Capsule Summary:
In this study, we validated a novel nanoparticle based in vitro diagnostic technique for drug allergies to two common platinum-based chemotherapeutics, oxaliplatin and carboplatin.
Keywords: oxaliplatin, carboplatin, drug allergy, diagnostic, nanoparticle, nanoallergen
To the Editor: Platinum based antineoplastic agents (platins) are the most frequently used chemotherapeutics in a variety of cancers such as ovarian, colorectal, endometrial, pancreatic cancer and others, and their use is associated with an increase in DHRs (drug hypersensitivity reactions).(1) In particular, two commonly used platins, oxaliplatin (oxpt) and carboplatin (cpt), have high rates of DHR: cpt DHR rates range from 9–27%, while oxpt DHR rates are as high as 25% and typically manifest between the 5th and 7th infusion. (2) Furthermore, the majority of DHRs to these drugs are IgE (immunoglobulin E) mediated, which typically results in severe and anaphylactic reactions.(3) An approach to prevent these IgE mediated DHRs is RDD (rapid drug desensitization). RDD induces temporary tolerization by delivering incremental suboptimal doses in a short period of time, allowing patients to receive the full therapeutic dose without or with minimal reactions.(4)
RDD has been proven to be highly effective; however, an additional challenge remains in identifying patients at risk. To date, no reliable biomarker or predictive diagnostic tests have been developed to identify patients at risk for IgE mediated DHR.(4) One significant complication of platin treatment is the myeloid suppressive effects of the drugs, which reduces both basophil counts and plasma cells. This is a contributing factor for low sensitivity (<50%) of platin specific IgE (sIgE).(3) Although skin testing has a high predictive value after patients have presented a reaction, its use requires delays in treatment of up to two weeks in order for non-specific swelling to reduce and can potentially induce severe reactions in highly sensitized patients.(3)
Here, we describe the development and application of a nanoparticle-based diagnostic method for identifying oncologic patients with positive skin testing for oxpt and cpt (the gold standard for demonstrating specific IgE antibodies) in a more rapid and reliable fashion.. This method uses nanoallergens(5), designer liposomes displaying metabolites of platin drugs, to induce in vitro degranulation of mast-cell-like cells (RBL-SX38) primed with serum from oncologic patients. Liposomes, spherical bi-layered vesicles of amphiphilic phospholipids, are well-suited as allergen display platforms due to their lack of innate immunogenicity and ability to display small molecules and peptides in a highly multivalent fashion. By introducing critical design features, such as linkers with specific length and polarity, we engineered nanoallergens that effectively display drug metabolites. (5)Effective multivalent binding of the haptens facilitate large sIgE-FcεRI clusters, which in turn trigger high levels of degranulation and function as a predictive marker for IgE dependent drug allergy.(5) In this study, we demonstrate that nanoallergens displaying platin-drug metabolites of oxpt and cpt trigger significant degranulation responses from mast-cell-like cells (RBL-SX38) primed with serum IgE from platin allergic patient.
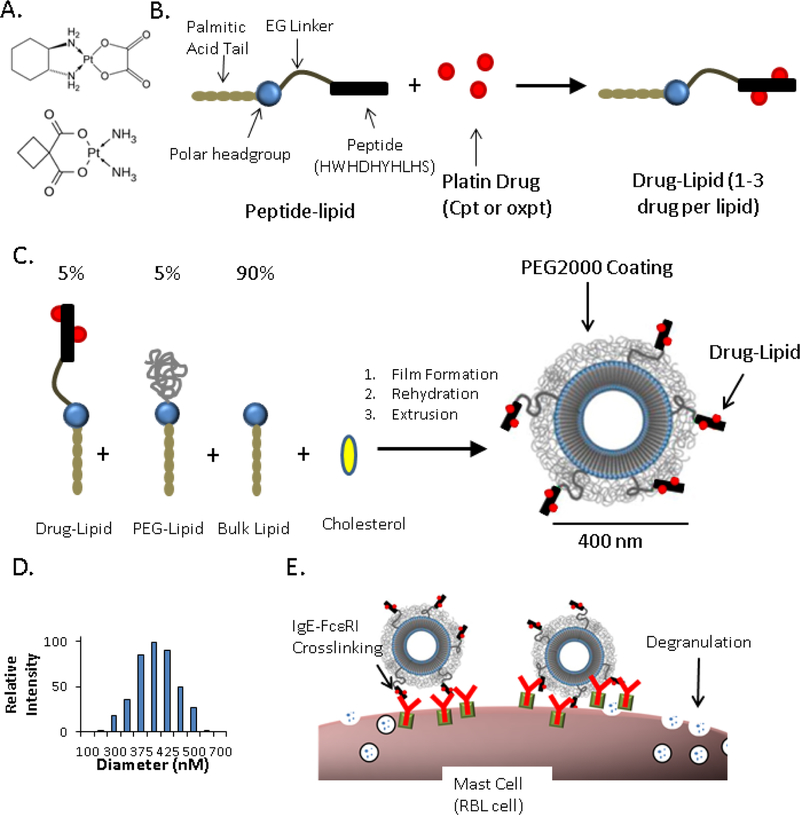
Nanoallergens are assembled using four highly pure molecular components mixed at precise stoichiometric ratios: (1) a drug-lipid conjugate, (2) a polyethylene glycol coated lipid (PEG2000-lipid), (3) a bulk lipid (DSPC) and (4) cholesterol (to improve particle stability).(6) While the latter three components are commercially available, the most critical component that carries the drug metabolites, the drug-lipid conjugate, was synthesized in our laboratory. This is a two-step synthesis, first a short peptide-lipid conjugate is synthesized using standard solid phase peptide synthesis protocols, where the peptide (HWHDHYHLHS) is rich in platinum-reactive nucleophilic residues (histidines) (Figure 1A, Figure E–1).(7) This molecule was purified by RP-HPLC and then reacted with either oxpt or cpt to form the corresponding drug-lipid conjugate, oxpt-lipid or cpt-lipid (Figure 1B). The reaction resulted in a heterogeneous mixture of drug-lipid molecules, varying in the number of drug conjugations per molecule as well as the platin substructure conjugated to histidines (Figure E–2). The drug-lipid mixture was purified using RP-HPLC to remove unreacted peptide-lipids or drugs. The average platin conjugation ratio for the purified samples was determined by measuring the platinum concentration using inductively coupled plasma (ICP) and relating it to total peptide-lipid concentration of as measured by tryptophan absorbance (Figure E–3).
Figure 1.
Nanoallergen Design and Characterization. (A) Chemical structure of the two platin drugs used in this study, oxaliplatin (top) and carboplatin (bottom). (B) Drug-lipid synthesis. A peptide (HWHDHYHLHS) was conjugated to a lipid tail via an ethylene glycol spacer and then reacted with free platin drug (oxaliplatin or carboplatin) to form drug-lipids. (C) Nanoallergen synthesis. Drug-lipids, PEG- lipid and a bulk lipid (DSPC) were mixed at 5:5:90 ratio and cholesterol was added for improved stability (50% of total lipid), rehydrated and extruded through filters with 400 nm pore sizes. (D) Dynamic light scattering analysis of oxpt loaded liposomes confirmed a diameter of ≈400 nm. (E) Cartoon demonstration describing how nanoallergens crosslink drug specific IgE-FcεRI complexes on mast cell surfaces and trigger degranulation.
Next, nanoallergens displaying the drugs were synthesized and characterized to ensure particle size and stability. Nanoallergens were synthesized via film extrusion to generate 400 nm liposomes with 5% molar ratio of oxpt-lipid (nanooxpt) or cpt-lipid (nanocpt) (Figure 1C,D). We determined the optimal formulation (400 nm and 5% platin-lipid) by testing several design features with RBL cell assays and allergic patient serum (Figure E–4). A major concern when synthesizing drug-loaded nanoallergens is stability of the nitrogen-platinum coordinate bond, potentially releasing cytotoxic platin metabolites and diminishing the platin presentation on the nanoparticle. To investigate this concern, we compared the concentration of drug-lipid in the lipid film before liposome extrusion and in the nanoallergen after extrusion via RP-HPLC and ICP analysis. We observed >90% retention of drug-lipid and appropriate particle size after nanoallergen formulation and storage for up to one week (Figure E–5, Table E–1). These results demonstrated that nanoallergens were stable and retained their platinum drug metabolite conjugates.
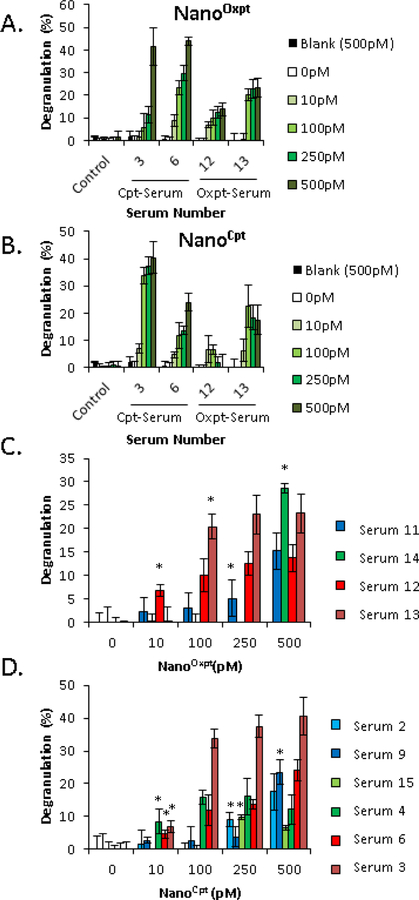
Serum from patients with ovarian and colon cancer presenting grade 2 and 3 reactions to carboplatin and oxaliplatin was obtain Brigham and Women’s Hospital (see supplement for further details) and used in the assays. Patient characteristics are described in Table E–2. Using nanoallergens, we triggered degranulation responses from mast-cell-like cells (RBL-SX38) that were primed with serum from platin drug allergic patients (Figure 1E). Human constant fragment epsilon (FcεRI) expressing mast-cell-like cells (RBL-SX38)(8) were incubated overnight with 4 different sera from patients presenting grade III reactions to either oxpt or cpt (2 for oxpt and 2 for cpt, Table E–2). All serum tested was drawn after RDD and we determined that RDD treatment did not have a significant effect on degranulation responses (Figure E–6). Cells were then washed and challenged with either nanooxp or nanocpt or a blank liposome at varying concentrations and degranulation measured using a beta-hexosaminidase assay (Figure 2A, B). No response was seen from RBL-SX38 cells challenged with unaltered drug molecules, blank liposomes, or from cells primed with serum from a healthy donor (Figure 2A, B, Figure E–7). Samples from allergic patients, generated robust responses at concentrations as low as 10 pM and demonstrated crossreactivity, indicating that oxpt-sensitive serum samples generated degranulation responses to nanoCpt and vice versa. This is not unexpected given previous reports of cross-reactive IgE between these two drugs.(3)
Figure 2.
Platin loaded nanoallergens trigger platinum specific degranulation in vitro. Human FcεRI expressing RBL-SX38 cells were primed with serum from patients (15% serum/85% media) overnight, washed and degranulation was triggered by incubating in (A) NanoOxpt or (B) NanoCpt at varying concentrations. The degranulation was measured using the beta-hexosaminidase assay. Blank indicates a drug-free 400 nm liposome at 500 pM concentration. Control serum data is an average of three different healthy volunteer controls each done in triplicate. (C) Serum from oxpt sensitive patients were similarly tested with nanooxpt at varying concentrations. Graphs are color coded in terms of reaction grade: grade I in blue, grade II in green and grade III in maroon or red. Grade III patient samples triggered degranulation responses at lower nanooxpt concentrations (D) Cpt sensitive patient samples were tested against nanocpt similarly as in part C. Serum from patients with grade I reactions are in light blue or dark blue, grade II reactions in light or dark green and serum from grade III reactions in red or maroon. Error bars indicate ± STD of triplicate experiments. *p<0.05, compared to control serum signal.
We similarly tested 6 additional serum samples from patients with grade I or grade II reactions to oxpt or cpt and compared their results to the grade III reactions (Table E?–2, Figure E–8 for all data). Oxpt-sensitive serum with grade III reactions (serum 12 and 13) trigger significantly higher degranulation than control patient serum at 10 pM and 100 pM, while serum from oxpt-sensitive patients with grade I (serum 11) or grade II (serum 12) reactions did not trigger significant degranulation until nanoallergen concentration was increased to 500 pM (Figure 2C, p<0.05). A similar result was observed from cpt- sensitive serum, where grade III reactions (serum 3 and 6) triggered significant responses at 10 pM, while serum from grade I (serum 2 and 9) and a grade II (serum 15) did not trigger reactions until 250 or 500 pM (Figure 2D). Surprisingly, serum 4 was the only grade II reaction to trigger a significant response at 10 pM nanocpt (Figure 2D). Although we expected nanooxpt to be a more potent simulator of degranulation given that there are more platin drug molecules on each nanoallergen (Figure E–3C), this data suggests that nanocpt triggers significant responses at lower concentrations (Figure 2D). Importantly, this outcome is in agreement with clinical data where cpt typically requires fewer exposures and lower concentrations than oxpt to trigger a response.(3) A potential explanation for the increased cpt sensitivity is that there is an increased sIgE antibody diversity for the smaller haptenized cpt substructure, leading to cross-reactivity of cpt sensitive patients to oxpt but not vice versa (Table E–1).(3,9) Additionally, there is a direct correlation between the nanoallergen concentration that triggers significant degranulation responses in vitro to severity of the in vivo reaction. The lower the nanoallergen concentration needed for in vitro degranulation, the higher the severity of the clinical reaction was, however, studies with larger patient populations with diverse clinical symptoms will be needed for further verification. Nevertheless, the data reported here establishes the nanoallergen system’s potential as an assay that can diagnose platinum allergies without the risks and delays inherent to skin testing and suggests that it can be implemented as a prognostic assay to monitor patients undergoing platinum therapies.
Supplementary Material
Acknowledgments
We would like to thank the University of Notre Dame Proteomics Facility and Notre Dame Center for Environmental Science and Technology for use of their mass spectrometry and ICP equipment.
We disclose funding from a private donor (Mr. Douglas Zych, Mr. and Mrs. Jim and Annette Lecinski) and the NIH (R01AI108884, Bilgicer) and Ovations for the Cure Fund (Castells).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- (1).Caiado J, Castells M. Presentation and Diagnosis of Hypersensitivity to Platinum Drugs. Current Allergy and Asthma Reports 2015. April;15(4):15. [DOI] [PubMed] [Google Scholar]
- (2).Parel M, Ranchon F, Nosbaum A, You B, Vantard N, Schwiertz V, et al. Hypersensitivity to oxaliplatin: clinical features and risk factors. Bmc Pharmacology & Toxicology 2014. January 13;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Caiado J, Venemalm L, Pereira-Santos MC, Costa L, Barbosa MP, Castells M. Carboplatin-, Oxaliplatin-, and Cisplatin-specific IgE: Cross-reactivity and Value in the Diagnosis of Carboplatin and Oxaliplatin Allergy. Journal of Allergy and Clinical Immunology-in Practice 2013. Sep-Oct;1(5):494–500. [DOI] [PubMed] [Google Scholar]
- (4).Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: Outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol 2008. September;122(3):574–580. [DOI] [PubMed] [Google Scholar]
- (5).Deak PE, Vrabel MR, Pizzuti VJ, Kiziltepe T, Bilgicer B. Nanoallergens: A multivalent platform for studying and evaluating potency of allergen epitopes in cellular degranulation. Experimental Biology and Medicine 2016. April 13. [DOI] [PMC free article] [PubMed]
- (6).Audera C, Ramírez J, Soler E, Carreira J. Liposomes as carriers for allergy immunotherapy. Clinical & Experimental Allergy 1991. 01;21(1):139–144. [DOI] [PubMed] [Google Scholar]
- (7).Sandlin RD, Whelan CJ, Bradley MS, Williams KM. Effects of amine ligand bulk and hydrogen bonding on the rate of reaction of platinum(II) diamine complexes with key nucleotide and amino acid residues. Inorg Chim Acta 2012. August 30;391:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ladics GS, van Bilsen JHM, Brouwer HMH, Vogel L, Vieths S, Knippels LMJ. Assessment of three human Fc epsilon RI-transfected RBL cell-lines for identifying IgE induced degranulation utilizing pea nut-allergic patient sera and peanut protein extract. Regulatory Toxicology and Pharmacology 2008. August;51(3):288–294. [DOI] [PubMed] [Google Scholar]
- (9).Wong JT, Ling M, Patil S, Banerji A, Long A. Oxaliplatin Hypersensitivity: Evaluation, Implications of Skin Testing, and Desensitization. Journal of Allergy and Clinical Immunology-in Practice 2014. Jan-Feb;2(1):40–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.