the study. Baseline clinical findings, the results of AVS, and the post-treatment outcomes were recorded electronically using the internet registry system (the EPS Corporation, Tokyo, Japan). Data security and maintenance of registered data were outsourced to the EPS Corporation. The study protocol was approved by the ethics committees of each center.
The diagnostic procedure for PA was based on the guidelines of both the Japan Endocrine Society [12] and the Japan Society of Hypertension [13]. The screening for PA was based on a ratio of plasma aldosterone concentration (PAC; in picograms per milliliter) to the plasma renin activity (PRA; measured as ng/mL/h) of >200, after the patients had changed medications from potentially interfering antihypertensive drugs to calcium channel blockers and/or α-blockers, where applicable. The diagnosis of PA was established by at least one positive result in confirmatory testing, including the captopril challenge test, the upright furosemide-loading test, and the saline-loading test.
B. Imaging
The CT images were interpreted by radiologists at each participating center. In this study, nodular lesions were defined as those ≥10 mm in diameter. Nodular lesions <10 mm and equivocal lesions were registered as “suspected nodular lesions,” and “enlarged lesions” were classified as normal.
C. AVS
Blood samples obtained via AVS were generally collected 30 minutes after administration of ACTH from both adrenal veins and the inferior vena cava (IVC) at a point distal to the renal vein. The sampling point of both adrenal veins was located at the central vein. The protocol for ACTH administration varied among centers. Eighteen centers used a bolus injection of 250 μg of cosyntropin and eight used a bolus injection of 200 μg (or 250 μg) followed by continuous infusion of 50 μg (or 100 μg, or 250 μg) of cosyntropin. One center used a continuous infusion of cosyntropin. Two centers sampled simultaneously from the left and right adrenal veins. In the remaining centers, sampling was carried out sequentially. Catheterization was considered successful if the selectivity index) was >5, calculated as the ratio of cortisol concentration between the adrenal vein and the IVC after administration of ACTH.
Unilateral disease was defined by a lateralization index >4, calculated as the ratio of aldosterone to cortisol between the dominant and nondominant side. Apparent bilateral aldosterone suppression was defined by an aldosterone-to-cortisol ratio in both adrenal veins bilaterally lower than that measured in the IVC. Patients with apparent bilateral aldosterone suppression according to AVS results were excluded from the study [14].
D. Analysis
The data from the JPAS were studied retrospectively. The patient characteristics and biochemical examinations at diagnosis were investigated. Hypokalemia was defined as either a serum potassium level <3.5 mmol/L or receiving potassium supplementation. The estimated glomerular filtration rate was calculated according to the formula for Japanese people [15]. Left-right differences in lateralization diagnoses were evaluated from the results of CT scans and AVS. In patients who underwent adrenalectomy, we estimated postoperative clinical and biochemical outcomes over either a 6- or 12- month follow-up, referring to the Primary Aldosteronism Surgery Outcome study [16].
E. Assay Methods
PAC and PRA were measured using commercially available kits. PAC was determined by RIA (SPAC-S Aldosterone kits [17]; Fuji Rebio, Tokyo, Japan) at all centers. The reference range of PAC in the supine position was 30 to 159 pg/mL. PRA was measured by RIA or enzyme immunoassay. The reference range of PRA in the supine position was 0.3 to 2.9 ng/mL/h (PRA-FR RIA kits [18]; Fuji Rebio) in 15 centers; 0.2 to 2.3 ng/mL/h (PRA enzyme immunoassay kits [19], Yamasa, Choshi, Japan) in seven centers; and 0.2 to 2.7 ng/mL/h [PRA RIA kits, Yamasa; or Renin RIA beads kits [20], Fuji Rebio; (N.B., these products are identical, because the vender has been replaced)] in four centers. Plasma active renin concentration (ARC) was measured by immunoradiometric assay (Renin IRMA-FR [21]; Fuji Rebio) in one center. The reference range of ARC in the supine position was 2.5 to 21.4 pg/mL. For statistical analysis, the ARC value was converted to PRA by dividing by 5, according to the Japan Endocrine Society guideline [12].
F. Statistics
The data were analyzed and compared using BellCurve for Excel (Social Survey Research Information, Tokyo, Japan). Continuous variables were expressed as either mean ± SD or median and interquartile range. Continuous variables were analyzed using either the one-way ANOVA or the Kruskal-Wallis test, as appropriate. Comparison of frequencies between two groups was estimated by either the χ2 test or the Fisher exact test, as appropriate. Statistical significance was achieved when the P value was <0.05.
2. Results
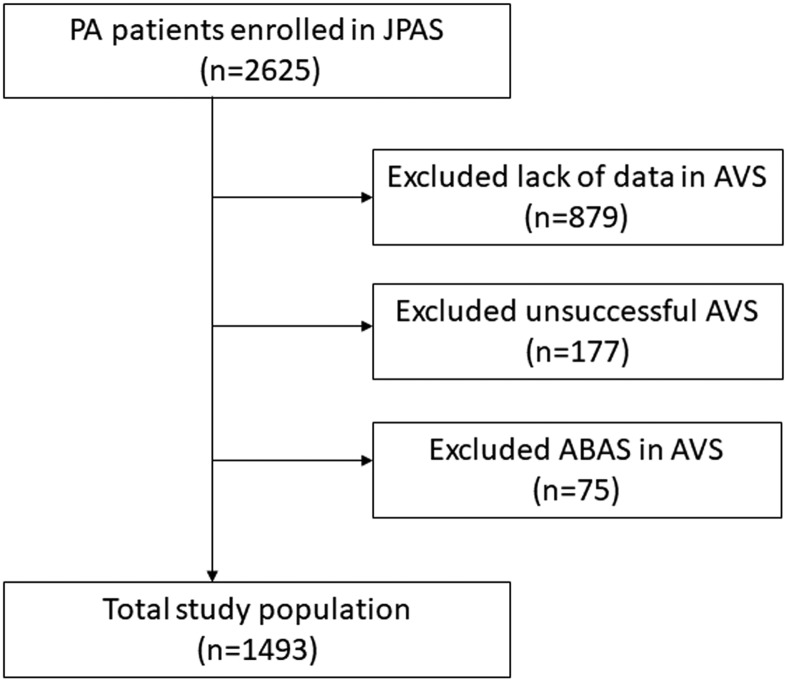
The flowchart of the study is shown in Fig. 1. A total of1493 patients who met the inclusion criteria were examined. Table 1 lists the baseline characteristics of the participants. The mean age was 53 years and 53.8% of the patients were female. The mean defined daily dose of antihypertensive drugs was 1.5. The median PAC-to-PRA ratio was 2.5 times higher than the cutoff value used for case detection in Japan. The prevalence of hypokalemia was relatively low at 35.2%.
Figure 1.
Flowchart of the study. ABAS, apparent bilateral aldosterone suppression.
Table 1.
Baseline Characteristics of the 1493 Study Participants
| Baseline Value | |
|---|---|
| Age, mean ± SD, y | 53 ± 11 |
| Sex, no. (%), male; female | 690 (46.2); 803 (53.8) |
| Duration of hypertension, mean ± SD, y | 8.5 ± 9.0 |
| Defined daily dose of antihypertensive drugs, mean ± SD | 1.5 ± 0.9 |
| Systolic blood pressure, mean ± SD, mm Hg | 142 ± 18 |
| Diastolic blood pressure, mean ± SD, mm Hg | 86 ± 13 |
| Body mass index, mean ± SD, kg/m2 | 24.8 ± 4.2 |
| Serum potassium, mean ± SD, mmol/L | 3.7 ± 0.5 |
| Hypokalemia, no. (%) | 525 (35.2) |
| Estimated glomerular filtration rate, mean ± SD, mL/min/1.73m2 | 79.5 ± 19.1 |
| PRA, median (IQR), ng/mL/h | 0.3 (0.2, 0.5) |
| PAC, median (IQR), pg/mL | 172 (123. 263) |
| Aldosterone renin ratio, median (IQR) | 532 (320, 1080) |
Abbreviation: IQR, interquartile range.
Table 2 lists the lateralizing diagnoses according to CT imaging and AVS. By CT imaging, adrenal nodular lesions were confirmed on the left side in 374 patients (25.1%), on the right side in 230 patients (15.4%), and bilaterally in 39 patients (2.6%). Nodular lesions were not found in bilateral adrenal glands in 850 patients (56.9%). By AVS, 258 patients (17.3%) had unilateral results on the left side, 202 patients (13.5%) had unilateral results on the right side, and 1032 patients (69.1%) had bilateral results. Similar results were observed for CT and AVS, with unilateral results more frequent on the left side than on the right side (P < 0.01 for both modalities).
Table 2.
Comparison of the Distribution of the Lateralizing Diagnoses Between CT and AVS
| Left Unilateral, no. (%) | Right Unilateral, no. (%) | P Valuea | Bilateral, no. (%) | |
|---|---|---|---|---|
| CT | 374 (25.1) | 230 (15.4) | <0.01 | 889 (56.0) |
| 39 (2.6)b | ||||
| 850 (53.4)c | ||||
| AVS | 258 (17.3) | 202 (13.5) | <0.01 | 1032 (69.1) |
P value was calculated for comparison of the frequency of left and right unilateral results on CT imaging and by AVS.
Bilateral nodular lesions.
Bilaterally normal image.
The left-to-right ratio was 1.63:1 for CT and 1.28:1 for AVS. The mean diameter of nodular lesions was not different between the left and right sides in patients with unilateral nodular lesions seen on CT (15.9 ± 6.4 mm vs 16.8 ± 7.3 mm; P = 0.10).
Table 3 shows the concordance rate of laterality diagnoses between CT and AVS. There was no significant difference in the concordance rate between laterality diagnoses of CT and AVS in patients with unilateral nodular lesions on the left and right sides (44.1% vs 50.9%; P = 0.15). In patients with nodular lesions <20 mm, the concordance rate was greater on the right side than the left side (45.8% vs 56.4%, respectively; P = 0.03). Including patients with bilateral nodular lesions and bilaterally normal images on CT scans, the discordance rate for all patients was 30.7%. In the 1032 patients with a bilateral result on AVS, left unilateral nodular lesions were detected by CT imaging more frequently than those located on the right side [n = 184 (17.8%) vs n = 97 (9.4%); P < 0.01]. In the 850 patients with a bilaterally normal image on CT, a left unilateral result by AVS tended to be more relevant but was not statistically significant [n = 65 (7.6%) vs n = 51 (6.0%); P = 0.18]. There were 41 patients (2.7% of all patients) with unilateral nodular lesions on CT imaging and a unilateral result according to AVS on the opposite side. Twenty-five patients (1.7% of all patients) had left unilateral nodular lesions on CT imaging and right unilateral disease by AVS, while 16 patients (1.1%) had right unilateral nodular lesions on CT and left unilateral disease according to AVS. Table 4 shows the comparison for the left-right distribution of nodular lesions on CT imaging in patients with unilateral nodular lesions grouped according to size categories. Significant differences in the frequency of nodular lesions on CT imaging were observed in smaller size categories (i.e., 10- to 15-mm and 15- to 20-mm groups; P < 0.01 for both).
Table 3.
Concordance Rate for the Lateralizing Diagnoses Between CT and AVS
| Left Unilateral Result According to AVS, no. (%) | Right Unilateral Result According to AVS, no. (%) | Bilateral Result According to AVS, no. (%) | Concordance Rate, % | |
|---|---|---|---|---|
| Left unilateral lesions on CT | 165 (44.1) a | 25 (6.7) | 184 (49.2) | 44.1 |
| Right unilateral lesions on CT | 16 (7.0) | 117 (50.9) | 97 (41.8) | 50.9 |
| Bilateral lesions on CT | 12 (30.7) | 9 (23.1) | 18 (46.2) | 46.2 |
| Bilateral normal on CT | 65 (7.6) | 51 (6.0) | 734 (86.4) | 86.4 |
The number of patients with concordant results between CT and AVS is indicated by bold type.
Table 4.
Distribution of Unilateral Nodular Lesions Grouped According to Size Categories on CT
| Nodular Lesion, mm | Distribution of Left Side Lesions, no. (%) | Distribution of Right-Side Lesions, no. (%) | P Value |
|---|---|---|---|
| ≥10 to <15 | 204 (66.2) | 104 (33.8) | <0.01 |
| ≥15 to <20 | 93 (60.4) | 61 (39.6) | <0.01 |
| ≥20 to <25 | 46 (51.7) | 43 (48.3) | 0.65 |
| ≥25 to <30 | 17 (60.7) | 11 (39.3) | 0.11 |
| ≥30 | 14 (56.0) | 11 (44.0) | 0.27 |
Adrenalectomy was performed in 418 patients (28.0%), with surgery on the left side in 252 patients (60.3%), on the right side in 159 patients (38.0%), and unknown in seven patients (1.7%). In accordance with categories based on CT findings, 188 (50.3%) of the 374 patients with left unilateral lesions, 117 (50.9%) of 230 patients with right unilateral lesions, 18 (46.2%) of 39 patients with bilateral lesions, and 95 (11.2%) of 850 patients with a bilaterally normal image received an adrenalectomy. Eleven of 118 patients with left unilateral lesions on CT imaging and nine of 117 patients with right unilateral lesions on CT imaging were resected on the opposite side.
Of the 406 patients with pathological findings, 249 had an operation on the left side, with findings of 228 adenomas, eight cases of hyperplasia, eight cases of equivocal findings, two cysts, and one myelolipoma. A total of 157 patients had an operation on the right side, with findings of 145 adenomas, four cases of hyperplasia, five cases of equivocal findings, one ganglioneuroma, one hematoma, and one metastasis of a papillary thyroid carcinoma. There were no differences in proportion of adenomas between the left and right side (92.0% vs 92.4%; P = 0.89).
We compared the clinical and biochemical outcomes after adrenalectomy between the patients with concordant results on CT and AVS and those with discordant results. In the concordant group, by CT imaging, complete, partial, and absent clinical successes were observed in 53 (34.0%), 60 (38.5%), and 41 (26.3%) patients, respectively. Complete, partial, and absent biochemical successes were observed in 92 (77.3%), 18 (15.1%), and 9 (7.6%) patients, respectively. In the discordant group, by CT imaging, complete, partial, and absent clinical successes were observed in 18 (19.8%), 43 (47.3%), and 30 (33.0%) patients, respectively. Complete, partial, and absent biochemical successes were observed in 51 (75.0%), 6 (8.8%), and 11 (16.2%) patients, respectively. The rate of overall clinical complete success was significantly higher in patients with concordant results of both CT imaging and AVS than in patients with discordant results (P = 0.01). In contrast, the rates of complete biochemical success were not different between patients with concordant results of both CT and AVS and those with discordant results (P = 0.72).
3. Discussion
In this study, we demonstrated that unilateral nodular lesions seen on CT imaging and unilateral disease according to AVS in patients with PA were observed more frequently on the left side than on the right side. We also showed a distinct divergence of lateralizing diagnoses between CT and AVS. When a patient has unilateral nodular lesions on CT imaging and a bilateral result according to AVS, it is thought that there are coexisting idiopathic hyperaldosteronism and nonfunctioning adrenal tumors. In addition, when a patient has unilateral disease according to AVS results and bilaterally normal image on CT imaging, it is thought that micro-APA may exist. Discordant results in laterality diagnoses between CT and AVS could be caused by nonfunctioning adrenal tumors and micro-APAs. When patients with unilateral nodular lesions on CT imaging and unilateral disease according to AVS on the opposite side were combined, 322 patients (21.6% of all patients) had a nonfunctioning adrenal tumor, 157 patients (10.5%) had micro-APA, and 41 patients (2.7%) had both a unilateral nonfunctioning tumor and micro-APA on the opposite side. When patients with a plasma cortisol concentration <1.8 μg/dL after a 1-mg dexamethasone suppression test were studied for the left-right distribution by both CT imaging and AVS, the concordance rate between CT and AVS was not different from the results in the total study cohort (data not shown). Therefore, it appears cosecretion of cortisol from adrenal tumors does not contribute to discordant laterality between CT and AVS.
A few studies have investigated the relationship between imaging and AVS used to diagnose patients with PA. Dekkers et al. [6] examined 90 patients with PA with results from both CT and AVS and showed unilateral nodular lesions on CT scans were significantly more frequent on the left side [42 (46.7%) vs 7 (7.8%); P < 0.01]. In contrast, unilateral results of AVS were slightly more frequent on the left side, although this difference was not statistically significant [26 (28.9%) vs 22 (24.4%); P = 0.50). Sam et al. [7] reported that in 342 patients with PA, unilateral lesions seen on CT or MRI were significantly more frequent on the left side [125 (36.5%) vs 66 (19.3%); P < 0.01), whereas unilateral results according to AVS showed a nonsignificant tendency for lesions to be on the right side more frequently when the lateralization index criterion was >4 [71 (20.8%) vs 87 (25.4%); P = 0.15). These three studies, including the current study, showed CT imaging enabled detection of significantly more lesions on the left side. The three studies, however, showed differences in the laterality of AVS. The current study and the Dekkers et al. study [6] used AVS results obtained after ACTH stimulation, whereas the Sam et al. study [7] measured AVS at baseline without ACTH stimulation. These differences in the AVS protocol may have influenced the distribution of the laterality diagnoses.
The results of the current study indicated that APAs, micro-APAs, and nonfunctioning adrenal tumors are more likely to occur on the left side, although reason for this skewed distribution remains unclear. We speculate that there may be two explanations for these findings. First, APAs and nonfunctioning tumors may occur in proportion to the volume of the left and right adrenal glands. Schneller et al. [22] reported that the volume of the adrenal glands measured using multidetector CT in 105 healthy volunteers was significantly greater on the left side than on the right side (4.84 ± 1.67 mL vs 3.62 ± 1.23 mL; P < 0.001). Carsin-Vu et al. [23] reported similar results in study of 154 healthy volunteers (4.5 ± 1.6 mL vs 3.8 ± 1.3 mL; P < 0.001). In contrast, Nougaret et al. [24] and Wang et al. [25] reported there were no significant differences in the volume of the left and right adrenal glands (3.8 ± 1.2 mL vs 3.4 ± 1.0 mL and 4.23 ± 0.74 mL vs 4.26 ± 0.86 mL, respectively). However, these two studies had relatively small sample sizes (40 and 80 subjects, respectively). Considering the results of the current study, APAs (>10 mm in diameter), micro-APAs, and nonfunctioning tumors were commonly more frequent on the left side because the volume of the left adrenal gland of a normal subject was greater than that of the right-sided gland.
Second, it may be easier to detect small tumors in the left adrenal gland using abdominal cross-sectional imaging, because of anatomical differences between the left and right adrenal glands and the different constitution of the surrounding organs. In addition, it appears that the compression direction is different between the left and right adrenal glands when imaging is performed in the supine position. Hao et al. [11] reported that in 1376 patients with incidentally discovered adrenal adenomas, excluding functioning tumors, 75% of unilateral adrenal adenomas were found on the left side. In addition, they showed that a higher proportion of small adrenal adenomas were found on the left side. The current study showed the same tendency, with left-sided nodular lesions being significantly more frequent in groups with small nodular lesions (<20 mm in diameter). This finding supports the hypothesis regarding anatomical differences between adrenal glands. Combining these two mechanisms, asymmetric left-side to right-side ratios of 1.63: 1 for CT imaging and 1.28:1 for AVS may have been achieved in the current study.
In the daily practice of managing PA, consideration should be given to the fact that APAs and nonfunctional tumors are more likely occur on the left side. In addition, it is important to consider that the concordance rate between CT imaging and AVS is low when unilateral nodular lesions are observed on CT scans. In particular, in patients with small unilateral tumors <20 mm in diameter on CT scans, it is also important to note that the concordance rate is lower on the left side than on the right side.
This study had several strengths. First, it was a multicenter collaborative study with a large sample size. Imaging diagnosis of all patients was obtained using CT imaging. Although AVS had a different detailed protocol, blood collection was performed after ACTH stimulation and a uniformed position used for blood sampling. Because the definition of a nodular lesion on CT imaging was set at ≥10 mm, bias between radiologists was less likely to have occurred.
There were some limitations to the study. First, we did not directly review CT images. Second, the unilateral results were underestimated because a stringent criterion was adopted for nodular lesions observed on CT scans and unilateral results according to AVS. However, because the determinations were made using the same criteria, judgment of the left-right distribution was thought to be appropriate. Third, the indication for surgery was different at each center, and because follow-up after surgery was not completed in all patients, we were unable to verify the validity of the criteria for subtype diagnosis.
In conclusion, our results suggest that APAs and nonfunctioning adrenal tumors develop more frequently on the left side in patients with PA and that misdiagnosis of CT-based lateralization may occur more frequently on the left side. These findings should be considered when subtyping of PA is performed.
Acknowledgments
We thank the JPAS/JRAS study group, Hiroshi Ito, Division of Endocrinology, Metabolism and Nephrology, Keio University; Yoshiyu Takeda, Division of Endocrinology and Hypertension, Department of Internal Medicine, Kanazawa University Graduate School of Medicine; Masanobu Yamada, Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine; Nobuya Inagaki, Department of Diabetes, Endocrinology and Nutrition, Kyoto University Graduate School of Medicine; Hiromi Rakugi and Koichi Yamamoto, Department of Geriatric and General Medicine, Osaka University Graduate School of Medicine; Masayoshi Soma, Division of General Medicine, Department of Internal Medicine, Nihon University School of Medicine; Toshihiko Yanase, Department of Endocrinology and Diabetes Mellitus, Faculty of Medicine, Fukuoka University; Hisashi Fukuda, Division of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine Yokohama City Seibu Hospital; Shigeatsu Hashimoto, Division of Nephrology, Hypertension, Endocrinology, and Diabetology/Metabolism, Fukushima Medical University Hospital; Yuichi Ohno, Department of Diabetes, Endocrinology and Nutrition, Kyoto University Graduate School of Medicine; Katsutoshi Takahashi, Department of Metabolism, Showa General Hospital; Hirotaka Shibata, Department of Endocrinology, Metabolism, Rheumatology and Nephrology, Faculty of Medicine, Oita University; Hironobu Umakoshi, Metabolism and Hypertension, Clinical Research Institute, National Hospital Organization Kyoto Medical Center; Yuichi Fujii, Department of Cardiology, JR Hiroshima Hospital; Tomoko Suzuki, Department of Public Health, School of Medicine, International University of Health and Welfare; Atsushi Ogo and Ryuichi Sakamoto, Department of Endocrinology and Metabolism, Clinical Research Institute, National Hospital Organization, Kyushu Medical Center Kyushu Medical Center; Tatsuya Kai, Department of Internal Medicine, Sakura Hospital, Tomikazu Fukuoka, Internal Medicine, Matsuyama Red-Cross Hospital; and Shozo Miyauchi, Uwajima City Hospital.
Financial Support: The study was supported, in part, by grants-in-aid for the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (Grants JP17ek0109122 and JP18ek0109352 to M.N.) and from the National Center for Global Health and Medicine (Grants 27-1402 and 30-1008 to M.N.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- APA
aldosterone-producing adenoma
- ARC
active renin concentration
- AVS
adrenal vein sampling
- IVC
inferior vena cava
- JPAS
Japan Primary Aldosterone Study
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
References and Notes
- 1. Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, Hermus AR, Lenders JW, Deinum J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826–2835. [DOI] [PubMed] [Google Scholar]
- 2. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. [DOI] [PubMed] [Google Scholar]
- 3. Mulatero P, Bertello C, Rossato D, Mengozzi G, Milan A, Garrone C, Giraudo G, Passarino G, Garabello D, Verhovez A, Rabbia F, Veglio F. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93(4):1366–1371. [DOI] [PubMed] [Google Scholar]
- 4. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329–337. [DOI] [PubMed] [Google Scholar]
- 5. Ladurner R, Sommerey S, Buechner S, Dietz A, Degenhart C, Hallfeldt K, Gallwas J. Accuracy of adrenal imaging and adrenal venous sampling in diagnosing unilateral primary aldosteronism. Eur J Clin Invest. 2017;47(5):372–377. [DOI] [PubMed] [Google Scholar]
- 6. Dekkers T, Prejbisz A, Kool LJS, Groenewoud HJMM, Velema M, Spiering W, Kołodziejczyk-Kruk S, Arntz M, Kądziela J, Langenhuijsen JF, Kerstens MN, van den Meiracker AH, van den Born BJ, Sweep FCGJ, Hermus ARMM, Januszewicz A, Ligthart-Naber AF, Makai P, van der Wilt GJ, Lenders JWM, Deinum J; SPARTACUS Investigators. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739–746. [DOI] [PubMed] [Google Scholar]
- 7. Sam D, Kline GA, So B, Leung AA. Discordance between imaging and adrenal vein sampling in primary aldosteronism irrespective of interpretation criteria. J Clin Endocrinol Metab. 2019;104(6):1900–1906. [DOI] [PubMed] [Google Scholar]
- 8. Kim J, Bae KH, Choi YK, Jeong JY, Park KG, Kim JG, Lee IK. Clinical characteristics for 348 patients with adrenal incidentaloma. Endocrinol Metab (Seoul). 2013;28(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sangwaiya MJ, Boland GW, Cronin CG, Blake MA, Halpern EF, Hahn PF. Incidental adrenal lesions: accuracy of characterization with contrast-enhanced washout multidetector CT--10-minute delayed imaging protocol revisited in a large patient cohort. Radiology. 2010;256(2):504–510. [DOI] [PubMed] [Google Scholar]
- 11. Hao M, Lopez D, Luque-Fernandez MA, Cote K, Newfield J, Connors M, Vaidya A. The lateralizing asymmetry of adrenal adenomas. J Endocr Soc. 2018;2(4):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J. 2011;58(9):711–721. [DOI] [PubMed] [Google Scholar]
- 13. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253–390. [DOI] [PubMed] [Google Scholar]
- 14. Shibayama Y, Wada N, Naruse M, Kurihara I, Ito H, Yoneda T, Takeda Y, Umakoshi H, Tsuiki M, Ichijo T, Fukuda H, Katabami T, Yoshimoto T, Ogawa Y, Kawashima J, Ohno Y, Sone M, Fujita M, Takahashi K, Shibata H, Kamemura K, Fujii Y, Yamamoto K, Suzuki T. The occurrence of apparent bilateral aldosterone suppression in adrenal vein sampling for primary aldosteronism. J Endocr Soc. 2018;2(5):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. [DOI] [PubMed] [Google Scholar]
- 16. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. RRID. AB_2801309, https://scicrunch.org/resolver/AB_2801309.
- 18. RRID. AB_2801311, https://scicrunch.org/resolver/AB_2801311.
- 19. RRID. AB_2801275, https://scicrunch.org/resolver/AB_2801275.
- 20. RRID. AB_2801312, https://scicrunch.org/resolver/AB_2801312.
- 21. RRID. AB_2801310, https://scicrunch.org/resolver/AB_2801310.
- 22. Schneller J, Reiser M, Beuschlein F, Osswald A, Pallauf A, Riester A, Tietze JK, Reincke M, Degenhart C. Linear and volumetric evaluation of the adrenal gland--MDCT-based measurements of the adrenals. Acad Radiol. 2014;21(11):1465–1474. [DOI] [PubMed] [Google Scholar]
- 23. Carsin-Vu A, Oubaya N, Mulé S, Janvier A, Delemer B, Soyer P, Hoeffel C. MDCT linear and volumetric analysis of adrenal glands: normative data and multiparametric assessment. Eur Radiol. 2016;26(8):2494–2501. [DOI] [PubMed] [Google Scholar]
- 24. Nougaret S, Jung B, Aufort S, Chanques G, Jaber S, Gallix B. Adrenal gland volume measurement in septic shock and control patients: a pilot study. Eur Radiol. 2010;20(10):2348–2357. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Jin ZY, Xue HD, Liu W, Sun H, Chen Y, Xu K. Evaluation of normal adrenal gland volume by 64-slice CT. Chin Med Sci J. 2013;27(4):220–224. [DOI] [PubMed] [Google Scholar]