Abstract
The triplet pair is the key functional unit in triplet–triplet annihilation photon upconversion. The same molecular properties that stabilize the triplet pair also allow dimers to form on the singlet energy surface, creating an unwanted energy relaxation pathway. Here we show that excimer formation most likely is a consequence of a triplet dimer formed before the annihilation event. Polarity-dependent studies were performed to elucidate how to promote wanted emission pathways over excimer formation. Furthermore, we show that the yield of triplet–triplet annihilation is increased in higher-viscosity solvents. The results will bring new insights in how to increase the upconversion efficiency and how to avoid energy-loss channels.
Introduction
Triplet–Triplet annihilation photon upconversion (TTA-UC) provides the possibility to convert low to high energy photons at low irradiation conditions.1,2 It is thus considered as a promising method to increase the efficiency of solar energy conversion systems like photocatalysis and solar cells.3−8 Insufficient utilization of the solar spectrum is one of the major hurdles for further development of solar energy conversion systems.9,10 Moreover, TTA-UC enables photocatalysis with low-energy photons, which increases selectivity and allows for reactions to be performed in turbid environments.11−13 Although the notion of triplet–triplet annihilation dates back more than 60 years, this field has gone through a fast development during the past decade.14−23
TTA-UC needs two basic molecular components, a sensitizer (S) and an annihilator (A). The sensitizer absorbs low-energy photons and transfers the excited-state energy to the annihilator via triplet energy transfer.24 Two annihilators, both in their excited triplet state, form a so-called triplet pair, where triplet–triplet annihilation (TTA) then occurs.2,25 As the terminal execution unit of TTA-UC, the properties of the annihilator are crucial for the overall system efficiency.26
Perylene is a frequently used annihilator, for instance, employed in TTA-UC schemes in light-emitting diodes, solar cells, photocatalysis, and bioimaging.27−29 Recently photoredox catalysis, using near-infrared light, has been demonstrated using perylene as the annihilator.13 Perylene is an effective annihilator due to its close to optimal energy level alignments and high fluorescence quantum efficiency. A record TTA-UC quantum yield of 38% has been recorded with this molecule.30 However, TTA-UC quantum yields ranging from 1 to 10% are typically reported in the literature.31−36 Different solvents, sensitizers, concentrations, and excitation sources can be the cause of this discrepancy. For practical high-efficiency TTA-UC systems to be constructed, a deeper mechanistic understanding of the processes occurring in the triplet pair is needed.
Dover et al. recently demonstrated the conversion between monomers and excimers during singlet fission and TTA-UC.37 Kinetic models were established, showing how excimers can be formed in geminate systems that involve singlet fission and TTA. The transformation of intermediate triplet pair species has also been elucidated in related works.38−41 Inspired by these findings, we examine the effect of environment on the excited states conversion in sensitized TTA-UC, which is nongeminate. By experimental and modeling investigations, we show that the solvent greatly effects the relationship between annihilation and excimer formation. Furthermore, we found a counterintuitive positive relationship between solvent viscosity and upconversion efficiency, indicating that higher inertia promotes TTA. These results give clear and practical insights in how to increase the performance of TTA-UC systems.
Results
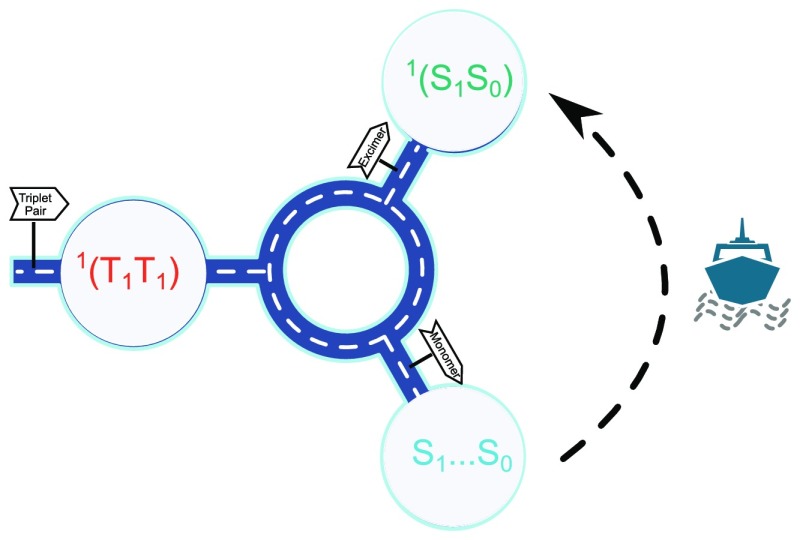
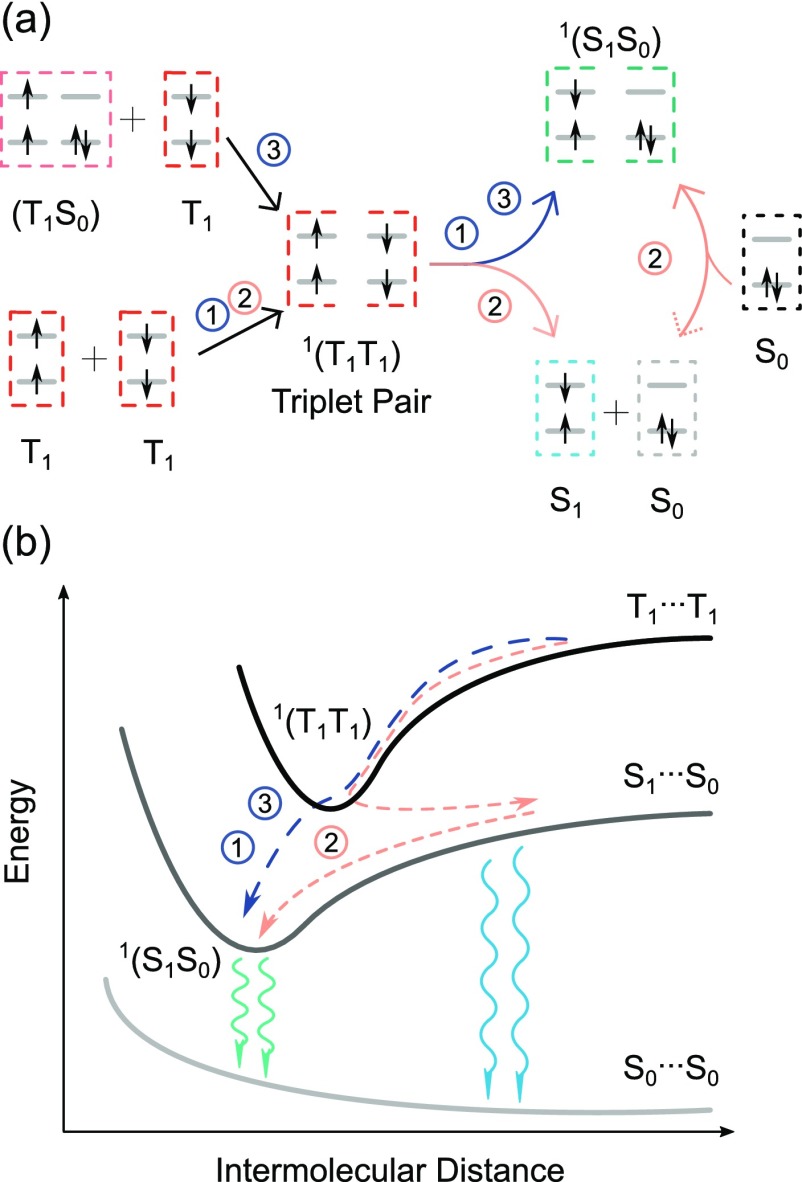
The process of triplet–triplet annihilation requires two annihilator molecules, both in their excited triplet state, to form a triplet pair. Typical annihilators like perylene, pyrene, rubrene, and diphenylanthracene have a large flat conjugated structure, thus having the ability to form favorable π–π and electronic interactions stabilizing the triplet pair.42,43 However, the same molecular properties that stabilize the triplet pair also allow dimers to form on the singlet energy surface.37,44−46 The excited dimer, consisting of one molecule in its ground state and one in its excited singlet state, is usually referred to as an excimer. Excimers can have high-emission quantum yields and may not affect the overall TTA-UC quantum yield.47 However, the lower photon energy of excimer emission reduces the overall energy conversion efficiency.31 Excimer formation in TTA-UC is consequently considered as an unfavorable process, especially when TTA-UC is applied in photocatalysis and solar cells, which have excitation energy thresholds. Three pathways are possible for the excimer formation. In the first pathway, excimers are directly produced from the triplet pair (mechanism 1; Scheme 1). In the second one, only monomers are formed from the triplet pair (mechanism 2; Scheme 1). The excimers are then formed through molecular diffusion; that is, an annihilator in its excited singlet state collides with another annihilator in its ground state forming the excimer. As such, the formation of monomer (S1...S0) and excimer (S1S0) are competitive processes. A third alternative would be if a stable dimer is already formed on the triplet surface (T1S0), before the annihilation event occurs. This could be considered as a special case of mechanism 1, with the difference being that the preformed dimer is suitably oriented to also form an excimer on the singlet surface after the annihilation event. Considering that excimer formation is an unwanted process, gaining knowledge of how this state is formed in TTA-UC systems will enable the construction of new improved systems with enhanced efficiencies.
Scheme 1.
(a) The proposed mechanism for excimer formation in TTA-UC. (b) Proposed energy conversion diagram for excimer formation in TTA-UC.
Perylene was used as annihilator, and two commercially available metal porphyrin complexes, namely, palladium(II) octaethylporphyrin (PdOEP) and platinum(II) tetrabenzotetraphenylporphyrin (PtTBTP), were used as sensitizers (Figure 1a). Both molecules are excellent triplet sensitizers with high molecular extinction coefficients, intersystem-crossing quantum yields near unity, and long triplet-state lifetimes.48Figure 1b displays the absorption and emission spectra of perylene and the two sensitizers. Perylene exhibits a structured absorption and emits in the blue part of the electromagnetic spectrum. PdOEP and PtTBTP have their lowest-energy maxima centered at 547 and 613 nm, respectively, offering a possible anti-Stokes shift of 0.54 and 0.79 eV (calculated from the E0–0 splitting of sensitizers and annihilator). Perylene can quench the phosphorescence of both these sensitizers, suggesting that the triplet-state energy can be transferred from either of these sensitizers to perylene (Figure S1). Figure 1c displays the TTA-UC emission from a mixture of sensitizer and acceptor, when the sensitizers are excited (in tetrahydrofuran, THF). Upconverted emission in the 400–500 nm range is clearly seen (the deviation between the upconverted emission and perylene emission in Figure 1b is due to inner filter effects). However, a large amount of emission around 565 nm is evident. On the grounds that this emission is sensitizer-independent, we exclude any sensitizer–annihilator complex responsible for the emission, and instead attribute it to emission from an excimer state.49,50 Excitation-dependent emission showed both linear and quadratic regions, indicating that both the monomer and excimer emission obey the dynamical model of TTA-UC (Figure S2).51 Furthermore, emission spectroscopy in the presence of oxygen showed neither monomer nor excimer emission (Figure S3), indicating that both emissive states are formed from the triplet state.
Figure 1.
(a) Molecular structure of perylene, PdOEP, and PtTBTP. (b) Normalized absorption and emission spectra of perylene, PdOEP, and PtTBTP. (c) Normalized emission spectra of 10 μM PdOEP (excited at 532 nm) or PtTBTP (excited at 617 nm) and 1 mM perylene in THF.
Molecular diffusion plays an important role in sensitized TTA-UC. The solvent environment will affect the bimolecular interaction and thus affect the TTA-UC performance. If both excimers and monomers are formed directly from the triplet pair (mechanism 1), the relative ratio between excimer and monomer will be dependent on the excimer formation possibility during annihilation. The relative ratio might increase with solvent viscosity. This is because molecular diffusion is slowed at high viscosities, enabling the two annihilators from the triplet pair to be in close contact for a longer period of time.52 The rate of dissociation is lower and therefore increases the possibility of excimer formation. However, if the excimer is formed due to an association of the monomer with an annihilator molecule (mechanism 2), which was not part of the original triplet pair, the excimer intensity will be enhanced in a low-viscosity environment. As the monomer needs to collide with a ground-state annihilator by means of diffusion, the rate of association is increased in a low-viscosity solvent, increasing the yield of excimers formed by mechanism 2. A preassociation mechanism (mechanism 3) is also proposed here, taking the possible association between a triplet excited annihilator with a ground-state annihilator into account. The solvent viscosity will affect bimolecular interaction rate constants and, thus, both the rate of excimer formation and the rate of annihilation in mechanism 3. The increases of these two rates work in opposite direction, and the influence of solvent viscosity on the excimer formation is therefore low in mechanism 3. Kinetic models have been constructed for the proposed mechanisms (see Note S1.6).
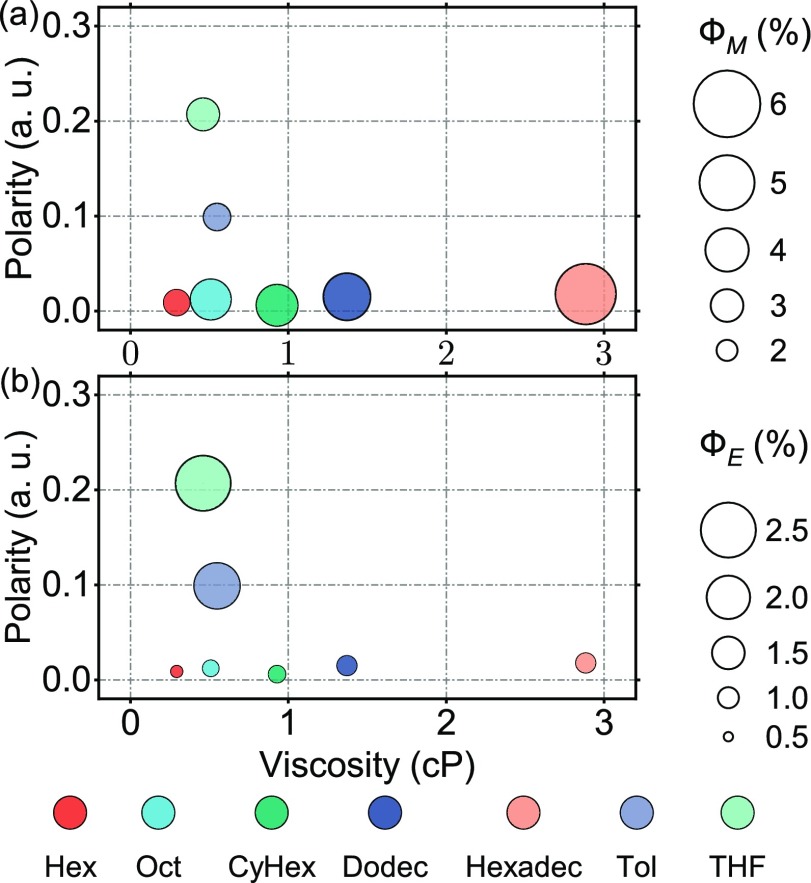
Figure 2 demonstrates the solvent dependence of monomer and excimer emission from TTA-UC. Absolute quantum yields were used to evaluate the photon upconversion efficiency (theoretical limit thus 50%), with inner filter effect not taken into account (Note S1.7). The values of viscosities and relative polarities were extracted from corresponding physical chemistry textbooks.53,54 Separated relationships of polarity versus quantum yield and viscosity versus quantum yield are displayed in Figure S4. From the sequence of alkane solvents, we found that the absolute quantum yield of both monomer (ΦM) and excimer (ΦE) emission increases with viscosity. An increase in excimer emission at higher viscosities is indicative of mechanism 1. However, also the monomer emission is increased at higher viscosities, indicating that the triplet pair is more easily formed (note that a large rate constant of diffusion in low-viscosity solvents should be kinetically beneficial for the TTA step). The increase in monomer emission will be further explored (vide infra). The ratio between monomer and excimer emission is actually decreasing slightly with viscosity, but not as much as expected for mechanism 2 (Note S1.9). Thus, viscosity-dependent upconversion reveals that TTA-UC counterintuitively is more efficient at higher viscosities. Comparing to previous upconversion work with the annihilator 9,10-bis(phenylethynyl)anthracene (BPEA) we note that in low-viscosity solvents the highest upconversion yield is less than 2%, whereas in high-viscosity solvents (poly(ethylene glycol) (PEG)) the yield is 15%.55−57 In this study, it was argued that the extended lifetime of the triplet pair intermediate allowed for a higher probability of obtaining a favorable geometry.56
Figure 2.
Quantum yield of (a) monomer emission (ΦM) and (b) excimer emission (ΦE) in different solvents with 10 μM PtTBTP and 1 mM perylene. Hex, Oct, CyHex, Dodec, Hexadec, Tol, and THF stand for hexane, octane, cyclohexane, dodecane, hexadecane, toluene, and tetrahydrofuran, respectively. Reported values are an average of three individual measurements.
The monomer emission does not seem to be related to solvent polarity, indicating that a possible charge-transfer state is not rate limiting in the upconversion process. However, we noticed that the excimer emission is strongly related to the increase in polarity, indicating that the excimer formation is favored compared to dissociative diffusion of the formed singlet ground-state pair in higher polar solvents. Note that direct excitation of perylene only shows a slight relationship between excimer emission and solvent polarity (Figure S5, Note S1.10). Earlier work has pointed out the possibility of charge-transfer states in TTA-UC, which can be affected by the solvent environment.58 Excimers of conjugated molecules like perylene or pentacene are known to exhibit charge-transfer character.59−61 Polar solvent can therefore stabilize the excimer state and reduce the energy barrier of excimer formation.62,63 Alternatively, the polarity dependence can be due to a hydrophobic effect, explaining this observation.
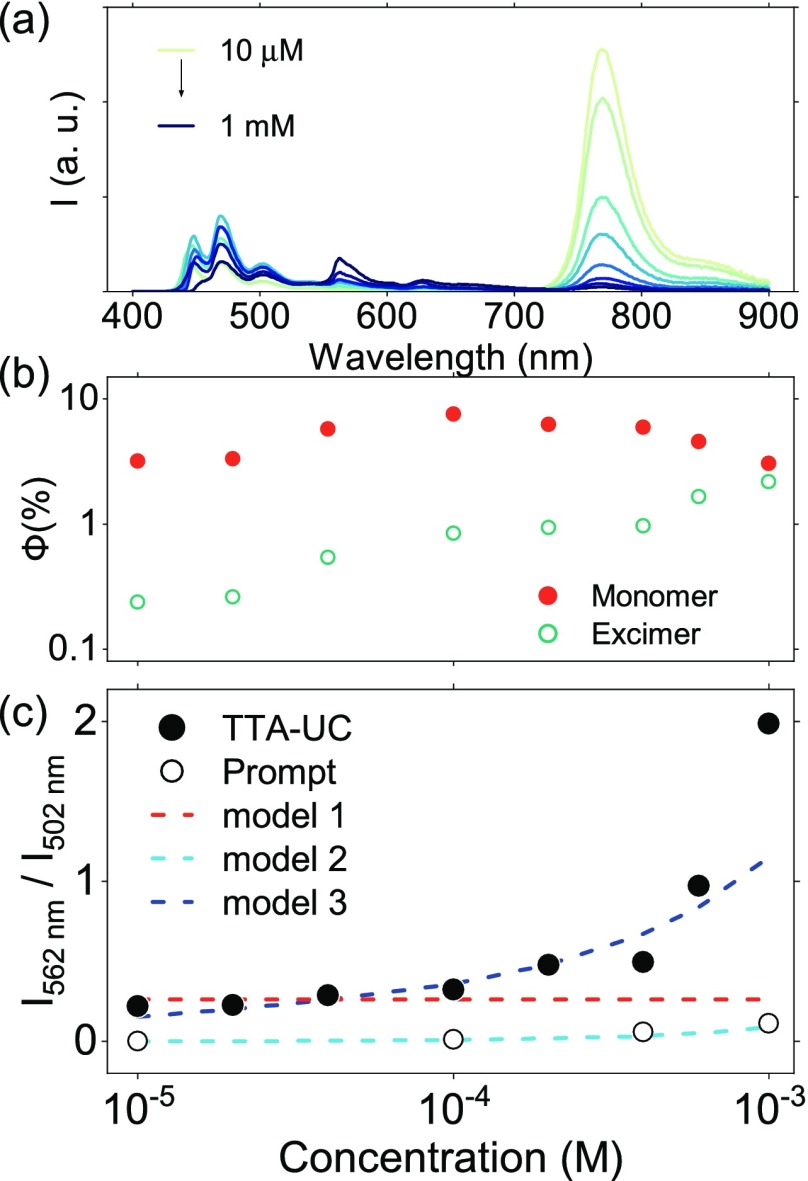
To gain further insights into the mechanism of excimer formation, we examined the TTA-UC efficiency at different annihilator concentrations. Of excimer mechanisms 1 and 2, only the latter should show a concentration dependence between the ratio of monomer and excimer emission. The higher the concentration, the lower the average annihilator–annihilator distance becomes, promoting processes involving diffusion. Studies of the concentration dependence of TTA-UC were performed with a constant concentration of PtTBTP and varying concentrations of perylene in THF (Figure 3). The ratio between excimer and monomer emission quantum yields is low but nonzero at low annihilator concentrations. When the annihilator concentration is increased, a dramatic increase of excimer emission intensity and a concurrent decrease of monomer emission were observed. The relative ratio between excimer and monomer emission is rather stable at low annihilator concentrations, but it increases fast at a few hundreds of micromolar of the annihilator. We also compared the emission spectra of the TTA-UC system with the prompt perylene emission of solutions of only annihilator having the same annihilator concentrations (Figure 3c). Stronger excimer emission was observed when generating the singlet excited state using TTA (Figure S6), indicating that excimer formation in TTA is inherited from the closely bound triplet pair. To start, the concentration-dependent data were fitted using kinetic models based on mechanisms 1 and 2 (Figure 3c; Note S1.6). On the one hand, the model based on mechanism 1 captures the low concentration regime well, but it is unable to describe any increase in excimer emission with concentration. The model based on mechanism 2, on the other hand, suggests that excimers do not form at low concentrations. This result is not surprising, since excimers are formed through diffusion. At higher concentrations, this model shows a slight increase in excimer emission with concentration. But the increase is not as large as the experimental result. However, this model successfully explains the excimer emission when directly exciting the annihilator (Figure 3c).
Figure 3.
(a) Emission spectra of mixtures of 10 μM of PtTBTP and 10, 20, 40, 100, 200, 400, 600, or 1000 μM of perylene in deoxygenated THF solution excited at 617 nm. (b) Quantum yield of monomer and excimer emission. (c) Ratios between excimer and monomer emission quantum yields. Experimental data are labeled with circles (excited at 617 or 380 nm), and lines show modeling using mechanisms 1–3.
Neither mechanism 1 nor 2 successfully explains the concentration dependence of the monomer–excimer emission ratio when upconverting. Furthermore, an excitation power dependence of the emission ratio was observed (Figure S7), which is not captured by these two mechanisms. An excitation power dependence indicates that the lifetime of the excited triplet state of the annihilator is of importance when modeling the emission ratio. The formation of an excited dimer on the triplet surface would be dependent on both the concentration and the lifetime of the excited triplet state. Furthermore, it can easily be envisioned that such a dimer would place the two annihilator molecules in a favorable geometry for excimer formation also on the singlet surface. Indeed, assuming that dimers can be formed on the triplet surface and that a triplet dimer–triplet annihilator encounter complex results in an excimer (note S1.6), both the concentration (Figure 3c) and power (Figure S7) dependence can successfully be explained. To summarize, studies of the concentration dependence of the excimer/monomer emission show that neither mechanism 1 nor 2 successfully explains the experimental results. However, also considering the power dependence of the excimer/monomer emission, it is likely that the excimer species originates from dimers already formed on the triplet surface before the annihilation event.
To show that the emission originates from the triplet pair, the excited-state energy was tracked from the excitation of the sensitizer to the final monomer and excimer emission. The dynamic information on triplet energy transfer from the sensitizer to the annihilator was obtained from the quenching effect of the sensitizer phosphorescence (Figure S8). The bimolecular quenching constant (the rate constant of triplet energy transfer) kTET was 1.38 × 109 M–1 s–1 in THF, which is in line with expectations for a diffusion-controlled reaction. Back energy transfer from excited singlet perylene to PtTBTP was also confirmed, where a long component of PtTBTP phosphorescence is present in the decay (Figure S9, Table S3). The back energy transfer reduces the overall upconversion quantum yield in TTA-UC systems, and strategies to minimize the effect are discussed in recent publications.20,23
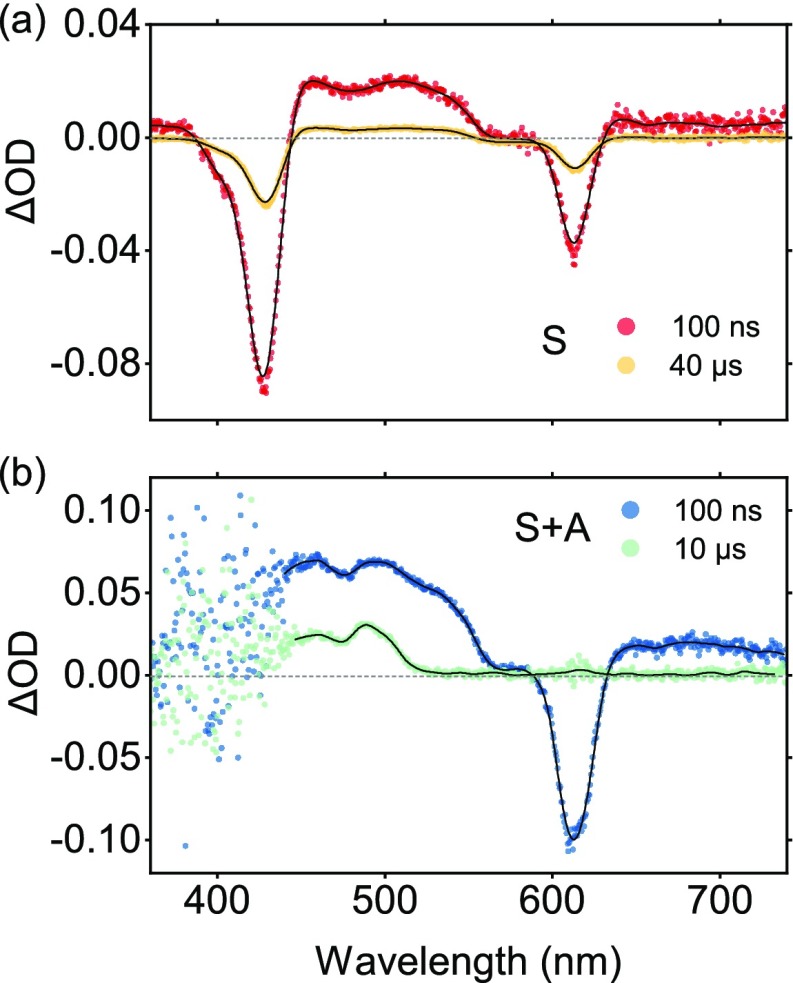
Because of the low quantum yield of the radiative decay of the triplet state of perylene, dynamic information cannot be directly obtained from emission. However, the high molar extinction coefficient of T1 – Tn transitions, allows the triplet state of perylene to be monitored with transient absorption spectroscopy. The comparison between sensitizer and sensitizer/annihilator transient spectra are illustrated in Figure 4. Two ground-state bleach (GSB) peaks can be observed at 430 and 610 nm for PtTBTP, which are in accordance with the S1 and S2 energies. Furthermore, PtTBTP also shows excited-state absorption at 460 and 512 nm due to T1 – Tn transitions (Figure 4a).64 All the GSB and excited state absorption peaks share the same decay kinetics, since all these transient signals are based on the T1 state of the sensitizer. In a sensitizer/annihilator solution, the decay of the triplet state of PtTBTP is dramatically accelerated (Figures 4b and S10). Furthermore, as the signal from PtTBTP diminishes, a new ESA signal appears at 485 nm (Figure 4b). This signal belongs to the absorption of the perylene triplet state, and it was used to monitor the decay of the triplet state of perylene.65
Figure 4.
Transient absorption spectra of (a) 10 μM PtTBTP or (b) 10 μM PtTBTP and 1 mM perylene in deoxygenated THF after excitation at 617 nm.
Perylene in its excited triplet state exhibits two possible classes of decay channels, single- or bimolecular.66,67 The intrinsic rate of relaxation (kT) is characteristic for the single-molecular decay channel, whereas the rate of triplet–triplet annihilation (kTTA) is characteristic for the bimolecular decay channel. The consumption rate of the annihilator in its excited triplet state can then be written as
| 1 |
where [3A*] is the concentration of the annihilator in its excited triplet state. The absorption of the annihilator in its triplet state was observed with transient absorption spectroscopy, and the concentration was calculated by the Beer–Lambert law using εT1_perylene(485 nm) = 13 400 M–1 cm–1.68 By solving the ordinary differential equation (eq 1), the rate of triplet–triplet annihilation was determined (Figure S10), resulting in a kTTA of 9.66 × 109 M–1 s–1 in THF, in line with a diffusion-controlled reaction.69 Monitoring the delayed fluorescence gave a similar value (Note S1.11).
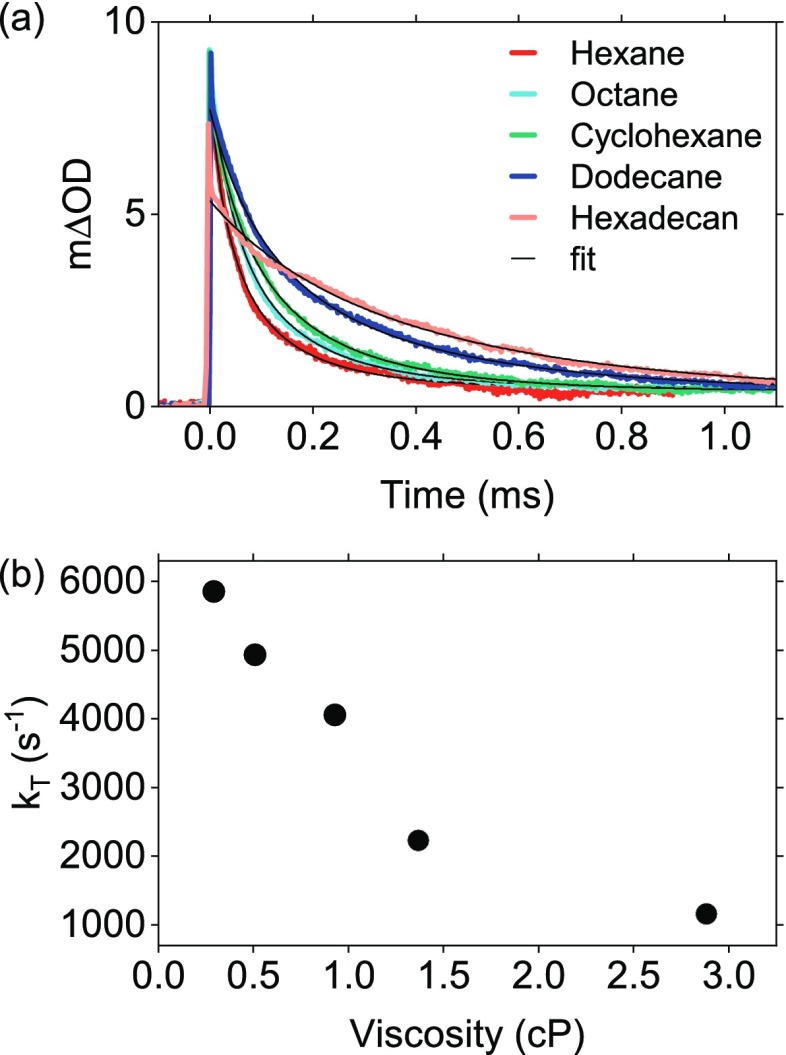
To get further insights into the surprisingly efficient upconversion in high-viscous solvents, transient absorption was performed using a set of alkane solvents (Figure 5). The calculated TTA rate constant deceases as expected when the solvent viscosity increases (Table S4). The TTA process is controlled by molecular diffusion and will therefore be restricted in viscous solvents. However, the TTA rate constant does not decease as much as the diffusion coefficient (Note S1.12, Table S4). This can be explained by a restriction of the diffuse dissociation and orientational relaxation of the triplet encounter complex in viscous solvents. Thus, the triplet pair encounter complex is kept at a favorable geometry for a longer time period. The intrinsic triplet decay rate constant of perylene is also reduced in viscous solvents (Figure 5b) due to a restriction of molecular vibrations.70 These effects will benefit the TTA-UC efficiency, explaining the observed high TTA-UC quantum yields in viscous solvents.
Figure 5.
(a) Transient absorption decays at 485 nm of 2 μM PtTBTP and 1 mM perylene in deoxygenated alkane solvents. (b) The calculated intrinsic triplet perylene decay rate constants vs solvent viscosity.
Conclusion
In conclusion, we have identified the mechanism of excimer formation during perylene-based photon upconversion. The excimer population is related to the annihilator concentration, irradiation power, and polarity of the solvent, indicating that the stability of the triplet pair and the excimer on both the singlet and triplet surfaces are a key factor for the construction of a high-efficiency upconversion systems. Furthermore, the upconversion efficiency is higher in viscous solvents, which is a surprising observation indicating that the rate of triplet pair formation is not solely determined by the rate of molecular diffusion. Further, we conclude from polarity-dependent upconversion measurements that low-polarity solvents are much preferred due to a lower amount of excimer emission. By investigating the mechanism of excimer formation in TTA-UC, we aim to bring more insight into the photophysical essence of this process, providing guidelines for achieving more efficient and reliable photon upconversion devices.
Acknowledgments
We gratefully acknowledge financial support from the Swedish Research council (2016-03354) and the European Research council (ERC-2017-StG-757733). M. Johnstone is acknowledged for proofreading the manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b02302.
Experimental section and instrument setup (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sugunan S. K.; Tripathy U.; Brunet S. M.; Paige M. F.; Steer R. P. Mechanisms of low-power noncoherent photon upconversion in metalloporphyrin-organic blue emitter systems in solution. J. Phys. Chem. A 2009, 113, 8548–8556. 10.1021/jp9034776. [DOI] [PubMed] [Google Scholar]
- Monguzzi A.; Tubino R.; Hoseinkhani S.; Campione M.; Meinardi F. Low power, non-coherent sensitized photon up-conversion: modelling and perspectives. Phys. Chem. Chem. Phys. 2012, 14, 4322–4332. 10.1039/c2cp23900k. [DOI] [PubMed] [Google Scholar]
- Singh-Rachford T. N.; Castellano F. N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. 10.1016/j.ccr.2010.01.003. [DOI] [Google Scholar]
- Pedrini J.; Monguzzi A. Recent advances in the application triplet-triplet annihilation-based photon upconversion systems to solar technologies. J. Photonics Energy 2018, 8, 022005. 10.1117/1.JPE.8.022005. [DOI] [Google Scholar]
- Frazer L.; Gallaher J. K.; Schmidt T. W. Optimizing the Efficiency of Solar Photon Upconversion. ACS Energy Lett. 2017, 2, 1346–1354. 10.1021/acsenergylett.7b00237. [DOI] [Google Scholar]
- Hill S. P.; Hanson K. Harnessing Molecular Photon Upconversion in a Solar Cell at Sub-solar Irradiance: Role of the Redox Mediator. J. Am. Chem. Soc. 2017, 139, 10988–10991. 10.1021/jacs.7b05462. [DOI] [PubMed] [Google Scholar]
- Tayebjee M. J. Y.; McCamey D. R.; Schmidt T. W. Beyond Shockley-Queisser: Molecular Approaches to High-Efficiency Photovoltaics. J. Phys. Chem. Lett. 2015, 6, 2367–2378. 10.1021/acs.jpclett.5b00716. [DOI] [PubMed] [Google Scholar]
- Dilbeck T.; Hanson K. Molecular Photon Upconversion Solar Cells Using Multilayer Assemblies: Progress and Prospects. J. Phys. Chem. Lett. 2018, 9, 5810–5821. 10.1021/acs.jpclett.8b02635. [DOI] [PubMed] [Google Scholar]
- Schulze T. F.; Schmidt T. W. Photochemical upconversion: present status and prospects for its application to solar energy conversion. Energy Environ. Sci. 2015, 8, 103–125. 10.1039/C4EE02481H. [DOI] [Google Scholar]
- Börjesson K.; Dzebo D.; Albinsson B.; Moth-Poulsen K. Photon upconversion facilitated molecular solar energy storage. J. Mater. Chem. A 2013, 1, 8521–8524. 10.1039/c3ta12002c. [DOI] [Google Scholar]
- Monguzzi A.; Oertel A.; Braga D.; Riedinger A.; Kim D. K.; Knusel P. N.; Bianchi A.; Mauri M.; Simonutti R.; Norris D. J.; Meinardi F. Photocatalytic Water-Splitting Enhancement by Sub-Bandgap Photon Harvesting. ACS Appl. Mater. Interfaces 2017, 9, 40180–40186. 10.1021/acsami.7b10829. [DOI] [PubMed] [Google Scholar]
- Barawi M.; Fresno F.; Pérez-Ruiz R.; de la Peña O’Shea V. A. Photoelectrochemical Hydrogen Evolution Driven by Visible-to-Ultraviolet Photon Upconversion. ACS Appl. Energy Mater. 2019, 2, 207–211. 10.1021/acsaem.8b01916. [DOI] [Google Scholar]
- Ravetz B. D.; Pun A. B.; Churchill E. M.; Congreve D. N.; Rovis T.; Campos L. M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. 10.1038/s41586-018-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. A.; Hatchard C. G.. Proc. R. Soc. London, Ser. A, 1962, 386–387 10.1098/rspa.1962.0197. [DOI] [Google Scholar]
- Duan P.; Yanai N.; Kimizuka N. Photon upconverting liquids: matrix-free molecular upconversion systems functioning in air. J. Am. Chem. Soc. 2013, 135, 19056–19059. 10.1021/ja411316s. [DOI] [PubMed] [Google Scholar]
- Börjesson K.; Rudquist P.; Gray V.; Moth-Poulsen K. Photon upconversion with directed emission. Nat. Commun. 2016, 7, 12689. 10.1038/ncomms12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamitsu S.; Yanai N.; Kimizuka N. Photon-Upconverting Ionic Liquids: Effective Triplet Energy Migration in Contiguous Ionic Chromophore Arrays. Angew. Chem., Int. Ed. 2015, 54, 11550–11554. 10.1002/anie.201505168. [DOI] [PubMed] [Google Scholar]
- Vadrucci R.; Monguzzi A.; Saenz F.; Wilts B. D.; Simon Y. C.; Weder C. Nanodroplet-Containing Polymers for Efficient Low-Power Light Upconversion. Adv. Mater. 2017, 29, 1702992. 10.1002/adma.201702992. [DOI] [PubMed] [Google Scholar]
- Wu M.; Congreve D. N.; Wilson M. W. B.; Jean J.; Geva N.; Welborn M.; Van Voorhis T.; Bulović V.; Bawendi M. G.; Baldo M. A. Solid-state infrared-to-visible upconversion sensitized by colloidal nanocrystals. Nat. Photonics 2016, 10, 31–34. 10.1038/nphoton.2015.226. [DOI] [Google Scholar]
- Gray V.; Börjesson K.; Dzebo D.; Abrahamsson M.; Albinsson B.; Moth-Poulsen K. Porphyrin-Anthracene Complexes: Potential in Triplet-Triplet Annihilation Upconversion. J. Phys. Chem. C 2016, 120, 19018–19026. 10.1021/acs.jpcc.6b06298. [DOI] [Google Scholar]
- Dzebo D.; Börjesson K.; Gray V.; Moth-Poulsen K.; Albinsson B. Intramolecular Triplet-Triplet Annihilation Upconversion in 9,10-Diphenylanthracene Oligomers and Dendrimers. J. Phys. Chem. C 2016, 120, 23397–23406. 10.1021/acs.jpcc.6b07920. [DOI] [Google Scholar]
- Bharmoria P.; Hisamitsu S.; Nagatomi H.; Ogawa T.; Morikawa M. A.; Yanai N.; Kimizuka N. Simple and Versatile Platform for Air-Tolerant Photon Upconverting Hydrogels by Biopolymer-Surfactant-Chromophore Co-assembly. J. Am. Chem. Soc. 2018, 140, 10848–10855. 10.1021/jacs.8b05821. [DOI] [PubMed] [Google Scholar]
- Ogawa T.; Hosoyamada M.; Yurash B.; Nguyen T. Q.; Yanai N.; Kimizuka N. Donor-Acceptor-Collector Ternary Crystalline Films for Efficient Solid-State Photon Upconversion. J. Am. Chem. Soc. 2018, 140, 8788–8796. 10.1021/jacs.8b04542. [DOI] [PubMed] [Google Scholar]
- Kitazawa M.; Yabe T.; Hirata Y.; Okada T. Solvent viscosity dependence of bimolecular reaction rate constant of the excited 9-cyanoanthracene quenched by 1,3-cyclohexadiene. J. Mol. Liq. 1995, 65–66, 321–324. 10.1016/0167-7322(95)00823-3. [DOI] [Google Scholar]
- Baluschev S.; Miteva T.; Yakutkin V.; Nelles G.; Yasuda A.; Wegner G. Up-conversion fluorescence: noncoherent excitation by sunlight. Phys. Rev. Lett. 2006, 97, 143903. 10.1103/PhysRevLett.97.143903. [DOI] [PubMed] [Google Scholar]
- Gray V.; Moth-Poulsen K.; Albinsson B.; Abrahamsson M. Towards efficient solid-state triplet-triplet annihilation based photon upconversion: Supramolecular, macromolecular and self-assembled systems. Coord. Chem. Rev. 2018, 362, 54–71. 10.1016/j.ccr.2018.02.011. [DOI] [Google Scholar]
- Di D.; Yang L.; Richter J. M.; Meraldi L.; Altamimi R. M.; Alyamani A. Y.; Credgington D.; Musselman K. P.; MacManus-Driscoll J. L.; Friend R. H. Efficient Triplet Exciton Fusion in Molecularly Doped Polymer Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605987. 10.1002/adma.201605987. [DOI] [PubMed] [Google Scholar]
- Cui X.; Zhao J.; Zhou Y.; Ma J.; Zhao Y. Reversible Photoswitching of Triplet-Triplet Annihilation Upconversion Using Dithienylethene Photochromic Switches. J. Am. Chem. Soc. 2014, 136, 9256–9259. 10.1021/ja504211y. [DOI] [PubMed] [Google Scholar]
- Askes S. H. C.; Bonnet S. Solving the oxygen sensitivity of sensitized photon upconversion in life science applications. Nature Reviews Chemistry 2018, 2, 437–452. 10.1038/s41570-018-0057-z. [DOI] [Google Scholar]
- Hoseinkhani S.; Tubino R.; Meinardi F.; Monguzzi A. Achieving the photon up-conversion thermodynamic yield upper limit by sensitized triplet-triplet annihilation. Phys. Chem. Chem. Phys. 2015, 17, 4020–4024. 10.1039/C4CP03936J. [DOI] [PubMed] [Google Scholar]
- Gray V.; Dzebo D.; Abrahamsson M.; Albinsson B.; Moth-Poulsen K. Triplet-triplet annihilation photon-upconversion: towards solar energy applications. Phys. Chem. Chem. Phys. 2014, 16, 10345–10352. 10.1039/C4CP00744A. [DOI] [PubMed] [Google Scholar]
- Cui X.; El-Zohry A. M.; Wang Z.; Zhao J.; Mohammed O. F. Homo- or Hetero-Triplet-Triplet Annihilation? A Case Study with Perylene-BODIPY Dyads/Triads. J. Phys. Chem. C 2017, 121, 16182–16192. 10.1021/acs.jpcc.7b05620. [DOI] [Google Scholar]
- Sasaki Y.; Amemori S.; Kouno H.; Yanai N.; Kimizuka N. Near infrared-to-blue photon upconversion by exploiting direct S-T absorption of a molecular sensitizer. J. Mater. Chem. C 2017, 5, 5063–5067. 10.1039/C7TC00827A. [DOI] [Google Scholar]
- Zhong F.; Zhao J. Phenyleneanthracene derivatives as triplet energy acceptor/emitter in red light excitable triplet-triplet-annihilation upconversion. Dyes Pigm. 2017, 136, 909–918. 10.1016/j.dyepig.2016.09.057. [DOI] [Google Scholar]
- Wang Z.; Zhao J. Bodipy-Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet-Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. 10.1021/acs.orglett.7b02047. [DOI] [PubMed] [Google Scholar]
- Liu L.; Guo S.; Ma J.; Xu K.; Zhao J.; Zhang T. Broadband Visible-Light-Harvesting trans-Bis(alkylphosphine) Platinum(II)-Alkynyl Complexes with Singlet Energy Transfer between BODIPY and Naphthalene Diimide Ligands. Chem. - Eur. J. 2014, 20, 14282–14295. 10.1002/chem.201403780. [DOI] [PubMed] [Google Scholar]
- Dover C. B.; Gallaher J. K.; Frazer L.; Tapping P. C.; Petty A. J.; Crossley M. J.; Anthony J. E.; Kee T. W.; Schmidt T. W. Endothermic singlet fission is hindered by excimer formation. Nat. Chem. 2018, 10, 305–310. 10.1038/nchem.2926. [DOI] [PubMed] [Google Scholar]
- Chan W. L.; Ligges M.; Jailaubekov A.; Kaake L.; Miaja-Avila L.; Zhu X. Y. Observing the multiexciton state in singlet fission and ensuing ultrafast multielectron transfer. Science 2011, 334, 1541–1545. 10.1126/science.1213986. [DOI] [PubMed] [Google Scholar]
- Monahan N. R.; Sun D.; Tamura H.; Williams K. W.; Xu B.; Zhong Y.; Kumar B.; Nuckolls C.; Harutyunyan A. R.; Chen G.; Dai H. L.; Beljonne D.; Rao Y.; Zhu X. Y. Dynamics of the triplet-pair state reveals the likely coexistence of coherent and incoherent singlet fission in crystalline hexacene. Nat. Chem. 2017, 9, 341–346. 10.1038/nchem.2665. [DOI] [PubMed] [Google Scholar]
- Bayliss S. L.; Weiss L. R.; Mitioglu A.; Galkowski K.; Yang Z.; Yunusova K.; Surrente A.; Thorley K. J.; Behrends J.; Bittl R.; Anthony J. E.; Rao A.; Friend R. H.; Plochocka P.; Christianen P. C. M.; Greenham N. C.; Chepelianskii A. D. Site-selective measurement of coupled spin pairs in an organic semiconductor. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 5077–5082. 10.1073/pnas.1718868115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel T.; Rinn A.; Sharifzadeh S.; da Jornada F. H.; Pick A.; Louie S. G.; Witte G.; Kronik L.; Neaton J. B.; Chatterjee S. Low-lying excited states in crystalline perylene. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 284–289. 10.1073/pnas.1711126115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco C.; Doucette G. S.; Munro J. M.; Kennehan E. R.; Lee Y.; Rimshaw A.; Payne M. M.; Wonderling N.; Anthony J. E.; Dabo I.; Gomez E. D.; Asbury J. B. Triplet Transfer Mediates Triplet Pair Separation during Singlet Fission in 6,13-Bis(triisopropylsilylethynyl)-Pentacene. Adv. Funct. Mater. 2017, 27, 1703929. 10.1002/adfm.201703929. [DOI] [Google Scholar]
- Sung J.; Kim P.; Fimmel B.; Wurthner F.; Kim D. Direct observation of ultrafast coherent exciton dynamics in helical pi-stacks of self-assembled perylene bisimides. Nat. Commun. 2015, 6, 8646. 10.1038/ncomms9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. B. Excimers and Exciplexes. Nature 1967, 214, 1187. 10.1038/2141187a0. [DOI] [Google Scholar]
- Mutsamwira S.; Ainscough E. W.; Partridge A. C.; Derrick P. J.; Filichev V. V. G-Quadruplex Supramolecular Assemblies in Photochemical Upconversion. Chem. - Eur. J. 2016, 22, 10376–10381. 10.1002/chem.201601353. [DOI] [PubMed] [Google Scholar]
- Casillas R.; Adam M.; Coto P. B.; Waterloo A. R.; Zirzlmeier J.; Reddy S. R.; Hampel F.; McDonald R.; Tykwinski R. R.; Thoss M.; Guldi D. M. Intermolecular Singlet Fission in Unsymmetrical Derivatives of Pentacene in Solution. Adv. Energy Mater. 2019, 9, 1802221. 10.1002/aenm.201802221. [DOI] [Google Scholar]
- Williams E. L.; Haavisto K.; Li J.; Jabbour G. E. Excimer-Based White Phosphorescent Organic Light-Emitting Diodes with Nearly 100% Internal Quantum Efficiency. Adv. Mater. 2007, 19, 197–202. 10.1002/adma.200602174. [DOI] [Google Scholar]
- Yanai N.; Kimizuka N. New Triplet Sensitization Routes for Photon Upconversion: Thermally Activated Delayed Fluorescence Molecules, Inorganic Nanocrystals, and Singlet-to-Triplet Absorption. Acc. Chem. Res. 2017, 50, 2487–2495. 10.1021/acs.accounts.7b00235. [DOI] [PubMed] [Google Scholar]
- Singh-Rachford T. N.; Castellano F. N. Triplet Sensitized Red-to-Blue Photon Upconversion. J. Phys. Chem. Lett. 2010, 1, 195–200. 10.1021/jz900170m. [DOI] [Google Scholar]
- Nakagawa K.; Numata Y.; Ishino H.; Tanaka D.; Kobayashi T.; Tokunaga E. Excimer Luminescence From Nonresonantly Excited Pyrene and Perylene Molecules in Solution. J. Phys. Chem. A 2013, 117, 11449–11455. 10.1021/jp402330n. [DOI] [PubMed] [Google Scholar]
- Monguzzi A.; Mezyk J.; Scotognella F.; Tubino R.; Meinardi F. Upconversion-induced fluorescence in multicomponent systems: Steady-state excitation power threshold. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 78, 78i. 10.1103/PhysRevB.78.195112. [DOI] [Google Scholar]
- Yokoyama K.; Wakikawa Y.; Miura T.; Fujimori J.; Ito F.; Ikoma T. Solvent Viscosity Effect on Triplet-Triplet Pair in Triplet Fusion. J. Phys. Chem. B 2015, 119, 15901–15908. 10.1021/acs.jpcb.5b11208. [DOI] [PubMed] [Google Scholar]
- Reichardt C.Appendix. In Solvents and Solvent Effects in Organic Chemistry ,3rd ed.; Wiley-VCH Verlag GmbH & Co., 2004; pp 471–507. [Google Scholar]
- Wypych G.Fundamental Principles Governing Solvents Use. In Handbook of Solvents ,3rd ed.; Wypych G., Ed. ChemTec Publishing, 2019; Vol. 1, pp 11–77. [Google Scholar]
- Murakami Y.; Das S. K.; Himuro Y.; Maeda S. Triplet-sensitized photon upconversion in deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 30603–30615. 10.1039/C7CP06494B. [DOI] [PubMed] [Google Scholar]
- Gray V.; Dreos A.; Erhart P.; Albinsson B.; Moth-Poulsen K.; Abrahamsson M. Loss channels in triplet-triplet annihilation photon upconversion: importance of annihilator singlet and triplet surface shapes. Phys. Chem. Chem. Phys. 2017, 19, 10931–10939. 10.1039/C7CP01368J. [DOI] [PubMed] [Google Scholar]
- Mongin C.; Golden J. H.; Castellano F. N. Liquid PEG Polymers Containing Antioxidants: A Versatile Platform for Studying Oxygen-Sensitive Photochemical Processes. ACS Appl. Mater. Interfaces 2016, 8, 24038–24048. 10.1021/acsami.6b05697. [DOI] [PubMed] [Google Scholar]
- Frink M. E.; Geiger D. K.; Ferraudi G. J. Excimer formation from triplet-triplet annihilation reactions of the lowest-lying triplet excited state in aluminum(III), silicon(IV), and metal-free phthalocyanines: medium and magnetic field effects on the rate of reaction. J. Phys. Chem. 1986, 90, 1924–1927. 10.1021/j100400a036. [DOI] [Google Scholar]
- Furube A.; Murai M.; Tamaki Y.; Watanabe S.; Katoh R. Effect of aggregation on the excited-state electronic structure of perylene studied by transient absorption spectroscopy. J. Phys. Chem. A 2006, 110, 6465–6471. 10.1021/jp060649b. [DOI] [PubMed] [Google Scholar]
- Cook R. E.; Phelan B. T.; Kamire R. J.; Majewski M. B.; Young R. M.; Wasielewski M. R. Excimer Formation and Symmetry-Breaking Charge Transfer in Cofacial Perylene Dimers. J. Phys. Chem. A 2017, 121, 1607–1615. 10.1021/acs.jpca.6b12644. [DOI] [PubMed] [Google Scholar]
- Basel B. S.; Zirzlmeier J.; Hetzer C.; Reddy S. R.; Phelan B. T.; Krzyaniak M. D.; Volland M. K.; Coto P. B.; Young R. M.; Clark T.; Thoss M.; Tykwinski R. R.; Wasielewski M. R.; Guldi D. M. Evidence for Charge-Transfer Mediation in the Primary Events of Singlet Fission in a Weakly Coupled Pentacene Dimer. Chem. 2018, 4, 1092–1111. 10.1016/j.chempr.2018.04.006. [DOI] [Google Scholar]
- Tamai Y.; Ohkita H.; Shimada J.; Benten H.; Ito S.; Yamanaka S.; Hisada K.; Tani K.; Kubono K.; Shinmyozu T. Dynamical Excimer Formation in Rigid Carbazolophane via Charge Transfer State. J. Phys. Chem. A 2013, 117, 7776–7785. 10.1021/jp402126a. [DOI] [PubMed] [Google Scholar]
- Brown K. E.; Salamant W. A.; Shoer L. E.; Young R. M.; Wasielewski M. R. Direct Observation of Ultrafast Excimer Formation in Covalent Perylenediimide Dimers Using Near-Infrared Transient Absorption Spectroscopy. J. Phys. Chem. Lett. 2014, 5, 2588–2593. 10.1021/jz5011797. [DOI] [PubMed] [Google Scholar]
- Magde D.; Brannon J. H.; Cremers T. L.; Olmsted J. Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 1979, 83, 696–699. 10.1021/j100469a012. [DOI] [Google Scholar]
- Steren C. A.; van Willigen H.; Biczók L.; Gupta N.; Linschitz H. C60 as a Photocatalyst of Electron-Transfer Processes: Reactions of Triplet C60 with Chloranil, Perylene, and Tritolylamine Studied by Flash Photolysis and FT-EPR. J. Phys. Chem. 1996, 100, 8920–8926. 10.1021/jp960640h. [DOI] [Google Scholar]
- Staroske W.; Pfeiffer M.; Leo K.; Hoffmann M. Single-step triplet-triplet annihilation: an intrinsic limit for the high brightness efficiency of phosphorescent organic light emitting diodes. Phys. Rev. Lett. 2007, 98, 197402. 10.1103/PhysRevLett.98.197402. [DOI] [PubMed] [Google Scholar]
- Schmidt T. W.; Castellano F. N. Photochemical Upconversion: The Primacy of Kinetics. J. Phys. Chem. Lett. 2014, 5, 4062–4072. 10.1021/jz501799m. [DOI] [PubMed] [Google Scholar]
- Carmichael I.; Helman W. P.; Hug G. L. Extinction Coefficients of Triplet-Triplet Absorption Spectra of Organic Molecules in Condensed Phases: A Least-Squares Analysis. J. Phys. Chem. Ref. Data 1987, 16, 239–260. 10.1063/1.555782. [DOI] [Google Scholar]
- Deng F.; Blumhoff J.; Castellano F. N. Annihilation limit of a visible-to-UV photon upconversion composition ascertained from transient absorption kinetics. J. Phys. Chem. A 2013, 117, 4412–4419. 10.1021/jp4022618. [DOI] [PubMed] [Google Scholar]
- Nickel B.; Wilhelm H. E.; Ruth A. A. Anti-Smoluchowski time dependence of the delayed fluorescence from anthracene in viscous solution due to triplet-triplet annihilation. Chem. Phys. 1994, 188, 267–287. 10.1016/0301-0104(94)00240-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.