Low back and neck pain are disabling conditions,124 projected to dramatically increase personal and public socioeconomic burden as the world ages.12; 26; 50 International experts have urgently called for renewed explanations and strategies to mitigate the persistence of spinal pain.12; 50 To that end, skeletal muscles, the spinal cord, and brain are receiving more attention as advancements in magnetic resonance imaging (MRI) technologies and analysis methods allow for improved visualization and quantification of their morphology.37 Growing in parallel is radiomics, the field of study that aims to mine large amounts of quantitative features from medical images using data characterization algorithms.51; 135 As such, large datasets characterizing both healthy and diseased soft tissues with the patient’s pain experience may add to the biopsychosocial model in understanding spinal pain.
Changes in soft tissue morphology may represent 1) biological markers of pathology/trauma/pain,31; 32; 59 2) ‘normal’ senescence,24; 122 or 3) an imaging variant with little, if any, prognostic or clinically relevant value.3; 16; 49; 87; 94; 120 Such conflicting evidence has resulted in suspicion of the value of imaging, particularly clinically where over-imaging has seeded doubt in the merit of image-identified (pathoanatomical) outcomes. However, readers will recognize that such findings may be influenced by inconsistent methods across research studies, limited image-resolution with conventional devices, use of historical imaging sequences, and the limitations of a purely biomedical approach for explaining persistent symptoms. As technologies advance, so too does our potential to understand the complexity of spinal disorders. MRI advancements27; 66 are allowing for prize-winning phenotypic discoveries regarding the vertebral column (intervertebral disc,81; 92; 108 vertebral end-plates,93; 139 and vertebral bone marrow.28; 102) Studies examining soft tissues are similarly promising.1; 31; 55; 56; 67; 70
The potential to bring together (and compare) distinct types of bio-psycho-social data, including the data generated by MRI, represents a substantial departure from the status quo of isolated pathoanatomical imaging. As clinical scientists employing MRI methodologies in our research, we recognize the exciting potential of exploring diverse data sources, bringing them into a common computational environment where big-data associations can be explored, further scrutinized, and settled.
Therefore, the purpose of this clinical commentary is two-fold; to summarize key and new literature and to highlight future directions in imaging spinal skeletal muscles, the spinal cord, and brain toward better understanding spinal conditions and the individual experience. We intend to make a case for the promise that advancing MRI technologies and radiomics have in bringing soft tissues forward in a collaborative clinical and research effort to limit the economic, societal, and personal burden of conditions affecting the spine.
SPINAL SKELETAL MUSCLE IMAGING
MRI is the gold standard for examining the integrity, size, and quality of spinal muscles due to its higher resolution, greater soft-tissue contrast, superior visualization of spinal landmarks, and potential for efficient semi- or automated segmentation. Water and fat images derived from multi-echo acquisitions (like Dixon) are superior in soft tissue analysis;40; 105; 136 yet, population-based ‘traditional’ T1-weighted and T2-weighted images remain widely used, and represent a valuable resource for investigators.48; 57; 91; 112; 113 Quantification techniques24 achieved by segregating representative fat pixels within a selected muscle region of interest, are more accurate and have higher reliability than earlier qualitative1 and semi-quantitative90 methods. Further, quantification permits improved longitudinal assessment of temporal changes in participants with varying levels of pain, disability, and function (FIGURE 1). A downside to such an approach remains the time required to manually segment muscles of interest in post-hoc fashion. Depending on the muscle and region of the body, it is not unusual for an experienced user to spend approximately 45 minutes to well over an hour to manually segment each targeted muscle (based on the authors’ personal experiences). However, a new landscape of semi-automated and automated platforms is becoming available69; 70; 72; 90; 134 and we are only recently realizing the potential of machine learning algorithms towards streamlining the measurement of soft-tissue morphology. We, and others, expect such applications to dramatically and positively change our research capacity, output, and potential translation to clinical practice.
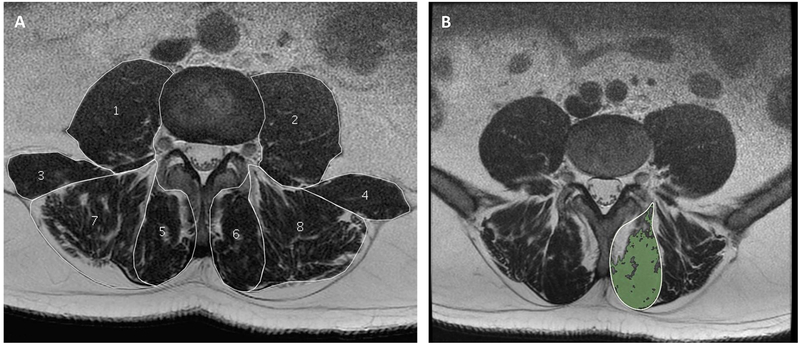
Figure 1a-b -.
Example of axial T2-weigthed lumbar paraspinal muscle morphology and composition measurements. a) illustrates the cross-sectional area measurements of the psoas (1,2), quadratus lumborum (3,4), multifidus (5,6) and erector spinae (7, 8), and also demonstrates current methodological differences in ROI definitions for the erector spinae muscle; the fat-filled “tent-region” under the lumbosacral fascia (posteriorly) was included in (7) and excluded in (8). b) illustrates the functional cross-sectional area (in green) representing the area of lean muscle mass (excluding fatty infiltration) of the multifidus muscle using a thresholding technique.
Change to paraspinal muscle composition (typically characterized by fatty infiltration) is widely examined and an accepted degenerative feature.38; 106; 112; 113 However, strong confounders like age,22–24; 122 and sex,24; 57; 109, in addition to inconsistencies in methodologies, confuse the clinical relevance to pain.53; 103 Body composition,48; 122, physical activity levels,49; 57 pain duration52; 127 and location (bilateral versus unilateral),14; 46 and co-existing pathology43; 100 may also influence spinal muscle composition. While limited evidence suggests that reduced multifidus cross-sectional area (CSA) predicts future (12 months) low back pain (LBP) in men,44; 103 their role and clinical relevance in the development of LBP and neck pain is far from explicit. Important limitations pertaining to understanding causation remain – does reduced muscle quality cause spinal pain, or vice versa? And further, can spinal muscle composition be modified with intervention(s) in health or disease, and if yes, what kind(s) of interventions, and at what age or injury-degenerative stage is intervention optimum?
Interestingly, non-uniform proportions and change to fatty infiltration between paraspinal muscles has been shown,22; 23; 48 alongside higher proportions of fatty infiltration in axial than peripheral skeletal muscles.25 These findings may have important implications for muscle function and targeted therapeutic interventions. While intuitive that greater paraspinal muscle fatty infiltration negatively impacts muscle function, previous studies showed no clear association with percent thickness change during submaximal voluntary contraction,76 muscle endurance25 or muscle structure.52 However, greater paraspinal fatty infiltration has been associated with lower physical function,45; 47; 58; 114 and lean muscle mass (excluding fatty infiltration) to muscle strength.42 Furthermore, better surgical outcome73; 117 and effective therapeutic exercise58 are achieved in those with superior muscle composition, which may reflect the superiority of muscle quality/composition rather than size in characterizing muscle tissues.33 Clarification is necessary.
Future directions for muscle imaging: measurement and analyses
Although highly reliable,21; 35 current MRI techniques and measurement methods of paraspinal muscle composition are time-consuming and not always feasible for clinical settings. Moreover, the use of proprietary image analysis software and insufficient description of measurement protocols hinders replication efforts. Currently, most manual segmentation techniques are tedious and rater-dependent, motivating the development of automated or semi-automated segmentation methods. Automated segmentation of spinal muscles lags behind that of other soft tissues like the brain71; 110 and larger (thigh) muscles.97 Automated thresholding algorithms and correction tools for lumbar paraspinal muscle analysis have been developed;47; 123 however, full automation requires advancements in computer-assisted imaging analysis involving atlas-based algorithms referencing a standardized coordinate system134 as used for the heart137 and brain.41; 133
The next generation of developments in spinal muscle phenotyping and analyses are atlases that preserve typical spine anatomy while largely reducing the individual differences in muscle shapes and composition.134 By way of example, lumbar multifidus is a complex transversospinales muscle comprising ‘typical’ orientation and length of fibers located superficial (approximately 4) and deep (approximately 2) to the intervertebral segments they span.20 While individual differences may exist in overall multifidus shape and composition, the impact on function is largely distilled to their anatomical fundament and therefore can be employed in prediction methods to understand healthy ageing and spine conditions.64; 65; 112; 113 FIGURE 2 provides examples of current use of MRI in examining and detailing the composition of muscles traversing the cervical spine.
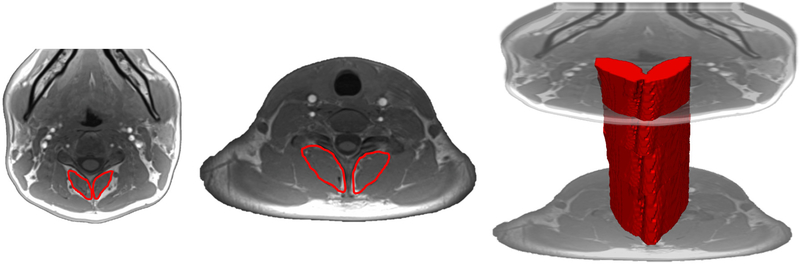
Figure 2 -.
Example of axial fat-only images examining composition of the multifidus and semispinalis cervicis traversing the cervical spine. a) illustrates the cross-sectional area measure of the multifidus and semispinalis muscles in the mid-cervical region (C3) and b) illustrates the cross-sectional area measure of the multifidus and semispinalis muscles in the lower-cervical region (C7). c) illustrates the propagated volume of the multifidus and semispinalis cervicis spanning those vertebral levels.
SPINAL CORD IMAGING
Conditions typically resulting from head/neck trauma (whiplash associated disorders (WAD)) from a motor vehicle crash (MVC), or spinal cord injury (SCI)), and degenerative cervical myelopathy (DCM) lead innovation for using MRI to examine spinal cord pathways. For example, the potential value of advanced MRI towards quantifying altered spinal cord anatomy has been found, albeit in a small number of patients, with chronic WAD36; 115 and incomplete SCI.115 Specifically, larger magnitudes of lower extremity muscle fat that seemed to correspond to altered spinal cord anatomy and reductions in the ability to maximally activate plantar flexor torques were identified in 3 patients with chronic WAD. In contrast, this was not found in a participant reporting full recovery.36 Alarmingly, the lower extremity structural changes and volitional weakness in a discrete number of individuals with chronic WAD were comparable to small number of participants with incomplete SCI.115 Larger scaled prospective studies probing the integrity of white matter pathways with spinal cord imaging applications and quantitative measurements are warranted before stronger conclusions can be drawn. Certainly, tract-specific volume losses identified with high-resolution MRI in patients with DCM have been demonstrated61 and specific regional changes of the anterior spinal cord are associated with clinical outcomes.17
Spinal cord imaging applications for WAD, DCM, and SCI
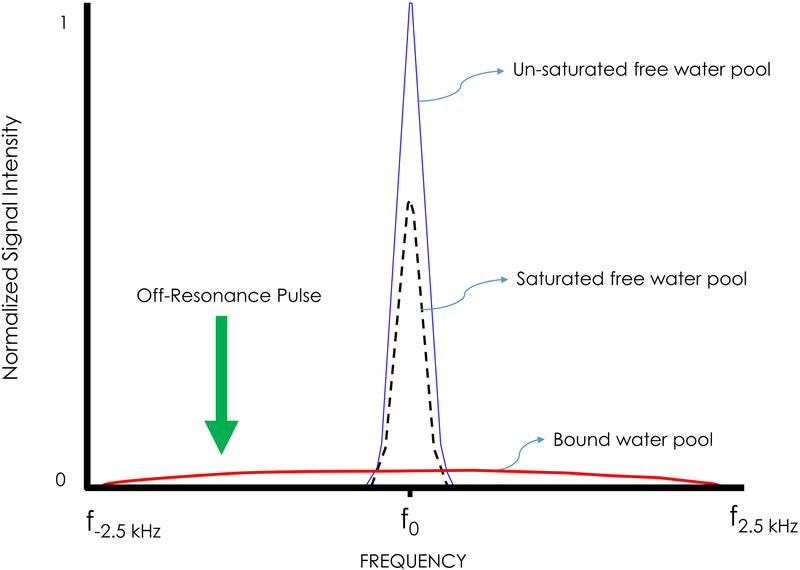
Seminal work by Wolff and Balaban furthered MRI’s sensitivity to tissue composition.131 This work, based on cross-relaxation and saturation transfer methods, originated in single voxel spectroscopy (SVS). Protons associated with free water (FIGURE 3; blue line) are distinguished from another broad-spectrum proton pool of other hydrophilic macromolecular surfaces (eg, proteins, lipids: FIGURE 3; red line). These two proton pools mix constantly, resulting in cross-relaxation29; 132 and probing of the bound and free water pools. By saturating the broad resonance with a powerful off-resonance (1–2 kHz) pulse (green arrow in FIGURE 3), the bound protons lose their magnetization and exchange with the free water pool resulting in macroscopic saturation exchange between the two (FIGURE 3; black dashed line). This magnetization transfer (MT) effect has demonstrated sensitivity to the macromolecular concentration and surface chemistry,18; 131; 132 and can be measured relative to a baseline condition (FIGURE 3, black dashed line relative to the blue line). This provides a semi-quantitative map of neuronal integrity of white matter pathways in the spinal cord. While this demonstrates potential prognostic value in traumatic neck pain,36,115 larger-scaled longitudinal work is required for translation to standard diagnostic practice.
Figure 3 –
Example of Magnetization Transfer Contrast
Ongoing research aims to standardize and automate MT measures throughout the cervical spinal cord, and to apply these methods to a large prospective dataset of individuals with WAD (ClinicalTrials.gov Identifier: NCT02157038). This approach may assist in and contribute to early prognosis of individuals at risk of poor functional recovery, and in particular, patients exposed to and presumably injured from a non-catastrophic MVC (eg, WAD), where evidence of salient tissue damage is rarely observed with conventional scans.
DCM is characterized as a narrowing of the cervical spinal canal leading to neurological impairment and spinal pain presumably due to damage of the spinal cord.96; 119 In individuals with DCM, abnormal levels of spinal cord metabolites were shown with magnetic resonance spectroscopy compared to matched controls.60; 107 One study revealed elevated lactate peaks, indicative of DCM-related spinal cord ischemia.[60] Abnormal spinal cord metabolites were associated with the modified Japanese Orthopedic Association score, highlighting a radiographical biomarker for the assessment (and informed management) of patients with advanced DCM.60; 107
Diffusion tensor imaging (DTI),68; 130 MT, and semi-automated atlas-based analyses are 3 other approaches permitting more in-depth investigation of the spinal cord and its pathways in DCM (FIGURE 4). In DCM, the cervical spinal cord tends to have reduced total density of tracts,130 yet elevated tract density at the site of cord compression.30 Spinal cord DTI holds potential as a prognostic/diagnostic imaging biomarker. Magnetization transfer ratios have also shown diagnostic promise in identifying cord abnormalities in individuals with DCM. While a trend towards decreased MT ratios was found at the cervical spinal cord cross section compared to controls, significant differences were revealed when analyzing the anterior portion of the cord.17 Decreased MT ratios were correlated with both clinical outcome scores17; 118 and a quantitative measure of upper extremity hyperreflexia.17
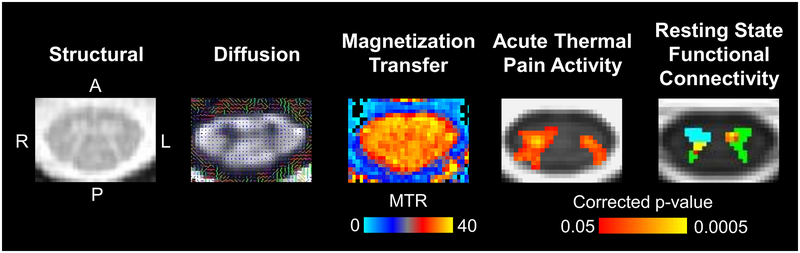
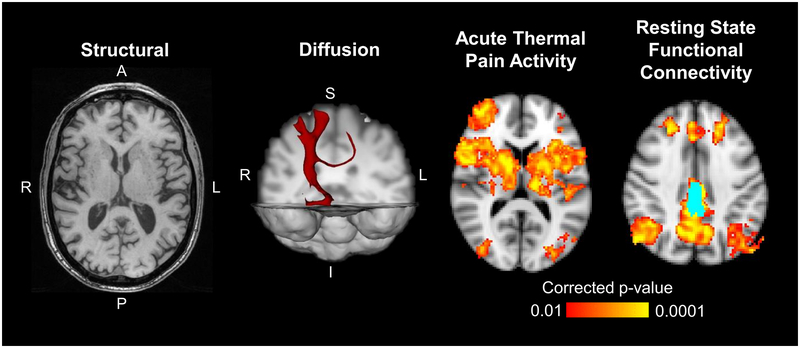
Figure 4-.
Example structural, diffusion, magnetization transfer, and functional axial cervical spinal cord images are shown. The structural image was acquired using a multi-echo gradient-echo sequence, which provides high white matter to gray matter contrast. In the diffusion image, the principle direction of diffusion in the spinal cord is through the axial plane as indicated by the colored lines. Magnetization transfer imaging can be used to calculate the magnetization transfer ratio (MTR), which provides a measure of tissue macromolecule content. The functional images show group average activation from an acute thermal pain stimulus applied to the right ventral forearm and group average connectivity to the C7 right anterior horn (light blue). Green = spinal cord gray matter.
Emerging evidence suggests spinal cord MRI data can be examined for structural abnormalities in specific gray matter regions and in the ascending and descending white matter tracts. As an example, reductions in spinal cord tract volumes and diameters were predictive of worsening clinical outcomes in individuals with DCM.62; 63 Atlas-based semi-automated approaches are also being applied, and potentially inform treatment schemes of traumatic SCI.116
Future directions for spinal cord imaging
Although currently pre-clinical, functional MRI (fMRI) shows promise for the study of neural activation. By measuring the blood oxygen level-dependent (BOLD) signal, an indirect measure of intrinsic neural activity can be obtained.
Recently, increased BOLD signal was found in the ipsilateral spinal cord corresponding to upper extremity muscle activation,129 while a similar specific BOLD signal fluctuation corresponding to increasing levels of thermal stimulus applied to the upper extremity was also reported.128 Early reports have shown promise that fMRI may be sensitive to differences in resting state spinal cord networks in DCM and other conditions afflicting spinal cord physiology, such as fibromyalgia and multiple sclerosis.19; 79; 85
Other exciting pre-clinical advanced MRI approaches include the use of high field strengths (7 Tesla) for mapping the spinal cord,86 and using machine-learning algorithms to both automatically segment white and gray matter of the cord99 and objectively detect focal and gross pathology.54 The future of advanced spinal cord imaging will likely involve combining sequences,82 such as DTI, MT, and atlas-based applications in conjunction with higher field strengths and machine-learning technology, to comprehensively characterize the spinal cord across a number of spinal disorders, such as WAD, DCM, and SCI.
BRAIN IMAGING:
Pain is acknowledged to be as much a psychological phenomenon as physiological; wherein, a complex integration of multiple physiological, and cognitive/emotional processes, as well as sociocultural exposures, are shaped by individual context, past experiences, perceived sense of self, and one’s expectation for recovery.104 Over the last 2 decades, brain neuroimaging has resulted in a shift towards characterizing the neural processing of a patient’s pain experience and how we consider the effect of pain, or the experience thereof,34 on the brain itself.83
fMRI has demonstrated that nociceptive processing and the subjective perception of pain is not encoded by a single brain region but distributed across a network of multiple brain regions, each with specific roles in the sensory and affective dimensions of the pain experience.4 To this end, MRI has been leveraged to study brain plasticity in clinical pain conditions towards identifying unique patterns of brain reorganization (ie, structural, diffusion, and functional) in patients with chronic pain; distinct from acute pain (FIGURE 5).84
Figure 5-.
Example structural, diffusion, and functional brain images are shown. The axial structural image was acquired using 3D MPRAGE T1-weighted gradient-echo sequence and can provide morphometric properties of the gray matter. The diffusion example shows a 3D tractography map using the right ventral posterolateral nucleus of the thalamus as a seed. The axial functional images show average group activation from an acute thermal pain stimulus applied to the lower back and group average connectivity to the bilateral posterior cingulate cortices (light blue).
Of the spinal pain conditions, chronic LBP has been the most studied using MRI to demonstrate differences in brain responses to experimentally evoked painful mechanical74; 80 and thermal7 stimuli. Baliki et al studied brain activity related to the spontaneous fluctuations in chronic LBP intensity and identified increased medial prefrontal cortex (mPFC) activity during periods of sustained LBP where the extent of mPFC activity correlated to symptom intensity.6
Excitingly, functional and structural MRI has demonstrated the brain structure is more plastic than previously understood. People with chronic LBP are shown to have reduced prefrontal and thalamic gray matter density,5 distinct spatial and temporal gray matter reorganization,8 and altered functional connectivity between the periaqueductal gray and the ventral medial prefrontal and rostral anterior cingulate cortices (rACC).138 Reduced functional connectivity between primary somatosensory cortex and the rest of the brain following provocative exercise has also been demonstrated.75 Collectively, these cross-sectional studies demonstrate co-localization of functional and structural brain changes, providing empirical evidence of abnormal cortical pain modulation in chronic LBP.
In longitudinal studies, Vachon-Presseau et al demonstrated that increased white matter and resting state functional connectivity within the corticolimbic circuitry can predict persistence of LBP at 3 years.121 Seminowicz et al reported that abnormalities in cortical thickness and brain activity in individuals with chronic LBP could be reversed following effective treatment, surgery or facet joint injections in this example.111 Determining which treatments are effective, and for whom, needs to be explored and established on a patient-by-patient basis.
Future directions for Brain imaging:
New directives for brain imaging are to determine the pathophysiological relevance of these changes and how they relate to the clinical presentation of both acute and chronic pain; that is, do these changes result from an acute injury or pain onset; do they have prognostic value; and/or do they reflect a vulnerable-phenotype at risk for chronicity, or a resilient-phenotype likely to recover spontaneously, and are the reported functional and structural brain abnormalities across common musculoskeletal conditions reversible with less invasive, non-surgical interventions?
WHAT TO DO WITH ALL OF THIS IMAGING (MUSCLE, SPINAL CORD, BRAIN) DATA?
Here, we will briefly highlight different data types that could be collected (eg, genomics, phenomics, physical activity, past medical history, previous experience with pain, pre-injury emotional status, or psychopathology, such as depression or related mental health disorder, even early life adversity) and how they may interact to affect the health of not only various soft-tissues, but in making the individual more or less resilient (or vulnerable) to a good (or adverse) outcome.125; 126
A growing body of evidence suggests various genetic influences on resilience or vulnerability to persistence of pain or heightened stress.9; 10; 77; 78; 88; 89 Relevant to our discussion are the common types of genetic variations called single nucleotide polymorphisms, or “SNPs”; some of which have demonstrated associations with the magnitude and persistence of pain complaints and distress following trauma exposure.10; 89 While exciting, establishing the cause-and-effect influence of genetic vulnerabilities based on polymorphisms and how this may/may not relate to findings from advanced imaging applications is complex and more work is needed in this area.
While the reader is cautioned when interpreting such results, it is difficult not to be excited by the potential value in combining quantitative data from radi-omics, phen-omics, gen-omics and, arguably the most common collection of clinical data; patient self-report outcome measures. Self-report variables could include cultural beliefs about the experience and expression of pain,11; 39; 95 the medicolegal context within which their experience is being scrutinized,15; 98 the amount of social support they receive,[101] and other stressors along the life-course.13 Importantly, the extent to which each of these factors are either resiliencies or vulnerabilities likely varies by context – a factor that may prove protective under one condition may be a vulnerability under different conditions.2 The interpretation of radiological abnormalities and the manner in which they are reported to and shared with the patient could also be influential.
Clearly, these situations cannot be ignored and may help provide some rationale behind recovery – or failure to recover. However, when combined with various data types, associations could be explored to help 1) define precision medicine, 2) produce more informed plans of care, and 3) improve our patient’s experience and hopefully, outcome.
CONCLUSION
We present observable and measurable changes in soft aqueous tissues of the spine, spinal cord, and brain that may contribute towards explaining persistent spinal disorders and its sequelae. This clinical commentary is not exhaustive; instead, we provide readers with a snapshot of what we know, what we don’t know, and what we need to know about the imaging of and characterization for common, but enigmatic neuromusculoskeletal pain conditions involving the spine. The long-term intent of our own research and that of our global colleagues in this area of imaging study should remain a continued line of focused interdisciplinary research towards exploring and establishing causal relationships between muscle, spinal cord, and brain morphometry and poor functional recovery for patients with spinal disorders (traumatic and non-traumatic). However, only an exploration of multiple sources of data can rationalize the dynamic pathways underlying recovery on a patient-by-patient basis. We assert that big data-driven identification of meaningful associations within and between disparate biopsychosocial data sources may unlock keys to understanding an individual’s pain experience like never before.
Synopsis:
The development of persistent spinal (traumatic and non-traumatic) pain is common and contributes to high societal and personal costs, globally. There is an acknowledged urgency for new and interdisciplinary approaches to the problem, and soft tissues including skeletal muscles, the spinal cord, and brain are rightly receiving increased attention as important biological contributors. In reaction to recent suspicion of and questioned value for imaging-based findings, this paper serves to recognize the promise that the technological evolution of imaging techniques, and particularly magnetic resonance imaging (MRI), is allowing in characterizing previously less visible morphology. We emphasize the value for quantification and data analysis of several contributors in the biopsychosocial model for understanding spinal pain. Further, we highlight emerging evidence regarding the pathobiology of changes to muscle composition (eg, atrophy, fatty infiltration) as well as advancements in neuro- and musculoskeletal imaging techniques (eg, fat/water imaging, functional MRI, diffusion imaging, magnetization transfer imaging) of these important soft tissues. These non-invasive and objective data sources may complement known prognostic factors of poor recovery, patient self-report, diagnostic tests, and the -omics fields. When combined, advanced ‘big-data’ analyses may assist in identifying associations previously not considered. Our clinical commentary is supported by empirical findings that may orient future efforts towards collaborative conversation and hypotheses-generation, interdisciplinary research, translating across a number of health fields. Our emphasis is that MRI technologies and research are crucial to the advancement of our understanding of the complexities of spinal conditions.
FUNDING SOURCES
JE and AC are supported by a National Institutes of Health award; National Institute of Child Health and Development - NIH R01HD079076
KW is supported by the National Institute on Drug Abuse – NIH T32DA035165] and National Institute of Neurological Disorders and Stroke- NIH K23NS104211]
AC, KW, and JE are supported by a National Institutes of Health award, National Institute of Child Health and Development – NIH R03HD094577
Footnotes
CONFLICT OF INTEREST STATEMENT
RC reports no conflict of interest
MF reports no conflict of interest
KW reports funding from the National Institutes of Health (NIH)
AC reports funding from NIH
JE reports funding from NIH; Advisory member for the Board of Directors, JOSPT; Editorial board member for JOSPT, Musculoskeletal Science & Practice; Advisory Board member the journal, Spine. Honoraria, conference travel, and accommodation for speaking engagements.
REFERENCES:
- [1].Abbott R, Peolsson A, West J, Elliott JM, Aslund U, Karlsson A, Leinhard O. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J 2018;18(5):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alschuler KN, Kratz AL, Ehde DM. Resilience and vulnerability in individuals with chronic pain and physical disability. Rehabil Psychol 2016;61(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anderson SE, Boesch C, Zimmermann H, Busato A, Hodler J, Bingisser R, Ulbrich EJ, Nidecker A, Buitrago-Tellez CH, Bonel HM, Heini P, Schaeren S, Sturzenegger M. Are There Cervical Spine Findings at MR Imaging That Are Specific to Acute Symptomatic Whiplash Injury? A Prospective Controlled Study with Four Experienced Blinded Readers. Radiology 2012;262(2):567–575. [DOI] [PubMed] [Google Scholar]
- [4].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9(4):463–484. [DOI] [PubMed] [Google Scholar]
- [5].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24(46):10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26(47):12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010;66(1):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 2011;6(10):e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-O-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular Med 2014;16(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain 2013;154(8):1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bostick GP, Ferrari R, Carroll LJ, Russell AS, Buchbinder R, Kawclw D, Gross DP. A population-based survey of beliefs about neck pain from whiplash injury, work-related neck pain, and work-related upper extremity pain. Eur J Pain 2009;13:300–304. [DOI] [PubMed] [Google Scholar]
- [12].Buchbinder R, van Tulder M, Oberg B, et al. Low back pain: a call for action. Lancet (London, England) 2018. 391(10137):2384–2388. [DOI] [PubMed] [Google Scholar]
- [13].Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res 2016. [DOI] [PubMed] [Google Scholar]
- [14].Campbell WW, Vasconcelos O, Laine FJ. Focal atrophy of the multifidus muscle in lumbosacral radiculopathy. Muscle & nerve 1998;21(10):1350–1353. [DOI] [PubMed] [Google Scholar]
- [15].Cassidy JD Carroll L, Cote P, et al. Effect of eliminating compensation for pain and suffering on the outcome of insurance claims for whiplash injury. N Engl J Med 2000;342:1179–1186. [DOI] [PubMed] [Google Scholar]
- [16].Chou R, Deyo RA, Jarvik JG. Appropriate Use of Lumbar Imaging for Evaluation of Low Back Pain. Radiologic Clinics of North America 2012; 50(4):569–85. [DOI] [PubMed] [Google Scholar]
- [17].Cloney MB, Smith ZA, Weber KA 2nd, Parrish TB. Quantitative Magnetization Transfer MRI Measurements of the Anterior Spinal Cord Region are Associated With Clinical Outcomes in Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2018;43(10):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011;55(3):1024–1033. [DOI] [PubMed] [Google Scholar]
- [19].Conrad BN, Barry RL, Rogers BP, Maki S, Mishra A, Thukral S, Sriram S, Bhatia A, Pawate S, Gore JC, Smith SA. Multiple sclerosis lesions affect intrinsic functional connectivity of the spinal cord. Brain 2018;141(6):1650–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cornwall J, Stringer MD, Duxson M. Functional morphology of the thoracolumbar transversospinal muscles. Spine 2011;36(16):E1053–1061. [DOI] [PubMed] [Google Scholar]
- [21].Crawford R, Cornwall J, Abbott R, Elliott J. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC musculoskeletal disorders 2017. 18(1):25. doi: 10.1186/s12891-016-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crawford R, Volken T, Valentin S, Melloh M, Elliott J. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: An age- aggregated cross-sectional simulation study. BMC Scoliosis and Spinal Disorders 2016. August 5;11:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crawford RJ, Elliott JM, Volken T. Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur Spine J 2017;26(12):3059–3067. [DOI] [PubMed] [Google Scholar]
- [24].Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. Am J Neuroradiol 2016;37(4):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dahlqvist JR, Vissing CR, Hedermann G, Thomsen C, Vissing J. Fat Replacement of Paraspinal Muscles with Aging in Healthy Adults. Medicine and science in sports and exercise 2017;49(3):595–601. [DOI] [PubMed] [Google Scholar]
- [26].Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, Lavado R, Lomsadze L, Reynolds A, Squires E, Campbell M, DeCenso B, Dicker D, Flaxman AD, Gabert R, Highfill T, Naghavi M, Nightingale N, Templin T, Tobias MI, Vos T, Murray CJ. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA 2016;316(24):2627–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dombrowski ME, Rynearson B, LeVasseur C, Adgate Z, Donaldson WF, Lee JY, Aiyangar A, Anderst WJ. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2018: dynamic imaging of degenerative spondylolisthesis reveals mid-range dynamic lumbar instability not evident on static clinical radiographs. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2018;27(4):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dudli S, Sing DC, Hu SS, Berven SH, Burch S, Deviren V, Cheng I, Tay BKB, Alamin TF, Ith MAM, Pietras EM, Lotz JC. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2017;26(5):1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duewell S, Wolff SD, Wen H, Balaban RS, Jezzard P. MR imaging contrast in human brain tissue: assessment and optimization at 4 T. 1996 1996;199(3):780–786. [DOI] [PubMed] [Google Scholar]
- [30].Ellingson BM, Salamon N, Woodworth DC, Holly L. Correlation between degree of subvoxel spinal cord compression measured with super-resolution tract density imaging and neurological impairment in cervical spondylotic myelopathy. J Neurosurg Spine 2015;22(6):631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS One 2011;6(6):e21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish T. The Rapid and Progressive Degeneration of the Cervical Multifidus in Whiplash: A MRI study of Fatty Infiltration. Spine (Phila Pa 1976) 2015;40(12):E694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Elliott JM, Kerry R, Flynn T, Parrish T. Content not quantity is a better measure of muscle degeneration in whiplash. Manual Therapy 2013;18(6):578–582. [DOI] [PubMed] [Google Scholar]
- [34].Elliott JM, Owen M, Bishop MD, Sparks C, Tsao H, Walton DM, Weber KA Jr, Wideman T. Measuring Pain for Patients Seeking Physical Therapy: Can Functional Magnetic Resonance Imaging (fMRI) Help? Physical Therapy 2017;97(1):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Elliott JM, Cornwall J, Kennedy E, Abbott R, Crawford RJ. Towards defining muscular regions of interest from axial magnetic resonance imaging with anatomical cross-reference: part II - cervical spine musculature. BMC Musculoskelet Disord 2018;19(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med 2014;15(11):1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Elliott JM, Hancock MJ, Crawford RJ, Smith AC, Walton DM. Advancing imaging technologies for patients with spinal pain: with a focus on whiplash injury. The spine journal. 2017. 18(8):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Elliott JM, Kerry R, Flynn T, Parrish TB. Content not quantity is a better measure of muscle degeneration in whiplash. Manual therapy 2013;18(6):578–582. [DOI] [PubMed] [Google Scholar]
- [39].Ferrari R, Constantoyannis C, Papadakis N. Laypersons’ expectation of the sequelae of whiplash injury: a cross-cultural comparative study between Canada and Greece. Med Sci Monit 2003;9(3):CR120–124. [PubMed] [Google Scholar]
- [40].Fischer MA, Nanz D, Shimakawa A, Schirmer T, Guggenberger R, Chhabra A, Carrino JA, Andreisek G. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology 2013;266(2):555–563. [DOI] [PubMed] [Google Scholar]
- [41].Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 2011;54(1):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fortin M, Wilk N, Dobrescu O, Martel P, antaguida C, Weber M. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract 2018:1–7. [DOI] [PubMed] [Google Scholar]
- [43].Fortin M, Dobrescu O, Courtemanche M, et al. Association Between Paraspinal Muscle Morphology, Clinical Symptoms and Functional Status in Patients With Degenerative Cervical Myelopathy. Spine 2017. 42(4):232–239. [DOI] [PubMed] [Google Scholar]
- [44].Fortin M, Gibbons LE, Videman T, Battie MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports 2015. 25(6):880–7. [DOI] [PubMed] [Google Scholar]
- [45].Fortin M, Lazary A, Varga PP, Battie MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. European spine journal. 2017;26(10):2543–2551. [DOI] [PubMed] [Google Scholar]
- [46].Fortin M, Macedo LG. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. Physical therapy 2013;93(7):873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fortin M, Omidyeganeh M, Battie MC, Ahmad O, Rivaz H. Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomedical engineering online 2017;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fortin M, Videman T, Gibbons LE, Battie MC. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Medicine and science in sports and exercise 2014;46(5):893–901. [DOI] [PubMed] [Google Scholar]
- [49].Fortin M, Yuan Y, Battie MC. Factors associated with paraspinal muscle asymmetry in size and composition in a general population sample of men. Physical therapy 2013;93(11):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet (London, England) 2018. 391(10137):2368–2383. [DOI] [PubMed] [Google Scholar]
- [51].Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, Van Oosterwijck J, Dhondt E, Danneels L. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J 2017;17(9):1285–1296. [DOI] [PubMed] [Google Scholar]
- [53].Goubert D, Meeus M, Willems T, De Pauw R, Coppieters I, Crombez G, Danneels L. The association between back muscle characteristics and pressure pain sensitivity in low back pain patients. Scandinavian journal of pain 2018;18(2):281–293. [DOI] [PubMed] [Google Scholar]
- [54].Gros C, De Leener B, Badji A, et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage 2019. 184:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hancock MJ, Kjaer P, Kent P, Jensen RK, Jensen T. Is the Number of Different MRI Findings More Strongly Associated with Low Back Pain than Single MRI Findings? Spine (Phila Pa 1976) 2017;42(17):1283–1288. [DOI] [PubMed] [Google Scholar]
- [56].Hancock MJ, Maher CM, Petocz P, Lin CW, Steffens D, Luque-Suarez A, Magnussen JS. Risk factors for a recurrence of low back pain. Spine J 2015;15(11):2360–2368. [DOI] [PubMed] [Google Scholar]
- [57].Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine 2014;39(17):1417–1425. [DOI] [PubMed] [Google Scholar]
- [58].Hebert JJ, Le Cara EC, Koppenhaver SL, Hoffman MD, Marcus RL, Dempsey AR, Albert WJ. Predictors of clinical success with stabilization exercise are associated with lower levels of lumbar multifidus intramuscular adipose tissue in patients with low back pain. Disabil Rehabil 2018:1–7. [DOI] [PubMed] [Google Scholar]
- [59].Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, Little C, Melrose J. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: Molecular and morphological evidence. Spine 2015;40(14):1057–1071. [DOI] [PubMed] [Google Scholar]
- [60].Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine 2009;10(3):194–200. [DOI] [PubMed] [Google Scholar]
- [61].Hopkins BS, Weber KA 2nd , Cloney MB, Paliwal M, Parrish TB, Smith ZA. Tract-Specific Volume Loss on 3T MRI in Patients With Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2018;43(20):E1204–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hopkins BS, Weber KA 2nd, Cloney MB, Paliwal M, Parrish TB, Smith ZA. Tract-Specific Volume Loss on 3T MRI in Patients with Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2018. 43(20):E1204–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hori M, Hagiwara A, Fukunaga I, Ueda R, Kamiya K, Suzuki Y, Liu W, Murata K, Takamura T, Hamasaki N, Irie R, Kamagata K, Kumamaru KK, Suzuki M, Aoki S. Application of Quantitative Microstructural MR Imaging with Atlas-based Analysis for the Spinal Cord in Cervical Spondylotic Myelopathy. Sci Rep 2018;8(1):5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ignasiak D, Rueger A, Sperr R, Ferguson SJ. Thoracolumbar spine loading associated with kinematics of the young and the elderly during activities of daily living. Journal of biomechanics 2018;70:175–184. [DOI] [PubMed] [Google Scholar]
- [65].Ignasiak D, Valenzuela W, Reyes M, Ferguson SJ. The effect of muscle ageing and sarcopenia on spinal segmental loads. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2018. 27(10):2650–2659. [DOI] [PubMed] [Google Scholar]
- [66].Jamaludin A, Lootus M, Kadir T, Zisserman A, Urban J, Battie MC, Fairbank J, McCall I. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2017: Automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. European spine journal. 2017;26(5):1374–1383. [DOI] [PubMed] [Google Scholar]
- [67].Jensen RK, Kent P, Jensen TS, Kjaer P. The association between subgroups of MRI findings identified with latent class analysis and low back pain in 40-year-old Danes. Bmc Musculoskeletal Disorders 2018;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. Am J Neuroradiol 2013;34(2):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kalin PS, Crawford RJ, Marcon M, Manoliu A, Bouaicha S, Fischer MA, Ulbrich EJ. Shoulder muscle volume and fat content in healthy adult volunteers: quantification with DIXON MRI to determine the influence of demographics and handedness. Skeletal Radiol 2018;47(10):1393–1402. [DOI] [PubMed] [Google Scholar]
- [70].Karlsson A, Leinhard OD, Aslund U, West J, Romu T, Smedby O, Zsigmond P, Peolsson A. An Investigation of Fat Infiltration of the Multifidus Muscle in Patients With Severe Neck Symptoms Associated With Chronic Whiplash-Associated Disorder. J Orthop Sports Phys Ther 2016;46(10):886–893. [DOI] [PubMed] [Google Scholar]
- [71].Kazemifar S, Drozd JJ, Rajakumar N, Borrie MJ, Bartha R. Automated algorithm to measure changes in medial temporal lobe volume in Alzheimer disease. Journal of neuroscience methods 2014;227:35–46. [DOI] [PubMed] [Google Scholar]
- [72].Kemnitz J, Eckstein F, Culvenor AG, Ruhdorfer A, Dannhauer T, Ring-Dimitriou S, Sanger AM, Wirth W. Validation of an active shape model-based semi-automated segmentation algorithm for the analysis of thigh muscle and adipose tissue cross-sectional areas. MAGMA 2017;30(5):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Khan AB, Weiss EH, Khan AW, Omeis I, Verla T. Back Muscle Morphometry: Effects on Outcomes of Spine Surgery. World neurosurgery 2017;103:174–179. [DOI] [PubMed] [Google Scholar]
- [74].Kobayashi Y, Kurata J, Sekiguchi M, Kokubun M, Akaishizawa T, Chiba Y, Konno S, Kikuchi S. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine (Phila Pa 1976) 2009;34(22):2431–2436. [DOI] [PubMed] [Google Scholar]
- [75].Kong J, Spaeth RB, Wey HY, Cheetham A, Cook AH, Jensen K, Tan Y, Liu H, Wang D, Loggia ML, Napadow V, Smoller JW, Wasan AD, Gollub R. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain 2013;9(43):doi: 10.1186/1744-8069-1189-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Le Cara E, Marcus RL, Dempsy AR, Hoffman MD, Hebert J. Morphology versus function: The relationship between lumbar multifidus intramuscular adipose tissue and muscle function among patients with low back pain. Arch Phys Med Rehabil 2014;95(10):1846–1852. [DOI] [PubMed] [Google Scholar]
- [77].Linnstaedt SD, Riker KD, Walker MG, Nyland JE, Zimny E, Lewandowski C, Hendry PL, Damiron K, Pearson C, Velilla MA, Jones J, Swor RA, Domeier R, McLean SA. MicroRNA 320a Predicts Chronic Axial and Widespread Pain Development Following Motor Vehicle Collision in a Stress-Dependent Manner. J Orthop Sport Phys 2016;46(10):911–919. [DOI] [PubMed] [Google Scholar]
- [78].Linnstaedt SD, Walker MG, Riker KD, Nyland JE, Hu J, Rossi C, Swor RA, Jones JS, Diatchenko L, Bortsov AV, Peak DA, McLean SA. Genetic variant rs3750625 in the 3’UTR of ADRA2A affects stress-dependent acute pain severity after trauma and alters a microRNA-34a regulatory site. Pain 2016. 158(2):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu X, Qian W, Jin R, Li X, Luk KD, Wu EX, Hu Y. Amplitude of Low Frequency Fluctuation (ALFF) in the Cervical Spinal Cord with Stenosis: A Resting State fMRI Study. PLoS One 2016;11(12):e0167279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lloyd D, Findlay G, Roberts N, Nurmikko T. Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine (Phila Pa 1976) 2008;33(12):1372–1377. [DOI] [PubMed] [Google Scholar]
- [81].Maatta J, Karppinen J, Luk KD, Cheung KM, Samartzis D. Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale, population-based study. The spine journal. 2015;15(9):1933–1942. [DOI] [PubMed] [Google Scholar]
- [82].Martin AR, De Leener B, Cohen-Adad J, Kalsi-Ryan S, Cadotte DW, Wilson JR, Tetreault L, Nouri A, Crawley A, Mikulis DJ, Ginsberg H, Massicotte EM, Fehlings MG. Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS One 2018;13(4):e0195733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Martucci KT, Mackey SC. Imaging Pain. Anesthesiol Clin 2016;34(2):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Martucci KT, Mackey SC. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology 2018;128(6):1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Martucci KT, Weber KA 2nd, Mackey SC. Altered Cervical Spinal Cord Resting State Activity in Fibromyalgia. Arthritis Rheumatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Massire A, Taso M, Besson P, Guye M, Ranjeva J-P, Callot V. High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. Neuroimage 2016;143:58–69. [DOI] [PubMed] [Google Scholar]
- [87].Matsumoto M, Ichihara D, Okada E, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Takahata T. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers - 10 year follow-up MR study. Injury 2012;43(6):912–916. [DOI] [PubMed] [Google Scholar]
- [88].McLean S The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine (Phila Pa 1976) 2011;36(25 Suppl):S226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain 2011;12(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mhuiris ÁN, Volken T, Elliott JM, Hoggarth MA, Samartzis D, Crawford R. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1-weighted MRI. BMC Musculoskeletal Disorders 2016;17:234. doi: 10.1186/s12891-016-1090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mhuiris AN, Volken T, Elliott JM, Hoggarth M, Samartzis D, Crawford RJ. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1-weighted MRI. BMC musculoskeletal disorders 2016;17(1):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. The spine journal. 2016;16(1):32–41. [DOI] [PubMed] [Google Scholar]
- [93].Mok FP, Samartzis D, Karppinen J, Luk KD, Fong DY, Cheung KM. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine 2010;35(21):1944–1952. [DOI] [PubMed] [Google Scholar]
- [94].Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine (Phila Pa 1976) 2015;40(6):392–398. [DOI] [PubMed] [Google Scholar]
- [95].Ng TS, Bostick G, Pedler A, Buchbinder R, Vicenzino B, Sterling M. Laypersons’ expectations of recovery and beliefs about whiplash injury: A cross-cultural comparison between Australians and Singaporeans. European Journal of Pain 2013;17(8):1234–1242. [DOI] [PubMed] [Google Scholar]
- [96].Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976) 2015;40(12):E675–693. [DOI] [PubMed] [Google Scholar]
- [97].Orgiu S, Lafortuna CL, Rastelli F, Cadioli M, Falini A, Rizzo G. Automatic muscle and fat segmentation in the thigh from T1-Weighted MRI. Journal of magnetic resonance imaging : JMRI 2016;43(3):601–610. [DOI] [PubMed] [Google Scholar]
- [98].Partheni M, Constantoyannis C, Ferrari R, Nikiforidis G, Voulgaris S, Papadakis N. A prospective cohort study of the outcome of acute whiplash injury in Greece. Clin Exp Rheumatol 2000;18(1):67–70. [PubMed] [Google Scholar]
- [99].Perone CS, Calabrese E, Cohen-Adad J. Spinal cord gray matter segmentation using deep dilated convolutions. Sci Rep 2018 2018;8(1):5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ploumis A, Michailidis N, Christodoulou P, Kalaitzoglou I, Gouvas G, Beris A. Ipsilateral atrophy of paraspinal and psoas muscle in unilateral back pain patients with monosegmental degenerative disc disease. Br J Radiol 2011;84(1004):709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Raichle K, Romano JM, Jensen M. Partner responses to patient pain and well behaviors and their relationship to patient pain behavior, functioning, and depression. Pain 2011;152(1):82–88. [DOI] [PubMed] [Google Scholar]
- [102].Rajasekaran S, Tangavel C, Aiyer SN, Nayagam SM, Raveendran M, Demonte NL, Subbaiah P, Kanna R, Shetty AP, Dharmalingam K. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. European spine journal. 2017;26(5):1384–1400. [DOI] [PubMed] [Google Scholar]
- [103].Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, Urquhart DM. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. The spine journal. 2017;17(11):1729–1748. [DOI] [PubMed] [Google Scholar]
- [104].Reddan MC, Wager T. Modeling Pain Using fMRI: From Regions to Biomarkers. Neurosci Bull 2018;34(1):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. Journal of magnetic resonance imaging : JMRI 2012;36(5):1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Resorlu H, Savas Y, Aylanc N, Gokmen F. Evaluation of paravertebral muscle atrophy and fatty degeneration in ankylosing spondylitis. Modern rheumatology 2017;27(4):683–687. [DOI] [PubMed] [Google Scholar]
- [107].Salamon N, Ellingson BM, Nagarajan R, Gebara N, Thomas A, Holly LT. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord 2013;51(7):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Samartzis D, Borthakur A, Belfer I, Bow C, Lotz JC, Wang HQ, Cheung KM, Carragee E, Karppinen J. Novel diagnostic and prognostic methods for disc degeneration and low back pain. The spine journal. 2015;15(9):1919–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sasaki T, Yoshimura N, Hashizume H, Yamada H, Oka H, Matsudaira K, Iwahashi H, Shinto K, Ishimoto Y, Nagata K, Teraguchi M, Kagotani R, Muraki S, Akune T, Tanaka S, Kawaguchi H, Nakamura K, Minamide A, Nakagawa Y, Yoshida M. MRI-defined paraspinal muscle morphology in Japanese population: The Wakayama Spine Study. PloS one 2017;12(11):e0187765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schick F Tissue segmentation: a crucial tool for quantitative MRI and visualization of anatomical structures. Magma (New York, NY) 2016;29(2):89–93. [DOI] [PubMed] [Google Scholar]
- [111].Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 2011;31(20):7540–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, Garfin SR, Ward SR. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 2017;35(12):2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, Allen RT, Hughes-Austin J, Garfin S, Ward SR. Contribution of Lumbar Spine Pathology and Age to Paraspinal Muscle Size and Fatty Infiltration. Spine (Phila Pa 1976) 2017;42(8):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sions JM, Coyle PC, Velasco TO, Elliott JM, Hicks GE. Multifidi Muscle Characteristics and Physical Function Among Older Adults With and Without Chronic Low Back Pain. Archives of physical medicine and rehabilitation 2017;98(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Smith AC, Parrish TB, Hoggarth M, McPherson J, Tysseling VM, Wasielewski M, Kim HE, Hornby TG, Elliott J. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases 2015;1 pii: 15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Smith AC, Weber KA 2nd, O’Dell DR, Parrish TB, Wasielewski M, Elliott JM. Lateral Corticospinal Tract Damage Correlates With Motor Output in Incomplete Spinal Cord Injury. Arch Phys Med Rehabil 2018;99(4):660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Storheim K, Berg L, Hellum C, Gjertsen O, Neckelmann G, Espeland A, Keller A. Fat in the lumbar multifidus muscles - predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC musculoskeletal disorders 2017;18(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Suleiman LI, Weber KA 2nd, Rosenthal BD, Bhatt SA, Savage JW, Hsu WK, Patel AA, Parrish TB. High-resolution magnetization transfer MRI in patients with cervical spondylotic myelopathy. J Clin Neurosci 2018;51:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tetreault L, Goldstein CL, Arnold P, Harrop J, Hilibrand A, Nouri A, Fehlings MG. Degenerative Cervical Myelopathy: A Spectrum of Related Disorders Affecting the Aging Spine. Neurosurgery 2015;77 Suppl 4:S51–67. [DOI] [PubMed] [Google Scholar]
- [120].Ulbrich EJ, Aeberhard R, Wetli S, Busato A, Boesch C, Zimmermann H, Hodler J, Anderson SE, Sturzenegger M. Cervical muscle area measurements in whiplash patients: Acute, 3, and 6 months of follow-up. J Magn Reson Imaging 2012;36(6):1413–1420. [DOI] [PubMed] [Google Scholar]
- [121].Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016;139(Pt 7):1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Manual therapy 2015;20(1):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Valenzuela W, Ferguson SJ, Ignasiak D, Diserens G, Hani L, Wiest R, Vermathen P, Boesch C, Reyes M. FISICO: Fast Image SegmentatIon Correction. PLoS One 2016;11(5):e0156035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Vos T et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015;385(9963):117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Walton DM, Elliott JM. An Integrated Model of Chronic Whiplash-Associated Disorder. J Orthop Sports Phys Ther 2017;47(7):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Walton DM, Elliott JM. A new clinical model for facilitating the development of pattern recognition skills in clinical pain assessment. Musculoskelet Sci Pract 2018;36:17–24. [DOI] [PubMed] [Google Scholar]
- [127].Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol 2015;88(1053):20140546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Weber KA 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. Functional magnetic resonance imaging of the cervical spinal cord during thermal stimulation across consecutive runs. Neuroimage 2016;143:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Weber KA 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. Lateralization of cervical spinal cord activity during an isometric upper extremity motor task with functional magnetic resonance imaging. Neuroimage 2016;125:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wen CY, Cui JL, Lee MP, Mak KC, Luk KD, Hu Y. Quantitative analysis of fiber tractography in cervical spondylotic myelopathy. Spine J 2013;13(6):697–705. [DOI] [PubMed] [Google Scholar]
- [131].Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10(1):135–144. [DOI] [PubMed] [Google Scholar]
- [132].Wolff SD, Eng J, B RS. Magnetization transfer contrast: method for improving contrast in gradient-recalled-echo images. Radiology 1991;179(1):133–137. [DOI] [PubMed] [Google Scholar]
- [133].Xiao Y, Fonov V, Beriault S, Al Subaie F, Chakravarty MM, Sadikot AF, Pike GB, Collins DL. Multi-contrast unbiased MRI atlas of a Parkinson’s disease population. International journal of computer assisted radiology and surgery 2015;10(3):329–341. [DOI] [PubMed] [Google Scholar]
- [134].Xiao Y, Fortin M, Battie MC, Rivaz H. Population-averaged MRI atlases for automated image processing and assessments of lumbar paraspinal muscles. Eur Spine J 2018. [DOI] [PubMed] [Google Scholar]
- [135].Yip SS, Aerts HJ. Applications and limitations of radiomics. Physics in medicine and biology 2016;61(13):R150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yoo YH, Kim HS, Lee YH, Yoon CS, Paek MY, Yoo H, Kannengiesser S, Chung TS, Song HT, Suh JS, Kim S. Comparison of Multi-Echo Dixon Methods with Volume Interpolated Breath-Hold Gradient Echo Magnetic Resonance Imaging in Fat-Signal Fraction Quantification of Paravertebral Muscle. Korean journal of radiology 2015;16(5):1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Young AA, Frangi AF. Computational cardiac atlases: from patient to population and back. Experimental physiology 2009;94(5):578–596. [DOI] [PubMed] [Google Scholar]
- [138].Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin 2014;6:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zehra U, Bow C, Lotz JC, Williams FMK, Rajasekaran S, Karppinen J, Luk KDK, Battie MC, Samartzis D. Structural vertebral endplate nomenclature and etiology: a study by the ISSLS Spinal Phenotype Focus Group. European spine journal. 2018;27(1):2–12. [DOI] [PubMed] [Google Scholar]