Abstract
Purpose
Prediction of clinical outcomes in patients with primary central nervous system lymphoma (PCNSL) is important for optimization of treatment planning. Quantitative imaging biomarkers for PCNSL have not yet been established. This study evaluated the prognostic value of pretreatment dynamic contrast-enhanced MRI and diffusion-weighted imaging for progression-free survival (PFS) in patients with PCNSL.
Methods
Pretreatment dynamic contrast-enhanced MRI and diffusion-weighted imaging were retrospectively analyzed in 18 immunocompetent patients with PCNSL. Volumes of interest encompassing the tumors were assessed for measurements of blood plasma volume (Vp), volume transfer constant (Ktrans), and apparent diffusion coefficient. Patients were divided into short and long PFS groups based on median PFS. Imaging and clinical variables were correlated with PFS.
Results
Median PFS was 19.6 months. Lower Vpmean and Ktransmean values increased risk for rapid progression (< 19.6 months). Receiver operating characteristic curve analysis demonstrated an optimal Vpmean cutoff value of 2.29 (area under the curve [AUC] = 0.74, sensitivity and specificity = 0.78, p = 0.023) for separating patients with short and long PFS. The optimal Ktransmean cutoff was 0.08 (AUC = 0.74, sensitivity = 0.67, specificity = 0.78, p = 0.025). Kaplan–Meier survival analysis with log-rank test demonstrated significantly (p = 0.015) increased risk of rapid progression for patients with Vpmean < 2.29. Vpmean was significantly (p = 0.03) associated with PFS on univariate Cox analysis. Apparent diffusion coefficient values and clinical factors did not influence PFS.
Conclusions
Pretreatment Vp and Ktrans derived from dynamic contrast-enhanced MRI may be novel prognostic quantitative imaging biomarkers of progression-free survival in patients with PCNSL. These data should be prospectively validated in larger patient cohorts.
Keywords: Primary CNS lymphoma, Prognostic biomarker, Perfusion MRI, Brain imaging, Survival
Introduction
Primary central nervous system lymphoma (PCNSL) is a malignant extranodal variant of non-Hodgkin lymphoma that is confined to the central nervous system. Although rare, its incidence is on the rise [1–3]. Physicians face difficulty in devising optimal treatment plans for patients with PCNSL because to date there is no standard therapeutic approach [4, 5]. Methotrexate-based chemotherapy has become widely accepted as being able to achieve high response rates; unfortunately, however, relapse is known to occur in many immunocompetent patients and prognosis is highly variable [6]. Thus, the development of a predictive model that can serve as a guideline for patient care is a crucial and unmet need. Knowing which patients are more likely to have a sustained response and who will progress early can optimize the initial treatment strategy. For example, lower risk patients may benefit from methotrexate alone, while those at higher risk for early progression may require additional chemotherapy and/or whole brain radiation therapy [7, 8]. While several clinical factors such as age and performance status have been identified, these have demonstrated variable degrees of reliability as prognostic markers [9, 10].
Patients with PCNSL typically undergo brain MRI examinations prior to treatment. As such, multiple groups have recently investigated the conventional and advanced MRI features of patients with PCNSL to assess their value as biomarkers for prognosis [10–13]. However, these data are relatively sparse and sometimes conflicting and therefore reliable and valuable imaging biomarkers have not yet been clearly established. For example, baseline tumor size and lesion location (infra- versus supra-tentorial) have been associated with poor overall survival and progression-free survival (PFS) in one group of patients [13] but were not significant in other PCNSL patient populations [10].
Advanced imaging techniques such as diffusion-weighted imaging (DWI) and MRI perfusion may offer improved prognostic markers. DWI measures the diffusion rate of unbound extracellular water molecules and yields apparent diffusion coefficient (ADC) values [14]. ADC values derived from DWI have been shown to inversely correlate with tumor cellularity in multiple cancers including PCNSL [14, 15]. When ADC values are correlated with patient outcomes, they have demonstrated predictive capabilities for PCNSL patients in some studies [10, 11] but not in others [8].
MRI perfusion is an advanced imaging technique that allows for quantitative assessment of tumor microvasculature by analysis of the distribution kinetics of an intravenously injected low-molecular-weight paramagnetic agent in the microvessels and extracellular extravascular space of tissue. Many studies have revealed that such analysis of MRI perfusion is useful for predicting patient outcomes in gliomas [16, 17]. Nevertheless, MRI perfusion for PCNSL prognosis has seldom been explored and its role is uncertain for these patients [11, 12].
Our investigation aimed to determine whether DWI and dynamic contrast-enhanced (DCE) MRI perfusion could provide valuable information about prognosis in treatment naïve PCNSL patients.
Materials and methods
This retrospective study was performed at a tertiary cancer institution in accordance with the Health Insurance Portability and Accountability Act and with local Institutional Review Board approval, including a waiver of informed consent.
Patient population
We queried departmental and institutional databases for all potentially eligible adult (18 years and older) patients with PCNSL between 2011 and 2015. Included patients met the following criteria: histopathologic diagnosis of PCNSL confirmed by a neuropathologist; no prior therapy for PCNSL; pretreatment MRI scan with both DCE and DWI; negative human immunodeficiency virus status; and no computed tomography evidence of systemic lymphoma in the chest, abdomen, and pelvis. We excluded patients with PCNSL limited to the eyes, leptomeninges, or spinal cord and who had no evidence of disease in the brain parenchyma. We recorded patient demographics, pretreatment baseline Karnofsky Performance scores (KPS), therapy regimens, and clinical outcomes from chart reviews.
MR imaging protocol
All sequences were acquired on 1.5T or 3T scanners (Signa Excite, HDx and Discovery 750, GE Healthcare, Milwaukee, WI) using an 8-channel head coil. Gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ) was injected via an intravenous catheter (18–21 gauge) at doses standardized by patient body weight (0.2 mL/kg body weight, maximum 20 mL) at 2–3 mL/s.
DWI was acquired in the transverse plane using a spin-echo, echo-planar imaging sequence with the following parameters: repetition time/echo time (TR/TE) 8000/104.2 ms; diffusion gradient encoding in 3 orthogonal directions; b = 0 and 1000 s/mm2; field-of-view 240 mm; matrix size 128 × 128 pixels; slice thickness 5 mm; section gap 1 mm; and number of average = 2.
T1-weighted DCE perfusion imaging was acquired using an axial 3D Spoiled Gradient Recalled sequence (TR 4–5 ms; TE 1–2 ms; section thickness 5 mm; flip angle 25°; field-of-view 240 mm; matrix 256 × 128). Ten phases were acquired for pre-injection time delay; and then 30 phases were acquired during the dynamic injection of intravenous contrast. This was followed by a 40-mL saline flush. The temporal resolution was 5–6 s (number of slices 10–15; total time 3.3–4 min). Matching post contrast T1-weighted (TR/ TE = 600/8 ms; slice thickness = 5 mm) spin-echo images were also obtained, along with standard T2-weighted images, FLAIR images, and susceptibility-weighted images. The native T1 was not measured and a fixed baseline value of 1000 ms was utilized. T1 mapping allows for the calculation of the T1 value for each voxel prior to contrast injection but has been shown to not significantly alter DCE quantification [18–20] and therefore is not performed at our institution.
MR imaging processing and analysis
The raw DCE-MRI perfusion data, contrast-enhanced T1-weighted images, and ADC maps were transferred to an off-line workstation. All imaging processing and analysis were performed blinded to clinical outcomes.
The ADC values were calculated utilizing the following parameters: ADC = [ln(S/S0)]/b, where S is the signal intensity (SI) of the region of interest obtained through 3 orthogonally oriented DWIs or diffusion trace images, S0 is the SI of the region of interest acquired through reference T2-weighted images, and b is the gradient factor with the highest value of 1000 s/mm2. ADC maps were calculated on a pixel-by-pixel basis. ADC values are reported as 100 × 10−6 mm2/s.
We processed raw DCE-MRI perfusion data with FDA-approved commercial software (NordicICE; Nordic Neuro Lab, Bergen, Norway). Preprocessing for the perfusion data included background noise adjustment and spatial and temporal smoothing for each patient. For the arterial input function (AIF), an appropriate artery was semi-automatically selected to characterize the input function curve and concentration–time curve. The linear assumption between change in signal intensity and gadolinium concentration was made to convert the signal intensity curve to a concentration–time curve. Curves displaying an optimal relationship between AIF and the concentration–time curve were carefully selected. We used the perfusion analysis method based on the 2-compartment model proposed by Tofts to calculate pharmacokinetic parameters, including blood plasma volume (Vp, unit: fractional) and volume transfer coefficient (Ktrans, unit: /min), and to display the results as parametric maps [21].
A trained operator (O.B.) manually outlined the regions of interest (ROIs) around the entire contrast-enhancing tumor on each transaxial T1-weighted slice. Volumes of interest (VOIs) were constructed for each patient by summing their ROIs. A board-certified attending neuroradiologist with a Certificate of Added Qualification in neuroradiology (V.H.) approved all VOIs.
Finally, for the purposes of statistical analysis, VOIs were subsequently transferred onto matching ADC, Vp, and Ktrans parametric maps and the corresponding ADCmean, Vpmean and Ktransmean values for each lesion were measured and recorded. Minimum values of zero pixels were removed.
Statistical analysis
The clinical endpoint analyzed in this study was PFS time which was measured in months from initiation of therapy to first tumor progression, death, or last follow-up. Tumor response and progression on MRI were assessed using the International PCNSL Collaborative Group criteria [22]. We divided patients into long and short PFS groups based on the median PFS. We then conducted receiver operating characteristic (ROC) curve analysis to evaluate the predictive capabilities of baseline tumor imaging variables (ADCmean, Vpmean and Ktransmean) and clinical variables (age and KPS) for PFS. Fisher’s exact test was used for biological sex. Optimal cutoff values for each variable were determined using Youden’s index. Based on the cutoff values, we dichotomized the variables and conducted Kaplan–Meier survival analysis with log-rank test to investigate if there was a difference in PFS time between the two groups. We also performed univariate Cox proportional hazards regression analysis for PFS using all dichotomized variables. All statistical analyses were performed using MATLAB (version 8.4; Mathworks, Natik, MA) and Stata (version 12.0; StataCorp, College Station, TX).
Results
Patients
Eighteen patients with PCNSL (10 males and 8 females) were included in the study. The mean patient age was 67.2 years (range 47–84 years) and the median KPS at diagnosis was 70 (range 50–90). All patients except one were treated with methotrexate-based chemotherapy as part of their initial management (1 patient received rituximab only). Only 1 patient had died at the time of analysis (mean followup time = 29.9 months). Median PFS time was 19.6 months (range 2.0–52.6 months). The patient demographics and baseline MR imaging characteristics are summarized in the Table 1.
Table 1.
Patient demographics and baseline MR imaging characteristics
| Patient no. | Age (year)/sex | KPS | Vpmean | Ktransmean | ADCmean | PFS group |
|---|---|---|---|---|---|---|
| 1 | 67/F | 90 | 6.53 | 0.15 | 1428.00 | High |
| 2 | 64/M | 60 | 2.29 | 0.08 | 1109.16 | High |
| 3 | 70/F | 70 | 1.56 | 0.04 | 1034.14 | High |
| 4 | 83/M | 50 | 1.72 | 0.08 | 984.53 | Low |
| 5 | 63/M | 70 | 4.84 | 0.08 | 1080.97 | Low |
| 6 | 66/M | 70 | 0.45 | 0.01 | 931.93 | High |
| 7 | 81/F | 70 | 2.99 | 0.06 | 1286.69 | High |
| 8 | 61/M | 80 | 3.53 | 0.09 | 912.91 | High |
| 9 | 78/F | 90 | 3.63 | 0.09 | 873.08 | High |
| 10 | 84/F | 70 | 2.62 | 0.08 | 1056.93 | Low |
| 11 | 67/M | 80 | 1.36 | 0.04 | 910.25 | Low |
| 12 | 62/F | 70 | 1.76 | 0.05 | 847.75 | Low |
| 13 | 52/F | 80 | 2.43 | 0.10 | 998.60 | High |
| 14 | 76/M | 60 | 0.81 | 0.02 | 1116.94 | Low |
| 15 | 47/M | 80 | 1.14 | 0.03 | 1180.98 | Low |
| 16 | 60/M | 80 | 0.73 | 0.01 | 849.90 | Low |
| 17 | 66/M | 70 | 1.30 | 0.05 | 1180.62 | Low |
| 18 | 62/F | 90 | 3.16 | 0.07 | 835.18 | High |
M male, F female, KPS Karnofsky performance score, Vp plasma volume, Ktrans volume transfer constant, ADC apparent diffusion coefficient, PFS progression-free survival
ROC curve analysis
Lower Vpmean and Ktransmean values increased risk for rapid progression (< 19.6 months). The optimal Vpmean cutoff was 2.29 (area under the curve (AUC) = 0.74, sensitivity and specificity = 0.78, p = 0.023) and the optimal Ktransmean cutoff was 0.08 (AUC = 0.74, sensitivity = 0.67, specificity = 0.78, p = 0.025) for separating patients into long and short PFS groups. The other variables we assessed were not statistically significant: ADCmean (cutoff = 849.9, AUC = 0.47, sensitivity = 1.00, specificity = 0.22, p = 0.579); KPS (cutoff = 60, AUC = 0.68, sensitivity = 1.00, specificity = 0.33, p = 0.082); age (cutoff = 81, AUC = 0.49, sensitivity = 0.22, specificity = 1.00, p = 0.522); biological sex (p = 0.153 using Fisher’s exact test).
Representative images of patients with long and short PFS are shown in Fig. 1.
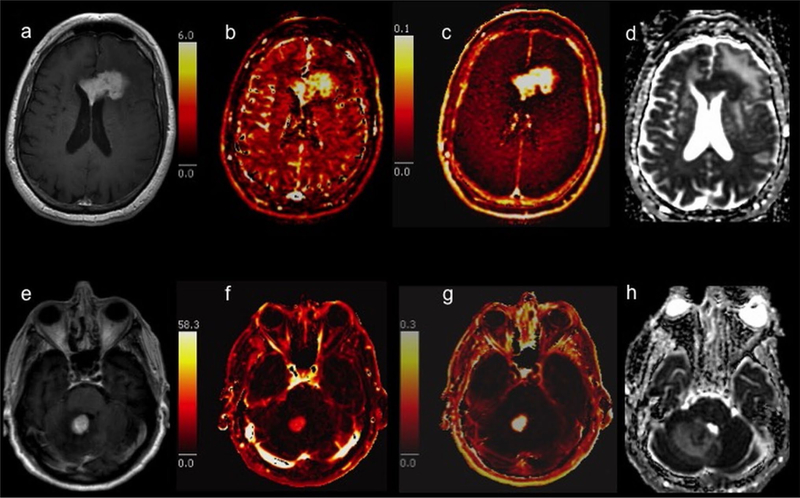
Fig. 1.
Representative pre-treatment MR images and parametric maps from patients with primary CNS lymphoma Top row: 61-year-old man with an enhancing corpus callosum mass on postcontrast T1-weighted imaging (a) had progression-free survival (PFS) > 30 months. Bottom row: 83-year-old man with a smaller enhancing mass in the cerebellar vermis (e) had tumor progression after 17.2 months. The patient with longer PFS had a higher Vp of 3.53 (b) compared to the patient with shorter PFS and Vp of 1.72 (f). The Ktrans (c and g) was slightly higher (0.09 versus 0.08) and the apparent diffusion coefficient (ADC) (d and h) was slightly lower (912.91 × 10−6 mm2/s versus 984.53 × 10−6 mm2/s) in the patient with longer PFS
Kaplan–Meier survival analysis
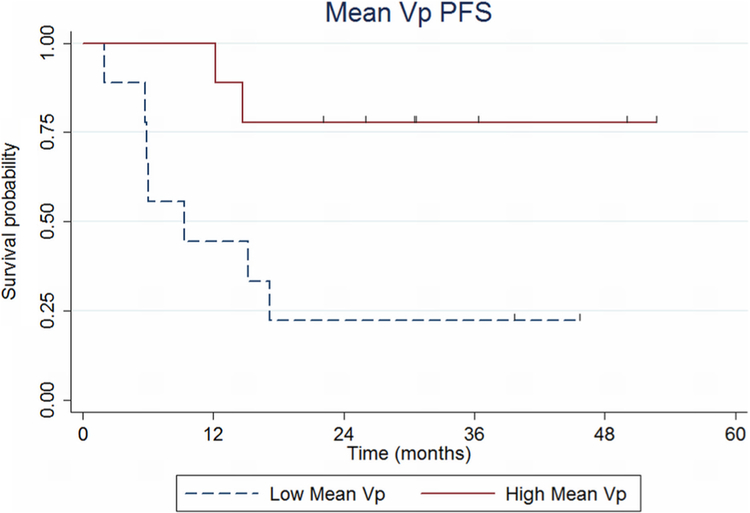
Patient outcome as a function of Vpmean is shown in Fig. 2. Patients in the low Vpmean group (< 2.29) had a significantly increased risk for early progression (logrank p = 0.015). Patients in the low Vpmean group experienced a shorter mean PFS time of 16.3 months compared with patients in the high Vpmean group who experienced a mean PFS time of 30.5 months (borderline significant at p = 0.050).
Fig. 2.
Progression-free survival (PFS) probability based on plasma volume, Vp
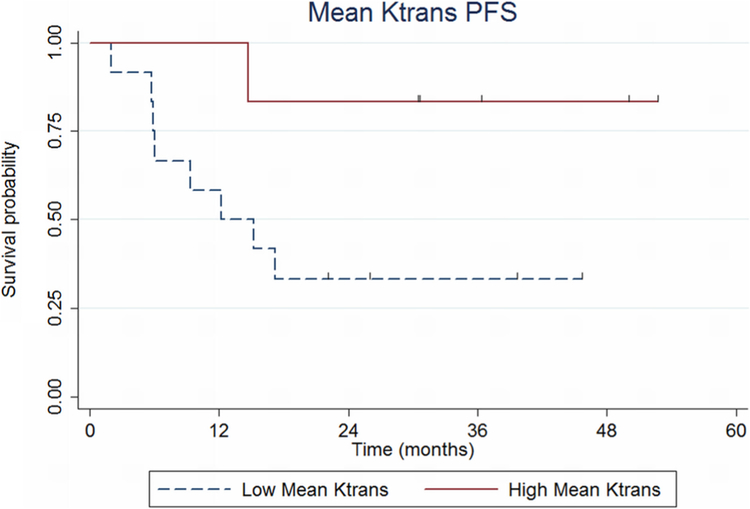
Patient outcome as a function of Ktransmean is shown in Fig. 3. Patients in the low Ktransmean group (< 0.08) had increased risk for early progression (log-rank p = 0.057). Patients in the low Ktransmean group experienced a shorter mean PFS time of 17.2 months compared with patients in the high Ktransmean group who experienced a mean PFS time of 35.7 months (significant at p = 0.025).
Fig. 3.
Progression-free survival (PFS) probability based on volume transfer constant, Ktrans
Univariate Cox proportional hazards regression analysis
The univariate Cox model results demonstrated that dichotomized Vpmean was significantly associated with PFS (p = 0.03, hazard ratio = 0.17), while dichotomized Ktransmean demonstrated borderline significance (p = 0.093, hazard ratio = 0.17). Baseline ADCmean, patient age, KPS and biological sex were not significantly associated with PFS (p > 0.1).
Discussion
This study involved the investigation of pretreatment DWI and DCE-MRI to predict PFS time in PCNSL patients. Our results demonstrate initial evidence that Vpmean and Ktransmean could be used as quantitative prognostic biomarkers for patients with PCNSL. Specifically, patients with lower Vpmean and Ktransmean values experienced shorter PFS than patients with higher values (16.3 months versus 30.5 months, and 17.2 months versus 35.7 months, respectively). The median PFS in our patient population was 19.6 months. This is similar to the median PFS of 17 months reported in a study of 338 patients with newly diagnosed PCNSL at Memorial Sloan Kettering Cancer Center [9]. Quantitative imaging biomarkers for clinical outcome in PCNSL are not yet established and further study and refinement could yield critical information on how to best manage upfront therapy in this population. For example, patients at increased risk for rapid progression based on advanced imaging can be offered additional therapy earlier in their treatment course than patients at lower risk.
In our study, we derived Vp from DCE-MRI which represents the blood plasma volume per unit volume of tissue. Vp is thought of as the physiologic equivalent of relative cerebral blood volume (rCBV) obtained from dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion imaging. Both Vp and rCBV correlate with histologic and angiographic assessment of vascular density in human brain tumors [23, 24] and have been successfully utilized to predict clinical outcomes in gliomas [16, 25]. However, at our institution we prefer DCE-MRI because rCBV measurements derived from DSC are semi-quantitative and can be influenced by multiple post-processing steps, including the choice of normal contralateral white matter and correction technique to address contrast extravasation. Additional drawbacks of DSC perfusion imaging include sensitivity to susceptibility effects from bone, calcification, and hemorrhage. DCE-MRI also provides better spatial resolution than DSC perfusion. Certain disadvantages of DCE are important to mention as well, including complexity in pharmacokinetic model post-processing, lack of widely available and easy-touse post-processing software, and difficulty in obtaining an accurate vascular input function. Also, DSC is more widely utilized than DCE and can be performed with shorter acquisition times.
In patients with PCNSL, rCBV has been shown to predict PFS in one study done by Valles et al. [11]. This study demonstrated that PCNSL patients (n = 25) with rCBVmean < 1.43 had worse PFS than patients with rCBVmean ≥ 1.43. The authors postulated that low rCBV values might be associated with unfavorable clinical outcomes for two main reasons. First, low rCBV could signify hypoxic tumors, which are known to be treatment resistant [26, 27]. Second, low rCBV may represent a decreased number of patent vessels that are able to deliver the intravenous chemotherapy required to successfully complete treatment. We agree with them and believe our findings using Vpmean further strengthen these assertions. To our knowledge, there are no additional studies that have assessed either rCBV or Vp for prognosis in PCNSL.
We also derived Ktrans from DCE-MRI. This parameter reflects the volume transfer constant of gadolinium from the intravascular compartment to the extracellular extravascular space and is partly dependent on vascular permeability. It is commonly understood to be a surrogate marker of vascular leakiness due to permeability and blood–brain barrier disruption. Our study showed that patients with lower Ktrans had increased risk for rapid progression. This finding suggests that optimal treatment of PCNSL is dependent not only on the increased vascularity (Vp) that allows intravenous chemotherapy to reach the tumor but also on vascular leakiness. Higher Ktrans possibly allows for sufficient concentrations of the intravenous drug to exit the blood vessels and interact with the tumor cells, but this is conjectural and further research is required. Chung et al. [12] demonstrated that PCNSL lesions with diffusely increased permeability on DCE-MRI were significantly associated with higher complete response rates than tumors with more heterogeneous patterns. Greater permeability was also associated with longer PFS but this reached only borderline significance. An important difference between our studies is that Chung et al. used qualitative instead of quantitative assessments of permeability, which may have introduced greater subjectivity in their results. We are not currently aware of any additional publications that have evaluated DCE-MRI for prognosis of PCNSL.
Along with Vp and Ktrans from DCE-MRI, we studied if ADC from DWI could be of potential prognostic value. ADC is inversely related to cellular density and has been shown to have predictive value for PCNSL prognosis in several studies [10, 11]. However, in our study we did not find ADCmean to be a significant predictor of PFS. Our result agrees with a study by Morris et al. [8] which evaluated pretreatment ADC values in 28 patients and compared patient outcomes (PFS and overall survival) based on the measurements. Huang et al. [28] also did not find pretherapeutic ADC values to be predictive of outcome in a study of 35 PCNSL patients. The reasons for the discrepancies in results are unclear at this time and further investigation will be necessary to better understand the role of ADC as a prognostic biomarker in PCNSL.
Our study has several limitations including small sample size and retrospective design. The small sample size was mainly due to the rarity of the disease and the requirement that perfusion data be captured prior to initiation of therapy. Another limitation is that we could not adequately assess the ability of pretreatment imaging to predict overall survival since only one patient had died at the time of analysis. Finally, we report our institutional protocol for capturing perfusion data, but the protocols vary among institutions limiting broad applicability until consensus protocols can be determined.
In conclusion, this study demonstrates promising evidence that DCE-MRI biomarkers measured prior to therapy for PCNSL can be utilized to predict PFS. Quantitative analysis of Vp and Ktrans has not been previously reported in this setting. Larger studies are needed to prospectively validate our results.
Acknowledgements
The authors thank Joanne Chin who provided editorial support.
Funding National Institutes of Health/National Cancer Institute (Cancer Center Support Grant P30 CA008748).
Footnotes
Data availability The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent For this type of study formal consent is not required.
Research involving human and animal rights This article does not contain any studies with animals performed by any of the authors.
References
- 1.Eloranta S, Branvall E, Celsing F, Papworth K, Ljungqvist M, Enblad G, Ekstrom-Smedby K (2018) Increasing incidence of primary central nervous system lymphoma but no improvement in survival in Sweden 2000–2013. Eur J Haematol 100(1):61–68. 10.1111/ejh.12980 [DOI] [PubMed] [Google Scholar]
- 2.Makino K, Nakamura H, Kino T, Takeshima H, Kuratsu J (2006) Rising incidence of primary central nervous system lymphoma in Kumamoto. Jpn Surg Neurol 66(5):503–506. 10.1016/j.surneu.2006.05.055 [DOI] [PubMed] [Google Scholar]
- 3.Grommes C, DeAngelis LM (2017) Primary CNS lymphoma. J Clin Oncol 35(21):2410–2418. 10.1200/jco.2017.72.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris PG, Abrey LE (2009) Therapeutic challenges in primary CNS lymphoma. Lancet Neurol 8(6):581–592. 10.1016/s1474-4422(09)70091-2 [DOI] [PubMed] [Google Scholar]
- 5.Qian L, Tomuleasa C, Florian IA, Shen J, Florian IS, Zdrenghea M, Dima D (2017) Advances in the treatment of newly diagnosed primary central nervous system lymphomas. Blood Res 52(3):159–166. 10.5045/br.2017.52.3.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS (2011) Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol 101(2):257–265. 10.1007/s11060-010-0252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C, Pentsova E, Grommes CC, Panageas KS, Baser RE, Faivre G, Abrey LE, Sauter CS (2015) R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 125(9):1403–1410. 10.1182/blood-2014-10-604561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, Karimi S, Curry R, Shah G, Abrey LE, DeAngelis LM, Omuro A (2013) Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 31(31):3971–3979. 10.1200/jco.2013.50.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, DeAngelis LM (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24(36):5711–5715. 10.1200/jco.2006.08.2941 [DOI] [PubMed] [Google Scholar]
- 10.Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S (2010) Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 31(1):60–66. 10.3174/ajnr.A1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valles FE, Perez-Valles CL, Regalado S, Barajas RF, Rubenstein JL, Cha S (2013) Combined diffusion and perfusion MR imaging as biomarkers of prognosis in immunocompetent patients with primary central nervous system lymphoma. AJNR Am J Neuroradiol 34(1):35–40. 10.3174/ajnr.A3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung SR, Choi YJ, Kim HS, Park JE, Shim WH, Kim SJ (2016) Tumor vascular permeability pattern is associated with complete response in immunocompetent patients with newly diagnosed primary central nervous system lymphoma: retrospective cohort study. Medicine 95(6):e2624 10.1097/md.0000000000002624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabouret E, Houillier C, Martin-Duverneuil N, Blonski M, Soussain C, Ghesquieres H, Houot R, Larrieu D, Soubeyran P, Gressin R, Gyan E, Chinot O, Taillandier L, Choquet S, Alentorn A, Leclercq D, Omuro A, Tanguy ML, Hoang-Xuan K (2017) Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neurooncology 19(3):422–429. 10.1093/neuonc/now238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo AC, Cummings TJ, Dash RC, Provenzale JM (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224(1):177–183. 10.1148/radiol.2241010637 [DOI] [PubMed] [Google Scholar]
- 15.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, Bedekar DP, Schmainda KM (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31(3):538–548. 10.1002/jmri.22068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spampinato MV, Schiarelli C, Cianfoni A, Giglio P, Welsh CT, Bisdas S, Rumboldt Z (2013) Correlation between cerebral blood volume measurements by perfusion-weighted magnetic resonance imaging and two-year progression-free survival in gliomas. Neuroradiol J 26(4):385–395. 10.1177/197140091302600404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law M, Oh S, Babb JS, Wang E, Inglese M, Zagzag D, Knopp EA, Johnson G (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging–prediction of patient clinical response. Radiology 238(2):658–667. 10.1148/radiol.2382042180 [DOI] [PubMed] [Google Scholar]
- 18.Haacke EM, Filleti CL, Gattu R, Ciulla C, Al-Bashir A, Suryanarayanan K, Li M, Latif Z, DelProposto Z, Sehgal V, Li T, Torquato V, Kanaparti R, Jiang J, Neelavalli J (2007) New algorithm for quantifying vascular changes in dynamic contrastenhanced MRI independent of absolute T1 values. Magn Reson Med 58(3):463–472. 10.1002/mrm.21358 [DOI] [PubMed] [Google Scholar]
- 19.Jung SC, Yeom JA, Kim JH, Ryoo I, Kim SC, Shin H, Lee AL, Yun TJ, Park CK, Sohn CH, Park SH, Choi SH (2014) Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol 35(6):1103–1110. 10.3174/ajnr.A3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V, Omuro A, Arevalo-Perez J, Thomas AA, Huse J, Peck K, Holodny AI, Young RJ (2017) Diagnostic accuracy of T1-weighted dynamic contrast-enhanced-MRI and DWI-ADC for differentiation of glioblastoma and primary CNS lymphoma. AJNR Am J Neuroradiol 38(3):485–491. 10.3174/ajnr.A5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergamino M, Bonzano L, Levrero F, Mancardi GL, Roccatagliata L (2014) A review of technical aspects of T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in human brain tumors. Phys Med 30(6):635–643. 10.1016/j.ejmp.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O’Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23(22):5034–5043. 10.1200/jco.2005.13.524 [DOI] [PubMed] [Google Scholar]
- 23.Aronen HJ, Pardo FS, Kennedy DN, Belliveau JW, Packard SD, Hsu DW, Hochberg FH, Fischman AJ, Rosen BR (2000) High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res 6(6):2189–2200 [PubMed] [Google Scholar]
- 24.Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH et al. (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191(1):41–51. 10.1148/radiology.191.1.8134596 [DOI] [PubMed] [Google Scholar]
- 25.Ulyte A, Katsaros VK, Liouta E, Stranjalis G, Boskos C, Papanikolaou N, Usinskiene J, Bisdas S (2016) Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology 58(12):1197–1208. 10.1007/s00234-016-1741-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteiro AR, Hill R, Pilkington GJ, Madureira PA (2017) The role of hypoxia in glioblastoma invasion. Cells 6 (4). 10.3390/cells6040045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahase S, Rattenni RN, Wesseling P, Leenders W, Baldotto C, Jain R, Zagzag D (2017) Hypoxia-mediated mechanisms associated with antiangiogenic treatment resistance in glioblastomas. Am J Pathol 187(5):940–953. 10.1016/j.ajpath.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang WY, Wen JB, Wu G, Yin B, Li JJ, Geng DY (2016) Diffusion-weighted imaging for predicting and monitoring primary central nervous system lymphoma treatment response. AJNR Am J Neuroradiol. 10.3174/ajnr.A4867 [DOI] [PMC free article] [PubMed] [Google Scholar]