Abstract
Pseudomyogenic hemangioendothelioma (PHE) is an uncommon, rarely metastasizing vascular neoplasm affecting with predilection young adults. The tumors often present as multiple nodules involving various tissue planes, including superficial and deep soft tissues as well as bone. Recurrent SERPINE1-FOSB gene fusions have been reported as the hallmark genetic abnormality in PHE, however, in our experience, a number of cases with typical histology lack this genetic abnormality. In this study, we identify a novel ACTB-FOSB gene fusion, which is as prevalent as the initial translocation reported. We selected 15 consecutive cases of PHE with typical morphologic features which had material for molecular testing. The cohort included 10 males and 5 females, ranging in age from 17 to 58 years (median age – 33 years; mean age – 35.3 years). Eight (53%) cases were located in the lower extremities (foot, calf, tibia, thigh), 5 (33%) were located in the trunk, abdomen or pelvis (abdominal wall – 2, shoulder, back, ischium) and 2 (13%) were located in the upper extremity (humerus and hand). Ten (67%) cases had multifocal presentation and 5 (33%) presented as solitary lesions. Three (20%) cases were located only in the superficial dermis and subcutaneous tissues, 4 (27%) involved the superficial and deep soft tissue and 8 (53%) cases involved only the deep soft tissue and bone. Using FISH and ARCHER fusionplex analysis we identified a novel ACTB-FOSB gene fusion in 7 cases, while the remaining 8 had the previously described SERPINE1-FOSB fusion. The clinicopathologic features and behavior of PHE associated with the ACTB-FOSB gene fusion were similar to those harboring the SERPINE1-FOSB; except that tumors with the ACTB variant were more often associated with solitary presentation. In conclusion, our results expand the spectrum of genetic alterations in PHE with a novel gene fusion identified in half of the cases. We speculate that some of the novel targeted therapies that have shown promise in SERPINE1-FOSB-positive PHE might also be beneficial in this molecular subset.
Keywords: ACTB, SERPINE1, FOSB, Pseudomyogenic Hemangioendothelioma
INTRODUCTION
Pseudomyogenic Hemangioendothelioma (PHE) is a rare vascular neoplasm, with only about hundred cases reported to date. [1–13] The tumors are often multicentric, involving multiple tissue planes and occur with predilection in young adults. In the latest WHO classification, PHE is categorized in the intermediate group, as a rarely metastasizing endothelial neoplasm.[14] The varying terminologies used reflect the diverse morphologic spectrum. In 2003, Billings et al.[15] were the first to describe this vascular neoplasm, using the term ‘epithelioid sarcoma-like hemangioendothelioma’, emphasizing its close resemblance to epithelioid sarcoma. Subsequently, Hornick and Fletcher [3] reported a large series of 50 cases expanding the histologic spectrum, to include cases with predominant spindle cell cytomorphology, reminiscent of myoid differentiation, and proposed the term ‘pseudomyogenic hemangioendothelioma’, currently the preferred designation used in the WHO.
The first reported genetic abnormality in PHE was a balanced t(7;19)(q22;q13) translocation in an index case of a 14 year old girl with multiple subcutaneous nodules in the chest wall.[16] Subsequently, Walther et al., using a combined molecular approach of chromosome banding analysis,fluorescence in situ hybridization (FISH), and mRNA sequencing, cloned the gene partners revealing a SERPINE1-FOSB fusion. [17]
Triggered by a group of PHE cases with classic histologic features lacking SERPINE1 gene rearrangement, we set out to perform a molecular evaluation using FISH and ARCHER assays to further unravel alternative novel gene abnormalities in this tumor.
MATERIALS AND METHODS
Patient Selection and Histologic Diagnosis
Archival and personal consultation material (CRA) from adult and pediatric patients with diagnosis of pseudomyogenic hemangioendothelioma or epithelioid sarcoma-like hemangioendothelioma were retrieved from the pathology files at Memorial Sloan Kettering Cancer Center, in which additional material was available for ancillary testing. Fourteen cases were identified in which the diagnosis of pseudomyogenic hemangioendothelioma was confirmed by re-review of the histologic slides. Formalin fixed paraffin embedded tissue was available on all the cases selected for the study. The study was approved by the Institutional Review Board at MSKCC.
FISH
FISH on interphase nuclei from paraffin-embedded 4-micron sections was performed applying custom probes using bacterial artificial chromosomes (BAC), covering and flanking genes FOSB, SERPINE1, ACTB, ZFP36, and WWTR1. BAC clones were chosen according to UCSC genome browser (http://genome.ucsc.edu), see Supplementary Table 1 and as previously described.[18] The BAC clones were obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI) (Oakland, CA) (http://bacpac.chori.org). DNA from individual BACs was isolated according to the manufacturer’s instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score.
ARCHER Assay
Archer FusionPlex™-targeted RNA sequencing analysis was performed in one case (case 2). The solid fusion assay is a targeted RNA-based panel that utilizes the Archer Anchored Multiplex PCR (AMP™) technology and next generation sequencing to detect gene fusions in solid tumor and sarcoma samples. The Archer™ custom solid panel targets 62 genes known to be recurrently involved in rearrangements associated with these tumors. RNA is extracted from formalin-fixed paraffin-embedded (FFPE) tumor material followed by cDNA synthesis. cDNA undergoes end repair, dA tailing, and ligation with Illumina molecular barcode adapters. SPRI-cleaned ligated fragments are subject to two rounds of PCR amplifications using two sets of gene specific primers (GSP1 used in PCR1 and a nested GSP2 pool used in PCR2) and a primer complementary to the Illumina adapter. At the end of two PCR steps, the final targeted amplicons are fully functional and ready for sequencing on an Illumina Miseq instrument (2×150bp). The Archer™ analysis software V5.0 used for data analysis to identify the fusion.
MSK-IMPACT Assay
Details of the MSK-IMPACT assay have been previously published. [20] Briefly, MSK-IMPACT is a comprehensive molecular profiling assay that involves hybridization capture and deep sequencing of all exons and selected introns of up to 468 oncogenes and tumor-suppressor genes, allowing the detection of point mutations, small and large insertions or deletions, and rearrangements.
RESULTS
Clinicopathologic Features
The clinicopathologic features of the 15 cases are summarized in Table 1. The tumors occurred in 10 males and 5 females and the patients ranged in age from 17 to 58 years (median age – 33 years; mean age – 35.3 years). Most (8, 53%) cases were located in the lower extremities (foot, calf, tibia, thigh), followed by the trunk, abdomen and pelvis (abdominal wall – 2, shoulder, back, ischium) in 5 (33%) cases and the upper extremity (humerus and hand) in 2 (13%). Two-thirds (10, 67%) of the cases had a multifocal presentation, while the remaining third (5, 33%) were solitary lesions. In 5 of the 10 (50%) cases with multifocal presentation, the lesions were located in different tissue planes including subcutaneous tissue, skeletal muscle and bone. The remaining 5 cases (50%) with multifocal presentation showed multiple lesions in the same tissue plane. Twelve (80%) cases involved the deep structures, of which 8 cases exclusively involved the deep tissues and the remaining 4 showed involvement of both superficial and deep tissues. All of the cases that involved deep structures, except one, involved the bone. Three cases (20%) were located exclusively in the superficial dermis and subcutaneous tissues.
Table 1.
Clinicopathologic features of PHE
| PHE | Age/Sex | Site | Gene fusion | Depth | Clinical Presentation | Mitoses /10HPF | FU duration | FU Status |
|---|---|---|---|---|---|---|---|---|
| 1# | 45/F | ischium, ankle | ACTB - FOSB | Deep - Bone | Multifocal | 25 | 26 | AWD |
| 2* | 54/F | shoulder | ACTB - FOSB | Sup - Dermis and SQ | Solitary | 0 | 4 | NED |
| 3 | 44/M | humerus | ACTB - FOSB | Deep - Bone | Solitary | 0 | 3 | NED |
| 4 | 25/M | foot | ACTB - FOSB | Deep - Bone | Solitary | 1 | na | na |
| 5 | 19M | calf (toe, tibia) | ACTB - FOSB | Deep - Bone | Multifocal | 1 | na | na |
| 6 | 24/M | calf | ACTB - FOSB | Deep - ST | Solitary | 0 | na | na |
| 7 | 38/M | abdominal wall | ACTB - FOSB | Sup - Dermis and SQ | Multifocal | 0 | 55 | NED |
| 8# | 40/F | thigh | SERPINE1 - FOSB | Sup -Dermis and SQ | Multifocal | 1 | 2 | NED |
| 9 | 33/M | tibia | SERPINE1 - FOSB | Deep - Bone | Multifocal | 2 | 12 | AWD |
| 10# | 17/M | foot | SERPINE1 - FOSB | Deep - Bone and ST | Multifocal | 0 | na | na |
| 11 | 30/F | hand/palm/ fingers | SERPINE1 - FOSB | Deep – Bone, Sup - Dermis and SQ | Multifocal | 1 | 6 | NED |
| 12 | 48/M | back | SERPINE1 - FOSB | Deep - Bone and ST, Sup - SQ | Multifocal | 1 | na | na |
| 13 | 58/M | abdominal wall | SERPINE1 - FOSB | Sup – SQ, Deep - ST | Solitary | 4 | 30 | NED |
| 14 | 25//M | calf | SERPINE1 - FOSB | Deep - Bone and ST | Multifocal | 0 | 66 | NED |
| 15 | 30 / M | leg / tibia/calcaneus | SERPINE1 - FOSB | Deep - Bone and ST, Sup - SQ | Multifocal | 0 | na | na |
analyzed by ARCHER fusionplex
analyzed by MSK-IMPACT
M-male, F-female; SUP-Superficial; ST – soft tissue; SQ-subcutaneous; NED, no evidence of disease; AWD, alive with disease; FU, follow up; na, not available.
The tumors showed a wide morphologic spectrum. (Figures 1 and 2) Most tumors showed a multinodular and infiltrative growth within the subcutaneous fat or skeletal muscle. They typically lacked a lobular growth pattern or encapsulation. The lesions were composed of a mixture of spindle and epithelioid cells in variable proportions. A subset of cases had mostly spindle cell morphology, arranged in short fascicles or patternless growth within a fibrotic stroma. These plump spindle cells had abundant dense eosinophilic cytoplasm and ovoid nuclei with open chromatin and mildly prominent nucleoli. In other cases, the epithelioid component predominated, being composed of relatively monomorphic epithelioid to rhabdoid cells with abundant densely eosinophilic cytoplasm and round nuclei with smooth contours and fine chromatin. Due to the eosinophilic quality and accentuated cell membranes, the tumors closely resembled the appearance of a smooth muscle neoplasm. Most tumors had a low level of cytologic atypia, except for Case 1, showing a moderate degree of nuclear pleomorphism in the primary lesion, which became severe in subsequent lesions after chemoradiation. This patient, a 45 year-old female presenting with a ischium bone lesion, was initially misdiagnosed as a sarcomatoid carcinoma of undetermined origin due to its strong cytokeratin positivity. (Figure 1) All except one case showed a low mitotic activity, of <5 MF/10 high power fields (HPFs). Case 1 described above had 25 MF/10 HPFs in the primary and subsequently developed lesions. No necrosis was identified in any of the tumors. A consistent finding seen in most of the tumors was the associated fibrotic stromal component. None of the cases showed a hemorrhagic background or any evidence of vascular lumen formation. Furthermore, blister cells, cells with intracytoplasmic vacuoles, were not present. In addition, there was a variable inflammatory infiltrate within some of the tumors, composed of either neutrophils or lymphocytes, but typically not eosinophils.
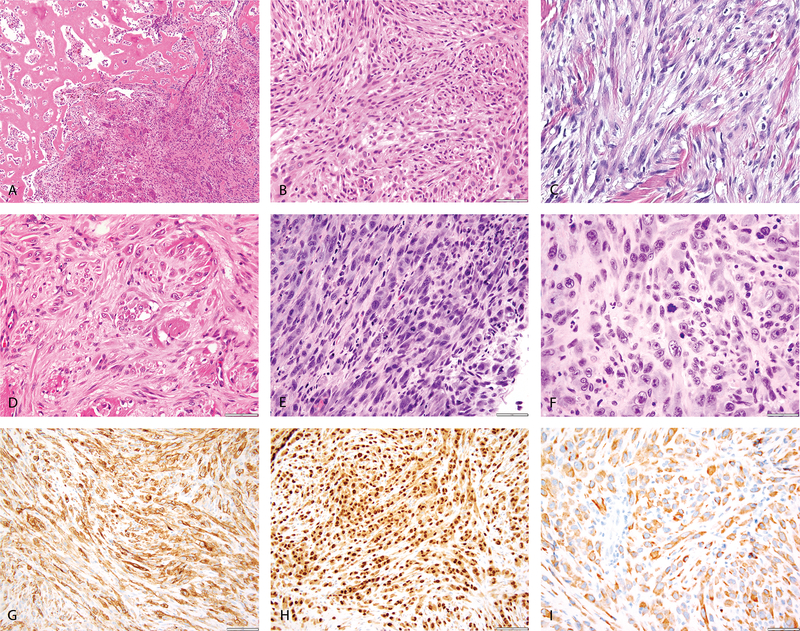
Figure 1: Morphologic spectrum of PHE with ACTB-FOSB fusion.
(A, B) Case 4 (25/M, foot) showing bone involvement by a cellular neoplasm (A), showing a mixture of epithelioid and spindle cells (B); (C) Case 2 (54/F, shoulder) showing involvement of the dermis and subcutaneous tissue with spindle cell neoplasm in a fascicular pattern; (D) Case 5 (19/M, calf) showing infiltration of the skeletal muscle by tumor cells with abundant eosinophilic cytoplasm. (E-I) Case 1 (45/F, ischium) showing cellular spindle cell areas with increased mitoses (E) and other areas showing pleomorphic epithelioid cells (F); immunohistochemical stains positive for CD31 (G), ERG (H) and Cytokeratin (I).
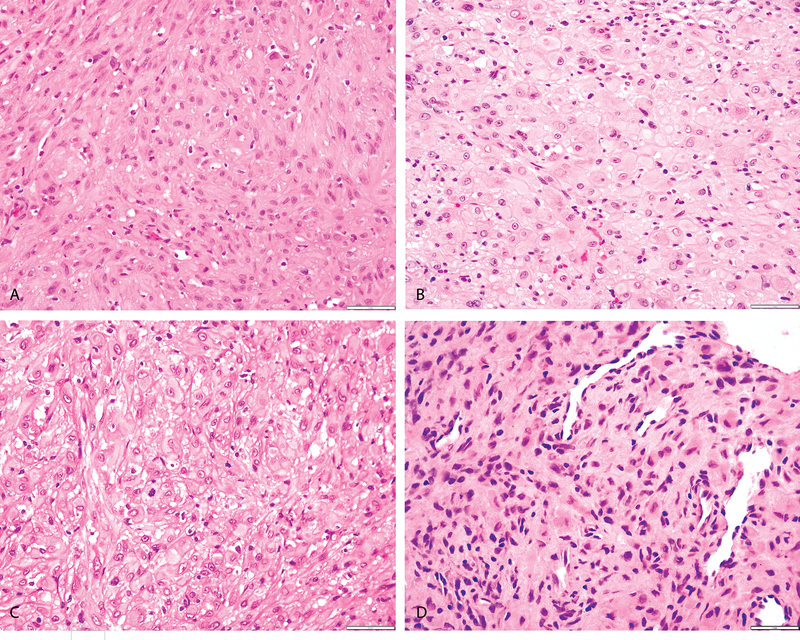
Figure 2: Morphologic spectrum of PHE with SERPINE1-FOSB fusion.
(A) Case 14 (25/M, calf) showing cells with abundant eosinophilic cytoplasm in a fibrotic stroma. (B, C) Case 12 (48/M, back) and Case 13 (58/M, abdominal wall) showing epithelioid cells with eosinophilic cytoplasm with accentuated membranes compactly arranged, suggestive of an epithelioid smooth muscle tumor. (D) Case 9 (33/M, tibia) showing spindle to epithelioid cells in a hyalinized stroma with prominent normal vasculature in the background.
Immunohistochemical staining results were available in all except one case. CD31 was positive in all 14 cases tested, however, in 5 cases showing only focal or patchy staining. In contrast, ERG, tested in 10 cases, was diffusely positive in all. (Figure 1) Cytokeratin, tested in 11 cases, was expressed in all, with half of the cases showing patchy positivity. Desmin and / or SMA stains were performed on 10 cases and were negative.
A novel recurrent ACTB-FOSB fusion was identified in half of the cases
FISH studies showed rearrangements of FOSB gene in all of the 15 cases, however, 7 cases (47%) lacked SERPINE1 gene abnormalities. These cases were further analyzed for other known gene partners of FOSB and FOS fusions described in various vascular neoplasms, including ZFP36, WWTR1 and ACTB. Interestingly, all of the 7 cases showed a rearrangement in the ACTB gene (Figure 3), in keeping with an ACTB-FOSB gene fusion. Furthermore, ARCHER FusionPlex RNA assay, performed in Case 2, showed a fusion transcript between exon 3 of ACTB gene and exon 2 of FOSB, thereby confirming the ACTB-FOSB gene fusion. (Figure 4)
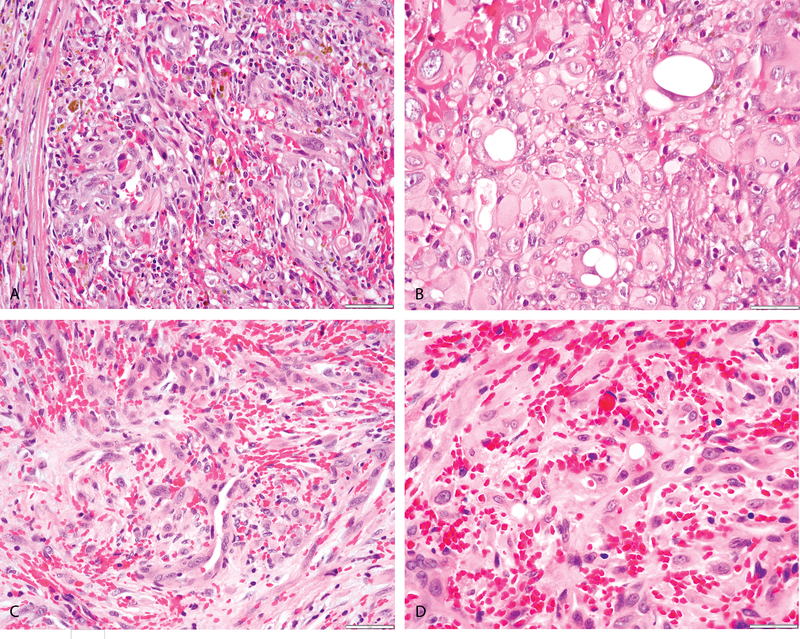
Figure 3: Morphologic features of ACTB-FOSB-positive epithelioid hemangioma.
(A-D) (A, B, 29/F, nasal cavity) Solid sheets of plump epithelioid cells with focal vasoformation (A) in a markedly hemorrhagic stroma; and cells with vacuolated cytoplasm (blister cells) (B). (C, D, 40/M, penis) plump spindle and epithelioid cells with focal vasoformation (C) and abundant extravasated RBCs; and cells with vacuolated cytoplasm (D), findings not seen in PHE cases with ACTB-FOSB fusion.
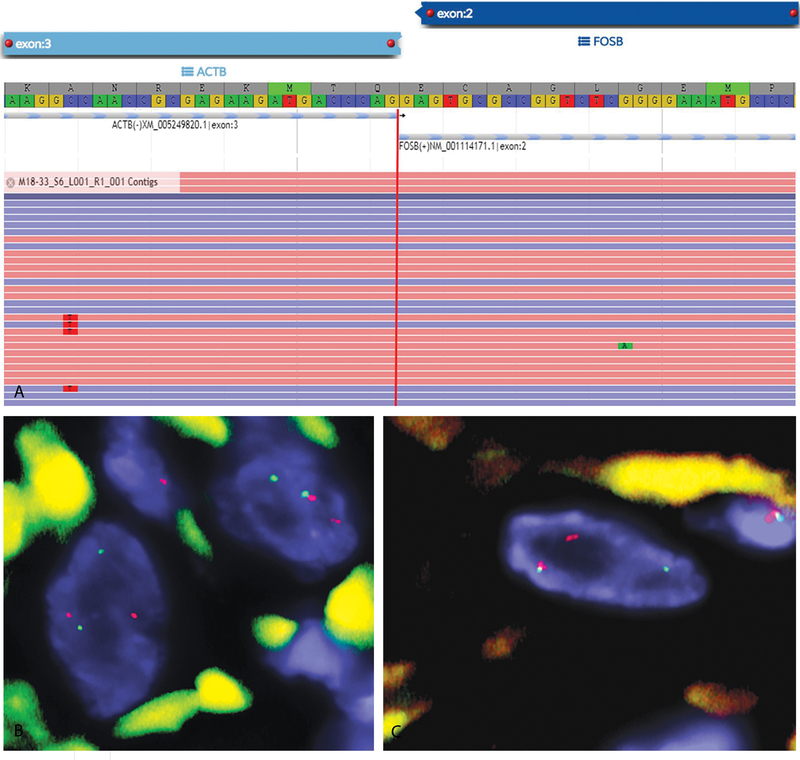
Figure 4:
(A) Schematic representation of the ACTB-FOSB fusion transcript identified by ARCHER fusionplex analysis, with fusion of ACTB exon 3 to FOSB exon 2 (case 2). (B-C) FISH analysis shows break-apart signals consistent with rearrangements of FOSB (B) and ACTB (C) genes (red, centromeric; green, telomeric; case 3).
The 7 tumors occurred in 5 males and 2 females, with ages ranging from 19 to 54 years (median – 38 years). More than half (4, 57%) of the tumors presented as solitary lesions and 3 (43%) showed multifocal presentation. The anatomic sites included lower extremity (4 cases), shoulder, humerus and anterior abdominal wall. Five (71%) tumors had a deep location with one involving the deep soft tissue and 4 cases involving the bone. Two (29%) cases were located in the superficial dermis and subcutaneous tissue.
The cohort of PHE with SERPINE1-FOSB gene fusions show similar clinicopathologic features
Eight (53%) cases showed SERPINE1 gene rearrangement, in keeping with a SERPINE1-FOSB gene fusion. The tumors occurred in 6 males and 2 females with ages ranging from 17 to 58 years (median age – 31.5 years). All except one showed multifocal presentation. The tumor sites included lower extremity in 5 cases, and one each involved the abdominal wall, back and hands. Three cases involved only the deep structures, 4 involved the deep and superficial tissues and 1 case involved only the superficial dermis and subcutaneous tissue.
Targeted Exome Sequencing showed no additional genetic alterations
MSK-IMPACT assay, a targeted exome sequencing next-generation assay of 468 genes, was performed in 4 cases (cases 1, 8, 10 and 15), one of which (case 1) had an ACTB-FOSB gene fusion and was associated with atypical histologic features and markedly increased mitotic activity. The other two (cases 8 and 10) had a SERPINE1-FOSB gene fusion. No additional genetic alterations (mutations and copy number alterations) were identified in any of the four cases.
Follow-up and management
Surgery was the main therapy applied, with 10 of the 11 cases, in which data was available, having undergone surgical excision. In case 1, in addition to surgery, the patient received chemotherapy and radiation. In one patient (case 9), a 33 year-old man with multiple bone lesions, radiofrequency ablation was performed instead. The treatment information was unavailable in the remaining 3 cases.
Follow-up data was available in 9 cases with duration ranging from 3 to 66 months. Seven (78%) patients had no evidence of disease (NED) and 2 (22%) were alive with disease (AWD).
DISCUSSION
Gene fusions involving FOS and FOSB have been identified recently in both benign and intermediate epithelioid vascular neoplasms, including epithelioid hemangioma and PHE. [17, 18, 20]. In epithelioid hemangioma, FOSB is commonly fused to ZFP36, with rare examples having WWTR1 and ACTB as the gene partners ([20] and CRA personal communications). This molecular subset of epithelioid hemangiomas with FOSB-gene rearrangements is often associated with worrisome histologic features, including increased cellularity, nuclear pleomorphism and focal necrosis [20]. An additional genetic group of epithelioid hemangiomas, preferentially occurring in skeletal anatomic sites, is characterized by recurrent gene fusions involving the FOS gene with a variety of gene partners, including LMNA and VIM [18, 21]. FOS and FOSB genes, mapping to chromosome 14q24.3 and 19q13, respectively, belong to the Fos gene family which also includes FOSL1 and FOSL2 genes. [22] The FOS and FOSB genes encode a transcription factor that dimerizes with members of the Jun family (c-Jun, JunB, and JunD), constituting the major components of the activating protein-1 (AP-1) complex. [23] The AP-1 transcription factor binds to TPA-responsive elements of the promoter and enhancer regions of target genes, thereby regulating a wide variety of physiological and tumorigenic processes including angiogenesis. [24–26] The mechanism through which an identical ACTB-FOSB fusion triggers the pathogenesis of two distinct vascular neoplasms, characterized by either benign or intermediate grande of malignancy, with or without vasoformative properties, remains to be determined.
The genetic alterations of PHE have only recently been identified. Trombetta et al., in 2011, identified a t(7;19)(q22;q13) translocation by conventional karyotyping.[16] Although FISH studies identified the breakpoint regions, RT-PCR studies failed to demonstrate the genes involved in the fusion. In a subsequent study, Walther et al.[17] identified a SERPINE1-FOSB fusion in two cases by using RNA-seq methodology. Further screening of 8 additional PHE cases by FISH showed evidence of the SERPINE1-FOSB fusion, in keeping with a highly recurrent genetic abnormality. However, in a recent study by Pradhan et al. only 3 of the 6 cases analyzed by FISH showed evidence of the SEPINE1-FOSB gene fusion. [7] Moreover, only 53% of the cases in our current study showed evidence of a SERPINE1-FOSB gene fusion. In the remaining 7 cases, SERPINE1 rearrangement was not present, prompting us to further investigate alternative gene partners, including those previously identified in epithelioid hemangioma, such as WWTR1 and ZFP36 [20] and ACTB (CRA personal communications). Interestingly, all 7 cases showed an ACTB gene rearrangement in keeping with an ACTB-FOSB fusion. ARCHER fusionplex assay performed in one of the cases confirmed an in-frame ACTB-FOSB fusion involving exon 3 of ACTB and exon 2 of FOSB gene. The breakpoints of FOSB gene seen in the ACTB-FOSB variant were similar to those previously reported in SERPINE1-FOSB and ZFP36-FOSB gene fusions, involving exon 2 of FOSB gene.[17, 20]
The FOSB transcription factor consists of an N-terminal FOS homology domain, a DNA binding bZIP (basic leucine zipper) domain and a C-terminal proline-rich transactivation domain. [26] The bZIP domain and the C-terminal transactivation domain are typically conserved in the FOSB fusion oncoprotein. [20] FOSB expression has been shown to be significantly increased in cases of epithelioid hemangioma and PHE with FOSB-associated gene fusions, compared to other tumors, which can be detected immunohistochemically. [17, 20]
Beta-actin (ACTB) is an ubiquitously expressed gene, which has been previously involved in other recurrent gene fusions in soft tissue neoplasms. It was initially reported to partner with the GLI1 gene in a distinctive lesion known as ‘pericytoma with t(7;12) translocation’.[28, 29] More recently, this peculiar group of tumors has been expanded to include a cohort of malignant epithelioid neoplasms defined by GLI1–associated fusions with various partners including ACTB, MALAT1 and PTCH1 genes.[30] The mechanism of GLI1 oncogenic activation in the ACTB-GLI1 fusion occurs by promoter swapping with the ubiquitously expressed ACTB, leading to the deregulation of GLI1 expression and its downstream targets. [30] We speculate that a similar mechanism of promoter swapping might be implicated in vascular tumors with ACTB-FOSB gene fusion, resulting in the overexpression and oncogenic activation of FOSB.
The evolving nomenclature of PHE reflects the morphologic overlap with other pathologic entities. The designation of ‘epithelioid sarcoma-like hemangioendothelioma’ was proposed by Billings et al. [15] Their study included 7 such cases, initially diagnosed as epithelioid sarcomas, which were composed of sheets, ill-defined nodules or fascicles of epithelioid cells in a desmoplastic stroma. They noted the distinct lack of vasoformation and hemorrhage in these tumors, despite being a vascular neoplasm. In keeping with these observations, none of the cases in our study, including the ACTB-FOSB fusion positive tumors, showed any vasoformation, putative primitive vascular features such as intracytoplasmic vacuoles, blister cells or presence of extravasated hemorrhage. Immunohistochemically, apart from cytokeratin positivity, all tumors showed positivity for vascular markers CD31 and FLI1 confirming the vascular origin. Based on their indolent clinical course, the tumors were regarded as tumors of intermediate malignancy (hemangioendothelioma category). The term ‘pseudomyogenic hemangioendothelioma’ was subsequently proposed by Hornick et al. [3] in a large study of 50 cases, due to their distinctive cytologic features with abundant brightly eosinophilic cytoplasm and rhabdomyoblast-like appearance. An associated stromal neutrophilic infiltrate was noted in one-third of the cases. Moreover, the authors highlighted the multifocal presentation in different tissue planes, the risk for locoregional recurrences and an overall indolent behavior with low risk of metastasis.
In addition to the lack of overt vasoformative properties, the PHE immunohistochemistry further adds to the morphologic non-conformity as a vascular neoplasm. In the study by Hornick et al., half of the cases were negative for CD31, while none were positive for CD34. FLI-1 was the only vascular marker consistently positive in all of the cases.[3] Our study confirmed these findings, CD31 showing only focal and patchy staining in most cases. However, ERG was diffusely and strongly positive in all of the cases, suggesting that ERG and/or FLI1 are better substitute vascular markers which can be used in the diagnosis of PHE. Interestingly, CD34 has been reported to be negative in all of the cases analyzed in different studies [3, 7, 15] and should not be used as a vascular marker in this setting. As previously reported myogenic markers, such as SMA and Desmin, were negative in all of the cases.
The consistent positivity for cytokeratin, typically with a strong and diffuse staining pattern, represents a significant pitfall in the diagnosis of PHE, especially in the context of a tumor lacking vascular channel formation and hemorrhagic stroma. [3, 15] The differential diagnoses vary depending on the anatomic location, with acral/superficial tumors raising the possibility of an epithelioid sarcoma. However, most, if not all, epithelioid sarcomas show loss of INI1 (SMARCB1) expression, [31] which is typically retained in PHE. In deep seated locations, including skeletal muscle or bone, especially in the context of multifocal presentation, the cytokeratin positivity raises the consideration of a metastatic/ sarcomatoid carcinoma. Two of the cases in this study were initially considered to be metastatic carcinoma displaying a prominent desmoplastic stroma. An extended panel of immunohistochemical stains, including vascular markers such as CD31, ERG and FLI1, should be performed in the setting of a sarcomatoid tumor in the absence of a clinically detectable primary site, to exclude the diagnosis of PHE. Furthermore, immunohistochemical stain for FOSB has recently been shown to be a sensitive immunohistochemical marker in the evaluation of PHE. [32, 33]
Additional considerations in the differential diagnosis of PHE are other vascular neoplasms, particularly epithelioid hemangioendothelioma, a low grade malignant neoplasm which also lacks mature vascular channel formation and stromal hemorrhage.[34] However, epithelioid hemangioendotheliomas show a variable number of blister cells with intra-cytoplasmic vacuoles and a distinct chondromyxoid stroma, which are not seen in PHE. Genetically, these tumors are characterized by a different chromosomal translocation, resulting in a WWTR1-CAMTA1 fusion in >95% of the cases.[35] In contrast, most epithelioid hemangiomas have a distinct morphologic appearance, with a lobular architecture of well-formed blood vessels lined by hobnailed endothelial cells, abundant hemorrhagic stroma and eosinophilic inflammatory infiltrate.[18, 20, 36] However, the presence of similar FOSB gene rearrangements, including 2 epithelioid hemangiomas with an identical ACTB-FOSB gene fusion (CRA unpublished observations), raise the possibility of a morphologic spectrum between epithelioid hemangioma and PHE. Certainly from a clinical standpoint, both pathologic entities have similar clinical presentations, with often multiple lesions involving various tissue planes. However, morphologically, as described above, the tumors show distinct microscopic features, with no significant overlap. (Figures 1 and 3)
The multifocal presentation of PHE in different tissue planes is rather intriguing, and similar to that seen in other benign and low grade vascular neoplasms such as epithelioid hemangiomas and epithelioid hemangioendotheliomas. One consideration is the so-called ‘field effect’, with multiple synchronous or metachronous tumors occurring in the same anatomic region, in keeping with a multiclonal process. Another possibility is locoregional metastasis from a single clone of disease within one area of the body, which might include various tissue planes, typically in the extremity. In the setting of multifocal epithelioid hemangioendothelioma, Errani et al [37] confirmed the monoclonality process by screening the fusion DNA breakpoints of separate nodules, which showed an identical WWTR1-CAMTA1 gene fusion break. These results support the loco-regional metastasis phenomenon, and suggest that multifocal PHE might evolve in a similar pattern.
PHE tend to behave in an indolent manner, with often local recurrences but rare distant metastases. In the report by Billings et al. [15], 2 of the 7 (29%) patients developed local recurrence, but none distant spread. In the study by Hornick et al. [3], 18 of the 31 (58%) patients with follow-up developed local recurrence, with 5 of them having multiple recurrences. One patient had a solitary regional lymph node metastasis and another patient developed distant metastasis 16 years after the primary lesion excision. Only one of the 31 patients in their series succumbed of disease. Rare cases with a more aggressive clinical course, including early multiple pulmonary metastases, have been reported.[38] None of the cases in our series showed distant spread, i.e. lymph node or lung metastatic implants, and all patients with available follow-up are still alive. The main therapy for PHEs has been surgical removal of solitary or pauci-multifocal lesions, with either en bloc or marginal excisions of tissue planes involved. Cytotoxic therapy has been attempted with little benefit.[6] Targeted therapy with inhibitors of mTOR like Sirolimus and Everolimus have shown benefit in some of the cases.[2, 6, 39] Recently, targeted therapy with Telatinib, a FLT1, FLT4 and PDGFRA receptor inhibitor, has been shown to be an effective therapy in PHE with a single case of a 17 year old male with multiple skin lesions in the head and neck showing complete remission after 4 years on the drug.[13] The study also showed that, in vitro, telatinib indirectly affects the expression of SERPINE1-FOSB, the driver genetic alteration in PHE, and thus could potentially be a highly specific therapy option in patients with multifocal/inoperable PHE. We speculate that this could be effective in the ACTB-FOSB fusion associated PHE.
In conclusion, our study expands the spectrum of genetic alterations in PHE, showing recurrent ACTB-FOSB fusions in half of the cases tested. The ACTB-FOSB molecular subset appears to have similar pathologic features and clinical outcome compared to the SERPINE1-FOSB fusion associated PHEs. It remains to be determined if the newer targeted therapies shown to have promising clinical benefit in the PHE with SERPINE1-FOSB fusion, will also be effective in this alternative ACTB-FOSB variant.
Supplementary Material
Supplementary Figure 1: (A) MRI image of a solitary PHE with an ACTB - FOSB fusion involving the calf muscle (case 6, 24/M). (B) Morphology from the same case showing spindle cells with abundant eosinophilic cytoplasm in a collagenous background. (C – E) Images from case 15 (30/M) illustrating the different tissue planes involved: (C) bone scan showing multiple lesions involving the tibia and the ankle; (D) image of a deep seated lesion showing epithelioid cells with abundant eosinophilic cytoplasm and; (E) superficial lesion of the dermis.
ACKNOWLEDGEMENTS
The authors would like to thank Jordana Shapiro for preparation of composite figures. They also thank the following pathologists and oncologists who kindly contributed case material and / or clinical follow-up information when available: Dr. Hsuan-Ting Huang, Taiwan.
Supported in part by: P50 CA 140146-01 (CRA), P30-CA008748 (CRA), Cycle for Survival (CRA), Kristin Ann Carr Foundation (CRA)
REFERENCES
- 1.Amary MF, O’Donnell P, Berisha F, Tirabosco R, Briggs T, Pollock R, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal radiology. 2013;42(7):947–57. [DOI] [PubMed] [Google Scholar]
- 2.Gabor KM, Sapi Z, Tiszlavicz LG, Fige A, Bereczki C, Bartyik K. Sirolimus therapy in the treatment of pseudomyogenic hemangioendothelioma. Pediatric blood & cancer. 2018;65(2). [DOI] [PubMed] [Google Scholar]
- 3.Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35(2):190–201. [DOI] [PubMed] [Google Scholar]
- 4.Ide YH, Tsukamoto Y, Ito T, Watanabe T, Nakagawa N, Haneda T, et al. Penile pseudomyogenic hemangioendothelioma/epithelioid sarcoma-like hemangioendothelioma with a novel pattern of SERPINE1-FOSB fusion detected by RT-PCR--report of a case. Pathol Res Pract. 2015;211(5):415–20. [DOI] [PubMed] [Google Scholar]
- 5.Inyang A, Mertens F, Puls F, Sumathi V, Inwards C, Folpe A, et al. Primary Pseudomyogenic Hemangioendothelioma of Bone. Am J Surg Pathol. 2016;40(5):587–98. [DOI] [PubMed] [Google Scholar]
- 6.Joseph J, Wang WL, Patnana M, Ramesh N, Benjamin R, Patel S, et al. Cytotoxic and targeted therapy for treatment of pseudomyogenic hemangioendothelioma. Clin Sarcoma Res. 2015;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradhan D, Schoedel K, McGough RL, Ranganathan S, Rao UNM. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue-a clinicopathological, immunohistochemical, and fluorescence in situ hybridization study. Hum Pathol. 2018;71:126–34. [DOI] [PubMed] [Google Scholar]
- 8.Rawal YB, Anderson KM, Dodson TB. Pseudomyogenic Hemangioendothelioma: A Vascular Tumor Previously Undescribed in the Oral Cavity. Head Neck Pathol. 2017;11(4):525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rekhi B, Gulia A, Rangarajan V. A rare case of multifocal pseudomyogenic hemangioendothelioma, involving soft tissues and bone, misdiagnosed as a rhabdomyosarcoma: Diagnostic and treatment implications. Indian J Pathol Microbiol. 2016;59(3):382–5. [DOI] [PubMed] [Google Scholar]
- 10.Requena L, Santonja C, Martinez-Amo JL, Saus C, Kutzner H. Cutaneous epithelioid sarcomalike (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149(4):459–65. [DOI] [PubMed] [Google Scholar]
- 11.Righi A, Gambarotti M, Picci P, Dei Tos AP, Vanel D. Primary pseudomyogenic haemangioendothelioma of bone: report of two cases. Skeletal radiology. 2015;44(5):727–31. [DOI] [PubMed] [Google Scholar]
- 12.Sheng WQ, Wang J. Primary pseudomyogenic haemangioendothelioma of bone. Histopathology. 2012;61(6):1219–24. [DOI] [PubMed] [Google Scholar]
- 13.van IDGP, Sleijfer S, Gelderblom H, Eskens F, van Leenders G, Szuhai K, et al. Telatinib Is an Effective Targeted Therapy for Pseudomyogenic Hemangioendothelioma. Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher C, Bridge JA, Hogendoorn PC, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone.: IARC: Lyon; 2013. [Google Scholar]
- 15.Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27(1):48–57. [DOI] [PubMed] [Google Scholar]
- 16.Trombetta D, Magnusson L, von Steyern FV, Hornick JL, Fletcher CD, Mertens F. Translocation t(7;19)(q22;q13)-a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer genetics. 2011;204(4):211–5. [DOI] [PubMed] [Google Scholar]
- 17.Walther C, Tayebwa J, Lilljebjorn H, Magnusson L, Nilsson J, von Steyern FV, et al. A novel SERPINE1-FOSB fusion gene results in transcriptional up-regulation of FOSB in pseudomyogenic haemangioendothelioma. J Pathol. 2014;232(5):534–40. [DOI] [PubMed] [Google Scholar]
- 18.Huang SC, Zhang L, Sung YS, Chen CL, Krausz T, Dickson BC, et al. Frequent FOS Gene Rearrangements in Epithelioid Hemangioma: A Molecular Study of 58 Cases With Morphologic Reappraisal. Am J Surg Pathol. 2015;39(10):1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonescu CR, Chen HW, Zhang L, Sung YS, Panicek D, Agaram NP, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer. 2014;53(11):951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van IDGP, de Jong D, Romagosa C, Picci P, Benassi MS, Gambarotti M, et al. Fusion events lead to truncation of FOS in Epithelioid Hamangioma of Bone. Genes Chromosomes Cancer. 2015;54(9):565–74. [DOI] [PubMed] [Google Scholar]
- 22.Milde-Langosch K The Fos family of transcription factors and their role in tumourigenesis. European journal of cancer. 2005;41(16):2449–61. [DOI] [PubMed] [Google Scholar]
- 23.Durchdewald M, Angel P, Hess J. The transcription factor Fos: a Janus-type regulator in health and disease. Histol Histopathol. 2009;24(11):1451–61. [DOI] [PubMed] [Google Scholar]
- 24.Catar R, Witowski J, Wagner P, Annett Schramm I, Kawka E, Philippe A, et al. The proto-oncogene c-Fos transcriptionally regulates VEGF production during peritoneal inflammation. Kidney Int. 2013;84(6):1119–28. [DOI] [PubMed] [Google Scholar]
- 25.Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, et al. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96(17):9671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Y, Wang S, Sun Y, Matt Y, Colburn NH, Shu Y, et al. JNK/AP-1 pathway is involved in tumor necrosis factor-alpha induced expression of vascular endothelial growth factor in MCF7 cells. Biomed Pharmacother. 2009;63(6):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nature medicine. 2000;6(9):985–90. [DOI] [PubMed] [Google Scholar]
- 28.Dahlen A, Mertens F, Mandahl N, Panagopoulos I. Molecular genetic characterization of the genomic ACTB-GLI fusion in pericytoma with t(7;12). Biochem Biophys Res Commun. 2004;325(4):1318–23. [DOI] [PubMed] [Google Scholar]
- 29.Bridge JA, Sanders K, Huang D, Nelson M, Neff JR, Muirhead D, et al. Pericytoma with t(7;12) and ACTB-GLI1 fusion arising in bone. Hum Pathol. 2012;43(9):1524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC. A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am J Surg Pathol. 2018;42(4):553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Loarer F, Zhang L, Fletcher CD, Ribeiro A, Singer S, Italiano A, et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer. 2014;53(6):475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung YP, Fletcher CD, Hornick JL. FOSB is a Useful Diagnostic Marker for Pseudomyogenic Hemangioendothelioma. Am J Surg Pathol. 2017;41(5):596–606. [DOI] [PubMed] [Google Scholar]
- 33.Sugita S, Hirano H, Kikuchi N, Kubo T, Asanuma H, Aoyama T, et al. Diagnostic utility of FOSB immunohistochemistry in pseudomyogenic hemangioendothelioma and its histological mimics. Diagn Pathol. 2016;11(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonescu C Malignant vascular tumors--an update. Mod Pathol. 2014;27 Suppl 1:S30–8. [DOI] [PubMed] [Google Scholar]
- 35.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50(8):644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Errani C, Zhang L, Panicek DM, Healey JH, Antonescu CR. Epithelioid hemangioma of bone and soft tissue: a reappraisal of a controversial entity. Clinical orthopaedics and related research. 2012;470(5):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Errani C, Sung YS, Zhang L, Healey JH, Antonescu CR. Monoclonality of multifocal epithelioid hemangioendothelioma of the liver by analysis of WWTR1-CAMTA1 breakpoints. Cancer genetics. 2012;205(1–2):12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. The American Journal of dermatopathology. 2013;35(5):597–600. [DOI] [PubMed] [Google Scholar]
- 39.Ozeki M, Nozawa A, Kanda K, Hori T, Nagano A, Shimada A, et al. Everolimus for Treatment of Pseudomyogenic Hemangioendothelioma. J Pediatr Hematol Oncol. 2017;39(6):e328–e31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: (A) MRI image of a solitary PHE with an ACTB - FOSB fusion involving the calf muscle (case 6, 24/M). (B) Morphology from the same case showing spindle cells with abundant eosinophilic cytoplasm in a collagenous background. (C – E) Images from case 15 (30/M) illustrating the different tissue planes involved: (C) bone scan showing multiple lesions involving the tibia and the ankle; (D) image of a deep seated lesion showing epithelioid cells with abundant eosinophilic cytoplasm and; (E) superficial lesion of the dermis.