Influenza viruses remain a severe threat to human health causing up to 650,000 deaths annually.1,2 Seasonal influenza virus vaccines can prevent infection, but are rendered ineffective by antigenic drift. To provide improved protection from infection, novel influenza virus vaccines that target conserved epitopes of influenza viruses, specifically those in the hemagglutinin (HA) stalk and neuraminidase (NA), are currently being developed.3
Antibodies against the HA stalk confer protection in animal studies.4–6 However, no data exist on natural infections in humans and these antibodies do not show activity in the hemagglutination inhibition (HI) assay, the HI titer being the current correlate of protection against influenza virus infection.7–9 While previous studies have investigated the protective effect of cellular immune responses and NA-inhibiting antibodies, additional serological correlates of protection from infection could aid the development of broadly protective or universal influenza virus vaccines.10–13 To address this gap, we performed a household transmission study to identify alternative correlates of protection from infection and disease in naturally exposed individuals. Using this study we determined 50% protective titers and levels for HI, full-length HA, NA, and HA stalk-specific antibodies. Further, we found that HA stalk antibodies independently correlated with protection from influenza virus infection.
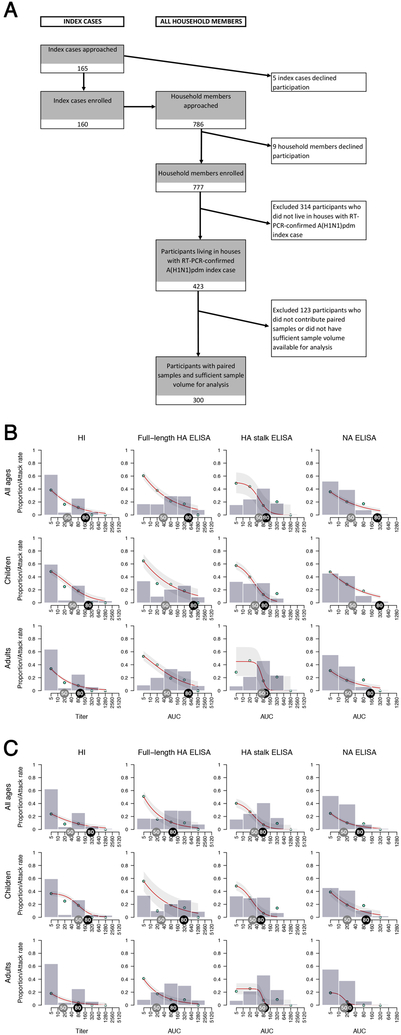
We followed 300 household members in a Nicaraguan family cohort who lived with one of 88 influenza-positive index cases for 3–5 weeks to test for infection and seroconversion (Figures 1A, Extended Data Fig. 1). The majority of households were recruited during the 2015 season (n=65) as pandemic H1N1 influenza virus activity was lower in 2013 (n=23). Only 10 household members were vaccinated for the concurrent influenza season (Table S1) which did not allow for detailed comparisons to unvaccinated individuals. Individuals who reported prior influenza virus vaccination were distributed evenly across antibody levels and two had PCR-confirmed influenza virus infection. Overall, 84 (28%) household members had a PCR-confirmed infection and approximately two-thirds (n=53) of the PCR-positive individuals developed symptomatic influenza.
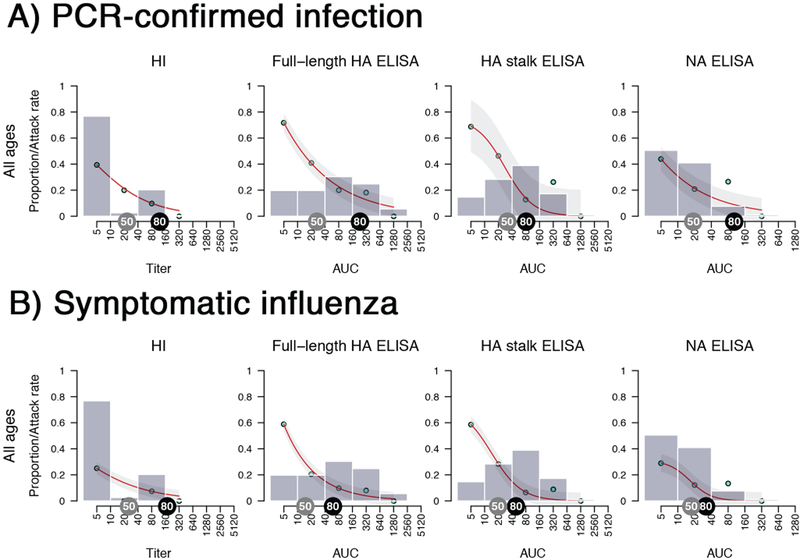
Figure 1. Study overview and antibody levels in relation to rates of infection.
A) Patient enrollment flow chart. B and C) Grey bars present the proportion of household contacts having a certain level of pre-existing antibody levels. The bars group individuals between the antibody levels covered by the bars on the x-axis (e.g. left-most bar includes all individuals with antibody levels <10, followed by 10 but less than 40, etc.). Red lines fit the antibody level-specific secondary attack rate based on the observed rates which are indicated as cyan points. The attack rate was calculated by dividing the number of infected contacts who had a specific baseline antibody level by the total number of contacts who had the same level of antibodies. Light grey shaded areas represent the 95% confidence intervals. Grey tags indicate a 50% protection antibody level and black tags indicate an 80% protection antibody level. Analyses were performed combined (all ages, n = 300 individuals) as well as separately for children (0–14 years old, n =101 individuals) and adults (15–85 years old, n=199 individuals).
To identify antibody levels associated with protection from infection and disease, we tested baseline (collected upon confirmed infection in a household) and follow-up (3–5 weeks post-enrollment) blood samples using the classical HI assay as well as in enzyme-linked immunosorbent assays (ELISAs) that measured antibodies against the full-length HA, the HA stalk domain or the NA.
As expected, we found that individuals with higher pre-exposure HI titers were less likely to become infected (Figure 1B). The 50% protective HI titer (i.e. the antibody level at which the risk of contracting influenza is reduced by 50% compared to individuals without detectable antibodies) was between 1:20 and 1:40, which is consistent with previous studies (Figure 1B, Table S2).14 High baseline ELISA levels measuring full-length HA antibodies in individuals who tested negative in the HI-assay indicate a strong prevalence of non-HI-active antibodies in the study participants. This can be explained by the presence of antibodies that bind the HA protein, but do not sterically interfere with receptor binding, which is the activity measured in the HI assay. ELISAs measuring full-length HA and NA antibodies correlated with protection from infection with narrow 95% confidence intervals (C.I.). The estimated 50% protective levels for both assays ranged between areas under the curve (AUCs) of 20 and 40 (Figure 1B, Table S2). The confidence intervals were wider for HA stalk antibodies, but similar 50% protective levels were between AUCs of 40 and 80.
We additionally estimated crude (i.e. not adjusted for age or other variables) 50% protection antibody levels against PCR-confirmed symptomatic influenza and found a similar good correlation with protection for all measured antibody levels. The antibody level associated with 50% protection was approximately 1:40 for HI antibodies and between AUCs of 20 and 40 for full-length HA, HA stalk and NA binding antibodies (Figure 1C, Table S3 and Extended Data Fig. 2, 3).
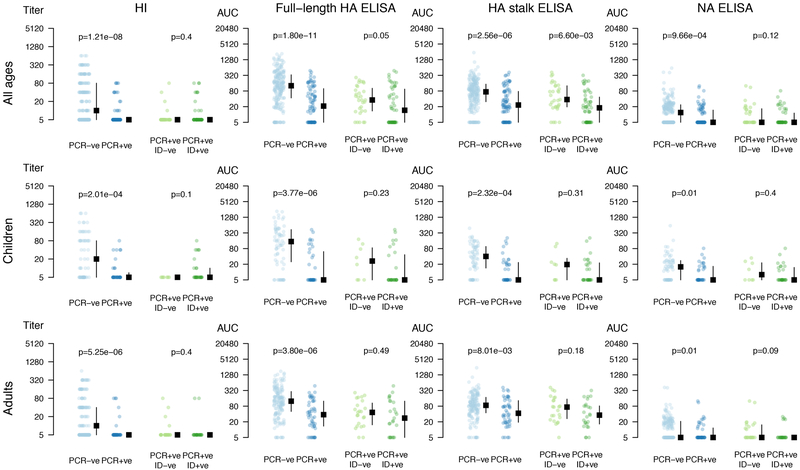
When pre-existing antibody levels of individuals who either were PCR-positive, or negative for influenza virus were plotted side-by-side, a clear trend could be observed (Figures 2, Extended Data Fig. 4 and Tables S4–S6). Participants who became infected had very low HA and NA antibody levels. In addition, HA stalk antibody levels measured by ELISA were lower in individuals who developed PCR-confirmed symptomatic influenza compared to PCR-confirmed asymptomatic individuals (p<0.01) suggesting that these antibodies correlated with protection from infection and disease.
Figure 2. Pre-existing antibody levels based on influenza outcomes.
Antibody levels for each individual, and the median and inter quantile range are shown. Y-axis indicates antibody levels. Individuals were separated by PCR-positivity status (blue dots) and by symptomatic influenza (green dots). Analyses were performed combined (all ages, n=300 individuals) as well as separately for children (0–14 years old, n=101 individuals) and adults (15–85 years old, n=199 individuals). Two-tailed Wilcoxon rank sum tests were used to calculate p-values. Please see Table S4 for false discovery rate analyses. Age groups and outcomes were pre-specified before analyses.
These crude analyses do not account for the effect of age and other antibodies in protected individuals. Thus, we adjusted for potential correlations by comparing the calculated protective effects associated with a 4-fold increase in antibody levels (seroconversion) in a single-assay model, to a multi-assay model that adjusts for correlation with other assays and age.
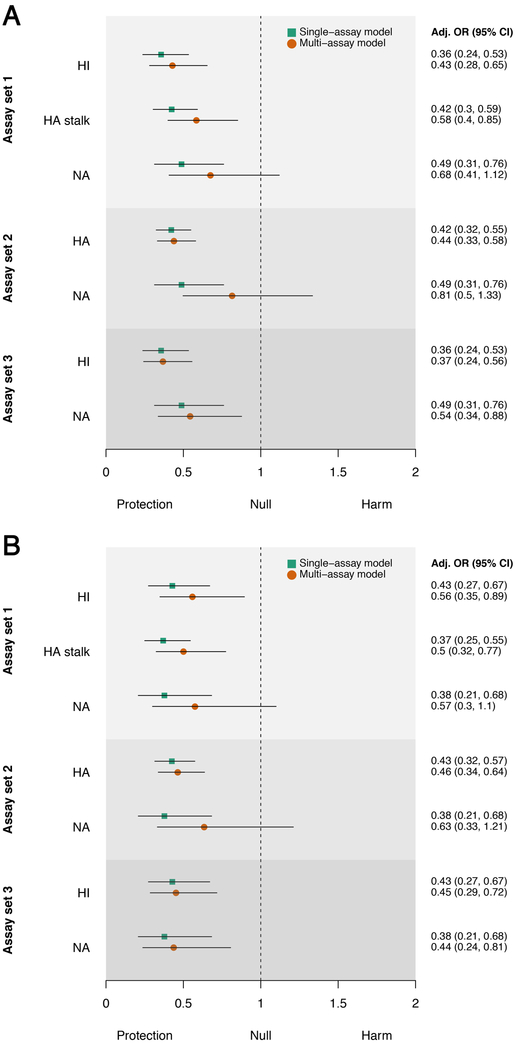
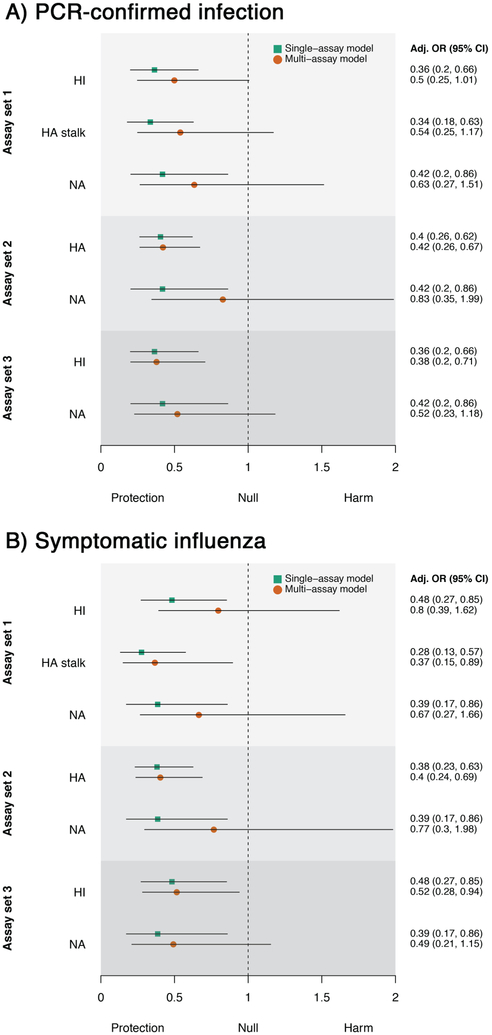
When adjusted for the effects of other measured antibody levels and age (multi-assay model), we found that HI, full-length HA, and HA stalk antibody levels remained independent predictors of protection against both PCR-confirmed infection and symptomatic influenza (Figure 3). Antibodies measured against NA showed a similar trend and were associated with protection against symptomatic influenza when adjusted for HI antibodies. However, they were not independently associated with protection when adjusted for both HI and HA stalk antibodies, indicating that NA antibodies in these individuals correlated with antibodies that were induced against HA. Using the multi-assay model, we found a four-fold increase in HA stalk antibodies to be associated with a 42% (C.I.,15%,60%) reduction in risk of being infected, which was slightly lower than the effect observed for a four-fold increase of HI antibodies (57%;C.I.,35%,72%). A similar reduction in infection risk was observed for symptomatic influenza. Age-stratified results are shown in extended data figures 5 and 6. These findings provide important support that non-HI active antibodies can be independently predictive of protection from influenza virus infection.
Figure 3. Protective effects associated with a 4-fold increase in antibody level.
Results are shown for three different sets of assays for A) PCR-confirmed infection and B) symptomatic influenza (n=300 individuals). Assay set 1 combines HI, HA stalk and NA ELISAs. Assay set 2 combines full-length HA and NA ELISAs. Assay set 3 combines HI and NA ELISAs. Adjusted odds ratios for the single-assay model are shown as green squares and the multi-assay model as orange circles. Black lines denote 95% confidence intervals.
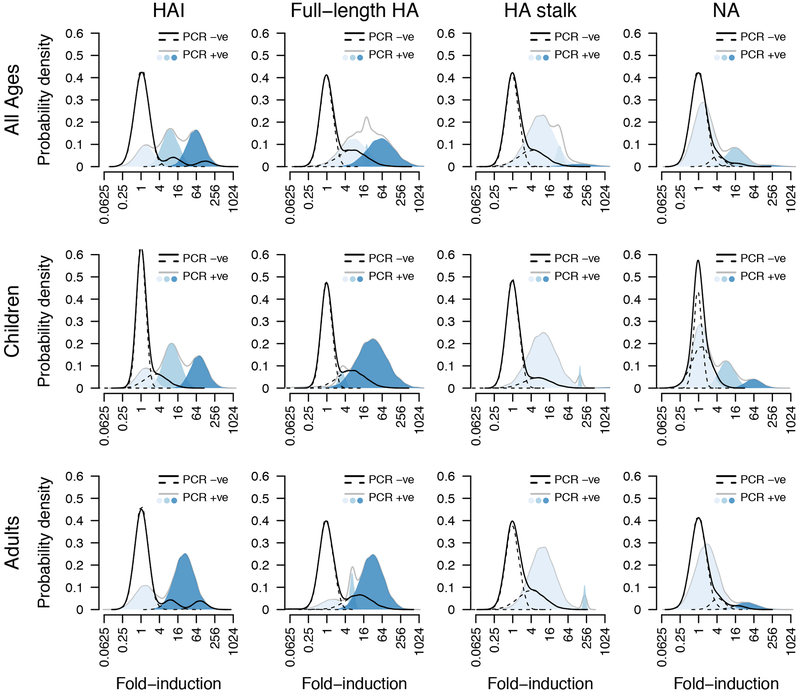
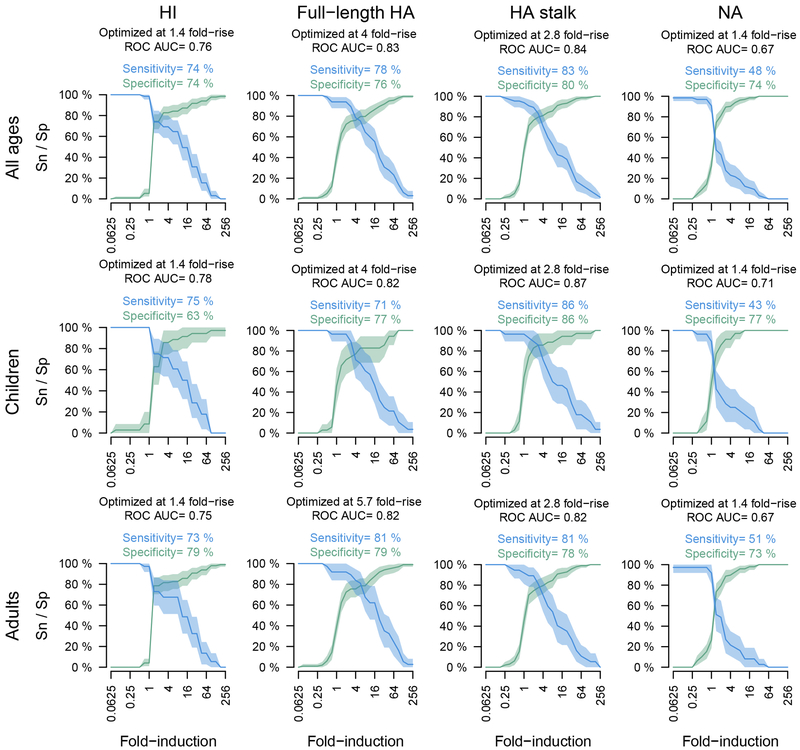
To compare the usefulness of ELISA-based readouts to HI for assessing seroconversion, we calculated fold-inductions of antibody levels post-infection for PCR-positive and PCR-negative cases (Figure 4). Consistent with previous studies, we found that 22% of individuals did not respond to infection as measured by HI (Figure 4, light-blue peak at 1-fold).15 Interestingly, we did not detect any apparent non-responders using ELISAs measuring full-length HA and HA stalk-specific antibodies. Many infected children (64%) did not show an increase in anti-NA antibody levels. Infection is generally thought to boost NA antibody levels,16 but measured responses against NA may be generally low as we found previously.17 A proportion of PCR-negative individuals seroconverted in all assays which might be attributable to these individuals not shedding enough virus for detection via PCR while still being infected. Additional sensitivity and specificity analyses were performed and indicate that ELISAs are useful to assess seroconversion in addition to HI assays (Extended Data Fig. 7, 8).
Figure 4. Non-responder subpopulation.
Black lines indicate probability density distribution of antibody response amongst non-infected members. Grey lines encompass the colored subpopulations of antibody response amongst infected members. The shades of blue indicate the 3 separate populations calculated by the model. Analyses were performed combined (all ages, n=300 individuals) as well as separately for children (0–14 years old, n=101 individuals) and adults (15–85 years old, n=199 individuals).
Novel universal influenza virus vaccines that elicit broadly-reactive antibodies against conserved epitopes in the HA stalk domain are currently in clinical development. However, HA stalk antibodies have not been shown to correlate with protection against natural influenza virus infection in humans. In this study, we used samples from a household transmission study to examine HI, full-length HA, HA stalk and NA antibodies as potential correlates of protection from influenza infection and disease. Importantly, using multiple statistical approaches we showed that HA stalk antibodies (which cannot be detected in HI assays10) were associated with protection against pandemic H1N1 influenza virus infection and disease.
Consistent with previous studies, a baseline HI titer between 1:20 and 1:40 was predictive of a 50% reduction in PCR-confirmed H1N1 influenza virus infection.14 Interestingly, only few individuals had baseline HI titers ranging from 1:10 to 1:40. Instead, titers were either undetectable or higher than 1:40 (Figure 1). A possible explanation for this could be that the antibodies measured in this largely unvaccinated population were elicited by recent infections, because the virus only circulated for 4–6 years prior to the study and it has been shown that that HI antibodies elicited by infection are maintained at titers >1:40 for many years.18 Using ELISAs that measured antibodies against full-length HA, NA or specifically the HA stalk, we were also able to identify crude estimates of protection. We found that these results were consistent between two influenza seasons (Extended Data Fig. 2, 3). Importantly, ELISAs against HA can measure antibody levels irrespective of the ability of the virus to agglutinate red blood cells, which is required for HI assays and has posed a problem for serology against recent H3N2 virus strains.19 These assays can furthermore detect non-neutralizing (but potentially protective) antibodies, which is of importance for anti-stalk antibodies that confer the majority of their protective effect through Fc-mediated functions.20,21
This study demonstrates that levels of HA stalk antibodies are a correlate of protection against natural pandemic H1N1 influenza virus infection. While previously published findings from a human challenge model did not find HA stalk antibodies to be predictive of protection from infection, they found an association with a reduction in viral shedding.22 A possible explanation for this difference is that human volunteers were intranasally inoculated with high doses of infectious particles (approximately 10^7), whereas natural infection is likely caused by much lower particle numbers.23 This difference is further highlighted by the fact that even individuals with high HI titers (> 1:1000) were not protected from infection in the challenge setting, which is not consistent with the findings of the majority of vaccine efficacy studies.24
Of note, the confidence intervals in this study for the predicted protective effect of HA stalk antibodies for PCR-confirmed infection were wider for adults compared to children (Figure 1A). Multiple factors may have contributed to this observation. There were few adult individuals who had baseline HA stalk antibody levels of <10, which may have contributed to the lower than expected number of cases. An important observation is the higher than expected number of infections at AUC levels from 160–640. This could be an indication that high titers of HA stalk antibodies are required for complete virus neutralization, which is consistent with previous observations that HA stalk antibodies have lower neutralizing activities compared to HI-active antibodies.25 Importantly, the correlation of HA stalk antibodies with protection from symptomatic influenza was consistent for both children and adults, which may indicate that HA stalk antibodies can reduce symptoms at sub-neutralizing levels.
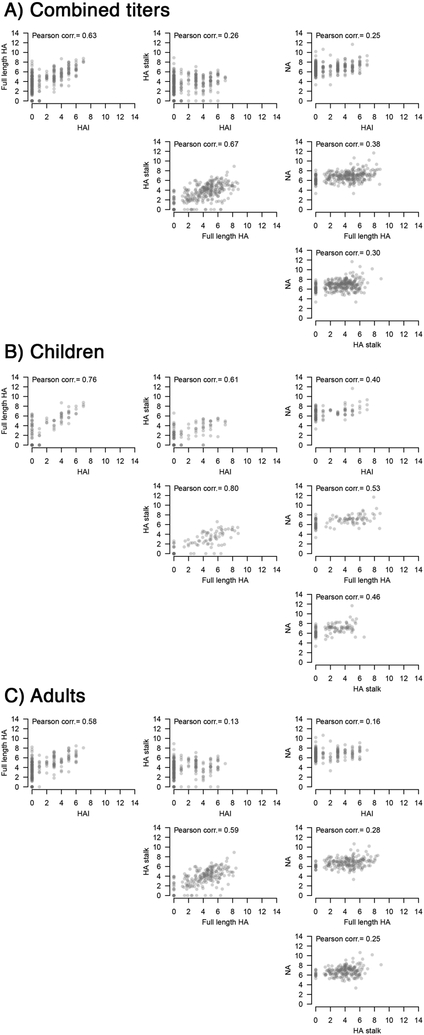
While NA antibody levels also correlated with protection, the majority of our subjects had low baseline NA antibody levels which limited the power of NA antibodies as an independent correlate of protection, after adjusting for age and HI titers. Furthermore, the results indicated that antibodies against NA correlated with HA antibodies in these individuals, potentially because the antibodies in this largely unvaccinated population were mainly elicited by infections, which would elicit antibodies against both HA and NA. NA antibodies correlated more with antibodies against the HA stalk (Pearson’s r = 0.35) compared to HI active antibodies (Pearson’s r = 0.25; Extended Data Fig. 9) Previous studies have shown that NA inhibition assays (NI) could be a useful correlate of protection from infection12,26 but HA stalk antibodies can contribute to NA inhibition measured in the traditional enzyme-linked lectin assay (ELLA).17,27–29 Based on these findings, it is possible that the correlation with protection reported for NI is partially conferred by HA stalk antibodies. Unfortunately, we did not have sufficient serum specimens to perform NI in this study. We also did not perform microneutralization assays, which have been previously shown to correlate with protection from infection, but may not fully capture the specific effects of HA stalk antibodies. The protective effect of these non-neutralizing antibodies should be investigated in future studies using assays that measure Fc-mediated functions of antibodies to dissect the mechanisms of antibody-mediated protection. Similarly, cell-mediated immunity could not be assessed here and will need to be further investigated. Importantly, these additional immune mechanisms could explain why a subset of adults did not have PCR-confirmed infection, despite low antibody levels.
ELISAs are used as standard assays for a number of other pathogens and are comparatively easy to standardize.30 While antibody binding as measured in these assays may not directly translate into functionality, our findings indicate that in a human cohort study setting with individuals who have acquired immunity primarily through virus exposure, results from binding assays could accurately predict protection from infection. We have also previously shown that ELISA antibody levels after vaccination can predict the protection of mice in a human serum transfer experiment.4 Furthermore, this study shows that these assays can be useful in combination with HI assays to assess seroconversion after influenza virus infection.
HA stalk antibodies were measured using a chimeric HA antigen, which has an exotic H6 head domain to which humans are generally naïve. However, some rare cross-reactive head antibodies have been previously described that could recognize conserved epitopes on this antigen.31,32 It can therefore not be excluded that part of the measured response is provided by non-HI active cross-reactive head antibodies. Since these antibodies have been rarely isolated from humans, the majority of the measured responses are likely HA stalk antibodies. Accordingly, antibody levels measured using a chimeric HA have been previously shown to correlate well with antibodies measured using headless HA probes in ferrets that were vaccinated with multiple heterologous HA head domains.5
A particular strength of our study was the intensive follow-up which allowed us to capture both symptomatic and asymptomatic individuals, which translated to a high number of observed infections and provided statistical power for our detailed analyses.
The study was performed in Nicaragua where influenza virus vaccination was introduced recently and is not widely used; therefore, the majority of the pre-existing antibody response was likely induced by repeated natural infections. This differs from the situation in some countries, where vaccination rates are high. Similar studies that test highly vaccinated individuals will be required to detect potential differences in protective antibody levels elicited by vaccination vs. infection.
In summary, we found that HA stalk antibodies are an independent correlate of protection from pandemic H1N1 infection and disease in a natural transmission setting. Further, antibodies measured by ELISA can be used as a powerful correlate of protection and to assess seroconversion, which will be important for novel universal influenza virus vaccine development.10,33,34 Additional resources should be allocated to standardize these assays to enable their use in both research and clinical settings. Further studies are required to examine the role of these antibodies as potential correlates of protection against influenza A(H3N2) and influenza B in natural transmission settings.
Methods
Participants and Study Procedures
As a part of an observational household transmission study in Nicaragua, members who lived with an influenza index case in their household were monitored for influenza virus infection. Daily symptoms were assessed, nasal and oropharyngeal swabs were taken every 2–3 days for 10–14 days and blood samples were collected at enrolment as well as 3–5 weeks later to determine infection outcomes and antibody responses. Eligible households included those that 1) had an index case that had a positive QuickVue Influenza A+B rapid test result and with acute respiratory infection (ARI) symptom onset within the previous 48 hours; and, 2) had at least one person living with the index case. Details of the study design are published.35,36 Participants were excluded from this analysis if sufficient blood samples were not available. The principles of the Declaration of Helsinki were strictly followed. Ethical approval was obtained from institutional review boards of the University of Michigan (HUM 00091392) and the Ministry of Health, Nicaragua (CIRE 06/07/10–025). Written informed consent was obtained from all adult participants and proxy written informed consent was obtained for all children. Assent was obtained from children aged 6 and older.
Laboratory Methods
Respiratory samples were tested in the Nicaraguan National Virology Laboratory by real-time reverse-transcription polymerase chain reaction (PCR) using standard protocols.37 HI assay38 was performed to determine HI titers while ELISA was performed to measure binding antibodies to full-length HA, HA stalk and NA. ELISAs were performed as described elsewhere.4 The HA full-length constructs corresponded to the vaccine strains during the respective seasons (H1 A/California/4/09 – 2013 season; H1 A/Michigan/45/15 – 2015 season). A chimeric HA expressing the head domain from an H6N1 virus (to which humans are naïve) and the stalk domain of pandemic H1N1 influenza virus A/California/4/09 was used to measure HA stalk antibodies. The HAs were expressed as soluble proteins with a trimerization domain to maintain correct protein folding and conformational epitopes as previously described.39 The NA of A/California/4/09 was used to measure NA-specific antibodies. The NA was expressed as soluble antigen with a tetramerization domain to maintain correct folding and enzymatic activity (as measured in NA star assays).40 ELISA values are reported as area under the curve (AUC). AUC was chosen, as it considers both the endpoint and the absolute levels of optical density measured at all tested serum dilutions. AUC calculation (optical density multiplied by serum dilution over the entire curve) was performed in GraphPad Prism. All assays were performed by personnel who were blinded to infection status.
Outcomes
The primary outcome was PCR-confirmed influenza and the secondary outcome was symptomatic influenza (PCR-confirmed infection with an episode of fever with cough or sore throat).41 Antibody response was measured by the ratio between post- and pre-exposure level (pre-existing antibody level).
Statistical Analyses
Antibody level-specific attack rates were calculated by dividing the number of infected contacts who had a specific baseline antibody titer by the total number of contacts who had the same level of antibody titer. To infer the crude estimates of the 50% and 80% protective levels, we used a 3-parameter logistic regression model (nplr R package) that allowed for a ≤ 1 probability of infection at the lowest detectable level, and a ≥0 probability of infection at the highest observable level, meaning that incomplete protection can occur at high levels and participants could have pre-existing antibodies at levels that were below what was required for complete protection. Two multivariable logistic regression models were used to study the effect of a 4-fold antibody level increase on infection outcome, including 1) a single-assay model in which levels of one serology assay and age are predictors and 2) a multi-assay model where levels of multiple assays and age are predictors. The level of pandemic H1N1 influenza virus activity differed between study years and was adjusted for in the analyses. In models 1 and 2, a smoothing spline function was used to model the effect of age on infection risk (mgcv R package). Antibody levels were log transformed for all analyses, levels below the lowest detectable limit of 1:10 were imputed as 1:5. Individual antibody titer data points were visualized and compared between disease outcome groups using a two-tailed Wilcoxon rank sum test. A finite mixture model was used to explore underlying non-responder sub-populations based on the observed distribution of the antibody response (mixtool R package). The model estimates the mean and standard deviation for each component of the Gaussian mixtures which were visualized to illustrate results on antibody response. Receiver Operating Characteristic Curve (ROC) analysis (pROC R package) was used to estimate the sensitivity and specificity of each assay. Classification and Regression Trees analysis (rpart R package) were performed to identify the best combination of assays indicated by their positive and negative predictive values in identifying PCR positive individuals. False discovery rates (FDR) were calculated in GraphPad Prism using the two-stage step-up method of Benjamini, Krieger and Yekutieli.42
Extended Data
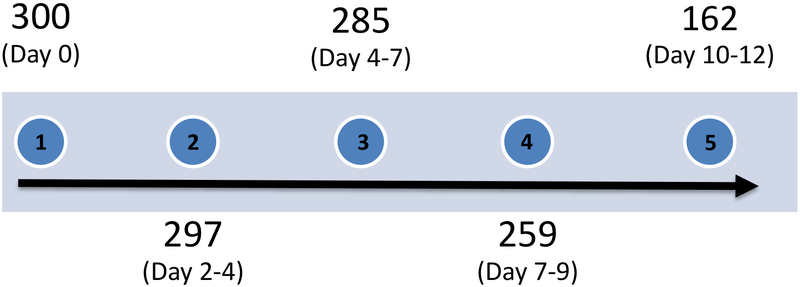
Extended Data Fig. 1. Participant follow-up timeline.
Participant sample collection timeline with number of samples collected from unique individuals (n=300 individuals). Day ranges are represented as quintiles.
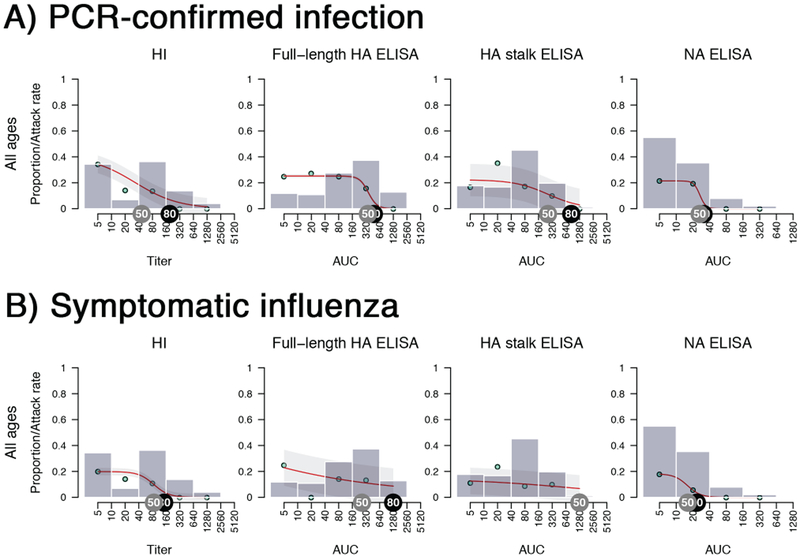
Extended Data Fig. 2. Pre-existing antibodies and corresponding secondary attack rates in 2015 (N=198 individuals).
Results are shown for A) PCR-confirmed infection and B) Symptomatic influenza. Note that geometric mean baseline HI titer for this year was 1:10. Grey tags indicate a 50% protection level and black tags indicate an 80% protection level. Grey bars present the proportion of household contacts having a certain level of pre-existing antibody levels. The bars group individuals between the antibody levels covered by the bars on the x-axis (e.g. left-most bar includes all individuals with antibody levels <10, followed by 10 but less than 40, etc.). Red lines fit the antibody level-specific secondary attack rate based on the observed rates which are indicated as cyan points. The attack rate was calculated by dividing the number of infected contacts who had a specific baseline antibody level by the total number of contacts who had the same level of antibodies. Shaded area represents the 95% confidence intervals.
Extended Data Fig. 3. Pre-existing antibodies and corresponding secondary attack rates in 2013 (N=102 individuals).
Results are shown for A) PCR-confirmed infection and B) Symptomatic influenza. Note that geometric mean baseline HI titer for this year was 1:34. Grey tags indicate a 50% protection level and black tags indicate an 80% protection level. Grey bars present the proportion of household contacts having a certain level of pre-existing antibody levels. The bars group individuals between the antibody levels covered by the bars on the x-axis (e.g. left-most bar includes all individuals with antibody levels <10, followed by 10 but less than 40, etc.). Red lines represent the sigmoid function fitted to the observed antibody level-specific secondary attack rates (SAR) which are indicated as cyan points. The attack rate was calculated by dividing the number of infected contacts who had a specific baseline antibody level by the total number of contacts who had the same level of antibodies. Shaded area represents the 95% confidence intervals for the predicted antibody level-specific SAR.
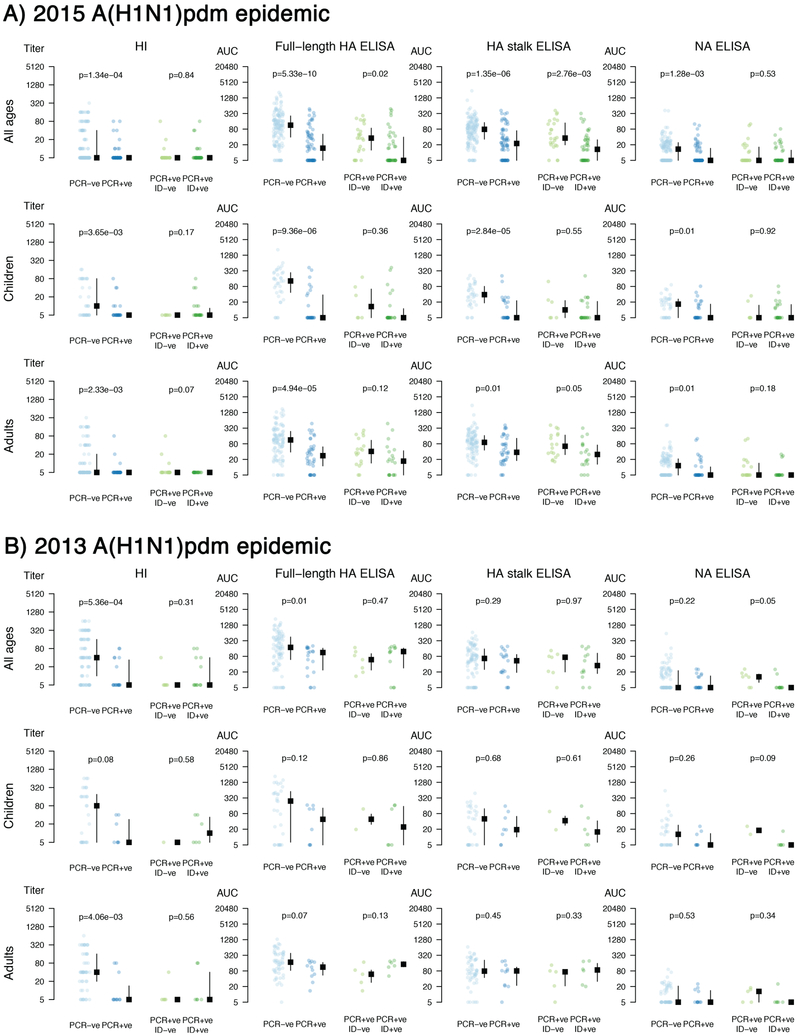
Extended Data Fig. 4. Influenza outcome specific distribution of pre-existing antibodies.
Results are shown for A) 2015 A(H1N1)pdm epidemic and B) 2013 A(H1N1)pdm epidemic. Antibody levels for each individual, and the median and inter quantile range are shown. Y-axis indicates antibody levels. Individuals were separated by PCR-positivity status (blue dots) and by symptomatic influenza (green dots). Individual antibody titer data points were compared between disease outcome groups using a two-tailed Wilcoxon rank sum test. Analyses were performed combined (all ages; 2013: n=102 individuals; 2015: n=198 individuals) as well as separately for children (0–14 years old; 2013: n=38 individuals; 2015: n=64 individuals) and adults (15–85 years old; 2013: n=63 individuals; 2015: n=135 individuals). Please see Table S5 and S6 for false discovery rate analyses. Age groups and outcomes were pre-specified before analyses.
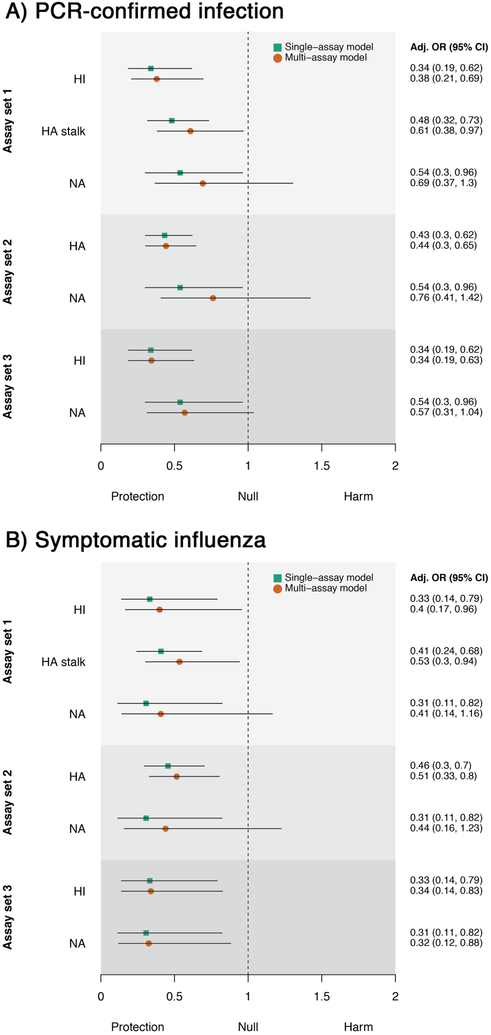
Extended Data Fig. 5. Protective effects associated with a 4-fold increase in antibody level amongst children.
Results are shown for three different sets of assays for A) PCR-confirmed infection and B) symptomatic influenza (n=101 individuals). Assay set 1 combines HI, HA stalk and NA ELISAs. Assay set 2 combines full-length HA and NA ELISAs. Assay set 3 combines HI and NA ELISAs. Adjusted odds ratios for the single-assay model are shown as green squares and the multi-assay model as orange circles. Black lines denote 95% confidence intervals. OR: odds ratio.
Extended Data Fig. 6. Protective effects associated with a 4-fold increase in antibody level amongst adults.
Results are shown for three different sets of assays for A) PCR-confirmed infection and B) symptomatic influenza (n=199 individuals). Assay set 1 combines HI, HA stalk and NA ELISAs. Assay set 2 combines full-length HA and NA ELISAs. Assay set 3 combines HI and NA ELISAs. Adjusted odds ratios for the single-assay model are shown as green squares and the multi-assay model as orange circles. Black lines denote 95% confidence intervals. OR: odds ratio.
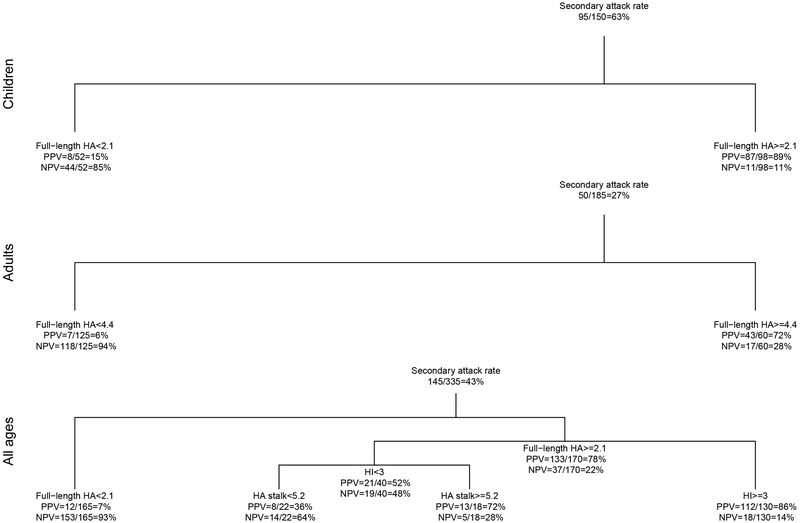
Extended Data Fig. 7. Positive and negative predictive values of the best serology testing strategy identified by decision tree analyses.
True positive cases were individuals who had PCR confirmed influenza virus infection. True negatives were individuals who had neither a positive PCR nor a four-fold rise in antibody serology tests. The model also suggested optimal cutoff points to use when defining seroconversion. PPV: positive predictive value; NPV: negative predictive value.
Extended Data Fig. 8. Sensitivity and Specificity of HI and ELISA in detecting PCR-confirmed Infections.
Curves are plotted as solid lines for sensitivity (Sn) in blue and specificity (Sp) in green. Shaded areas indicate 95% confidence intervals. X-axes show fold induction for the respective assay. Analyses were performed combined (all ages, n=300) as well as separately for children (0–14 years old, n=101) and adults (15–85 years old, n=199).
Extended Data Fig. 9. Antibody titer correlations.
Correlation analyses for antibody titers were performed A) combined (all ages, n=300 individuals) as well as separately for B) children (0–14 years old, n=101 individuals) and C) adults (15–85 years old, n=199 individuals). Pearson’s r value is plotted in each figure.
Supplementary Material
Acknowledgements
The authors thank past and present members of the study team based at the Health Center Soócrates Flores Vivas, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute in Nicaragua for their dedication and high-quality work. We are grateful to the study participants and their families. Lastly, we would like to thank F. Busto Carrillo for assistance in generating figures.
Funding
This work was supported by the National Institute for Allergy and Infectious Diseases (award R01 AI120997 to A.G. and contract numbers HHSN272201400008C to F.K. and HHSN272201400006C to A.G.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines. The Krammer laboratory receives funding for universal influenza virus vaccine projects from the Department of Defense, PATH, the Bill and Melinda Gates Foundation and GlaxoSmithKline.
Footnotes
Data availability
De-identified data sets used for the study are available on ImmPort (study SDY1436). Identifying data required to replicate the study analyses, such as exact age, are available by request as required by the institutional review board-approved protocol for the Nicaraguan Household Influenza Transmission Study.
Code Availability
Code to understand and assess the conclusions of this research is available via ImmPort (study SDY1436).
References
- 1.Nair H, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378, 1917–1930 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses 3, 37–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachbagauer R & Krammer F Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect 23, 222–228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen H, et al. Influenza Virus Hemagglutinin Stalk-Specific Antibodies in Human Serum are a Surrogate Marker for In Vivo Protection in a Serum Transfer Mouse Challenge Model. MBio 8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachbagauer R, et al. A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines 2, 26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachbagauer R & Palese P Development of next generation hemagglutinin-based broadly protective influenza virus vaccines. Curr. Opin. Immunol 53, 51–57 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Hobson D, Curry RL, Beare AS & Ward-Gardner A The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 70, 767–777 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohmit SE, Petrie JG, Cross RT, Johnson E & Monto AS Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis 204, 1879–1885 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Potter CW & Oxford JS Determinants of immunity to influenza infection in man. Br. Med. Bull 35, 69–75 (1979). [DOI] [PubMed] [Google Scholar]
- 10.Erbelding EJ, et al. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis 218, 347–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElhaney JE, Coler RN & Baldwin SL Immunologic correlates of protection and potential role for adjuvants to improve influenza vaccines in older adults. Expert Rev Vaccines 12, 759–766 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Monto AS, et al. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. J. Infect. Dis 212, 1191–1199 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Sridhar S, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med 19, 1305–1312 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Coudeville L, et al. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 10, 18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauchemez S, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLoS Pathog. 8, e1003061 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y-Q, et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 173, 417–429.e410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran M, et al. Analysis of Anti-Influenza Virus Neuraminidase Antibodies in Children, Adults, and the Elderly by ELISA and Enzyme Inhibition: Evidence for Original Antigenic Sin. MBio 8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babu TM, et al. Population Serologic Immunity to Human and Avian H2N2 Viruses in the United States and Hong Kong for Pandemic Risk Assessment. J. Infect. Dis 218, 1054–1060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zost SJ, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. U.S.A 114, 12578–12583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries RD, et al. Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 35, 238–247 (2017). [DOI] [PubMed] [Google Scholar]
- 21.DiLillo DJ, Tan GS, Palese P & Ravetch JV Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med 20, 143–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J-K, et al. Evaluation of Preexisting Anti-Hemagglutinin Stalk Antibody as a Correlate of Protection in a Healthy Volunteer Challenge with Influenza A/H1N1pdm Virus. MBio 9(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varble A, et al. Influenza A virus transmission bottlenecks are defined by infection route and recipient host. Cell Host Microbe 16, 691–700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng S, et al. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J. Infect. Dis 208, 1320–1324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, et al. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J. Virol 89, 3610–3618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couch RB, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis 207, 974–981 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y-Q, Lan LY-L, Huang M, Henry C & Wilson PC Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J. Virol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosik I, et al. Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies. J. Exp. Med 216, 304–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohlbold TJ, et al. Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice. J Virol 90, 851–861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotkin SA Correlates of protection induced by vaccination. Clin. Vaccine Immunol 17, 1055–1065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekiert DC, et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat. Med 22, 1456–1464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paules CI, Marston HD, Eisinger RW, Baltimore D & Fauci AS The Pathway to a Universal Influenza Vaccine. Immunity 47, 599–603 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Paules CI, Sullivan SG, Subbarao K & Fauci AS Chasing Seasonal Influenza - The Need for a Universal Influenza Vaccine. N. Engl. J. Med 378, 7–9 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Ng S, et al. The Timeline of Influenza Virus Shedding in Children and Adults in a Household Transmission Study of Influenza in Managua, Nicaragua. Pediatr. Infect. Dis. J 35, 583–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng S, et al. Association between Haemagglutination inhibiting antibodies and protection against clade 6B viruses in 2013 and 2015. Vaccine 35, 6202–6207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novel Swine-Origin Influenza, A.V.I.T., et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med 360, 2605–2615 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Manual for the laboratory diagnosis and virological surveillance of influenza, (World Health Organization, Geneva, 2011). [Google Scholar]
- 39.Margine I, Palese P & Krammer F Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp, e51112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wohlbold TJ, et al. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6, e02556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budd A, et al. Manual for the Surveillance of Vaccine-Preventable Diseases. (Centers for Disease Control and Prevention, 2017). [Google Scholar]
- 42.Benjamini Y, Krieger AM & Yekutieli D Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.