Abstract
Background:
Since respiratory tract infections (RTIs) precede most exacerbations, better understanding of the risk factors of RTIs and RTI-associated exacerbations in patients with asthma is a pressing public health need. Obesity in patients with asthma is associated with worse asthma control and higher asthma-associated healthcare utilization but its effect on RTI risk is unknown.
Objective:
We aimed to study the association of BMI classification on the risk of self-reported RTIs and related asthma morbidity among adults and children with asthma.
Methods:
This post-hoc analysis of five large asthma trials involving 747 children and 1287 adults compared BMI classification, defined as lean, overweight and obese based on age-appropriate body mass index (BMI) and BMI-percentile conventions. The primary outcome was rate of visits with RTIs. Secondary asthma outcomes included upper respiratory infection (URI) severity, systemic steroid use, and healthcare contact.
Results:
Children had 1.4 times the rate of RTI compared to adults (95% CI 1.27-1.56). In all participants, BMI classification did not affect the rate of visits with RTI. In children, BMI classification did not affect URI severity, all-cause asthma events or RTI-associated asthma events. However, in adults, higher BMI classification was associated with an increase in moderate/severe URI (p=0.02). Adults with higher BMI classification also had increased rates of all-cause and RTI-associated asthma exacerbations requiring systemic steroids and healthcare contact.
Conclusions:
BMI classification was not associated with increased risk of RTIs in children or adults. In adults only, obesity was associated with increased URI severity and all-cause and RTI-associated asthma morbidity.
Keywords: obesity, asthma, respiratory tract infections
Introduction
Asthma affects 25.9 million Americans and frequently is poorly controlled. About 50% of children have a severe attack each year.1 In patients with asthma, obesity is associated with significantly greater symptoms, loss of asthma control, poor response to conventional ICS therapy, and higher asthma-associated healthcare utilization.2–4 How obesity leads to poorly controlled asthma is not fully understood, and proposed mechanisms include airway mechanics, genetic factors or obesity-related comorbidities such as metabolic disease or gastroesophageal reflux.5 However, respiratory tract infections (RTIs) trigger most asthma exacerbations6 and there are few data on the effects of obesity on the risk and severity of respiratory tract infections in patients with asthma. This knowledge gap is critical to asthma care given demonstrated associations of obesity to increased risk for RTIs in the general population. During the 2009 H1N1 influenza pandemic, obesity was identified as a major independent risk factor for infection, hospitalization, and death.7, 8 In cross-sectional studies, obesity was associated with more frequent RTIs in children and adults.9–11 Mechanisms may include immunomodulatory effects of adipose tissue, exaggerated microvascular inflammatory and thrombogenic responses, increased pathogen acquisition and carriage, altered lung mechanics and augmented airway resistance.12
Thus, poor asthma control in obesity may be mediated by increased RTIs and related exacerbations. If true, treatment of obese asthmatics could be improved by preventing respiratory tract infections and ameliorating their effects on asthma morbidity. Using data from five large clinical trials in the American Lung Association Airways Clinical Research Centers (ALA-ACRC) network, we evaluated the effect of BMI classification on respiratory tract infections and associated exacerbations. We hypothesized that higher BMI classification would increase susceptibility to RTIs and RTI-related asthma morbidity in adults and children.
Methods
Participant Selection
The inclusion and exclusion criteria have been published for the five individual studies: Long Acting Beta Agonist Step Down Study (LASST, NCT01437995); Leukotriene Modifier or Corticosteroid or Corticosteroid Salmeterol Trial (LOCCS, NCT00156819); Study of Acid Reflux in Children with Asthma (SARCA, NCT00604851); Study of Soy Isoflavones in Asthma (SOYA, NCT01052116); Study of Asthma and Nasal Steroids (STAN, NCT01118312).13–17 Two of the trials, LASST and LOCCS, recruited adults and children with well controlled asthma to evaluate step-down therapies. The other three trials focused on therapies for asthmatics with poor control: SARCA treated children ages 6-17 years old with lansoprazole; SOYA treated adults and children age 12 or older with soy isoflavone and STAN treated adults and children with rhinosinusitis with intranasal mometasone. All participants had asthma confirmed by physician diagnosis. All caregivers or participants signed written informed consent. The present post hoc study was approved by the Duke institutional review board (Pro00089972). We included data from 747 children (age<18 years) and 1287 adults (age≥18 years). Clinic visit data were collected over 12 years (LOCCS 2003-2005; SARCA 2006-2011; SOYA 2010-2012; STAN 2010-2014; LASST 2012-2015). Observation periods of the studies ranged from 16 weeks (LOCCS) to 48 weeks (LASST). Specific details and characteristics are included in the appendix and Table E1.
Clinical Data
We analyzed demographic, medical and environmental histories, pulmonary function testing, asthma scores and asthma-related utilization. Height and weight were measured during baseline visits to determine body mass index (BMI) and weight-to-height ratio (WHR). Age and sex-adjusted BMI percentiles were calculated using Centers for Disease Control (CDC) normative data and assigned a BMI classification category: lean (in children, BMI <85 percentile; in adults, BMI <25), overweight (in children, 85≤BMI<95 percentile; in adults, 25≤BMI<30); and obese (in children, BMI ≥95 percentile; in adults, BMI ≥30). Analyses were also run using waist-to-height ratio (WHR) ≥0.5 and ≥0.55 as previously determined pediatric measures of central obesity.18, 19 GERD and secondhand smoke (SHS) exposure was determined by self or caregiver report in standardized interviews. PPI use was defined in SARCA by treatment allocation to daily lansoprazole, otherwise by self-reported use of PPIs daily or multiple times per week. The presence of atopy was defined by self-reported allergic rhinitis, atopic dermatitis, or food allergy. RTIs were reported by caregivers and participants during the observation period at clinic visit interviews which were asked about in a standardized fashion in all studies. The rate of visits with RTIs was determined for each participant. Respiratory tract infections were further described as upper respiratory tract infections (URTI; upper respiratory infection (URI), otitis media, sinusitis, or strep throat) or lower respiratory tract infections (LRTI; bronchitis or pneumonia).Participants were asked during standardized interviews to rate the severity of each URI as mild, moderate, or severe. Asthma-related healthcare contact and exacerbations were collected from standardized interviews at scheduled study visits. Exacerbations were defined as an increase in asthma symptoms requiring systemic corticosteroids during the observation period. Additional analyses were performed for RTI-associated events by only analyzing data at interval visits where a RTI was reported. The RTI-associated change in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) was determined among participants with a recent RTI by comparing baseline to visit spirometry measures. Further details regarding data collection and differences in variables between trials can be found in the online repository.
Statistical Analysis
Baseline data were summarized by BMI classification and study. Chi-square and Kruskal-Wallis tests were used for comparing categorical and continuous variables, respectively. The primary analysis compared the rate of visits with RTIs among BMI classification groups. Trends among BMI classifications for the rate of visits with events were investigated using negative binomial regression models. Since participants were stratified into children (age < 18 years old) and adults (age ≥ 18 years old), the models were assessed for an interaction between child status and BMI classification. Secondary analyses of FEV1 and FVC change and URI severity score were performed using simple linear regression and chi-square testing. Missing data are summarized in Table E5. Multivariable generalized linear regression under the negative binomial likelihood was used to compare main outcomes between BMI classification and respiratory tract infections. Covariates included in the final model were gender, ethnicity, race, GERD, PPI, SHS, and atopy. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were two-tailed at a level of significance of 0.05.
Results
Baseline Characteristics
Baseline characteristics for children (<18 years of age) and adults (≥18 years of age) are shown by BMI classification in Tables Ia and Ib, respectively.
Table Ia:
Baseline Characteristics in Children by BMI Classification
| BMI classification | |||||
|---|---|---|---|---|---|
| Total (n=747) | Lean <85% (n=405) | Overweight 85-94% (n=130) | Obese ≥95% (n=212) | p-value | |
| Age (yrs) | 12.1 (3.0) | 12.1 (3.1) | 12.0 (2.9) | 12.0 (3.0) | 0.90 |
| Female, n (%) | 297 (39.8%) | 164 (40.5%) | 52 (40.0%) | 81 (38.2%) | 0.86 |
| Hispanic/Latino, n (%) | 147 (19.7%) | 78 (19.3%) | 24 (18.5%) | 45 (21.2%) | 0.78 |
| Race, n (%) | 0.38 | ||||
| Caucasian | 334 (44.7%) | 189 (46.7%) | 58 (44.6%) | 87 (41.0%) | |
| African-American | 333 (44.6%) | 166 (41.0%) | 63 (48.5%) | 104 (49.1%) | |
| Asian | 8 (1.1%) | 5 (1.2%) | 1 (0.8%) | 2 (0.9%) | |
| Other | 72 (9.6%) | 45 (11.1%) | 8 (6.2%) | 19 (9.0%) | |
| Age of asthma onset (yrs) | 3.3 (3.1) | 3.2 (3.1) | 3.6 (3.4) | 3.4 (2.9) | 0.47 |
| Atopy, n (%) | 598 (80.1%) | 325 (80.2%) | 104 (80.0%) | 169 (79.7%) | 0.99 |
| URI Trigger, n (%) | 312 (91.2%) | 160 (89.9%) | 64 (90.1%) | 88 (94.6%) | 0.01 |
| Steroid courses in last yr | 1.4 (1.8) | 1.3 (1.7) | 1.6 (2.0) | 1.5 (1.8) | 0.24 |
| Controller Therapy, n (%) | |||||
| Inhaled corticosteroid | 339 (45.4%) | 189 (46.7%) | 56 (43.1%) | 94 (44.5%) | 0.74 |
| Inhaled combo | 430 (57.6%) | 225 (55.6%) | 81 (62.3%) | 124 (58.5%) | 0.38 |
| Oral anti-leukotriene | 330 (44.2%) | 169 (41.7%) | 68 (52.3%) | 93 (43.9%) | 0.11 |
| Pre-Bronchodilator | |||||
| FVC (L) | 3.052 (1.1) | 2.940 (1.1) | 3.144 (1.1) | 3.208 (1.1) | 0.01 |
| FEV1 (L) | 2.407 (0.9) | 2.368 (0.9) | 2.444 (0.9) | 2.458 (0.8) | 0.20 |
| FEV1/FVC (L) | 0.794 (0.1) | 0.809 (0.1) | 0.781 (0.1) | 0.774 (0.1) | <0.001 |
| FEV1 % predicted | 92.4 (15.8) | 92.5 (16.5) | 92.9 (15.2) | 91.9 (14.9) | 0.75 |
| % change in FEV1 | 9.1 (10.1) | 8.9 (10.3) | 10.3 (10.8) | 8.8 (9.1) | 0.52 |
| Asthma Scales | |||||
| ACT | 19.6 (3.8) | 19.8 (3.7) | 19.2 (3.6) | 19.4 (4.1) | 0.51 |
| cACT | 18.6 (4.1) | 18.6 (3.7) | 18.4 (4.2) | 18.7 (4.4) | 0.77 |
| ACQ | 1.031 (0.736) | 0.983 (0.698) | 1.125 (0.743) | 1.077 (0.799) | 0.38 |
| ASUI | 0.842 (0.140) | 0.852 (0.128) | 0.837 (0.141) | 0.828 (0.158) | 0.32 |
| GERD, n (%) | 62 (8.3%) | 29 (7.2%) | 6 (4.6%) | 27 (12.8%) | 0.01 |
| Regular PPI use, n (%) | 159 (29.2%) | 80 (28.7%) | 21 (20.4%) | 58 (35.6%) | 0.03 |
| OSA, n (%) | 19 (4.6%) | 8 (3.9%) | 2 (2.9%) | 9 (7.1%) | 0.26 |
| Diabetes Mellitus, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Immunodeficiency, n (%) | 5 (0.8%) | 3 (0.9%) | 1 (0.8%) | 1 (0.5%) | 0.90 |
| Smoking, n (%) | |||||
| Current/Former | 2 (0.5%) | 1 (0.4%) | 0 (0.0%) | 1 (0.9%) | 0.66 |
| Never | 441 (99.5%) | 248 (99.6%) | 79 (100.0%) | 114 (99.1%) | |
| SHS, n (%) | 238 (31.9%) | 119 (29.4%) | 43 (33.1%) | 76 (35.8%) | 0.25 |
URI – upper respiratory infection; FVC – forced vital capacity; FEV1 – forced expiratory volume in one second; ACT – Asthma Control Test; cACT – Childhood Asthma Control Test; ACQ – Asthma Control Questionnaire; ASUI – Asthma Symptom Utility Index; GERD – Gastroesophageal reflux disease; PPI – proton pump inhibitor; OSA – obstructive sleep apnea, SHS – secondhand smoke. Bold indicates statistical significance (p<0.05). Results are expressed as number of participants (percentage) for categorical variables and mean (standard deviation) for continuous variables. P-values determined by chi-square test for categorical variables and Kruskal-Wallis for continuous variables.
Table Ib:
Baseline Characteristics in Adults by BMI classification
| BMI classification | |||||
|---|---|---|---|---|---|
| Total (n=1287) | Lean <24 (n=364) | Overweight 25-29 (n=363) | Obese ≥30 (n=560) | p-value | |
| Age (yrs) | 39.8 (13.7) | 34.5 (13.6) | 41.7 (13.8) | 42.0 (12.7) | <0.001 |
| Female, n (%) | 873 (67.8%) | 245 (67.3%) | 197 (54.3%) | 431 (77.0%) | <0.001 |
| Hispanic/Latino, n (%) | 162 (12.6%) | 31 (8.5%) | 53 (14.6%) | 78 (14.0%) | 0.02 |
| Race, n (%) | <0.001 | ||||
| Caucasian | 743 (57.8%) | 254 (69.8%) | 224 (61.7%) | 265 (47.4%) | |
| African-American | 439 (34.1%) | 76 (20.9%) | 102 (28.1%) | 261 (46.7%) | |
| Asian | 33 (2.6%) | 17 (4.7%) | 11 (3.0%) | 5 (0.9%) | |
| Other | 71 (5.5%) | 17 (4.7%) | 26 (7.2%) | 28 (5.0%) | |
| Age of asthma onset (yrs) | 15.9 (15.0) | 13.1 (13.2) | 16.1 (15.2) | 17.6 (15.7) | 0.001 |
| Atopy, n (%) | 897 (69.7%) | 260 (71.4%) | 254 (70.0%) | 383 (68.4%) | 0.61 |
| URI trigger, n (%) | 732 (82.6%) | 173 (84.8%) | 202 (81.8%) | 357 (82.1%) | 0.84 |
| Steroid courses in last yr | 0.6 (1.0) | 0.4 (0.8) | 0.5 (1.0) | 0.7 (1.2) | <0.001 |
| Controller therapy, n (%) | |||||
| Inhaled corticosteroid | 267 (20.8%) | 73 (20.1%) | 83 (22.9%) | 111 (19.8%) | 0.49 |
| Inhaled combo | 837 (65.1%) | 202 (55.5%) | 237 (65.5%) | 398 (71.1%) | <0.001 |
| Oral anti-leukotriene | 277 (21.5%) | 74 (20.3%) | 76 (21.0%) | 127 (22.7%) | 0.67 |
| Pre-Bronchodilator | |||||
| FVC (L) | 3.780 (1.1) | 4.096 (1.0) | 3.982 (1.1) | 3.443 (1.0) | <0.001 |
| FEV1 (L) | 2.787 (0.8) | 3.067 (0.8) | 2.879 (0.9) | 2.546 (0.7) | <0.001 |
| FEV1/FVC (L) | 0.770 (0.1) | 0.792 (0.1) | 0.754 (0.1) | 0.765 (0.1) | <0.001 |
| FEV1 % predicted | 86.4 (14.4) | 89.8 (13.3) | 85.9 (14.8) | 84.5 (14.4) | <0.001 |
| % change in FEV1 | 6.6 (8.2) | 7.4 (7.9) | 7.0 (7.5) | 5.9 (8.7) | 0.02 |
| Asthma Scales | |||||
| ACT | 18.7 (4.6) | 19.3 (4.2) | 18.7 (4.7) | 18.4 (4.8) | 0.16 |
| ACQ | 0.7 (0.373) | 0.721 (0.391) | 0.711 (0.358) | 0.750 (0.365) | 0.68 |
| ASUI | 0.847 (0.149) | 0.862 (0.122) | 0.849 (0.156) | 0.835 (0.159) | 0.14 |
| GERD, n (%) | 318 (24.7%) | 46 (12.6%) | 90 (24.9%) | 182 (32.6%) | <0.001 |
| Regular PPI use, n (%) | 77 (14.5%) | 8 (6.7%) | 14 (9.3%) | 55 (21.2%) | <0.001 |
| OSA, n (%) | 31 (8.7%) | 2 (2.4%) | 5 (5.2%) | 24 (13.6%) | 0.004 |
| Diabetes Mellitus, n (%) | 43 (4.9%) | 0 (0.0%) | 6 (2.4%) | 37 (8.5%) | <0.001 |
| Immunodeficiency, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Smokins, n (%) | 0.054 | ||||
| Current/Former | 236 (18.3%) | 61 (16.8%) | 56 (15.4%) | 119 (21.3%) | |
| Never | 1051 (81.7%) | 303 (83.2%) | 307 (84.6%) | 441 (78.8%) | |
| SHS, n (%) | 437 (34.0%) | 104 (28.6%) | 115 (31.7%) | 218 (38.9%) | 0.003 |
URI – upper respiratory infection; FVC – forced vital capacity; FEV1 – forced expiratory volume in one second; ACT – Asthma Control Test; ACQ – Asthma Control Questionnaire; ASUI – Asthma Symptom Utility Index; GERD – Gastroesophageal reflux disease; PPI – proton pump inhibitor; OSA – obstructive sleep apnea, SHS – secondhand smoke. Bold indicates statistical significance (p<0.05). Results are expressed as number of participants (percentage) for categorical variables and mean (standard deviation) for continuous variables. P-values determined by chi-square test for categorical variableps and Kruskal-Wallis for continuous variables.
In children, demographic characteristics were generally similar across BMI classification groups. Higher BMI classification was associated with an increased FVC and decreased FEV1/FVC before and after bronchodilator. Higher BMI classification was associated with more frequent gastroesophageal reflux disease (GERD) and proton-pump inhibitor (PPI) use. Overweight/obese participants more commonly reported respiratory infection as an asthma trigger.
In adults, higher BMI classification was associated with older age, female sex, African American race, later asthma onset and greater utilization of inhaled combination corticosteroid-long acting beta agonist medication use. Overweight/obesity was associated with decreased FVC, FEV1, FEV1/FVC, and FEV1 percent-predicted pre- and post-bronchodilator. Lastly, higher BMI classification was associated with more frequent GERD, PPI use, obstructive sleep apnea, diabetes, and secondhand smoke exposure.
Risk and severity of RTI:
In all participants, BMI classification did not affect the rate of visits with RTI (see rate ratios in Table II, visit and participant numbers in Table E3, and event rates in Table E4). Children reported RTIs at 20.5% of visits compared to adults at 14.8% (rate ratio 1.41, 95% CI 1.27-1.56, p<0.001); therefore RTI analyses among adult and children were conducted separately. In unadjusted analyses, risk factors for RTI included PPI use in children and SHS and atopy in adults. In children, after adjusting for gender, ethnicity, and race, PPI use remained an independent risk factor for RTI; BMI classification, GERD, SHS, and atopy were not. In adults, after adjusting for gender, ethnicity, and race, atopy remained an independent risk factor for RTI; BMI classification, GERD, PPI use, and SHS were not. In all participants, BMI classification did not affect the rate of visits with URTI or LRTI (Figure 1). Among children with URIs, BMI classification did not affect the self-reported severity of infections (p=0.51). However, among adults with URIs, there is an association between BMI classification and URI severity. The prevalence of moderate/severe URI was increased in obese adults compared to lean and overweight adults (p-value=0.03, Figure 2). Analyses using WHR to determine obesity yielded similar results. We found no effect of WHR on rate of RTIs; however, a higher WHR associated with increased URI severity in adults. The individual studies were also analyzed and demonstrated no effect of BMI classification on rate of RTIs and a trend toward increased URI severity in participants with higher BMI classification compared to lean participants.
Table IIa:
Univariable and multivariable negative binomial models of respiratory tract infections in Children
| Univariable analysis | Multivariable analysis* | |||
|---|---|---|---|---|
| Rate ratio (95% CI) | p-value | Rate ratio (95% CI) | p-value | |
| BMI classification | OW 1.11 (0.91-1.37) | 0.30 | OW 1.11 (0.91-1.37) | 0.30 |
| OB 1.04 (0.87-1.25) | 0.69 | OB 1.04 (0.87-1.25) | 0.67 | |
| GERD | 1.02 (0.77-1.35) | 0.91 | 0.89 (0.64 -1.26) | 0.52 |
| PPI | 1.41 (1.20-1.66) | <0.001 | 1.40 (1.19 -1.65) | <0.001 |
| SHS | 1.07 (0.91-1.26) | 0.40 | 1.15 (0.97-1.35) | 0.11 |
| Atopy | 1.01 (0.83-1.22) | 0.94 | 0.96 (0.79-1.17) | 0.69 |
OW – overweight; OB – obese; GERD – gastroesophageal reflux disease; PPI – proton pump inhibitor; SHS – secondhand smoke. Bold indicates statistical significance (p<0.05).
adjusting for gender, ethnicity, race, and the other covariance variables in the table. Analysis sample size: BMI classification – 747, GERD – 746, PPI – 545, SHS – 747, atopy – 747, multivariable – 544. P-values determined by negative binomial regression.
Figure 1: The risk of Respiratory Tract Infection (RTI) and RTI-associated asthma morbidity in obese vs lean participants.
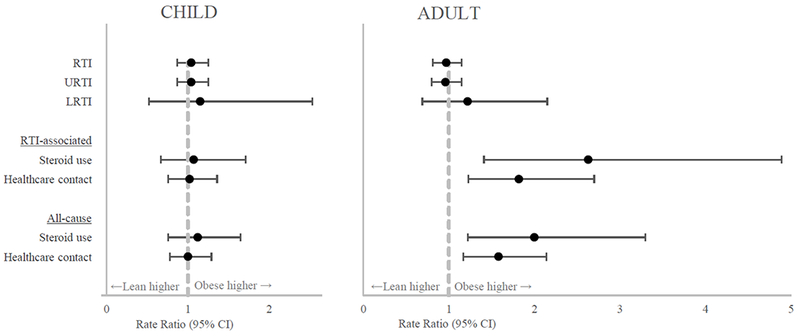
RTI – respiratory tract infection; URTI – upper respiratory tract infection; LRTI – lower respiratory tract infection. Bold indicates statistical significance (p<0.05). Points are rate ratios comparing obese and lean participants with bars indicating the 95% confidence interval. Dashed gray line is the reference rate ratio of 1.0. P-values determined by negative binomial regression.
Figure 2: The effect of BMI classification on Upper Respiratory Infection (URI) severity in Adults.
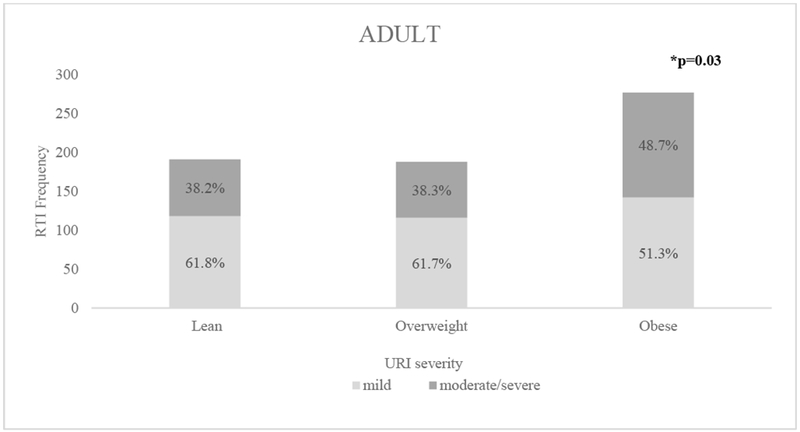
Bold indicates statistical significance (p<0.05). *P-value determined by chi-square test.
Asthma-related events and lung function:
In children, BMI classification did not affect the rate of all-cause or RTI-associated asthma events. However, in adults, higher BMI classification was associated with significantly increasing rates of allcause and RTI-associated exacerbations requiring systemic steroids and all-cause and RTI-associated healthcare contact for asthma (see Figure 1, rate ratios in Table III). There was a significant statistical interaction between being a child and BMI classification on RTI-associated healthcare contact for asthma (p=0.01, effect sizes in Table III). Among participants with a recent RTI, BMI classification was not associated with a subsequent significant decline in lung function (all p>0.05 for changes in FEV1 and FVC in children and adults). Analysis with WHR showed similar trends with an increased rate of asthma-related events in obese adults compared to lean adults and no effect of obesity on asthma-related events in children. The individual studies were also analyzed and showed similar trends in increased allcause and RTI-related morbidity in adults with higher BMI classifications.
Table III:
Summary of BMI classification effects on selected outcomes
| CHILD | ADULT | |||||
|---|---|---|---|---|---|---|
| RTI-related | Overweight | Obese | p-value | Overweight | Obese | p-value |
| Risk of RTI | 1.10 | 1.04 | 0.64 | 1.05 | 0.97 | 0.61 |
| (0.90-1.36) | (0.87-1.25) | (0.91-1.22) | (0.81-1.15) | |||
| Steroid use | 0.93 | 1.07 | 0.77 | 1.68 | 2.63 | 0.001 |
| (0.54-1.63) | (0.67-1.71) | (0.84-3.36) | (1.41-4.89) | |||
| Healthcare | 1.15 | 1.02 | 0.89 | 1.03 | 1.82 | 0.002 |
| contact | (0.83-1.56) | (0.76-1.36) | (0.65-1.64) | (1.23-2.70) | ||
| All-cause | ||||||
| Steroid use | 0.86 | 1.12 | 0.58 | 1.37 | 2.00 | 0.005 |
| (0.53-1.40) | (0.76-1.65) | (0.78-2.43) | (1.22-3.30) | |||
| Healthcare | 1.11 | 1.00 | 1.00 | 1.21 | 1.58 | 0.003 |
| contact | (0.83-1.49) | (0.78-1.29) | (0.86-1.70) | (1.17-2.14) | ||
Values are rate ratio (95% CI) compared to lean as a reference. RTI – respiratory tract infection; URI – upper respiratory infection; FVC – forced vital capacity; FEV1 – forced expiratory volume in one second. Bold indicates statistical significance (p<0.05). P-values determined by negative binomial regression test for trend.
Discussion:
In this observational study of five large ALA-ACRC trials, BMI classification did not identify increased rates of RTIs in adults or children with asthma. As expected, children had higher rates of RTIs compared to adults. However, in adults, higher BMI classification was associated with more severe URIs and higher rates of all-cause and RTI-associated asthma exacerbations requiring systemic steroids and allcause and RTI-associated asthma-related healthcare contact. In addition, PPI use was independently associated with higher rates of RTIs in children whereas atopy was independently associated with higher rates of RTIs in adults. We conclude that obesity was not associated with increased RTIs but with increased RTI-associated asthma morbidity in adults only.
Previous cross-sectional studies have shown increased respiratory tract infections in obese children and adults. In a field health survey of 1129 preadolescent children in Krakow, children with a body mass index of 20 or higher had twice the risk of infection than children with a body mass index less than 20 (OR 2.02, 95% CI 1.13-3.59).10 In a prospective cohort study of 75,000 women enrolled in the Danish National Birth Cohort from 1996 to 2002, obesity was associated with increased risk of acute respiratory tract infections (HR 3.64, 95% CI 1.62-8.18).9 In an analysis of participants in two large cohort studies, the Health Professionals Follow-up Study and the Nurses’ Health Study II, men who gained 40 pounds or more were at increased risk for community acquired pneumonia.11 These studies did not examine the relationship between respiratory tract infection and obesity in participants with asthma.
There is increasing evidence that obesity increases susceptibility to severe respiratory infections through an impaired immune response. In studies of influenza-vaccinated adults, obesity did not affect influenza-specific antibody responses but doubled the risk of influenza or influenza-like illness suggesting a deficiency in cellular immune response.20 Leptin, a pro-inflammatory adipokine which is increased in obesity, and even more so in patients with obesity and asthma, may be a mediator for the effects of obesity.21 Leptin metabolically regulates proliferation and cytokine production of effector T cells and may impair anti-viral interferon responses through inhibition of signaling through the JAK-STAT pathway.7, 22, 23 In a preclinical diet-induced obesity (DIO) mouse model, leptin inhibition in obese mice prior to viral infection led to improved survival.24 In human epidemiological studies, plasma leptin levels have been positively correlated with annual risk of respiratory infection and mortality in patients with severe pneumonia.25 In patients with asthma and obesity, increased leptin levels may lead to impaired cellular immunity resulting in more frequent and severe respiratory infections, associated asthma exacerbations, and worse asthma control.
Obesity may modify susceptibility to RTIs differently in asthmatics compared to non-asthmatics. The relationship between asthma and RTIs is complex as viruses and bacteria have been implicated in both the development and exacerbation of asthma. Corne et al. studied 76 cohabitating couples consisting of an individual with atopic asthma and a healthy individual (without atopy or asthma) and found that risk of rhinovirus infection did not differ between the asthma and the healthy group adjusting for sex and use of inhaled steroids. However, the severity and duration of lower respiratory tract symptoms were significantly greater in the asthma group than the healthy group.26 Thus, patients with asthma may not have more viral infections than healthy individuals, but greater duration and severity especially of lower respiratory symptoms.27 In our study, in adults, obesity had a similar relationship with RTIs – more RTI-associated asthma morbidity without an increase in susceptibility.
Notably, the effect of obesity on RTI-associated asthma morbidity was different between children and adults. Obesity affected RTI-associated asthma morbidity in adults but not children. Children had higher rates of reporting RTIs than adults after controlling for many covariates including BMI classification. In our studies, fewer children than adults were obese (28% vs 43% respectively) and obesity in children may be associated with other disease states such as early onset puberty. Thus, it was more challenging to show an effect of obesity on RTI in children with asthma. Further investigations comparing asthmatic and nonasthmatic children may help elucidate the true effect of obesity on RTIs in children with asthma. Moreover, obesity was associated with increased URI severity in adults but not in children. Children are likely to have more URIs of all severity – the effect of being a child was greater than the effect of obesity. However, obese adults were more likely to have moderate or severe URIs, which likely placed them at increased risk for RTI-associated asthma morbidity. Obese adults may also be more vulnerable to age effects on airway function and immune response compared to their lean counterparts leading to poorer respiratory outcomes.27, 28 We were unable to analyze adolescents (age 12-18) given our sample size.
In the multivariable model, we found that the use of PPIs increased risk for RTIs in children. Increased rates of URI, sore throat, and bronchitis had been previously shown in SARCA, in which children were placed on a PPI as the study intervention.17 The proportion of participants on a PPI was much lower in the other studies compared to SARCA, so further studies are needed to externally validate the risk of PPIs on RTIs seen in SARCA. PPIs have been associated with increased risk for community-acquired pneumonia.29, 30 Research into pathogenesis has found pH dysregulation increases bacterial survival and PPIs appear to directly inhibit neutrophil and natural killer cell activity.31, 32 GERD symptoms are common in asthmatics and are associated with poor asthma control33, 34; however, treatment with PPI do not consistently improve asthma control.17, 35–37 PPIs are metabolized by hepatic enzymes coded by the highly polymorphic CYP2C19. The rate of PPI metabolism in the population can vary widely.38 Post-hoc analysis has shown that children identified as intermediate or poor metabolizers receiving conventional doses of lansoprazole have higher serum PPI levels and, compared to normal or extensive metabolizers, are at greater risk for RTIs and worse asthma control.38, 39 In our data, controlling for GERD or PPI status did not significantly change the effect of obesity on RTIs, suggesting that greater GERD or GERD treatment in adults would not explain the greater RTI-associated asthma morbidity in that population.
This study’s strengths include large numbers of participants with well characterized asthma followed prospectively in five controlled trials conducted by experienced asthma centers participating in the ALA-ACRC research network. Participants were recruited from 20 sites around the US and made up a diverse population of patients with asthma. The study also included longitudinal data involving several structured clinic visits spanning over 3-6 months. These five trials had similar reporting of RTIs that allowed consolidation of data. The current study has several limitations, including its post-hoc nature. This retrospective analysis was limited by the fact that it assessed RTIs by report at visits rather than a true count and without microbiological confirmation of RTI. The majority of RTIs in asthmatics have been found to be caused by respiratory viruses like rhinovirus, influenza, respiratory syncytial virus, parainfluenza, and adenovirus. However, patients with asthma are also at increased risk for pneumococcal disease and atypical bacteria and polymicrobial infections involving viral and bacterial pathogens increase the risk for severe exacerbations. Studies of respiratory tract infections in obesity have been focused on an increased susceptibility and morbidity with viral infections. Therefore, further studies are needed to investigate the relationships between asthma, pathogen type, and obesity. We also did not have available data on socioeconomic status (such as income or health literacy) which could be confounding factors for risk of respiratory tract infection, although we did adjust for ethnicity, race, and SHS. In addition, our analysis was limited by the fact that we combined data from five studies with different inclusion and exclusion criteria. However, as noted in the results, analysis of the individual studies showed similar trends in increased URI severity and RTI-related morbidity in obese adults.
In summary, higher BMI classification was not associated with an increased rate of RTIs in children or adults but was associated with increased RTI-associated morbidity in adults. A better understanding of he mechanisms of poor control help identify new treatment strategies for this high risk population that does not respond to conventional therapies. Overweight and obese adult asthmatics are likely to benefit from interventions aimed at reducing obesity. Additionally, difficult to control overweight and obese asthmatics may benefit from strategies to prevent RTIs. Further studies are needed to better understand the complex relationship between obesity, RTIs, and asthma.
Supplementary Material
Table IIB:
Univariable and multivariable negative binomial models of respiratory tract infections in Adults
| Univariable analysis | Multivariable analysis* | |||
|---|---|---|---|---|
| Rate ratio (95% CI) | p-value | Rate ratio (95% CI) | p-value | |
| BMI classification | OW 1.11 (0.91-1.37) | 0.30 | OW 1.28 (0.92-1.79) | 0.15 |
| OB 1.04 (0.87-1.25) | 0.67 | OB 1.33 (0.96-1.83) | 0.08 | |
| GERD | 1.10 (0.94-1.29) | 0.23 | 1.26 (0.94 -1.69) | 0.13 |
| PPI | 1.13 (0.82-1.55) | 0.45 | 0.80 (0.55 -1.16) | 0.25 |
| SHS | 1.18 (1.02-1.37) | 0.03 | 1.02 (0.79-1.30) | 0.90 |
| Atopy | 1.51 (1.29-1.77) | <0.001 | 1.64 (1.22-2.19) | 0.001 |
OW – overweight; OB – obese; GERD – gastroesophageal reflux disease; PPI – proton pump inhibitor; SHS – secondhand smoke. Bold indicates statistical significance (p<0.05).
adjusting for gender, ethnicity, race, and the other covariance variables in the table. Analysis sample size: BMI classification – 1287, GERD – 1285, PPI – 530, SHS – 1287, atopy – 1287, multivariable – 527. P-values determined by negative binomial regression.
Highlights box:
1. What is already known about this topic?
Obesity in patients with asthma is associated with severe and poorly controlled asthma.
2. What does this article add to our knowledge?
Higher BMI classification is not associated with increased risk of respiratory tract infections in children or adults with asthma but is associated with increased illness severity and infection-associated asthma morbidity in adults.
3. How does this study impact current management guidelines
Adults with obesity and asthma may improve their asthma control by using interventions targeting weight reduction and prevention of respiratory tract infections.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health (NIH) [grant no. T32 AI007062-39]. The associated parent studies were supported by the American Lung Association, GlaxoSmithKline (LOCCS, LASST), and the NIH (grant no. U01 HL080450 and U01 HL080433 for SARCA, U01 HL087987, U01 HL 0088367, and U54TR001018 for SOYA, U01HL089464, U01 HL089510, UL1 TR000448 for STAN).
Abbreviations:
- BMI
Body mass index
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GERD
Gastroesophageal reflux disease
- LASST
Long Acting Beta Agonist Step Down Study
- LOCCS
Leukotriene Modifier or Corticosteroid or Corticosteroid Salmeterol Trial
- LRTI
Lower respiratory tract infection
- PPI
Proton pump inhibitor
- RTI
Respiratory tract infection
- SARCA
Study of Acid Reflux in Children with Asthma
- SHS
Secondhand smoke
- SOYA
Study of Soy Isoflavones in Asthma
- STAN
Study of Asthma and Nasal Steroids
- URI
Upper respiratory infection
- URTI
Upper respiratory tract infection
- WHR
Waist-height ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: R.A. Wise reports grants and personal fees from AstraZeneca / Medimmune, grants and personal fees from Boehringer Ingelheim, personal fees from Contrafect, personal fees from Pulmonx, personal fees from Roche, personal fees from Spiration, personal fees from Sunovion, grants from Pearl Therapeutics, personal fees from Merck, personal fees from Circassia, grants and personal fees from GSK, personal fees from Pneuma, personal fees from Verona, personal fees from Bonti, personal fees from Denali, personal fees from Aradigm, outside the submitted work; Johns Hopkins University manages conflicts of interest. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.NHLBI Fact Book, Fiscal Year 2012. National Heart, Lung, and Blood Institute, NIH, 2013. [Google Scholar]
- 2.Boulet LP. Asthma and obesity. Clin Exp Allergy 2013; 43:8–21. [DOI] [PubMed] [Google Scholar]
- 3.Lang JE. Obesity, Nutrition, and Asthma in Children. Pediatr Allergy Immunol Pulmonol 2012; 25:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stukus DR. Obesity and asthma: the chicken or the egg? J Allergy Clin Immunol 2015; 135:894–5. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol 2014; 14:35–43. [DOI] [PubMed] [Google Scholar]
- 6.Bardin PG, Johnston SL, Pattemore PK. Viruses as precipitants of asthma symptoms. II. Physiology and mechanisms. Clin Exp Allergy 1992; 22:809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almond MH, Edwards MR, Barclay WS, Johnston SL. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax 2013; 68:684–6. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Chaves SS. Obesity and influenza. Clin Infect Dis 2011; 53:422–4. [DOI] [PubMed] [Google Scholar]
- 9.Harpsoe MC, Nielsen NM, Friis-Moller N, Andersson M, Wohlfahrt J, Linneberg A, et al. Body Mass Index and Risk of Infections Among Women in the Danish National Birth Cohort. Am J Epidemiol 2016; 183:1008–17. [DOI] [PubMed] [Google Scholar]
- 10.Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health 1998; 112:189–95. [DOI] [PubMed] [Google Scholar]
- 11.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 2000; 160:3082–8. [DOI] [PubMed] [Google Scholar]
- 12.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006; 6:438–46. [DOI] [PubMed] [Google Scholar]
- 13.American Lung Association Asthma Clinical Research C, Peters SP, Anthonisen N, Castro M, Holbrook JT, Irvin CG, et al. Randomized comparison of strategies for reducing treatment in mild persistent asthma. N Engl J Med 2007; 356:2027–39. [DOI] [PubMed] [Google Scholar]
- 14.American Lung Association-Asthma Clinical Research Centers’ Writing C, Dixon AE, Castro M, Cohen RI, Gerald LB, olbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. J Allergy Clin Immunol 2015; 135:701–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers L, Sugar EA, Blake K, Castro M, Dimango E, Hanania NA, et al. Step-Down Therapy for Asthma Well Controlled on Inhaled Corticosteroid and Long-Acting Beta-Agonist: A Randomized Clinical Trial. J Allergy Clin Immunol Pract 2017. [DOI] [PubMed] [Google Scholar]
- 16.Smith LJ, Kalhan R, Wise RA, Sugar EA, Lima JJ, Irvin CG, et al. Effect of a soy isoflavone supplement on lung function and clinical outcomes in patients with poorly controlled asthma: a randomized clinical trial. JAMA 2015; 313:2033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Writing Committee for the American Lung Association Asthma Clinical Research C, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012; 307:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno E, Han YY, Libman IM, Muzumdar RH, Celedon JC. Adiposity and Asthma in a Nationwide Study of Children and Adults in the United States. Ann Am Thorac Soc 2018; 15:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, et al. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. J Allergy Clin Immunol 2009; 123:1321–7 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 2017; 41:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 2012; 186:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, MacIver NJ. Leptin Metabolically Licenses T Cells for Activation To Link Nutrition and Immunity. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teran-Cabanillas E, Montalvo-Corral M, Caire-Juvera G, Moya-Camarena SY, Hernandez J. Decreased interferon-alpha and interferon-beta production in obesity and expression of suppressor of cytokine signaling. Nutrition 2013; 29:207–12. [DOI] [PubMed] [Google Scholar]
- 24.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis 2013; 207:1270–80. [DOI] [PubMed] [Google Scholar]
- 25.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 2002; 359:831–4. [DOI] [PubMed] [Google Scholar]
- 27.Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010; 376:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. In: Clin Interv Aging; 2006. p. 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics 2006; 117:e817–20. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One 2015; 10:e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci 2011; 56:931–50. [DOI] [PubMed] [Google Scholar]
- 32.Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut 1992; 33:617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang JE, Hossain MJ, Lima JJ. Overweight children report qualitatively distinct asthma symptoms: analysis of validated symptom measures. J Allergy Clin Immunol 2015; 135:886–93 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field SK, Underwood M, Brant R, Cowie RL. Prevalence of gastroesophageal reflux symptoms in asthma. Chest 1996; 109:316–22. [DOI] [PubMed] [Google Scholar]
- 35.American Lung Association Asthma Clinical Research C, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360:1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med 2011; 171:620–9. [DOI] [PubMed] [Google Scholar]
- 37.Kiljander TO, Junghard O, Beckman O, Lind T. Effect of esomeprazole 40 mg once or twice daily on asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2010; 181:1042–8. [DOI] [PubMed] [Google Scholar]
- 38.Lang JE, Holbrook JT, Mougey EB, Wei CY, Wise RA, Teague WG, et al. Lansoprazole Is Associated with Worsening Asthma Control in Children with the CYP2C19 Poor Metabolizer Phenotype. Ann Am Thorac Soc 2015; 12:878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima JJ, Lang JE, Mougey EB, Blake KB, Gong Y, Holbrook JT, et al. Association of CYP2C19 polymorphisms and lansoprazole-associated respiratory adverse effects in children. J Pediatr 2013; 163:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


