Abstract
Engineering three-dimensional (3D) implantable tissue constructs is a promising strategy for replacing damaged or diseased tissues and organs with functional replacements. However, the efficient vascularization of new 3D organs is a major scientific and technical challenge since large tissue constructs or organs require a constant blood supply to survive in vivo. Current approaches to solving this problem generally fall into the following three major categories: (a) cell-based, (b) angiogenic factor-based, and (c) scaffold-based. In this review, we summarize state-of-the-art technologies that are used to develop complex, stable, and functional vasculature for engineered 3D tissue constructs and organs; additionally, we have suggested directions for future research.
Keywords: Bioengineering, Angiogenesis, Tissue scaffolds
INTRODUCTION
The demand for organ transplants continues to grow, with the number of active patients on the waiting list for a solid organ transplant in the United States reaching 80,000 in 2015 [1]. In addition to the increasing demand for transplants, the disparity between the number of patients awaiting organ transplantation and the number of available donated organs has dramatically widened, with organ shortage causing numerous patient deaths and increased social burden [2,3]. Tissue engineering technologies combining chemicals, biocompatible materials, and cells have made continuing progress to address this issue. However, the most common challenge for the clinical translation of three-dimensional (3D) tissue-engineered constructs is their requirement for vascularization. In general, living cells must be within 200 μm of a blood supply to acquire sufficient oxygen and nutrients and to remove waste, ensuring long-term survival and functionality [4–6]. Due to the oxygen diffusion limit from the periphery, most 3D constructs at a physiologically relevant scale require vascularization in order to deliver oxygen and nutrients throughout the engineered tissue. Therefore, achieving adequate vascularization is the main therapeutic goal when designing 3D constructs in vitro to prevent hypoxia and cellular necrosis. There have been many efforts to create vascular networks or promote vascularization within 3D engineered tissue constructs [7]. Robust, efficient, and reproducible vascularization strategies could be developed based on the physiological process in vivo, with successful translation depending on the ability of the vascularization strategies to replicate in vivo phenomena. Therefore, it is crucial to thoroughly understand vascular network development in vivo. This review will highlight our current understanding of the physiological development of human vasculature and the most promising vascularization strategies in the field of tissue engineering.
PHYSIOLOGICAL DEVELOPMENT OF HUMAN VASCULATURE
The following two mechanisms are generally involved in the generation of a vasculature in vivo: vasculogenesis and angiogenesis [8]. Vasculogenesis is the process that initiates blood vessel formation, primarily at the embryo stage. Endothelial precursor cells (EPCs) (“angioblasts” in embryos and “endothelial progenitor cells” in adults) migrate, differentiate, and assemble to form a primary vascular labyrinth [9]. EPCs migrate in response to chemo-attractants such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and placental growth factor [10,11]. Further blood vessel development occurs by extending the pre-existing vascular network through the process of sprouting or intussusception, also known as angiogenesis [12,13]. During sprouting, the tip cells of existing blood vessels are extended by multiple filopodia guided by angiogenic stimuli such as VEGF, FGF, and EGF via Notch signaling; thus, the vascular network is extended [12]. Growth factor gradients guide endothelial cell (EC) migration by signaling via receptors on the filopodia such as VEGFR-2. The sprouts can extend to neighboring microvessels and integrate with them via a process called inosculation. Vascular networks can be further extended by splitting or intussusception, in which the intussusceptive pillar is extended by duplicating existing vessels [14]. The recruitment of pericytes and vascular smooth muscle cells by platelet-derived growth factor (PDGF)-BB and angiopoietin-1 (ANG-1), and the generation of an extracellular matrix (ECM) mature and stabilize the nascent vasculature and regulate vessel function (arteriogenesis) [15,16]. In addition to this soluble signaling pathway, angiogenesis is also highly influenced and regulated by cell-ECM and cell-cell interactions. The ECM provides guidance cues for the proliferation, migration, and differentiation of mural cells and ECs. Cell-ECM and cell-cell interactions have been reviewed in detail elsewhere [17–19]. The arterial or venous fate of ECs is regulated via specific molecular identities; for instance, activation of the Notch signaling pathway by VEGF binding to its receptors, such as Flk1 and neuropilin 1, promotes arterial specification, whereas repressing Notch signaling through the orphan receptor COUP-TFII promotes venous specification [20,21]. The regulation of vessel specialization has been reviewed in depth elsewhere [22]. Considering the physiological process of vascular network generation in vivo, we will highlight state-of-the-art approaches to vascularization in tissue engineering.
VASCULARIZATION APPROACHES FOR TISSUE-ENGINEERED 3D CONSTRUCTS
In thin or avascular tissues such as skin and cartilage, implanted cells can acquire oxygen and nutrients through diffusion to maintain their survival [23]; however, slow or insufficient vascularization has remained a critical challenge in thicker constructs with a physiologically relevant size. As host vessel ingrowth proceeds slowly, at a rate of less than several tenths of a micrometer per day, complications can arise including avascular necrosis in the core region and eventual failure to engraft; therefore, achieving a well-distributed and interconnected 3D vascular network is necessary to maintain cell viability in tissue-engineered constructs at a clinically-relevant size [24–26]. The current strategies for achieving functional vascularization in 3D tissue-engineered constructs can be categorized as cell-based, angiogenic factor-based, or scaffold-based approaches. Scaffold-based approaches include the construction of tissues with decellularized grafts, sacrificial scaffolds, spatial micropatterning, biomimetic scaffolds using vascular corrosion casting, and 3D printing techniques.
1) Cell-based vascularization approaches
① Vascularization by vascular cell co-cultures
Many early studies have shown that co-culturing cells of endothelial origin can create a vascular network [27–29]; consequently, this technique has been used for a long time as a starting point for vascularization in a variety of tissues. Co-culturing with ECs in vitro has enabled the formation of stable vascular network that maintained its stability and anastomosed with host vasculature when implanted in vivo [24,30]. Fibroblasts, which provide structural support to blood vessels by synthesizing ECM proteins, were initially used in this co-culturing system. ECs co-cultured with fibroblasts in a collagen gel became spindle shaped and reorganized into capillary-like structures in 3 to 5 days [31]. Another study co-cultivated human umbilical vein endothelial cells (HUVECs) with fibroblasts to engineer well-formed capillaries in a fibrin gel which anastomosed with the host vasculature within 4 to 5 days of implantation in immunodeficient mice [30]. Several different cells of endothelial origin have also been used in the co-culturing system. The ability of endothelial progenitor cells, outgrowth ECs (also termed endothelial colony-forming cells), and outgrowth endothelial progenitor cells to form capillary-like structures have been investigated and were found to accelerate vasculature formation [32–38]. In the co-cultivation system, cells which provide vascular wall structures, including fibroblasts, keratinocytes, pericytes, and vascular smooth muscle cells, have been used to enhance EC differentiation and promote vascular network formation [39–42]. Recently, mesenchymal stem cells (MSCs), which are well known potent producers of VEGF-A [43], have been shown to improve vessel formation in 2D and 3D in vitro culture systems as well as when implanted in vivo [44]. When co-cultured with MSCs, ECs exhibited increased endothelial specific ANG-1 expression and decreased ANG-2 expression, which mediate vascular maturation via EC stabilization by increasing the binding of ANG-1 to the Tie 2 receptor. Consequently, coculturing MSCs and ECs increased the density of mature vasculature in vitro and in vivo [45,46]. Despite the promising results of co-culture techniques and the resulting de novo vascularization, the inability to control the geometry of the vascular network is a major limitation for achieving 3D vascularization in the engineered organ. Furthermore, culturing cells in vitro is expensive and time consuming.
② Cell sheet vascularization
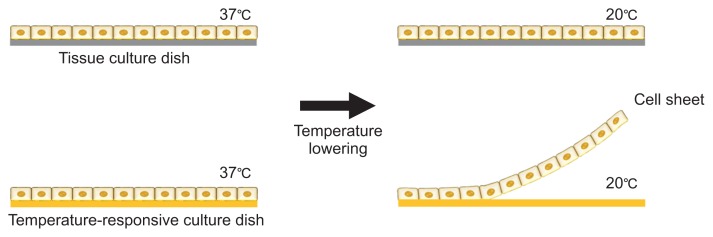
Another promising technology is cell sheet engineering (CSE), developed by Yang et al. [47], which allows prevascularized networks to be fabricated without the need of a scaffold. The basic principle of CSE is a monolayer cell culture on a temperature-responsive culture dish coated with poly(N-isopropylacrylamide) (PIPAAm) [48] which promotes the attachment and proliferation of cells at 37°C. Lowering the temperature to <32°C causes the PIPAAm to become highly hydrophilic, making the surface anti-adhesive due to the rapid hydration and swelling of PIPAAm. Consequently, the monolayer of cultivated cells detaches from the surface as a confluent cell sheet with intact cell-to-cell junctions and deposited ECM (Fig. 1) [49].
Fig. 1.
Generating confluent cell sheets using temperature-responsive culture dishes.
ECs in a single layer cell sheet are known to spontaneously form a vascular network. Co-cultivating tissue-specific cells and ECs as a confluent cell sheet with multiple layers generates a thicker prevascularized tissue construct [50–52]. Sekine et al. [53] created triple-layer cardiac cell sheets produced from neonatal rat cardiac cells cocultured with ECs overlaid on the vascular bed. After 3 days of culture in a bioreactor with added FGF-2, the co-cultured constructs were thoroughly perfused and many tubular blood vessels had been formed [54]. Thicker tissue constructs have been successfully made by stacking twelve cell sheets, with the preformed vascular network effectively connecting to host capillaries and surviving after transplantation [55]. Overall, CSE is a very promising approach for fabricating thin vascularized tissue; however, it has limitations when constructing viable thick tissue and fabricating well-organized vascular networks suitable for organs at a clinically translatable scale.
③ Promoting vascularization by progressive layering
Despite recent advances in engineering transplantable tissues, reconstructing highly vascularized volumetric tissues and organs on a large scale (>mm to cm) remains challenging. To address this issue, the progressive layering technique has been developed. Kim et al. [56] injected muscle cells into the tibialis anterior muscle defect site every week for 4 weeks in a progressive manner. Large-scale (>mm) muscle tissue was successfully reconstructed and host vasculature and neurons were integrated well. Moreover, the technique enhanced muscle volume and improved functional recovery compared with single cell injections of the same volume. The idea of ‘multiple and progressive layering’ has also been applied to engineered cell sheets and has facilitated the construction of thicker 3D tissues. Shimizu et al. [57] transplanted three-layer cell sheets derived from neonatal rat cardiomyocytes into the dorsum of nude rats. The repeated transplantation of triple-layer sheets at the same site resulted in well-synchronized grafts and ~1 mm thick cardiac tissue with a well-organized microvascular network, permitting whole myocardial survival without necrosis. In vivo vascularization with ‘multiple and progressive layering’ utilizes the natural host regeneration process to its advantage; however, multiple cell injections or poly-surgery is time consuming and requires multiple patient interventions which would not be clinically acceptable.
2) Angiogenesis with growth factors
① Angiogenic effect of growth factors
Functionalized biomaterial matrices or scaffolds with angiogenic growth factors have been widely used to promote vascularization. Up to twenty angiogenic growth factors such as VEGF, PDGF-BB, bFGF, hepatocyte growth factor (HGF), insulin like growth factor (IGF), and transforming growth factor-β (TGF-β) have been widely used to promote vascularization in a variety of ECM proteins and disease models [58–60].
All key angiogenic growth factors (VEGF, FGF-2, IGF, HGF, PDGF-BB, and TGF-β1) have the ability to bind specific sites in the ECM; once bound, their release kinetics are dependent on their binding affinity and the action of proteases which cleave the ECM itself or the ECM-binding domain of the growth factors [61]. It has been demonstrated that insufficient exposure to angiogenic growth factors inhibits appropriate angiogenesis [62–64]. Growth factor overexpression may also lead to immature and unstable vessels by inhibiting the function of vascular smooth muscle cells and pericytes, and even vascular tumors [65,66]. Consequently, for therapeutic purposes the dose and duration of growth factor release must be carefully controlled. Tremendous efforts have been made to enable the controlled release of growth factors from different biodegradable materials. Heparin or heparan sulfate-mimetic molecules can be covalently crosslinked with the collagen type I scaffold via 1-ethyl-3-dimethyl aminopropyl carbodiimide (EDC) and N-hydroxysuccinimide (NHS) to control the release of heparin-binding growth factors, resulting in enhanced angiogenesis [67]. In addition, combining VEGF and FGF with a heparin-immobilized scaffold increased angiogenesis compared with a single growth factor [68,69]. Biomaterial scaffolds have also been functionalized by surface modifications [70] or incorporating the growth factor- or heparin-binding ECM domain [61,71,72]. For example, a fibrin matrix covalently crosslinked with multifunctional recombinant fibronectin (FN) fragments including both its 12th and 14th type III repeats (FN III12-14) and FN III9-10 allowed multiple growth factors (VEGF-A165, PDGF-BB, and BMP-2) to be sequestered and enhanced the angiogenic effects of the growth factors in a mouse model of chronic wounds [61]. Angiogenic growth factors themselves can also be modified to enhance their binding affinity to biomaterials instead of engineering the biomaterials to increase their affinity with growth factors [73–76]. Sacchi et al. [74] showed that fibrin hydrogels covalently crosslinked with VEGF164 fused to a sequence derived from α2-plasmin inhibitor (α2-PI1–8) could release growth factors by enzymatic cleavage. This allowed the VEGF dose and delivery duration to be precisely controlled by the α2-PI1–8-fused variant of the fibrinolysis inhibitor aprotinin, which efficiently induced stable and functional angiogenesis. Mittermayr et al. [75] used the specific binding technology TG-hook and showed that PDGF-AB modified with a TG-hook enables growth factors to be retained within the fibrin matrix, subsequently increasing functional angiogenesis. Another approach for increasing the binding affinity of growth factors to biomaterials is engineering ‘super-affinity’ growth factors [77]. A domain in placenta growth factor-2 (PIGF-2123-144) which has an exceptionally strong binding affinity to ECM proteins was fused to VEGF-A and PDGF-BB. These super-affinity growth factors significantly increased angiogenesis in vivo at low doses compared to their wild-type forms. A variety of strategies have been developed to control the local delivery of angiogenic growth factors in order to facilitate and promote angiogenesis; however, their inherent inability to control the geometric architecture of vascular network has limited their applications in 3D tissue construction.
② Bioactive motif immobilization to promote angiogenesis
Incorporating short bioactive peptides onto 3D hydrogels for tissue engineering has been an effective method for enhancing vascularization [78]. Recent studies have demonstrated the effects of the immobilized bioactive peptides on vascularization. The binding of integrin to short peptide adhesive sequences derived from ECM proteins such as collagen (Arg-Gly-Asp [RGD]), laminin (e.g., Tyr-Ile-Gly-Ser-Arg [YIGSR] and Ser-Ile-Lys-Val-Ala-Val [SIKVAV]), and FN (e.g., RGD and Arg-Glu-Asp-Val [REDV]) enhanced EC attachment and migration, and thus angiogenesis [79–83]. Short peptide-functionalized hydrogels exhibit BM-like activities, such as directing cell attachment, spreading, invading, and differentiation. Hydrogel bioactivation by including functional RGD and REDV sequences in an elastin-Like recombinamer-based hydrogel enhanced EC adhesion and improved in vivo angiogenic potential at the earliest time point via general cell adhesion (RGD) and specific endothelial cell adhesion (REDV) [84].
Despite the promise of growth factor- or bioactive peptide-guided vascular network formation, this approach is limited by the lack of ability to control network geometry which inhibits the generation of a spatially-controllable 3D vascular network. Furthermore, the uncontrolled delivery of angiogenic growth factors inhibits ECs from forming mature vasculature, leading to a leaky and disorganized vascular network. For this reason, tissue engineering has attempted to fabricate precisely-controllable, mature 3D vasculature using scaffolds; some promising techniques are described below.
3) Scaffold-based approaches
① Decellularized tissues
The decellularization of a vascularized organ or tissue to make a 3D structure of a vascular network has been extensively investigated [85,86]. Naturally-derived 3D vasculature can be obtained by decellularizing native tissues or whole organs (Fig. 2) to form an acellular matrix, which often preserves tissue-specific vascular structures, cell-matrix interaction, and functional molecules that regulate cellular functions, phenotype, and signaling [87]. This approach can eliminate the need to design 3D vascular networks in vitro and can be followed by repopulation with desirable human primary cells to build perfusable constructs with native 3D vasculature.
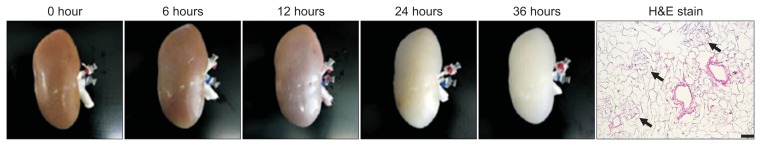
Fig. 2.
Time lapse images of pig kidney decellularization using 0.5% sodium dodecyl sulfate and a representative H&E image (arrows indicate preserved glomerular structure). Scale bar=50 μm. Part of figure reproduced with permission [87].
Removing all cellular remnants, the biocompatibility of the preserved tissue matrices, and scaffold reproducibility are crucial to the success of the decellularization approach. There are numerous decellularization protocols which typically use physical, chemical, and biological agents. The perfusion of ionic (e.g., sodium dodecyl sulfate [SDS] and sodium deoxycholate) or non-ionic (e.g., Triton X-100) detergents and enzymes (e.g., DNase) through the vasculature of an organ is an efficient method for removing its cellular contents [87–89]. These chemical or biological agents are routinely combined with physical methods such as whole organ perfusion and shear stress. Regardless of the protocol, a decellularized scaffold should fulfill the following current pre-set criteria for clinical use: 1) complete or near complete removal of native cellular materials (DNA content less than 50 ng/mg dry tissues); 2) preservation of the native vascular structure, and 3) preservation of native extracellular components and ultrastructure [88,90–92].
Repopulating the decellularized organ matrix with organ-specific cells (recellularization) is required to build a functional implantable organ. Current decellularization/recelluarization techniques have enabled less complex tissue matrices such as heart valves, small intestinal submucosa for the femoral artery, and dermal matrices to be used clinically [93–95]. These achievements provide proof-of-concept that a new functional bioengineered organ could be built from a native organ via decellularization/recellularization. A number of whole organs including hearts, lungs, livers, and kidneys have been successfully bioengineered in vitro using current decellularization/recellularization techniques, with preclinical in vivo studies underway using small and large animal models [96–100]. Song et al. [96] decellularized rat kidneys using renal artery perfusion with 1% SDS and repopulated the acellular matrix with HUVECs via renal artery perfusion and with rat neonatal kidney cells via the ureter. After culturing the perfused organ in the bioreactor for 3 to 5 days, the bioengineered kidney was able to generate concentrated urine in vitro, and the graft integrated with the host circulation and produced urine when transplanted into a rat.
Despite the successes of decellularization/recellularization techniques in generating transplantable organs, a major hurdle for long-term in vivo success is vascular patency. Without complete endothelial reseeding of the vascular tree, the implanted graft experiences vascular thrombosis which inevitably leads to the failure of the recellularized graft. Ko et al. [97,98] improved re-endothelization by conjugating anti-CD31 antibodies with the vasculature of the acellular matrix, promoting EC coverage and resulting in homogenous endothelium formation in acellular porcine livers and kidneys. When transplanted into healthy Yorkshire pigs, the endothelia of the Ab-conjugated constructs effectively prevented platelet adhesion and were able to maintain blood flow for 24 hours.
Since the decellularized organ and tissue matrices closely mimic the vascular network of an organ, the decellularization/recellularization technique is the most clinically translatable approach for building vasculature in bioengineered tissue and organ constructs. However, long-term vascular patency should be addressed by uniformly and homogenously re-endothelizing onto the vascular tree and reducing residual antigenicity before widespread clinical application [101].
② Sacrificial template for vascular channel/network formation
Sacrificial materials have been used to create desired geometric vascular channels/networks by molding a non-sacrificial material around a sacrificial component, and then removing the sacrificial material. Hollow channels are then lined by ECs to form predesigned vascular networks within the constructs. Sacrificial template methods were initially used to form microvascular tubes in vitro. Chrobak et al. [102] made a 120 μm diameter channel in collagen hydrogel using a 120 μm diameter stainless steel needle, then removed the needle after gelation of the collagen. Seeding human ECs and perivascular cells into the hollow channels yielded an EC tube that mimicked a constantly perfusable microvessel. Although non-sacrificial materials (stainless steel needles) can define the geometry of a channel, the destruction of constructs during the removal of spacers and the un-branched pattern produced are drawbacks of the method. To reduce the possibility of destroying the integrity of the constructs during manual or mechanical spacer removal, thermoresponsive or glucose-sensitive materials have been investigated as sacrificial components [103]. To fabricate a patterned vascular network, Miller et al. [104] printed carbohydrate-glass lattices (channel diameter of 150–750 μm) as the sacrificial element and encapsulated them with ECM along with a suspension of living cells. After ECM crosslinking, carbohydrates were dissolved in the culture medium to yield an open, interconnected, and perfusable channel in the gel.
Strategies using sacrificial materials to construct vascular networks have successfully fabricated a functional, interconnected, vascular channel inside a hydrogel; however, no studies have yet demonstrated how to build a complex vascular network within thick 3D-engineered tissue constructs to create a clinically translatable tissue or organ. In addition, the sacrificial material must be completely removed since remnants could be cytotoxic or harmful to the recipient.
③ Spatial micropatterning
The spatial micropatterning approach to vascular network formation has attracted attention since it has a spatial resolution of less than 10 μm. The detailed methods of EC micropatterning have been reviewed in detail elsewhere [105]. This approach generally involves microfabrication technologies, such as soft lithography and photopolymerization, to engineer spatially organized EC positioning. Both technologies depend on the following four major steps which are essentially based on printing, molding, and embossing: 1) pattern design; 2) photomask and master fabrication; 3) polydimethylsiloxane (PDMS) stamp fabrication; and 4) micro- and nano-structure fabrication using the stamp [106]. Raghavan et al. [107] used soft lithography techniques on microfabricated PDMS templates with intended geometries. Introducing a suspension of ECs in collagen gel into the channel and stimulation with VEGF and bFGF resulted in the formation of spatially arranged endothelial cords. Baranski et al. [108] implanted the micropatterned EC cords with human hepatocytes into nude mice and found that the implanted cords acted as a guide for a rapid vascularization response, leading the cords to anastomose with the host vasculature. Laser guided direct writing (LGDW) is another method for depositing cells on matrices with micrometer accuracy [109], which was developed based on methods for optically trapping cells. Like optical tweezers, cells are forced into a position using laser beams; however, in LGDW the laser beam is weakly focused on a spot, allowing cells to be pushed along the beam axis onto an arbitrary surface. Using LGDW, Nahmias et al. [110] created an endothelial vascular structure and showed that it could recruit primary mature hepatocytes in a HGF-dependent manner to form liver sinusoid-like structures in vitro.
The high spatial resolution and simplicity of the micropatterning approach make this strategy a very powerful tool for fabricating vascular structures at a micro- or nanoscale. Despite its many advantages, this technology has not yet been used to create 3D structures with other cell types through stacking or rolling. In addition, the complexity of the vascular structure and the size of the micropatterned substrates are currently limited.
④ Biomimetic scaffolds using vascular corrosion casts
Vascular corrosion casting is a well-established methodology for creating a complete replica of the vascular lumen of an organ and has provided detailed morphologies of the vascular luminal structures of the kidney, pancreas, uterus, liver, lung, and placenta [111–116].
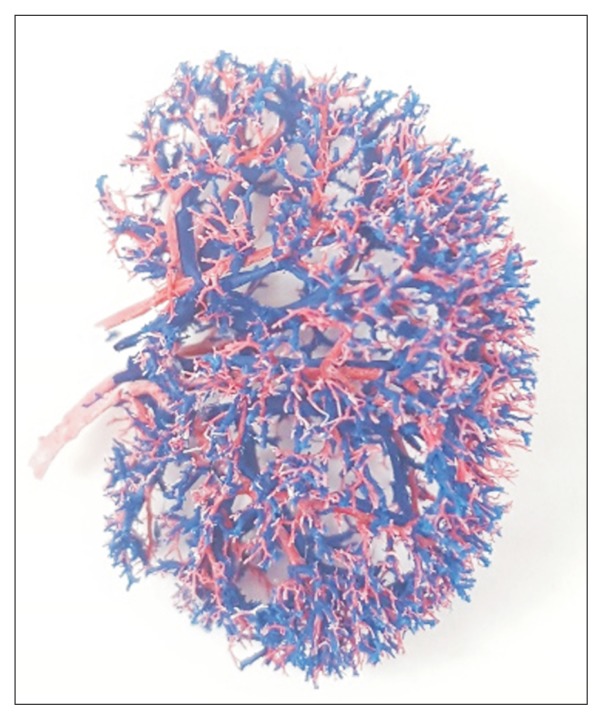
We created rat kidney vascular casts by perfusing 10 % polycaprolactone (PCL) dissolved in acetone (Fig. 3). Most of the renal vasculature and glomerular capillaries were preserved after digesting the native tissue with 20% sodium hydroxide. The PCL casts were then used as a template to create biomimetic vascular scaffolds by coating the casts with type I collagen solution, cross-linking, and removing the PCL with acetone. The resultant vascular scaffolds had a similar 3D branching architecture when observed by scanning electron microscopy. EC-seeded scaffolds were embedded in the collagen hydrogel to produce a consistent vessel-like structure formed by the EC-covered vascular scaffold [117].
Fig. 3.
Renal vascular corrosion cast made using polycaprolactone perfusion.
Fabricating vascular scaffolds using vascular corrosion casting techniques could be a powerful tool for creating truly biomimetic and tissue-specific 3D vascular scaffolds. This technique is also simple and cost effective [118]; however, the endothelialization of the fabricated scaffold must be precisely tuned and the strategy must be scaled up to enable clinically translatable constructs to be fabricated.
⑤ Three-dimensional bioprinting
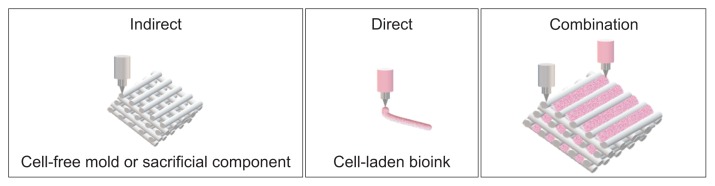
Three-dimensional bioprinting is a multidisciplinary process that spatially patterns living cells and other biologics by stacking them using a computer-aided layer-by-layer deposition approach to fabricate 3D organs and tissue constructs [119]. Recently, 3D bioprinting has been increasingly used to fabricate vascularized 3D constructs as it enables geometrically-complex, anatomically-precise structures to be created and has shown promising results when creating complex composite tissue constructs. Bioprinting utilizes two manufacturing concepts to fabricate constructs: direct and indirect printing. Direct printing involves actively bioprinting bioink (cell-laden hydrogels) into desired vascular structures, whereas indirect printing involves bioprinting cell-laden hydrogel layers onto a cell-free mold or sacrificial component (Fig. 4).
Fig. 4.
Manufacturing concepts for printing constructs. Indirect printing involves printing a mold or sacrificial component for subsequent direct printing with cell-loaded bioink.
The most commonly used bioprinting approaches nowadays are based on jetting, extrusion, and lasers. In jetting methods, the print-head is positioned over the printing bed and bioink droplets made by thermal, electrostatic, or piezoelectric inkjet bioprinters [120–122] are released to yield a 3D tissue construct [123]. Jetting methods have the ability to produce picoliter scale drops and have a high printing resolution of ~30 to 60 μm [124]. Using modified thermal inkjet printers, Cui and Boland [125] printed human microvascular ECs simultaneously alongside fibrin to fabricate microvasculature. The printed ECs proliferated to form a tubular structure inside the fibrin scaffold after 21 days of culture; however, since the hydrogel concentration is low, the thickness of constructs printed using jetting methods may be limited by low levels of structural support [126]. Bioprinting using extrusion-based bioprinters is one of the most commonly utilized techniques, wherein bioink is deposited layer-by-layer using a syringe and piston system to dispense material through microscale nozzles [127]. Extrusion-based methods use high concentrations of hydrogels such as alginate and Pluronic F-127 to produce more stable 3D cell-laden structures [128,129]. Gao et al. [130] recently developed a coaxial extrusion nozzle to allow an interior flow of calcium solution alongside an exterior flow of alginate solution. Using this coaxial extrusion bioprinting, they were able to create hollow calcium alginate filaments which were high strength, cell-laden, 3D hydrogel structures with perfusable endogenous microchannels. Unfortunately, due to inadequate mechanical stability and structural integrity, it is difficult to print clinically scalable tissue structures. Recently, our group demonstrated the ability of the integrated tissue-organ printer (ITOP), one of the most sophisticated 3D bioprinters, to fabricate stable, human-scale tissue constructs in any shape [131]. The ITOP patterns multiple cell-laden composite hydrogels consisting of gelatin, fibrinogen, hyaluronic acid, and glycerol while delivering a supporting PCL polymer and a sacrificial Pluronic F-127 hydrogel to achieve mechanical stability. The ability of ITOP to fabricate a human-scale mandible, calvarial bone, cartilage, and skeletal muscle was demonstrated, with evaluation of these tissues in vivo showing tissue maturation and large blood vessel formation within the implanted tissues. The ITOP is perhaps the most advanced 3D bioprinter allowing the clinical application of 3D bioprinting techniques to date.
Lastly, laser-assisted bioprinters are an alternative method for bioprinting a precise microvasculature, although few studies have been published. Wu and Ringeisen [132] fabricated branch/stem structures with HUVECs using biological laser printing and deposited human umbilical vein smooth muscle cells on top and around the printed HUVEC structures after 1 day. The resulting microvasculature had two stems and the branches that connect the stems had stable lumina and closely mimicked native vascular network in size.
Although 3D bioprinting technology has led to enormous advances in the fabrication of vascular structures, building clinically relevant vascularized tissue and organs remains a significant challenge. Three-dimensional bioprinting has the advantages of precision, reproducibility, and relatively low operational cost, while innovations in hardware, bioink formulation, and printing strategy are rapid and will facilitate vascularized thick tissues to be 3D bioprinted at clinically relevant volumes in the near future.
CONCLUSION AND FUTURE PERSPECTIVES
Vascularization is one of the most pressing scientific and technical challenges facing the engineering of 3D tissue and organs. Successful vascularization has paved the way for implantable 3D constructs at a clinically-relevant scale. Although significant progress has been made during the last decade in the area of vascularized tissue engineering, building scaffolds with vascular networks that mimic the complexity, ultrastructure, geometry, biochemical cues, and cellular density and distribution of organs remains a challenge. Furthermore, the appropriate and timely vascularization of the implanted 3D constructs has yet to be achieved. Although the direct anastomosis of preformed microvascular networks with host microvasculature is the most rapid reperfusion process for implanted 3D constructs, it is almost impossible. Therefore, newer approaches for faster vascularization are required. The rapid, external inosculation of preformed vascular networks with host vessels may ensure adequate angiogenesis and survival of the implanted cells [133], which is thought to be promoted by cultivating prevascularized tissue constructs in an angiogenic ECM [134].
No single vascularization approach discussed in this article can produce a functional, stable, and scalable vascular structure by itself, although each has fabricated thin, simple vascular networks successfully. A better approach consisting of a tailored, synergistic combination of multiple methods is required to engineer improved vascular networks and promote external inosculation. Improving our understanding of normal angiogenesis could allow the growth and development of vessels in clinically translatable 3D constructs to be optimized.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.OPTN/SRTR 2015 annual data report: introduction. Am J Transplant. 2017;17(Suppl 1):11–20. doi: 10.1111/ajt.14123. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee P, Venkataramani AS, Vijayan A, Wellen JR, Martin EG. The effect of state policies on organ donation and transplantation in the United States. JAMA Intern Med. 2015;175:1323–1329. doi: 10.1001/jamainternmed.2015.2194. [DOI] [PubMed] [Google Scholar]
- 3.Delmonico FL, Domínguez-Gil B, Matesanz R, Noel L. A call for government accountability to achieve national self-sufficiency in organ donation and transplantation. Lancet. 2011;378:1414–1418. doi: 10.1016/S0140-6736(11)61486-4. [DOI] [PubMed] [Google Scholar]
- 4.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of microvascular networks: a review. Biomaterials. 2005;26:1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Kim JJ, Hou L, Huang NF. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. 2016;41:17–26. doi: 10.1016/j.actbio.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Swift MR, Weinstein BM. Arterialvenous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 11.Peplow PV. Influence of growth factors and cytokines on angiogenic function of endothelial progenitor cells: a review of in vitro human studies. Growth Factors. 2014;32:83–116. doi: 10.3109/08977194.2014.904300. [DOI] [PubMed] [Google Scholar]
- 12.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 13.Mentzer SJ, Konerding MA. Intussusceptive angiogenesis: expansion and remodeling of microvascular networks. Angiogenesis. 2014;17:499–509. doi: 10.1007/s10456-014-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–123. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 17.Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163–174. doi: 10.1159/000362276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar LT, Hoying JB, Utzinger U, Underwood CJ, Krishnan L, Baggett BK, et al. Mechanical interaction of angiogenic microvessels with the extracellular matrix. J Biomech Eng. 2014;136:021001. doi: 10.1115/1.4026471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bersini S, Yazdi IK, Talò G, Shin SR, Moretti M, Khademhosseini A. Cell-microenvironment interactions and architectures in microvascular systems. Biotechnol Adv. 2016;34:1113–1130. doi: 10.1016/j.biotechadv.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 21.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 22.Corada M, Morini MF, Dejana E. Signaling pathways in the specification of arteries and veins. Arterioscler Thromb Vasc Biol. 2014;34:2372–2377. doi: 10.1161/ATVBAHA.114.303218. [DOI] [PubMed] [Google Scholar]
- 23.Atala A. Advances in tissue and organ replacement. Curr Stem Cell Res Ther. 2008;3:21–31. doi: 10.2174/157488808783489435. [DOI] [PubMed] [Google Scholar]
- 24.Kang KT, Allen P, Bischoff J. Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood. 2011;118:6718–6721. doi: 10.1182/blood-2011-08-375188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborn SL, So M, Hambro S, Nolta JA, Kurzrock EA. Inosculation of blood vessels allows early perfusion and vitality of bladder grafts--implications for bioengineered bladder wall. Tissue Eng Part A. 2015;21:1906–1915. doi: 10.1089/ten.tea.2014.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–301. doi: 10.1002/aja.1000640203. [DOI] [Google Scholar]
- 27.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 28.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97(5 Pt 1):1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx M, Perlmutter RA, Madri JA. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1994;93:131–139. doi: 10.1172/JCI116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuzuya M, Kinsella JL. Induction of endothelial cell differentiation in vitro by fibroblast-derived soluble factors. Exp Cell Res. 1994;215:310–318. doi: 10.1006/excr.1994.1347. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16:585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieminski AL, Hebbel RP, Gooch KJ. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11:1332–1345. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- 35.Patel J, Wong HY, Wang W, Alexis J, Shafiee A, Stevenson AJ, et al. Self-renewal and high proliferative colony forming capacity of late-outgrowth endothelial progenitors is regulated by cyclin-dependent kinase inhibitors driven by Notch signaling. Stem Cells. 2016;34:902–912. doi: 10.1002/stem.2262. [DOI] [PubMed] [Google Scholar]
- 36.Fu J, Wiraja C, Muhammad HB, Xu C, Wang DA. Improvement of endothelial progenitor outgrowth cell (EPOC)-mediated vascularization in gelatin-based hydrogels through pore size manipulation. Acta Biomater. 2017;58:225–237. doi: 10.1016/j.actbio.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 38.Minami Y, Nakajima T, Ikutomi M, Morita T, Komuro I, Sata M, et al. Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. Int J Cardiol. 2015;186:305–314. doi: 10.1016/j.ijcard.2015.03.166. [DOI] [PubMed] [Google Scholar]
- 39.Black AF, Berthod F, L’heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, Kim SH, Leong KW, Jung Y. Nanografted substrata and triculture of human pericytes, fibroblasts, and endothelial cells for studying the effects on angiogenesis. Tissue Eng Part A. 2016;22:698–706. doi: 10.1089/ten.tea.2015.0461. [DOI] [PubMed] [Google Scholar]
- 41.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25–C38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elbjeirami WM, West JL. Angiogenesis-like activity of endothelial cells co-cultured with VEGF-producing smooth muscle cells. Tissue Eng. 2006;12:381–390. doi: 10.1089/ten.2006.12.381. [DOI] [PubMed] [Google Scholar]
- 43.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, et al. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther. 2016;7:5. doi: 10.1186/s13287-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 46.Boyd NL, Nunes SS, Krishnan L, Jokinen JD, Ramakrishnan VM, Bugg AR, et al. Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in three-dimensional environments. Tissue Eng Part A. 2013;19:211–223. doi: 10.1089/ten.tea.2011.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 48.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(Nisopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 49.Ide T, Nishida K, Yamato M, Sumide T, Utsumi M, Nozaki T, et al. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials. 2006;27:607–614. doi: 10.1016/j.biomaterials.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341:573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 51.Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31:3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- 52.Ren L, Kang Y, Browne C, Bishop J, Yang Y. Fabrication, vascularization and osteogenic properties of a novel synthetic biomimetic induced membrane for the treatment of large bone defects. Bone. 2014;64:173–182. doi: 10.1016/j.bone.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118(14 Suppl 1):S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 54.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi K, Shimizu T, Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release. 2015;205:83–88. doi: 10.1016/j.jconrel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Ko IK, Atala A, Yoo JJ. Progressive muscle cell delivery as a solution for volumetric muscle defect repair. Sci Rep. 2016;6:38754. doi: 10.1038/srep38754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 59.Martino MM, Brkic S, Bovo E, Burger M, Schaefer DJ, Wolff T, et al. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front Bioeng Biotechnol. 2015;3:45. doi: 10.3389/fbioe.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Almubarak S, Nethercott H, Freeberg M, Beaudon C, Jha A, Jackson W, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016;83:197–209. doi: 10.1016/j.bone.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 62.Isner JM, Walsh K, Symes J, Pieczek A, Takeshita S, Lowry J, et al. Arterial gene therapy for therapeutic angiogenesis in patients with peripheral artery disease. Circulation. 1995;91:2687–2692. doi: 10.1161/01.CIR.91.11.2687. [DOI] [PubMed] [Google Scholar]
- 63.Akimoto T, Liapis H, Hammerman MR. Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol Regul Integr Comp Physiol. 2002;283:R487–R495. doi: 10.1152/ajpregu.00699.2001. [DOI] [PubMed] [Google Scholar]
- 64.Karvinen H, Ylä-Herttuala S. New aspects in vascular gene therapy. Curr Opin Pharmacol. 2010;10:208–211. doi: 10.1016/j.coph.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gianni-Barrera R, Burger M, Wolff T, Heberer M, Schaefer DJ, Gürke L, et al. Long-term safety and stability of angiogenesis induced by balanced single-vector co-expression of PDGF-BB and VEGF164 in skeletal muscle. Sci Rep. 2016;6:21546. doi: 10.1038/srep21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 68.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 70.Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253–1259. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 71.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chem Commun (Camb) 2014;50:15651–15668. doi: 10.1039/C4CC04317K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anisimov A, Tvorogov D, Alitalo A, Leppänen VM, An Y, Han EC, et al. Vascular endothelial growth factor-angiopoietin chimera with improved properties for therapeutic angiogenesis. Circulation. 2013;127:424–434. doi: 10.1161/CIRCULATIONAHA.112.127472. [DOI] [PubMed] [Google Scholar]
- 74.Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci U S A. 2014;111:6952–6957. doi: 10.1073/pnas.1404605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mittermayr R, Slezak P, Haffner N, Smolen D, Hartinger J, Hofmann A, et al. Controlled release of fibrin matrix-conjugated platelet derived growth factor improves ischemic tissue regeneration by functional angiogenesis. Acta Biomater. 2016;29:11–20. doi: 10.1016/j.actbio.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Ding L, Zhao Y, Sun W, Chen B, Lin H, et al. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009;119:1776–1784. doi: 10.1161/CIRCULATIONAHA.108.800565. [DOI] [PubMed] [Google Scholar]
- 77.Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 78.Cruz-Acuña R, García AJ. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 2017;57–58:324–333. doi: 10.1016/j.matbio.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, et al. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992;153:614–625. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- 80.Lin YS, Wang SS, Chung TW, Wang YH, Chiou SH, Hsu JJ, et al. Growth of endothelial cells on different concentrations of Gly-Arg-Gly-Asp photochemically grafted in polyethylene glycol modified polyurethane. Artif Organs. 2001;25:617–621. doi: 10.1046/j.1525-1594.2001.025008617.x. [DOI] [PubMed] [Google Scholar]
- 81.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26:167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Choi WS, Joung YK, Lee Y, Bae JW, Park HK, Park YH, et al. Enhanced patency and endothelialization of small-caliber vascular grafts fabricated by coimmobilization of heparin and cell-adhesive peptides. ACS Appl Mater Interfaces. 2016;8:4336–4346. doi: 10.1021/acsami.5b12052. [DOI] [PubMed] [Google Scholar]
- 83.Wang W, Guo L, Yu Y, Chen Z, Zhou R, Yuan Z. Peptide REDV-modified polysaccharide hydrogel with endothelial cell selectivity for the promotion of angiogenesis. J Biomed Mater Res A. 2015;103:1703–1712. doi: 10.1002/jbm.a.35306. [DOI] [PubMed] [Google Scholar]
- 84.Staubli SM, Cerino G, Gonzalez De Torre I, Alonso M, Oertli D, Eckstein F, et al. Control of angiogenesis and host response by modulating the cell adhesion properties of an Elastin-Like Recombinamer-based hydrogel. Biomaterials. 2017;135:30–41. doi: 10.1016/j.biomaterials.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 85.Yesmin S, Paget MB, Murray HE, Downing R. Bio-scaffolds in organ-regeneration: clinical potential and current challenges. Curr Res Transl Med. 2017;65:103–113. doi: 10.1016/j.retram.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 87.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 88.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawecki M, Łabuś W, Klama-Baryla A, Kitala D, Kraut M, Glik J, et al. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine. J Biomed Mater Res B Appl Biomater. 2018;106:909–923. doi: 10.1002/jbm.b.33865. [DOI] [PubMed] [Google Scholar]
- 90.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 91.Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134–136. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Padalino MA, Quarti A, Angeli E, Frigo AC, Vida VL, Pozzi M, et al. Early and mid-term clinical experience with extracellular matrix scaffold for congenital cardiac and vascular reconstructive surgery: a multicentric Italian study. Interact Cardiovasc Thorac Surg. 2015;21:40–49. doi: 10.1093/icvts/ivv076. discussion 49. [DOI] [PubMed] [Google Scholar]
- 94.Dobrilovic N, Soukas P, Sadiq I, Goldstein L, Raman J. Early complications of biologic extracellular matrix patch after use for femoral artery repair. J Vasc Surg. 2017;65:705–710. doi: 10.1016/j.jvs.2016.07.131. [DOI] [PubMed] [Google Scholar]
- 95.Li X, Meng X, Wang X, Li Y, Li W, Lv X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689–699. doi: 10.1016/j.burns.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko IK, Abolbashari M, Huling J, Kim C, Mirmalek-Sani SH, Moradi M, et al. Enhanced re-endothelialization of acellular kidney scaffolds for whole organ engineering via antibody conjugation of vasculatures. Technol. 2014;2:243–253. doi: 10.1142/S2339547814500228. [DOI] [Google Scholar]
- 98.Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–79. doi: 10.1016/j.biomaterials.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 99.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 100.Zhou H, Kitano K, Ren X, Rajab TK, Wu M, Gilpin SE, et al. Bioengineering human lung grafts on porcine matrix. Ann Surg. 2018;267:590–598. doi: 10.1097/SLA.0000000000002129. [DOI] [PubMed] [Google Scholar]
- 101.Wong ML, Wong JL, Vapniarsky N, Griffiths LG. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials. 2016;92:1–12. doi: 10.1016/j.biomaterials.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Tseng TC, Hsieh FY, Theato P, Wei Y, Hsu SH. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials. 2017;133:20–28. doi: 10.1016/j.biomaterials.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson DE, Hinds MT. Endothelial cell micropatterning: methods, effects, and applications. Ann Biomed Eng. 2011;39:2329–2345. doi: 10.1007/s10439-011-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 107.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A. 2010;16:2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A. 2013;110:7586–7591. doi: 10.1073/pnas.1217796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nahmias Y, Odde DJ. Micropatterning of living cells by laser-guided direct writing: application to fabrication of hepatic-endothelial sinusoid-like structures. Nat Protoc. 2006;1:2288–2296. doi: 10.1038/nprot.2006.386. [DOI] [PubMed] [Google Scholar]
- 110.Nahmias Y, Schwartz RE, Hu WS, Verfaillie CM, Odde DJ. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. 2006;12:1627–1638. doi: 10.1089/ten.2006.12.1627. [DOI] [PubMed] [Google Scholar]
- 111.Wei W, Popov V, Walocha JA, Wen J, Bello-Reuss E. Evidence of angiogenesis and microvascular regression in autosomal-dominant polycystic kidney disease kidneys: a corrosion cast study. Kidney Int. 2006;70:1261–1268. doi: 10.1038/sj.ki.5001725. [DOI] [PubMed] [Google Scholar]
- 112.Gorczyca J, Tomaszewski KA, Henry BM, Pękala PA, Pasternak A, Mizia E, et al. The vascular microarchitecture of the human fetal pancreas: a corrosion casting and scanning electron microscopy study. Pancreas. 2017;46:124–130. doi: 10.1097/MPA.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 113.Wrona AN, Konarska M, Aleksandrovych V, Bereza T, Sajewicz M, Mróz I, et al. Certain remarks on blood supply of normal human uterine corpus - corrosion casting and SEM study. Folia Med Cracov. 2015;55:91–97. [PubMed] [Google Scholar]
- 114.Németh K, Deshpande R, Máthé Z, Szuák A, Kiss M, Korom C, et al. Extrahepatic arteries of the human liver - anatomical variants and surgical relevancies. Transpl Int. 2015;28:1216–1226. doi: 10.1111/tri.12630. [DOI] [PubMed] [Google Scholar]
- 115.Strek P, Nowogrodzka-Zagórska M, Litwin JA, Miodoński AJ. The lung in closeview: a corrosion casting study on the vascular system of human foetal trachea. Eur Respir J. 1994;7:1669–1672. doi: 10.1183/09031936.94.07091669. [DOI] [PubMed] [Google Scholar]
- 116.Wee LY, Taylor M, Watkins N, Franke V, Parker K, Fisk NM. Characterisation of deep arterio-venous anastomoses within monochorionic placentae by vascular casting. Placenta. 2005;26:19–24. doi: 10.1016/j.placenta.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 117.Huling J, Ko IK, Atala A, Yoo JJ. Fabrication of biomimetic vascular scaffolds for 3D tissue constructs using vascular corrosion casts. Acta Biomater. 2016;32:190–197. doi: 10.1016/j.actbio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 118.Huling J, Min SI, Kim DS, Ko IK, Atala A, Yoo JJ. Kidney regeneration with biomimetic vascular scaffolds based on vascular corrosion casts. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 119.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 120.Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, Nakagawa H, et al. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131:035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 121.Wijshoff H. The dynamics of the piezo inkjet printhead operation. Phys Rep. 2010;491:77–177. doi: 10.1016/j.physrep.2010.03.003. [DOI] [Google Scholar]
- 122.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 123.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 124.Xu T, Kincaid H, Atala A, Yoo JJ. High-throughput production of single-cell microparticles using an inkjet printing technology. J Manuf Sci Eng. 2008;130:0210171–0210175. doi: 10.1115/1.2903064. [DOI] [Google Scholar]
- 125.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 126.Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011;98:160–170. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 128.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater. 2011;23:H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 130.Gao Q, He Y, Fu JZ, Liu A, Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 131.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 132.Wu PK, Ringeisen BR. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP) Biofabrication. 2010;2:014111. doi: 10.1088/1758-5082/2/1/014111. [DOI] [PubMed] [Google Scholar]
- 133.Kuehl AR, Abshagen K, Eipel C, Laschke MW, Menger MD, Laue M, et al. External inosculation as a feature of revascularization occurs after free transplantation of murine liver grafts. Am J Transplant. 2013;13:286–298. doi: 10.1111/j.1600-6143.2012.04336.x. [DOI] [PubMed] [Google Scholar]
- 134.Laschke MW, Mussawy H, Schuler S, Eglin D, Alini M, Menger MD. Promoting external inosculation of prevascularised tissue constructs by pre-cultivation in an angiogenic extracellular matrix. Eur Cell Mater. 2010;20:356–366. doi: 10.22203/eCM.v020a29. [DOI] [PubMed] [Google Scholar]