Abstract
Background
MRSA strains of clonal complexes (CCs) 5, 8, 30 and 45 are leading causes of complicated endovascular infections associated with suboptimal clinical outcomes. Telavancin is a novel anti-MRSA agent that both inhibits bacterial cell wall synthesis and disrupts membranes by depolarization.
Methods
In this study, we compared the in vitro susceptibility and in vivo efficacy of telavancin versus daptomycin in an experimental rabbit infective endocarditis (IE) model caused by four MRSA strains representing each of the above CC types.
Results
All study strains were susceptible to telavancin (MICs of ≤0.12 mg/L) and daptomycin (MICs of ≤0.5 mg/L). In vitro time–kill analyses revealed that supra-MIC levels of telavancin were effective at preventing regrowth at 24 h of incubation. In the IE animal model for all CC types, treatment with telavancin produced significantly greater reductions in MRSA counts as compared with daptomycin-treated animals in all target tissues. Moreover, telavancin-treated animals had a significantly higher percentage of sterile tissue cultures versus daptomycin-treated animals (e.g. 78%–100% versus 0% sterile vegetations and 100% versus 0%–11% sterile kidneys and spleen, in the telavancin- and daptomycin-treated animals, respectively).
Conclusions
These results suggest that telavancin exhibits significantly greater efficacies versus daptomycin in treating experimental IE caused by MRSA clinical isolates across four common CC types.
Introduction
Staphylococcus aureus is the most common cause of endovascular infections, including infective endocarditis (IE).1 In addition, S. aureus IE is a life-threatening infection that is often caused by MRSA strains.1 Despite the use of ‘gold-standard’ anti-MRSA antibiotics, such as vancomycin and daptomycin, morbidity and mortality associated with such infections remain unacceptably high.2,3 Given this potentially serious public health problem, there is a critical and urgent need to evaluate alternative anti-MRSA bactericidal agents, such as telavancin, for these invasive infections.
Telavancin is a semi-synthetic bactericidal lipoglycopeptide approved for use in Gram-positive bacterial infections including MRSA.4,5 It exhibits a dual mechanism of action: inhibition of bacterial cell wall synthesis and disruption of the bacterial membranes.4 The FDA approved telavancin in 2009 for complicated skin and skin structure infections and in 2013 for hospital-acquired and ventilator-associated bacterial pneumonia caused by S. aureus.6
In the current investigation, we evaluated the in vitro activity and in vivo efficacy of telavancin versus daptomycin in a prototypic experimental model of IE in rabbits caused by multi-clonotypic MRSA strains. The strain-set used in these studies represents the most common clonal complex (CC) types associated with invasive S. aureus endovascular infections, such as IE (CC5, CC8, CC30 and CC45).7,8
Materials and methods
Bacterial strains and growth conditions
The MRSA strains employed in this study were from a multinational S. aureus bacteraemia clinical trial collection, as well as from a collection at the Public Health Research Institute (Newark, NJ, USA) (courtesy of Dr Barry Kreiswirth).9–13 All S. aureus strains were routinely grown in tryptic soy broth (TSB; Disco Laboratories, Detroit, MI, USA) or Mueller–Hinton broth (MHB; Disco Laboratories).
Antibiotics
Telavancin was provided by Theravance Biopharma US, Inc. (South San Francisco, CA, USA). Daptomycin was purchased from Cubist Pharmaceuticals (Lexington, MA, USA). The antibiotics were reconstituted according to the manufacturers' recommendations.
MICs
The MICs of telavancin were determined by JMI Laboratories (North Liberty, IA, USA).14 The MICs of daptomycin were determined by the standard Etest method (bioMérieux, La Balme-les-Grottes, France).
In vitro time–kill curves
Time–kill experiments were performed in accordance with CLSI guidelines, with initial inocula of 105 or 107 cfu/mL in the presence of 1×, 2× and 5× MICs of telavancin or daptomycin. The two different inocula were chosen to encompass bacterial counts commonly achieved in all target tissues of animals with experimental IE.3
Experimental IE model
A well-characterized rabbit model of aortic IE was used.3,4 Briefly, female New Zealand White rabbits (2.2–2.5 kg body weight; Harlan Laboratories, Placentia, CA, USA) underwent indwelling transcarotid–transaortic valve catheterization and were infected intravenously (iv) with ∼105 cfu/animal, an ID95 dose established previously.10,11 At 24 h post-infection, animals were randomized to receive: (i) no therapy (control); (ii) telavancin at 30 mg/kg, iv, twice daily; or (iii) daptomycin at 12 mg/kg, iv, once daily. The telavancin and daptomycin doses were selected because they approximate the pharmacokinetic profiles of recommended human clinical doses (10 mg/kg, iv, once daily for telavancin, and 6 mg/kg once daily for daptomycin).15–17 Treatments lasted for 3 days. Control and antibiotic-treated animals were sacrificed by rapid iv injection of sodium pentobarbital (200 mg/kg; Abbott Laboratories, Lake Bluff, IL, USA) at either 24 h post-infection (untreated controls) or 24 h after the last antibiotic dose, respectively. At the time of sacrifice, cardiac vegetations, kidneys and spleen were sterilely removed and quantitatively cultured.10,11 The mean log10 cfu/g of tissue (±SD) was calculated for statistical comparisons.
Ethics
Animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care criteria and were cared for in accordance with national guidance. The Animal Research Committee (IACUC) of the LABioMed at Harbor-UCLA Medical Center approved these studies (#21294-01).
Statistical analysis
To compare tissue MRSA counts of control and antibiotic-treated animals, and between the different antibiotic treatment regimens (telavancin versus daptomycin), Student's t-test was employed.4P values of <0.05 were considered statistically significant.
Results
Telavancin and daptomycin MICs
The MICs of telavancin/daptomycin for the CC5, CC8, CC30 and CC45 strains were 0.12/0.38, 0.06/0.5, 0.12/0.5 and 0.06/0.38 mg/L, respectively.
In vitro telavancin and daptomycin time–kill curves
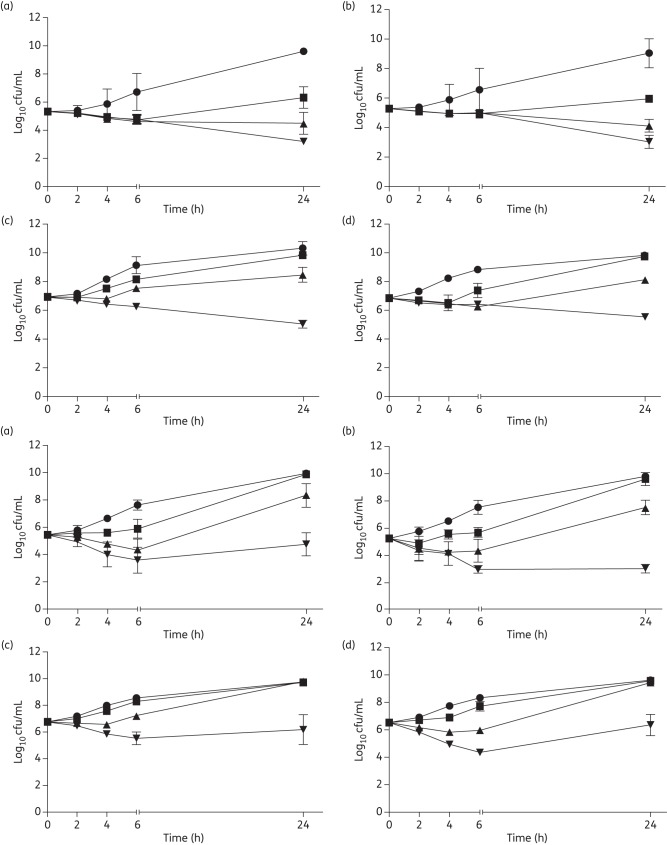
Figure 1 shows the in vitro killing curves of telavancin (top panel) and daptomycin (bottom panel) on two representative MRSA strains (CC5 and CC45). All telavancin concentrations yielded an early decrease in cfu, while supra-MIC telavancin concentrations (2× and 5× MICs) prevented regrowth at 24 h of incubation in both MRSA strains at 105 cfu/mL initial inocula (Figure 1, top panel, a and b). At the 107 cfu/mL initial inocula, only 5× MICs of telavancin prevented regrowth at 24 h of incubation for study MRSA strains (Figure 1, top panel, c and d).
Figure 1.
In vitro telavancin (top panel) and daptomycin (bottom panel) time–kill curves. CC45 300-169 strain (a) and CC5 300-246 strain (b) at 105 cfu/mL. CC45 300-169 strain (c) and CC5 300-246 strain (d) at 107 cfu/mL. Time–kill experiments were performed using Mueller–Hinton broth in the presence of 0 (circles), 1× (squares), 2× (triangles) and 5× (inverted triangles) MIC of telavancin or daptomycin.
A concentration-dependent killing effect of DAP was observed during the early incubation time period (Figure 1, bottom panel, a and b), but only daptomycin at 5× MICs was effective in preventing regrowth at 24 h of incubation in the CC5 strain at 5 × 105 cfu/mL initial inocula (Figure 1, bottom panel, b). However, regrowth was observed at 24 h of incubation at the 107 cfu/mL initial inocula, even at 5× MIC of daptomycin for both MRSA strains.
Rabbit IE model
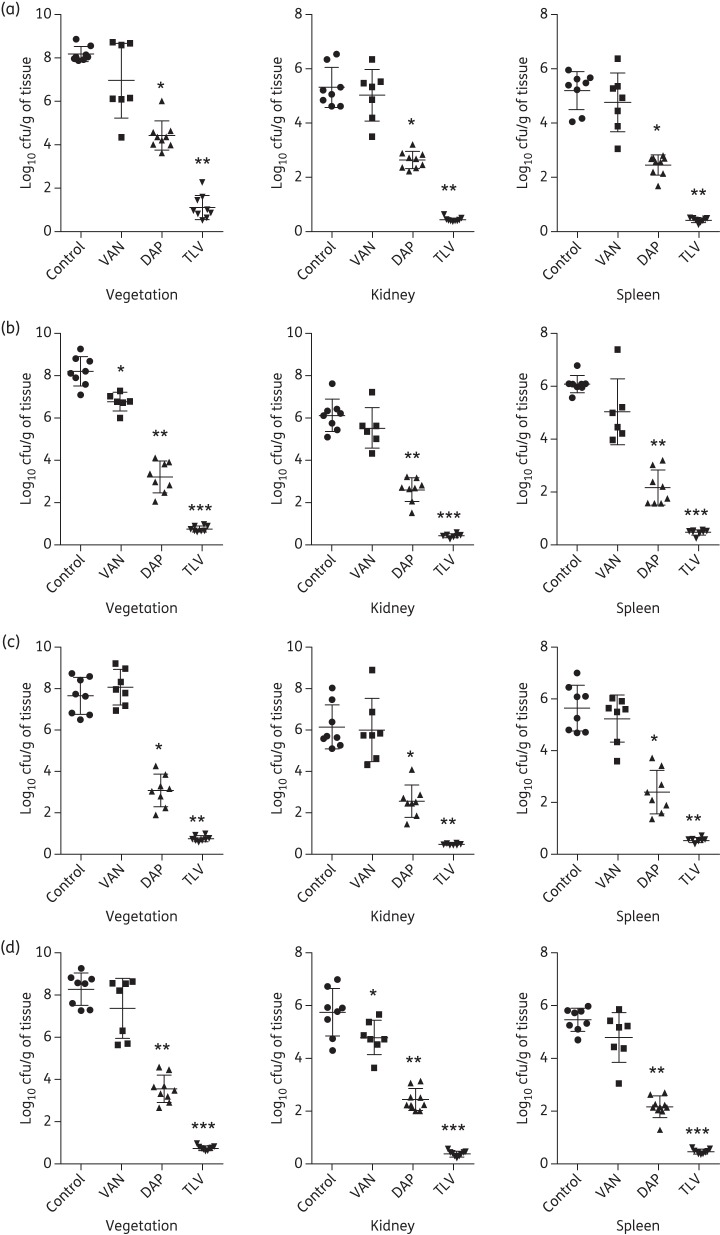
Both telavancin and daptomycin treatments significantly reduced MRSA densities of the four common CC types in all three target tissues versus untreated controls (Figure 2). Historical data with vancomycin treatment10,11 are also shown for comparative purposes. As previously reported, animals with IE caused by these study MRSA strains showed one of two outcomes following vancomycin treatment: (i) no response to vancomycin treatment; or (ii) a statistically relevant, but relatively small, reduction in target tissue counts versus corresponding untreated controls.10,11 Importantly, telavancin treatment had significantly better efficacy as compared with both vancomycin and daptomycin therapy (Figure 2; P < 0.001). The magnitude of these reductions in target tissue MRSA counts was dramatic, with at least 5 log10 cfu/g decreases observed versus controls. Additionally, telavancin-treated animals had a significantly higher percentage of culture-negative target tissues (78%–100% in vegetations and 100% in kidneys and spleen) versus daptomycin therapy (0% in vegetations and kidneys and 0%–11% in spleen).
Figure 2.
Densities of MRSA strains CC5 300-246 (a), CC8 BK19069 (b), CC30 BK33367 (c) and CC45 300-169 (d) (log10 cfu/g) in target tissues (cardiac vegetations, kidneys and spleen) in the IE model with and without antibiotic treatment. For each group, individual data for each rabbit are represented by a dot. The means and SD are represented by horizontal and vertical bars, respectively. *P < 0.05, **P < 0.00001 and ***P < 0.000001 versus untreated controls in all target tissues. P < 0.001 telavancin versus daptomycin in all target tissues. Historical vancomycin data are reproduced from Abdelhady et al.10 and Seidl et al.11 VAN, vancomycin; DAP, daptomycin; TLV, telavancin.
Discussion
Multiple factors have been implicated in the therapeutic challenge of invasive MRSA infections, including the dynamic epidemiology of genotypic lineages.18,19 Of note, CC5, CC8, CC30 and CC45 are among the most frequently isolated strains from complicated MRSA infections. Although vancomycin and daptomycin are standard therapeutic options, treatment failures with these agents have been increasingly reported even with ‘susceptible’ strains based on in vitro CLSI breakpoints.10,11 Therefore, these results have exposed the need for the development of new and improved antimicrobial agents in the treatment of persistent MRSA infections.
Given its dual mechanism of action, telavancin is a potent bactericidal agent both in vitro and in vivo in animal models, and has a low potential for resistance emergence.4,15,20 For instance, Madrigal et al.15 demonstrated that telavancin was significantly more effective than vancomycin in clearing vancomycin-intermediate S. aureus (VISA) isolates from cardiac vegetations in a rabbit IE model. In addition, Miro et al.20 also reported that telavancin was efficacious in the same model caused by VISA isolates, without the emergence of telavancin-resistant clones. Recently, we demonstrated that telavancin significantly reduced MRSA densities in all target tissues versus daptomycin-treated animals in the same rabbit IE model caused by a daptomycin-non-susceptible MRSA strain.4 Taken together, these prior investigations suggested that telavancin may be an effective alternative to vancomycin and/or daptomycin in the treatment of serious staphylococcal infections caused by MRSA strains. However, to the best of our knowledge, there are no studies of telavancin efficacies in experimental IE across a ‘relevant clonotype range’ of MRSA strains representing the most common genotypes encountered in clinical practice today. Therefore, in the current studies, we focused on the four most common CC types (CC5, CC8, CC30 and CC45) predominating in complicated MRSA endovascular infections.7 We demonstrated that all four of these study strains were highly susceptible to telavancin and daptomycin in vitro. In addition, telavancin exhibited an impressive early killing effect against these MRSA strains at both 105 and 107 cfu/mL initial inocula. Importantly, telavancin showed significantly greater efficacies versus daptomycin and vancomycin both in terms of decreasing MRSA target tissue densities and in sterilization of such target organs, regardless of CC type.
It is important to recognize the limitations of the present study. Our data are somewhat restricted in that only a ‘standard’ daptomycin dose was investigated. It will be important to test higher daptomycin doses in this model to simulate doses in humans of 10 mg/kg, which may be important in clinical settings for IE treatment. In addition, we did not query whether the development of daptomycin resistance occurred after treatment in the IE model. Moreover, we did not study whether there was relapse after completion of telavancin therapy. Nonetheless, the results of the current study reveal key insights regarding the potent bactericidal activity of telavancin in the experimental IE model in terms of MRSA clearance and sterilization across four major CC types.
Funding
This study was supported by a research grant from Theravance Biopharma Antibiotics, Inc. (to Y. Q. X.).
Transparency declarations
None to declare.
Acknowledgements
We thank Dr Barry Kreiswirth for providing the CC8 and CC30 MRSA strains.
References
- 1. Fowler VG Jr, Miro JM, Hoen B et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293: 3012–21. [DOI] [PubMed] [Google Scholar]
- 2. Khatib R, Johnson LB, Fakih MG et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scan J Infect Dis 2006; 38: 7–14. [DOI] [PubMed] [Google Scholar]
- 3. Xiong YQ, Fowler VG, Yeaman MR et al. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 2009; 199: 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiong YQ, Hady WA, Bayer AS et al. Telavancin in therapy of experimental aortic valve endocarditis in rabbits due to daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2012; 56: 5528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Hal SJ, Paterson DL. New Gram-positive antibiotics: better than vancomycin? Curr Opin Infect Dis 2011; 24: 515–20. [DOI] [PubMed] [Google Scholar]
- 6. Corey GR, Kollef MH, Shorr AF et al. Telavancin for hospital-acquired pneumonia: clinical response and 28-day survival. Antimicrob Agents Chemother 2014; 58: 2030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fowler VG Jr, Nelson CL, McIntyre LM et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 2007; 196: 738–47. [DOI] [PubMed] [Google Scholar]
- 8. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M et al. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis 2011; 204: 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fowler VG Jr, Boucher HW, Corey GR et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. New Eng J Med 2006; 355: 653–65. [DOI] [PubMed] [Google Scholar]
- 10. Abdelhady W, Chen L, Bayer AS et al. Early agr activation correlates with vancomycin treatment failure in multi-clonotype MRSA endovascular infections. J Antimicrob Chemother 2015; 70: 1443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seidl K, Chen L, Bayer AS et al. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55: 5631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seidl K, Bayer AS, Fowler VG Jr et al. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother 2011; 55: 575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diep BA, Gill SR, Chang RF et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006; 367: 731–9. [DOI] [PubMed] [Google Scholar]
- 14. Farrell DJ, Mendes RE, Rhomberg PR et al. Revised reference broth microdilution method for testing telavancin: effect on MIC results and correlation with other testing methodologies. Antimicrob Agents Chemother 2014; 58: 5547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madrigal AG, Basuino L, Chambers HF. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49: 3163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darouiche RO, Mansouri MD, Schneidkraut MJ. Comparative efficacies of telavancin and vancomycin in preventing device-associated colonization and infection by Staphylococcus aureus in rabbits. Antimicrob Agents Chemother 2009; 53: 2626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chambers HF, Basuino L, Diep BA et al. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother 2009; 53: 1463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiology 2013; 5: 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews 2009; 7: 629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miro JM, Garcia-de-la-Maria C, Armero Y et al. Efficacy of telavancin in the treatment of experimental endocarditis due to glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51: 2373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]