Abstract
Background:
Lung cancer is an important public health issue, particularly among American Indians (Als). The reported decline in tobacco use for most racial/ethnic groups is not observed among Als. This project was designed to address the research question, “Why don’t more Northern Plains American Indians alter tobacco use behaviors known to increase the risk of cancer?”
Methods:
Guided by the Theory of Planned Behavior, a multi-component intervention study was implemented. Adult AIs, age 18years or older and currently smoking, were enrolled. Eligible subjects were randomized to one of 15 groups and exposed to either a MINIMAL or an INTENSE level of 4 intervention components. The intervention was delivered face-to-face or via telephone by Patient Navigators (PN). The primary outcome was self-reported abstinence from smoking verified by carbon monoxide measurement.
Results:
At18 months post-quit date, 88% of those who were still in the study were abstinent. This included 6% of all participants who enrolled in the study (14/254) and 13% of those who made it to the quit date (14/108). No intervention groups were found to have significant proportions of participants who were abstinent from smoking at the quit date (visit 5) or primary outcome visit (18 months post-quit date, visit 11), but use of pharmacologic support for abstinence was found to be an effective strategy for individuals who continued participation throughout the study. Those who remained in the study received more visits and were more likely to be abstinent.
Conclusions:
Use of NRT increased the odds of not smoking, as assessed at the 18-month follow-up visit, but no other interventions were found to significantly contribute to abstinence from smoking. Although the intervention protocol included numerous points of contact between CRRs and participants (11 visits) loss to follow-up was extensive with only 16/254 remaining enrolled. Additional research is needed to improve understanding of factors that influence enrollment and retention in smoking cessation interventions for AI and other populations.
1. Introduction
Smoking is the leading cause of preventable death worldwide and a leading cause of many types of cancer [1]. Smoking-related morbidity and mortality are especially important in American Indian (AI) communities where the daily cigarette use is nearly double that of non-Hispanic Whites [2] and the declines in tobacco use reported for most racial/ethnic groups are not seen in AIs [3]. Additionally, AIs diagnosed with lung cancer are more likely to die and to die more rapidly than non-Hispanic Whites [4]. The profound health, economic, and human costs of tobacco use and dependence make prevention and cessation a top priority.
The overall smoking rates in AIs mask dramatic variation by region and tribe and suggest that community-specific factors may be important. Data from the 2016 BRFSS showed an AI smoking prevalence rate of 13.8% in New Mexico, 38.9%in Minnesota, 24.3% in Oklahoma, and 48.7% in South Dakota, compared to 17% over all race/ethnic groups in the nation [5]. Of particular concern are age-related patterns of smoking initiation during adolescence and young adulthood seen among Northern Plains AIs in the EARTH study birth cohorts [6]. This study revealed a consistent increase in the incidence of smoking initiation by age 18 which is more likely to lead to long-term tobacco use, future nicotine dependence, and an increased risk for lung cancer.
AIs persist in using commercial tobacco for a variety of reasons including reluctance to tell others not to smoke, liking the effects of smoking, and norms about the acceptance of smoking even during ceremonies [7]. A study of Northern Plains AIs showed that many AIs have lenient attitudes about cigarette smoking, perceive smoking to be less harmful than identified, and engage in smoking for pleasure, taste, and enjoyment of the rituals associated with smoking [8]. In addition, AIs have negative attitudes about modern Western medicine, including cessation medications [9].
Careful integration of cultural characteristics in smoking cessation programs has been widely cited in the literature in the recent past. In the first known smoking cessation trial conducted in an American Indian/Alaska Native tribal community that combined FDA-approved cessation medication (varenicline) and culturally specific intervention components, 6 months abstinence rates of 20% were found among all subjects with a 42% abstinence rate in the responder-only analysis [10]. In a study to assess the relative effectiveness of a culturally tailored smoking cessation program compared to a non-tailored current best practices approach, self-reported abstinence rates at 12 weeks and 6 months were higher in the culturally-tailored arm, but no differences were founds for cotinine-verified abstinence rates [11].
This study was designed to focus on the NCI Provocative Question “Why don’t more people alter behaviors known to increase the risk of cancers?” through research questions focusing on the impact of culturally appropriate tobacco use education and support interventions. These include multiple forms of social support and education on smoking cessation among Northern Plains American Indians. The study focused on the effectiveness of 4 smoking cessation strategies: 1) Nicotine Replacement Therapy; 2) pre-cessation counseling education sessions; 3) post-cessation counseling education sessions; and, (4) mHealth education and support through text messages. Guided by the Theory of Planned Behavior (TPB) [12] and organized using the Phase-based Framework for Smoking Cessation [13], a multi-component intervention study was carried out. The TPB is a model that helps identify how behavior change is influenced by intentions and social norms. We used this model to identify AI specific variables that contribute to explaining smoking behavior and challenges to abstaining from tobacco use. We describe here the study design, intervention components, baseline characteristics, and results from this study.
2. Methods
The study design and intervention have been described in a previous publication [14]. In brief, the study was implemented with 254 AI smokers residing in 3 South Dakota sites: Pine Ridge (Oglala Sioux Tribe) and Rosebud (Rosebud Sioux Tribe) Reservations and Rapid City (see Fig. 1). The study was approved by the Regional Health Institutional Review Board (IRB), July 30, 2013), Avera Health IRB (February 9, 2016), Great Plains Area Indian Health Service IRB (August 16, 2013) Rosebud Sioux Tribe Health Board and the Oglala Sioux Tribe Research and Review Board (August 30, 2013). All subjects provided written informed consent. Subjects were assigned at random to one of 15 groups that include combinations of minimal or intense levels of 4 interventions including Nicotine Replacement Therapy (NRT), Pre-cessation Counseling, Post-cessation Counseling, and mHealth text messages (Table 1). Power analysis was used to guide the number of participants assigned to each treatment group. The primary outcome selected for power calculations was abstinence from smoking at the quit date (visit 5). The power analysis showed that at least 240 participants were needed to provide 80% power at a 2-sided significance level of 5%, assuming an ICC as high as 0.025 for detecting absolute differences of 20% in the proportion of subjects abstaining from smoking. Each participant was provided with a study cell phone and phone service to receive and respond to mHealth text messages. Thus, the study design was implemented as a 2×2×2×2 incomplete factorial design with 15 possible treatment combinations. The effect of the incomplete factorial design on factorial contrasts is minimal [15].
Fig. 1.
Areas where the study was implemented.
Table 1.
Study design: intervention components and interventions by study group.
| Study group | Component 1 | Component 2 | Component 3 | Component 4 | All Groupsa |
|---|---|---|---|---|---|
| Cessation NRT | Precessation telephone counseling | Cessation in-person counseling | mHealth text messaging | Maintenance medication for 16weeks | |
| 1 | Intense | Intense | Intense | Intense | |
| 2 | Intense | Intense | Intense | Minimal | |
| 3 | Intense | Intense | Minimal | Minimal | |
| 4 | Intense | Intense | Minimal | Intense | |
| 5 | Intense | Minimal | Intense | Intense | |
| 6 | Intense | Minimal | Intense | Minimal | |
| 7 | Intense | Minimal | Minimal | Intense | |
| 8 | Intense | Minimal | Minimal | Minimal | |
| 9 | Minimal | Intense | Intense | Intense | |
| 10 | Minimal | Intense | Intense | Minimal | |
| 11 | Minimal | Intense | Minimal | Minimal | |
| 12 | Minimal | Intense | Minimal | Intense | |
| 13 | Minimal | Minimal | Intense | Intense | |
| 14 | Minimal | Minimal | Minimal | Intense | |
| 15 | Minimal | Minimal | Minimal | Minimal | |
All groups because research shows maintenance medication is recommended for all cessation participants.
2.1. Recruitment procedures
Potential subjects self-identified and self-referred following promotions disseminated via radio, newspaper, and flyers at community and social events, at activities sponsored by the Northern Plains Comprehensive Cancer Control Program, at Indian Health Service and Tribal and Urban Indian clinics, and at markets, casinos, tribal head-quarters, and chapter houses. Subjects on Rosebud Reservation were also referred by pharmacists and healthcare providers. All promotional materials referred the potential subject to a Community Research Representative (CRR, referred to as Patient Navigators in other programs) at each of the 3 study sites. The CRRs met with subjects face-to-face where they explained the study, answered questions, obtained informed consent for participation and administered a baseline carbon monoxide breath test to assess smoking status. The initial visit concluded with administration of a 178 -item baseline survey that contained demographic, smoking history, nicotine dependence as measured by the Fagerström Test for Nicotine Dependence (FTND) [16] and attitudes, beliefs, and cultural questions.
2.2. Intervention delivery
All intervention visits were conducted by the CRR either in person or by telephone. Each CRR was equipped with an iPad pre-loaded with informed consent documents, instructions for each study visit by study group, and all survey items and process evaluation queries. The CRR also had hard copies of the outlines used for the counseling sessions as well as copies of the study visit schedules and the protocol. Once the CRR completed the baseline visit, the participant was randomized into one of the 15 groups.
Significant cultural modifications were made to the survey items, the mHealth text messaging and to the Motivational Interviewing (MI) counseling outlines. Each setting (see Fig. 1) had a community advisory committee that provided guidance throughout the first 18 months of the study. Additionally, two series of usability tests were conducted in each of the three project settings: one on survey items and the other on text messages to ensure materials were culturally congruent with the cultural beliefs and norms of the AIs residing in the study settings. Three focus groups were conducted to review the draft survey items, mHealth messages and/or MI Counseling outlines. All text messages were categorized into (1) generation information/statistics, (2) general motivation, (3) strategies and (4) traditional American Indian perspectives” and cross-listed by the “type of message” (e.g., crave, tips, slip, mood, motivation and quotes and for phase of quitting (pre-cessation, cessation, post-cessation, maintenance)). The majority of AI-focused messages were within “motivation”, but others were integrated within the other categories (see Fig. 1 for examples). The revised list of text messages was approximately 438 of which about one-half were AI-specific.
Table 2 shows the schema for delivery of the 11-visit intervention. The MINIMAL and INTENSE levels of intervention delivery were as follows: for NRT, the MINIMAL level provided 1 NRT product and the INTENSE level 2 NRT products; for pre-cessation counseling, the MINIMAL level was 2 counseling sessions and the INTENSE level was 3 counseling sessions; for post-cessation counseling, the MINIMAL level was counseling on the quit date plus 2 additional sessions and INTENSE was counseling on the quit date plus 3 more sessions. The MINIMAL level of the mHealth intervention component was 2 text messages daily (a morning query requiring a response and an additional message) and the INTENSE level was 4 text messages daily (morning query plus 3 additional messages). For the NRT intervention, the original plan had been to include prescription smoking cessation medications, however their lack of availability for free on the reservations, precluded this option, so only nicotine gum, lozenge and/or patch were used.
Table 2.
Intervention schema.
| Visit | When | Format | Time (minutes) | aIntervention Levels | mHealth | Measures | |
|---|---|---|---|---|---|---|---|
| None | Survey | Cotinine | |||||
| 1 | Baseline | In-person | 45 | M & I | MINIMAL =2/day | Full | Yes |
| 2 | 3 weeks before quit date | In-person | 45 | M & I | INTENSE = 4/day | Interim | Yes |
| 3 | 1 week before quit date | Telephone | 45 | M&I | MINIMAL =2/day | Interim | No |
| 4 | 2 days before quit date | Telephone | 45 | I only | INTENSE = 4/day | Interim | No |
| 5 | Quit date | In-person | 45 | M&I | INTENSE = 4/day | Interim | Yes |
| 6 | 7 days after quit date | In-person | 45 | M&I | MINIMAL =2/day | Interim | Yes |
| 7 | 14days after quit date | In-person | 45 | M&I | INTENSE = 4/day | Interim | Yes |
| 8 | 3 months after quit date | Telephone | 10 | I only | MINIMAL =2/day | Interim | No |
| 9 | 6 months after quit date | In-person | 45 | M&I | INTENSE = 4/day | Follow-up | Yes |
| 10 | 12months after quit date | In-person | 45 | M&I | INTENSE = 4/day | Follow-up | Yes |
| 11 | 18 months after quit date | In-person | 45 | M&I | INTENSE = 4/day | Follow-up | Yes |
Full survey included 178 items with skip items, Interim and follow-up survey included between 15 and 60 items.
Intervention Levels; M = MINIMAL I = INTENSE.
2.3. Primary and secondary outcome measures
The primary outcome measure to assess intervention efficacy was smoking cessation (abstinence). This was determined by asking subjects if they smoked cigarettes on follow-up visits, starting with the quit date (visit 5) through the 18-month follow-up visit. Participants were asked “Have you smoked any cigarettes today, even a single puff?” at the quit date (visit 5) and each follow-up visit (visit 6–11). The treatment period for this project spanned from the initial visit (visit 1) to the 18-month follow-up visit (visit 11). The follow-up assessment period overlapped the treatment period and spanned from the quit date (visit 5) to the 18-month follow-up visit (visit 11). The smoking status was also biochemically verified by CO monitoring for cotinine level during selected visits (see Table 2). Values of < 10 ppm were considered confirmatory of abstinence from smoking. Secondary outcome measures were levels of receipt of the 4 intervention strategy combinations associated with smoking cessation.
2.4. Analysis
Multivariate logistic regression was used with effect coding (where Minimal = −1 and Intense = 1) to evaluate the main effects and interactions (2-way, 3-way, and 4-way) on the primary outcome of smoking cessation (where not smoking = 1 and smoking = 0) using SAS software version 9.3. Analyses were carried out with and without adjusting for gender, age group, education and psychosocial factors (motivation, confidence and stress). To compare the success of the mHealth AI tobacco study with other research, the Research Team used “cold turkey” 3–5% and “prescription” 14% success rates at 6-months as benchmarks for comparison [17]. Subjects with missing data for smoking status at the visits when smoking was assessed (visits 5, 6, 7, 9, 10, and 11) were assigned a smoking status of “smoking” to comply with an intent-to-treat analysis.
3. Results
3.1. Participant demographics
A total of 254 subjects were enrolled in the study. Table 3 presents demographic and baseline characteristics of the study population. Subjects ranged in age from 18 to 80 years and 63% were under age 50 years. Majority of the subjects were female and 78% reported education at the high school/GED level or greater. The mean age of smoking onset was 16 years (SD = 6) with an average smoking duration of 24 (SD = 16) years. FTND scores and CO levels were also collected at baseline.
Table 3.
Demographic, behavioral and smoking history at baseline.
| Baseline (n = 254) | |
|---|---|
| Gender, females n (%) | 161 (64%) |
| Education, n (%) | |
| Less than HS/GED | 55 (22%) |
| HS/GED | 61 (24%) |
| Some college | 75 (30%) |
| College/Tech degree | 61 (24%) |
| Current cigarettes per day mean (SD) | 13 (SD = 8) |
| CO level mean (SD) | 14 (SD = 12) ppm |
| FTND score mean | 6 (SD = 2) |
| FTND level of nicotine dependence | |
| Low | 6% |
| Low to moderate | 20% |
| Moderate to high | 54% |
| High | 20% |
3.2. Intervention effectiveness
Table 4 shows the percentage of participants who were abstinent from smoking at the quit date (visit 5) and 18-month follow-up assessment (visit 11). At the quit date, the percentage of participants who were abstinent was highest (35%) among three groups: 1) those who received a combination of INTENSE NRT, and MINIMAL remaining interventions, 2) a combination of MINIMAL NRT and INTENSE pre-cessation counseling and mHealth, and 3) a combination of INTENSE pre-cessation counseling and MINIMAL remaining interventions (Fisher’s Exact p = .04). It is important to note that at the time of the quit date participants had not received post-cessation counseling. The total number of participants completing visit 11 was dramatically reduced from 254 at visit 1 to 16. The percentage of participants who were abstinent was highest among those who received INTENSE NRT and MINIMAL intensity for the remaining interventions (18%). Abstinence from smoking was next highest among those receiving INTENSE NRT, MINIMAL pre-cessation counseling, INTENSE post-cessation counseling, and MINIMAL mHealth text messages (17%).
Table 4.
Number and percent of participants abstinent from smoking at the quit date and 18-month follow-up by intervention group, ITT analysis.
| Intervention groupa | Abstinent visit 5 (%) | Abstinent visit 11 (%) |
|---|---|---|
| IIII (n = 17) 5 (29%) | 5 (29%) | 2 (12%) |
| IIIM (n = 16) 2 (13%) | 2 (13%) | 0 (0%) |
| IIMI (n = 16) 3 (19%) | 3 (19%) | 1 (6%) |
| IIMM (n = 17) 5 (29%) | 5 (29%) | 1 (6%) |
| IMII (n = 17) 5 (29%) | 5 (29%) | 0 (0%) |
| IMIM (n = 18) 5 (28%) | 5 (28%) | 3 (17%) |
| IMMI (n = 18) 6 (33%) | 6 (33%) | 2 (11%) |
| IMMM (n = 17) 6 (35%) | 6 (35%) | 3 (18%) |
| MIII (n = 16) 3 (19%) | 3 (19%) | 0 (0%) |
| MIIM (n = 15) 4 (25%) | 4 (25%) | 1 (6%) |
| MIMI (n = 17) 6 (35%) | 6 (35%) | 0 (0%) |
| MIMM (n = 17) 6 (35%) | 6 (35%) | 0 (0%) |
| MMII (n = 17) 2 (12%) | 2 (12%) | 0 (0%) |
| MMMI (n = 18) 5 (28%) | 5 (28%) | 1 (6%) |
| MMMM (n = 17) 3 (18%) | 3 (18%) | 0 (0%) |
| Total (n = 254) | 66 (26%) | 14 (6%) |
Intervention group level of intensity is identified with I=INTENSE or M=MINIMAL with the first letter for NRT, second letter for pre-cessation counseling, third letter for post-cessation counseling, and fourth letter for mHealth text messages.
Table 5 shows logistic regression results by intervention component for the quit date (visit 5) and 18-month follow-up assessment (visit 11). The logistic regression was adjusted for demographic variables including gender, age, education and ethnicity, and as shown, receiving NRT was associated with increased odds of having stopped smoking at the 18-month follow-up assessment (visit 11). No post-cessation counseling variables were included in the visit 5 model because post-cessation counseling did not start until after visit 5.
Table 5.
Logistic regression models for visits 5 and 11 as outcomes, intent-to-treat analysis. Probability modeled: smoking = 0.
| Estimate (β) | Chi square | p-value | |
|---|---|---|---|
| Visit 5 - quit date | |||
| NRT | −0.09 | 0.32 | 0.57 |
| Pre-cessation counseling | −0.02 | 0.01 | 0.92 |
| mHealth | 0.03 | 0.04 | 0.85 |
| NRT*Pre-cessation counseling | 0.27 | 2.90 | 0.09 |
| NRT*mHealth | −0.03 | 0.03 | 0.87 |
| Pre-cessation counseling*mHealth | 0.03 | 0.03 | 0.87 |
| NRT*mHealth*Pre-cessation counseling | −0.10 | 0.42 | 0.51 |
| Visit 11–18months follow-up | |||
| NRT | −0.79 | 3.77 | 0.05 |
| Pre-cessation counseling | 0.47 | 0.93 | 0.34 |
| Post-cessation counseling | −0.31 | 0.38 | 0.54 |
| mHealth | 0.24 | 0.33 | 0.57 |
| NRT*Pre-cessation counseling | 0.02 | 0.00 | 0.96 |
| NRT*Post-cessation counseling | 0.33 | 0.43 | 0.51 |
| NRT*mHealth | −0.05 | 0.01 | 0.91 |
| Pre-cessation counseling*Post-cessation counseling | −0.25 | 0.48 | 0.49 |
| Pre-cessation counseling*mHealth | −0.47 | 1.64 | 0.20 |
| Post-cessation counseling*mHealth | 0.38 | 1.13 | 0.29 |
3.3. Responder-only analysis
To further investigate the effect of the intervention on smoking cessation, the research team conducted analyses limited to those who responded [18]. Using this approach, smoking status of those that remained in the study at the quit date (visit 5) and at subsequent visits beyond was investigated and dropouts were deleted from the analyses. Table 6 shows the smoking status at each visit that smoking was assessed by intervention group. Of 108 subjects that remained in the study at the quit date (visit 5), 61% were found to be abstinent. Of the 16 subjects that remained in the study at the 18-month follow-up (visit 11), 88% were abstinent (Fisher exact p = .049).
Table 6.
Number and percent of participants abstinent from smoking at the quit date and 18-month follow-up by intervention group, Responder Only analysis.
| Intervention groupa | N at visit 5 |
Abstinent visit 5 (%) |
N at visit 11 |
Abstinent visit 11 (%) |
|---|---|---|---|---|
| IIII | 7 | 5 (71%) | 2 | 1 (100%) |
| IIIM | 3 | 2 (67%) | 0 | 0 (0%) |
| IIMI | 6 | 3 (50% | 1 | 1 (100%) |
| IIMM | 7 | 5 (71%) | 1 | 1 (100%) |
| IMII | 7 | 5 (71%) | 1 | 0 (0%) |
| IMIM | 7 | 5 (71%) | 3 | 3 (100%) |
| IMMI | 6 | 6 (100%) | 2 | 2 (100%) |
| IMDMM | 7 | 6 (86%) | 3 | 3 (100%) |
| MIII | 8 | 3 (38%) | 0 | 0 (0%) |
| MIIM | 8 | 4 (50%) | 1 | 1 (100%) |
| MIMI | 7 | 6 (86%) | 0 | 0 (0%) |
| MIMM | 7 | 6 (86%) | 0 | 0 (0%) |
| MMII | 10 | 2 (20%) | 0 | 0 (0%) |
| MMMI | 8 | 5 (63%) | 1 | 1 (100%) |
| MMMM | 10 | 3 (30%) | 1 | 0 (0%) |
| Total | 108 | 66 (61%) | 16 | 14 (88%) |
Intervention group level of intensity is identified with I = INTENSE or M = MINIMAL with the first letter for NRT, second letter for pre-cessation counseling, third letter for post-cessation counseling, and fourth letter for mHealth text messages.
3.4. Impact of level of participation
Data on the number of visits between CRRs and subjects were used to assess level of participation and smoking cessation. Study subjects were scheduled to take part in 10 or 11 CRR visits (only those receiving INTENSE post-cessation counseling were eligible for Visit 8) that included receiving intervention components and data collection. Because participants dropped out at different visits, we grouped those that remained in the program for 7 or fewer visits into a low level participation group and those that attended 8 to 11 visits into a high level participation group. Of those in the high level group, 87% were abstinent at their last visit compared to 68% abstinent in the low level group, Fisher’s Exact Test e = 3.9, p ≤ .05.
4. Discussion
This study used a randomized trial to assess the efficacy of four well-established smoking cessation strategies among Northern Plains American Indians. At 18 months post-quit date, the primary outcome visit (visit 11), 88% of those who were still in the study were abstinent. This includes 6% of all participants who enrolled in the study (14/254) and 13% of those who made it to the quit date (14/108). As shown in Table 5, no intervention groups were found to have significant pro-portions of participants who were abstinent from smoking at the quit date (visit 5) or primary outcome visit (18 months post-quit date, visit 11), but use of pharmacologic support (NRT) for abstinence was found to be an effective strategy for individuals who continued participation throughout the study (18 months post-quit date, visit 11). Those who dropped out of the study were likely smokers. Those who remained in the study received more visits and were more likely to be abstinent.
Previous research has shown that individuals who quit smoking on their own (cold turkey) have 3–5% success in stopping smoking past 6 months [19]. While a 2011 study found that only 14% of Chantix (Varenicline) users were still not smoking at 6 months [20]. Participants in this study were not allowed to use Chantix and had a slightly less successful quit rate.
The results from this study suggest that having access to NRT was a primary factor supporting successful smoking cessation. Although NRT was effective for most of those who succeeded, for many of the dropouts, NRT was insufficient and/or the subjects experienced too many uncomfortable side effects from the NRT to continue using it. Based on anecdotal information, some of the drop-outs had private insurance and were able to obtain prescription (e.g., Varenicline) and based on self-reports, were successful in stopping smoking. The research team chose to not provide smoking cessation prescription medications because of the inconsistency in its availability on the reservations as well as the expense. The former is a disparity issue, the latter was that the study could not afford to purchase the smoking cessation prescription medications; hence prescription medications were not part of the study design.
Contrary to expectations, mHealth as a means of providing social support was not associated with smoking cessation in this study. Of note, all groups (those receiving both the MINIMAL and the INTENSE level of mHealth) were positive about mHealth messaging and continued to use them throughout the study which suggests that receiving mHealth inadvertently became a “constant” rather than a separate intervention component. It is likely that mHealth functioned as a moderator or an advantage that the people who continued to use mHealth tended to remain in the study (and subsequently more likely to quit smoking/stay quit).
Additional analyses to explore the influence of text message suggest that the messages may play different roles depending on the status of the participant in terms of cessation. Fig. 2 shows the overall mean number of responses to the MINIMAL or INTENSE level of text messages by those still smoking and not smoking at each visit when smoking was assessed. As the figure shows, subjects not smoking at the quit date (visit 5) responded to the morning query at higher rates than those still smoking. Those not smoking and in the INTENSE level for receiving text messages had a higher overall mean of replies than those in the MINIMAL group. This pattern suggests that the text messages may play a role in assisting with quitting smoking. For those unable to stop smoking on the planned quit date, continuation of daily text messages may serve as reminders and keep an emphasis on quit attempts resulting in a higher number of responses. Further research on the role of text messages in smoking cessation is needed.
Fig. 2.
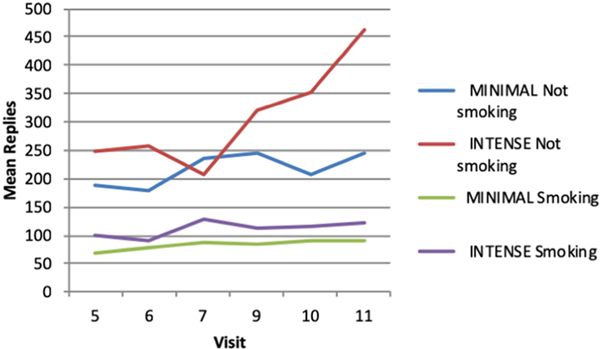
Mean number of text message replies by smoking status at each visit and level of intensity of text message delivery.
Given that a large proportion of the subjects did not stop smoking, it is important to understand the underlying reasons for this. Process evaluation data collected in the bi-weekly webinars with the Project Team indicate that many of these subjects were more interested in receiving phones and phone service than smoking cessation. Some subjects attempted to confuse the CRRs about their smoking status by going to the local casinos to inhale tobacco contaminated air and then visiting the CRR to take the CO test. For many others, their level of readiness to quit may not have been as high as that self-reported during baseline. Other factors that contributed were being in situations where others were smoking, having too much stress or feeling too much anxiety, having a bad event occur, feeling too much anger or irritability, and gaining weight. Some subjects requested up to five delays in their visit 5 (quit date and visit) prior to becoming “lost to follow-up.
4.1. Conclusion
In conclusion this project was designed to test a multi-component intervention to achieve cessation among Northern plains AIs who were current smokers. While use of NRT increased the odds of not smoking as assessed at the 18-month follow-up visit, no other interventions were found to significantly contribute to abstinence from smoking. Although the intervention protocol included numerous points of contact between CRRs and participants (11 visits) loss to follow-up was extensive with only 16/254 remaining enrolled. Additional research is needed to improve understanding of factors that influence enrollment and retention in smoking cessation interventions for AI and other populations.
5. Limitations
Limitations of the intent-to-treat analysis, which assigns a value of smoking to subjects with missing smoking status, include under-estimation of intervention effects because this method assumes that the intervention was ineffective for drop-outs. However, some subjects may have quit smoking and remained abstinent and decided not to continue with the study for other unknown reasons. Limitations of the secondary analysis, which excludes subjects with missing smoking status, includes potentially biased (overestimated) smoking abstinence rates because it only includes those who completed follow-up visits. Other limitations include subjects who were more motivated to participate in the study to get a free cell phone than to stop smoking which may have caused limited smoking abstinence rates and uncertainty of when NRT was used or started among subjects. Another limitation was the inability to provide prescriptive cessation medications to subjects due to costs and inaccessibility through some of the relevant IHS pharmacies. Finally, the high rate of attrition from enrollment (n = 254) to the quit date visit (n = 108) raises concern that selection bias may have influenced the study outcomes.
Acknowledgments
Funding
National Cancer Institute, United States of America, R01CA170336.
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- [1].U.S. Department of Health and Human Services, The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, 2014. [Google Scholar]
- [2].Cobb N, Espey D, King J, Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010, Am. J. Public Health 104 (Suppl. 3) (2014. June) S481–S489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K, Lung cancer deaths among American Indians and Alaska natives, 1990–2009, Am. J. Public Health 104 (Suppl. 3) (2014) S388–S395 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS, Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States, Am. J. Public Health 104 (Suppl. 3) (2014. June) S377–S387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention (CDC), Behavioral Risk Factor Surveillance System Survey Data, (2016). [Google Scholar]
- [6].Henderson PN, Kanekar S, Wen Y, Buchwald D, Goldberg J, Choi W, Okuyemi KS, Ahluwalia J, Henderson J, Am J Public Health. 99 (11) (2009. November) 2020–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jamal A, King BA, Neff LJ, et al. , Current cigarette smoking among adults—United States, 2005–2015, MMWR Morb. Mortal. Wkly Rep. 65 (2016) 1205–1211. [DOI] [PubMed] [Google Scholar]
- [8].Hodge F, Struthers R, Persistent smoking among Northern Plains Indians: lenient attitudes, low harm value, and partiality towards cigarette smoking, J. Cult. Divers. (2006) 181–185. [PubMed] [Google Scholar]
- [9].Burgess D, Fu SS, Joseph AM, Hatsukami DK, Solomon J, van Ryn M, Beliefs and experiences regarding smoking cessation among American Indians, Nicotine Tob Res. 9 (Suppl. 1) (2007. January) S19–S28. [DOI] [PubMed] [Google Scholar]
- [10].Smith SS, Rouse LM, Caskey M, Fossum J, Strickland R, Culhane JK, Waukau J, Culturally-tailored smoking cessation for adult American Indian smokers: a clinical trial, Couns. Psychol. 42 (2014) 852–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choi WS, Beebe LA, Nazir N, Kaur B, Hopkins M, Talawyma M, Shireman TI, Yeh HW, Greiner KA, Daley CM, All nations breath of life: A randomized trial of smoking cessation for American Indians, Am. J. Prev. Med. 51 (2016) 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ajzen I, The theory of planned behavior, Organ. Behav. Hum. Decis. Process. 50 (1991) 179–211. [Google Scholar]
- [13].Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, Christiansen BA, Schlam TR, Cook JW, Fiore MC, The multiphase optimization strategy for engineering effective tobacco use interventions, Ann. Behav. Med. 41 (2) (2011) 208–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burhansstipanov L, Krebs LU, Petereit D, Dignan MB, Ahamed SI, Sargent M, Cina K, Crawford K, Thibeault D, Bordeaux S, Kanekar S, Ahsan GST, Williams D, Addo I, Reality versus grant application ‘plans’, Health Promot. Pract. 19 (4) (2018. July) 566–572, 10.1177/1524839917700892 Epub 2017 Jul 2 28669241 [DOI] [PubMed] [Google Scholar]
- [15].Gerami A, Incomplete factorial experiments in completely randomized and randomized complete block designs, Stat. Probab. Lett. 78 (14) (2008) 2058–2065. [Google Scholar]
- [16].Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire, Br. J. Addict. 86 (1991) 1119–1127. [DOI] [PubMed] [Google Scholar]
- [17].Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO, Efficacy of Varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial, Nicotine Tob. Res. 13 (10) (2011) 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith SS, Rouse LM, Caskey M, Fossum J, Strickland R, Culhane JK, Waukau J, Culturally-tailored smoking cessation for adult American Indian smokers: a clinical trial, Couns. Psychol. 42 (2014) 852–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].https://truthinitiative.org/news/read-trying-quit-smoking-cold-turkey, Accessed date: 28 May 2018.
- [20].Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO, Efficacy of Varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial, Nicotine Tob. Res. 13 (10) (2011) 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]


