Abstract
Background
Individuals with fragile X syndrome (FXS), the most common known inherited form of intellectual disability (ID), are at increased risk for showing specific forms of self-injurious behaviour (SIB) such as hand biting and head hitting, suggesting that biological factors associated with the syndrome confers increased risk for SIB. Few studies, however, have examined the extent to which social-environmental variables can influence the occurrence of these behaviours in this population.
Method
Twenty-two adolescent boys with FXS, aged 10 to 18 years were systematically exposed to seven environmental conditions in functional analyses of SIB conducted over 2 days at our research centre.
Results
Fourteen (63.6%) boys with FXS engaged in SIB during the functional analyses. Ten (45.5%) boys engaged in SIB that was maintained by social-environmental variables, that is, gaining access to attention/tangibles and/or escaping from social interaction, task demands and/or transition demands. For two boys, SIB was undifferentiated across conditions, and for two boys, SIB appeared to be maintained by automatic reinforcement.
Conclusions
Social-environmental variables appeared to maintain SIB in a significant proportion of boys with FXS. Given that pharmacological treatments for SIB have limited efficacy in this population, the potential role of social-environmental factors on SIB should be examined before pharmacological treatments are implemented for these behaviours.
Keywords: aggression, fragile X syndrome, functional analysis, self-injury
Introduction
A large proportion of individuals diagnosed with fragile X syndrome (FXS), the most common known form of inherited ID, display specific forms of self-injurious behaviour (SIB) such as self-hitting and self-biting behaviours that can cause a significant impact on the individual’s well-being and educational opportunities. For example, studies conducted over the past few decades indicate that as many as 60–80% of individuals with FXS display these behaviours to the extent that tissue damage or minor injury occurs (Hall et al. 2008; Hessl et al., 2008; Langthorne & McGill 2012; Symons et al. 2003). Given that the prevalence of these behaviours in FXS is generally higher compared with that observed in individuals with ID in general, it seems likely that genetic factors associated with the syndrome may confer increased risk for the occurrence of SIB in FXS (Hall et al. 2008; Hessl et al., 2008; Symons et al. 2003).
Fragile X syndrome is caused by mutations to the FMRI gene at location 27.3 on the X chromosome (Verkerk et al., 1991) resulting in transcriptional silencing and reduced or absent FMRP, the protein product of the gene. Several theories have therefore been advanced to link the genetic mutation responsible for causing FXS to the phenotypic expression. For example, one prominent theory suggests that SIB in FXS may result from hypothalamic–pituitary–adrenal axis dysfunction (Hessl et al. 2004). This theory stems from observations that individuals with FXS, particularly boys, have an abnormally strong physiological and behavioural response to novel situations, commonly termed ‘hyperarousal’ (Roberts et al. 2001; Hall et al. 2009). There is some evidence, for example, that increased levels of salivary cortisol, a measure of hypothalamic–pituitary–adrenal axis dysfunction, may be associated with increased levels of SIB, particularly in boys with FXS (Hessl et al. 2002; Hall et al. 2006). Studies have also shown that boys with FXS evidence increased heart rate and reduced vagal tone during challenging situations compared with typically developing individuals (Hall et al. 2009; Klusek et al. 2015). It has also been reported that enlargement of specific regions of the brain such as the caudate nucleus, a region linked to reduced expression of FMRP, may be associated with SIB in FXS (Wolff et al. 2013).
Alongside potential biological explanations for SIB in FXS, other investigators have suggested that SIB in FXS may be influenced by social-environmental variables (Symons et al. 2003; Hall et al. 2006; Langthorne & McGill 2012). In a systematic review of studies employing both direct and indirect approaches to functional assessment, Hardiman and McGill (2017) reported that individuals with FXS appear more likely to engage in problem behaviour to escape from social interactions with others, task demands and/or unexpected transitions and that individuals with FXS are less likely to engage in problem behaviour to gain access to attention. These data suggest that the motivation to escape from unwanted situations may therefore be heightened in FXS, whereas the motivation to gain access to adult attention may be diminished (Hardiman & McGill, 2017).
One way to examine the impact of these variables on SIB is to conduct a functional analysis (Iwata et al. 1994a). A functional analysis (FA) involves repeatedly exposing the individual to several specific test and control conditions that simulate the natural environment and examining the effect of these manipulations on the occurrence of the target behaviour. Within each condition, consequent events that may serve to maintain SIB are systematically presented and withdrawn. For example, in a ‘demand escape’ condition, antecedent task demands are repeatedly presented to a subject and contingent on the occurrence of the target behaviour, the task demands are subsequently removed for a brief period of time. If the target behaviour occurs at higher rates in this condition, then it can be inferred that the target behaviour is maintained by escape from task demands. In an ‘attention’ condition, the subject is placed in a situation where attention from others is low or absent (the antecedent). When the target behaviour occurs, attention is subsequently given (the consequence). This condition is designed to test whether the behaviour is maintained by attention. In an ‘alone’ (or ‘ignore’) condition, the subject is left alone (or ignored) with nothing to do. If the target behaviour continues to occur in this condition, then the behaviour may have an automatic reinforcement function because it persists in the absence of any social consequences. In an epidemiological analysis of 152 cases of individuals with ID who showed SIB, Iwata et al. (1994c) found that SIB occurred most often in the demand escape condition for 38.1% of cases, in the attention condition for 26.3% of cases and in the alone condition for 25.7% of cases. For the remaining cases, SIB was found to occur in more than one condition (5.3%), or there was an undifferentiated pattern across conditions, which precluded any definitive conclusions regarding behavioural function to be made (4.6% of cases). Taken together, these data indicate that for the majority of cases, social-environmental influences on SIB could be identified. Since the seminal study of Iwata et al. (1994a), FA methodology has been employed successfully in hundreds of studies across various forms of behaviours and populations (Beavers et al. 2013) and is considered the gold standard approach to functional assessment. When treatments tailored to the function of an individual’s problem behaviour – such as noncontingent reinforcement (Vollmer et al. 1993), extinction (Iwata et al. 1994b) and differential reinforcement (Vollmer & Iwata 1992) – are implemented, significant reductions in the frequency and severity of those behaviours are observed (Hanley et al. 2003). Given these considerations, it seems prudent to examine whether FA methodology can be employed to examine the extent to which social-environmental factors may be influential in maintaining SIB commonly exhibited by boys with FXS.
To date, however, only three studies have directly examined the influence of social-environmental factors on problem behaviours shown by individuals with FXS. In a study conducted by Langthorne et al. (2011), functional analyses were conducted for eight boys with FXS, aged 8 to 15 years, who were reported to exhibit a number of problem behaviours including SIB, aggression and destructive behaviours. The FA conditions were implemented either at home or at school and included the standard set of conditions (i.e. ignore, attention, academic demand and play) and two additional conditions – social avoidance (i.e. removing social interaction contingent on problem behaviour) and tangible (providing access to preferred items contingent on problem behaviour). These authors reported that social functions could be identified in 100% of cases, namely, escaping from demands (four cases), gaining access to tangible items (three cases) and by multiple sources of social reinforcement including social avoidance (one case). In another study, Machalicek et al. (2014) conducted functional analyses on 12 boys with FXS, aged 2 to 4 years, who were reported to exhibit a number of forms of problem behaviour including SIB, aggression and disruptive behaviours. In that study, caregivers were coached to implement the various conditions (i.e. attention, social avoidance, demand escape, tangible and play) with their child in a clinic setting, and the children were exposed to the various conditions only briefly. These authors reported that social functions could be identified in 11 of the 12 cases, namely, gaining access to tangible items (three cases), escaping from demands (one case) and by multiple sources of social reinforcement (seven cases). Finally, in a study conducted by Kurtz et al. (2015), functional analyses were conducted on nine children with FXS (eight boys and one girl) aged 6 to 15 years who showed SIB, aggression and destructive behaviours. These functional analyses were conducted on an inpatient or outpatient basis with a variety of conditions being implemented on a case-by-case basis including attention, complying with the child’s demands for ‘playing his way’ demand escape, tangible, alone and ignore. These authors identified social functions for problem behaviour in 8 of the 9 cases in the form of escaping from demands (two cases), gaining attention (one case), complying with the child’s demands (two cases), gaining access to tangibles (one case) and by multiple sources of social reinforcement (three cases).
Taken together, data from previous studies suggest that problem behaviours shown by individuals with FXS are often maintained by social-environmental factors. However, these data should be interpreted cautiously for several reasons. First, not all children were reported to exhibit SIB in each study. For example, in the study conducted by Machalicek et al. (2014), only 6 of the 12 children showed SIB in the functional analysis. Second, in all three studies, the individual response classes observed in each study (i.e. SIB, aggression and destructive behaviours) were aggregated into a single response class for analysis. Thus, it is unknown whether the variables maintaining SIB were similar to those identified for the aggregated response class. Finally, the participants in each study were each exposed to a variety of different conditions and number of exposures to each condition. For example, in the study conducted by Kurtz et al. (2015), a social avoidance condition was not included, and in the study conducted by Machalicek et al. (2014), participants were exposed to the various conditions only once or twice. These methodological variations could have affected the outcome of each functional analysis.
To overcome these issues, in the present study, we identified a sample of boys with FXS from a larger cohort of boys who had been reported to show SIB, aged 10 to 18 years (Hall et al. 2016). The rationale was to focus on the functions for SIB in FXS and on employing FXS-specific conditions in the functional analysis. Each individual was therefore exposed to the same number of environmental conditions including tangible, social escape and transition escape in addition to the standard FA conditions to ensure that potential functions were not missed. These conditions were included because previous studies have indicated that transitions, social interaction and the removal of tangible items might be more likely to occasion SIB in FXS. Given that some boys also showed aggressive behaviour in addition to SIB, we conducted separate analyses for each response class. Given previous research, we hypothesised that social functions for SIB and aggressive behaviour, particularly escape and tangible functions, would be identified in a significant proportion of individuals with FXS.
Method
Participants
Participants were recruited via a series of emails sent out to members of the National Fragile X Foundation and local support groups serving individuals with ID. The email invited caregivers to complete a short online survey to obtain information about their child’s age, sex, diagnosis, the frequency and severity of their child’s SIB, other forms of problem behaviour and current treatments (Hall et al. 2016).
Participants were selected for inclusion in the present study if they were boys with FXS aged between 10 and 18 years, were able to travel to Stanford University, and had been reported to display SIB on at least a weekly basis. DNA reports were obtained from parents prior to enrolment to confirm the diagnosis of FXS (i.e. >200 cytosine-guanine-guanine (CGG) repeats within the FMR1 gene with evidence of aberrant methylation). Twenty-two boys with FXS met the study inclusion criteria and travelled to Stanford University to take part in the study. Table 1 shows the demographic characteristics of the participants.
Table 1.
Demographic characteristics
| Subject | Age (years) | Vineland-2 composite† | ABC-C total‡ | Frequency of SIB | Current treatments |
|---|---|---|---|---|---|
| 1 | 13.9 | 48 | 77 | Daily | Response blocking, chewable toy |
| 2 | 12.3 | 54 | 76 | Hourly | Medications |
| 3 | 13.4 | 60 | 77 | Daily | Medications, token economy |
| 4 | 11.3 | 44 | 68 | Daily | No treatments |
| 5 | 13.3 | 46 | 116 | Weekly | Medications, time-out |
| 6 | 17.5 | 37 | 36 | Daily | No treatments |
| 7 | 11.5 | 68 | 51 | Daily | Medications, redirection |
| 8 | 12.9 | 37 | 75 | Daily | Time-out, reprimanding |
| 9 | 12.5 | 51 | 74 | Daily | Medications, calming strategies |
| 10 | 11.9 | 55 | 86 | Hourly | Medications |
| 11 | 16.3 | 58 | 54 | Daily | Medications, calming strategies |
| 12 | 13.6 | 43 | 87 | Hourly | Reprimanding |
| 13 | 15.4 | 61 | 68 | Hourly | Medications, chewable toy |
| 14 | 12.8 | 58 | 102 | Weekly | Medications, visual schedule |
| 15 | 12.4 | 36 | 123 | Weekly | Medications, compliance with requests |
| 16 | 10.6 | 71 | 24 | Daily | Chewable toy |
| 17 | 13.5 | 55 | 80 | Daily | Medications, calming strategies |
| 18 | 11.5 | 55 | 78 | Daily | Medications, calming strategies |
| 19 | 15.4 | 59 | 33 | Daily | Medications |
| 20 | 14.1 | 47 | 65 | Daily | Applying lotion |
| 21 | 14.4 | 54 | 18 | Daily | Reprimand |
| 22 | 15.6 | 42 | 27 | Daily | Medication, reprimanding, calming strategies |
Vineland Adaptive Behavior Scales, Second Edition (Sparrow, Cicchetti & Balla, 2005).
Aberrant Behavior Checklist – Community (Aman & Singh 1994). SIB, self-injurious behaviour.
The mean age of the participants was 13.5 years (SD = 1.8 years, range = 10.6 to 17.5 years), and the mean composite score obtained on the Vineland Adaptive Behavior Scales, Second Edition (Sparrow, Cicchetti & Balla, 2005) was 51.8 (SD = 9.6, range = 36 to 71). The mean total score obtained on the Aberrant Behavior Checklist – Community (Aman & Singh 1994) was 68.0 (SD = 28.1, range = 18 to 123). In terms of the reported frequency of SIB, 4 (18.2%) participants were reported to exhibit the behaviour on an hourly basis, 14 (63.6%) on a daily basis and 4 (18.2%) on at least a weekly basis. Fourteen (63.6%) boys were taking psychoactive medications primarily in the form of antipsychotics, antidepressants, stimulants and anticonvulsants. Behavioural strategies included implementing calming strategies (five boys), reprimanding the behaviour (four boys), introducing chewable toys (three boys) and complying with requests (one boy). There were no differences between those who were taking medications and those who were not taking medications in terms of age, adaptive behaviour, Aberrant Behavior Checklist – Community total score and frequency of SIB.
Procedure
All procedures were approved by the Institutional Review Board at Stanford University, and parental consent was obtained in all cases prior to study onset. Functional analysis sessions were conducted over two consecutive days at the research centre in a large padded room equipped with a one-way observation window. All sessions were recorded through the oneway window via a digital video camera for later coding. In addition to the standard FA conditions (i.e. attention, demand and play), we also included a tangible condition to determine whether SIB was maintained by access to preferred items (Shirley et al. 1999), a social escape condition to determine whether SIB was maintained by escape from social interaction (Hagopian et al. 2001) and a transition escape condition to evaluate whether SIB was maintained by escape from transitions (i.e. the termination of an activity, location change and initiation of a different activity) (McCord et al. 2001). As described earlier, these conditions were included because previous studies have suggested that these variables may be likely to reinforce SIB in individuals with FXS. An ignore condition was included instead of an alone condition because the majority of boys with FXS were unable to tolerate being in the room on their own and would spend the session attempting to leave the room unless an adult was present. The seven conditions were conducted in the same sequence: ignore, attention, tangible, social escape, demand escape, transition escape and play in order to maximise the motivating operations for SIB across conditions (Hammond et al. 2013), and each participant was repeatedly exposed to the sequence of conditions 6 to 7 times. Each session lasted for 5 min in duration to minimise fatigue effects. Each FA was preceded by a preference assessment that was conducted to determine whether items such as magazines, video games or activities were highly preferred or moderately preferred. Task demands that were difficult for the child to complete were identified via caregiver interview.
Conditions
In the ignore condition, a therapist sat behind the participant out of direct view, no materials were present, and no consequences for problem behaviour were delivered. This condition was designed to determine whether SIB persisted in the absence of social consequences (automatic reinforcement). In the attention condition, the therapist was present in the room but seated at a distance with a laptop or smart phone and told the participant that she was busy responding to emails. The participant was given access to moderately preferred materials (magazines). If SIB occurred, the therapist delivered statements of concern (e.g. ‘that looks like it hurts’). This condition was designed to determine whether SIB was maintained by attention. In the tangible condition, the participant was given approximately 90-s free access to a highly preferred leisure item immediately prior to session onset. The experimenter then removed the item at the start of session. Contingent on the occurrence of SIB, the participant was allowed access to the item for ~20 s. This condition was designed to determine whether SIB was maintained by access to tangibles. In the social escape condition, the experimenter presented social interaction trials to the participant using a system of least prompts. The experimenter first initiated interaction by asking the participant an open-ended question (e.g. ‘what’s your favorite food?’) and allowed the participant 5 s to respond. If the participant failed to initiate an appropriate response after 5 s, the experimenter repeated the question and prompted the participant to look her in the eyes when answering and waited an additional 5 s. If no response occurred at that point, the experimenter repeated the question, modelled an example of a response and physically guided the participant to look at her using the least amount of contact necessary. A new social interaction was initiated contingent upon a reciprocal social response. Contingent on the occurrence of SIB, the experimenter terminated the trial and moved away from the participant for ~20 s. The experimenter based the questions on the interests of the participant and asked further questions based on participant responses. Eye contact was included as a specific prompt in this condition because previous studies have indicated that a high proportion of boys with FXS engages in eye contact avoidance during social interactions (Hall et al. 2009). This condition was designed to determine if SIB was maintained by social escape. In the demand escape condition, the therapist was present and in close proximity to the participant and presented low-probability task demands (i.e. those which the participant did not initiate without prompts) using a system of least prompts. Compliance with the task resulted in praise. If SIB occurred, the therapist said, ‘Let’s take a break’, removed the task materials and stepped away from the participant for ~20 s. This condition was designed to determine if SIB was maintained by escape from task demands. In the transition escape condition, the experimenter prompted the participant to initiate a moderate-preferred activity. After approximately 30 s of engagement, the experimenter prompted the participant to terminate the activity, move to another location and initiate a different moderate-preferred activity. If the participant did not readily engage in the activity, terminate the activity or move to the new location within 5 s of the instruction, the experimenter used a system of least prompts to gain compliance with that component of the transition. If the participant engaged in SIB at any time during the transition condition, he was given a brief (~20-s) break from the transition, after which time the last instruction given before the break was delivered again. This condition was designed to determine if SIB was maintained by escape from transitions. In the play condition, the therapist was present and in close proximity to the participant, preferred materials (e.g. computer games and YouTube videos) and ongoing interaction with the therapist and materials were available, but no consequences were delivered for problem behaviour. This condition served as a control condition and was designed to determine if SIB was suppressed when the participant had free access to preferred leisure items and therapist attention, and in the absence of social, task and transition demands. The same contingencies described in each condition for SIB were also applied if the participant engaged in aggressive behaviour. For example, if aggressive behaviour occurred in the attention condition, the therapist delivered other appropriate statements of concern such as ‘please don’t hurt me’.
The second and third authors served as the therapists for each FA. As a safety precaution, termination criteria were outlined prior to the outset of the assessment, and if a participant engaged in SIB or aggression that presented a threat to the safety of the participant or to the therapist, then the session was discontinued. The termination criteria were implemented if any cuts, bruising or bleeding occurred to the individual or the therapist as a result of the behaviour. To minimise carryover effects between the conditions, 5-min breaks were implemented between sessions, and problem behaviour was required to have ceased for at least 2 min before beginning the next session.
Response measurement and reliability
Observations were recorded from the digital video files using ObsWin software that allowed multiple topographies of problem behaviour to be coded in real time (Martin et al. 1998). Self-injury was defined as any behaviour directed toward the child’s own body that could result in physical harm (e.g. hand biting, head hitting and body hitting); aggression was defined as any behaviour directed toward the therapist that could result in physical harm (e.g. hitting, pushing and biting). The primary dependent variable was the frequency per minute of SIB observed in each session. The secondary dependent variable was the frequency per minute of aggression observed in each session. Observers were graduate students in psychology who were blind to the study hypotheses and trained to press a key on the keyboard associated with each behaviour to indicate its occurrence. For 25% of the sessions for each participant, a second observer also collected data. Agreement was calculated on a 10-s interval-by-interval basis using Cohen’s kappa, a statistic that corrects for chance agreements (Hartmann 1977). If two observers obtained a reliability coefficient for a given behaviour that was less than 0.60, the observers received additional training and familiarity with the definitions of each behaviour, and the video was recoded. The mean level of agreement across participants was 0.82 (range = 0.61 to 0.93) for SIB and 0.80 (range .64 to .90) for aggression, an acceptable level of agreement.
Data analysis
Previous studies have employed visual analysis or modified structured visual-inspection criteria (Roane et al. 2013) to determine whether a test condition is differentiated from the play condition. To evaluate the utility of this method, we applied the modified structured visual-inspection criteria to, a large sample of FA multielement graphs published in several volumes of the Journal of Applied Behavior Analysis. When the structured criteria method was applied to the datasets, the outcomes were not always concordant with the published outcomes. Further analysis indicated that the modified structured criteria method was more likely to miss a potential function when outlier points were present in the data. This is because the modified structured criteria method assumes that the data are normally distributed, an assumption that may be violated in some cases.
To provide a more objective method to compare the levels of SIB and aggression observed across conditions for each participant, we calculated the median levels of SIB and aggression observed in each condition and obtained a 95% confidence interval for each median using the method outlined in Hogg et al. (2015, p. 333). A test condition was considered to meet the initial criteria for differentiation if the median of the test condition was higher than the upper 95% confidence limit of the play condition. Each test condition was then checked for the presence of an upward or downward trend by calculating a Kendall’s Tau coefficient for each time series (Fisch 2001). If a test condition met the initial criteria for differentiation, but Kendall’s Tau coefficient was significantly lower than zero (i.e. a decreasing trend), then that condition was no longer differentiated, unless responding was decreasing to response efficiency (i.e. two responses per minute). Conversely, if a test condition had not met the initial criteria for differentiation, but Kendall’s Tau coefficient was significantly higher than zero (i.e. an increasing trend), that condition was differentiated. The outcome of each FA was then determined using the rules specified by Roane et al. (2013). For example, if the median level of responding was high (defined as greater than 1.5 responses per minute) across all conditions (including play), or if the ignore condition was the only condition that met the criteria for differentiation, the outcome was classified as ‘automatic reinforcement’. If more than one social condition met the criteria for differentiation, the outcome was classified as ‘multiple social variables’. If one of those conditions included the ignore condition, automatic reinforcement was included in the list of functions only if the median level of responding in the ignore condition was the first or second highest, and there was no downward trend in the ignore condition. If none of the test conditions met criteria for differentiation, then the outcome was classified as undifferentiated (Roane et al. 2013).
Results
Functional analyses
Of the 22 boys who received a functional analysis, 11 (50%) boys displayed SIB only, 5 (22.7%) boys displayed aggression only, and 3 (13.6%) boys displayed both SIB and aggression. For three (13.6%) boys, neither SIB nor aggression occurred during the functional analyses.
Self-injurious behaviour
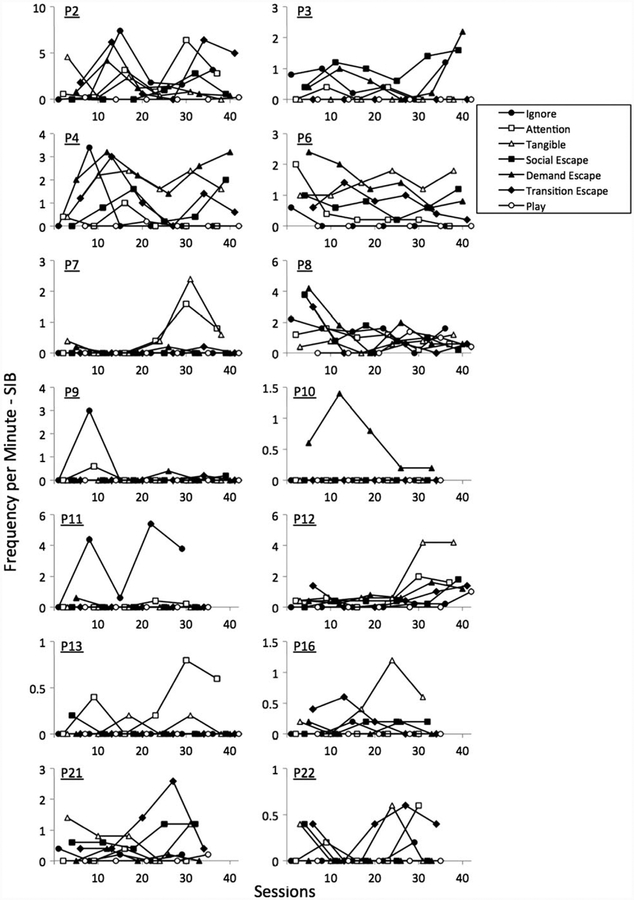
Figure 1 shows the rates of SIB observed in each condition for the 14 boys who displayed SIB in the functional analyses.
Figure 1.
Frequency per minute of self-injurious behaviour (SIB) observed across sessions in each condition of the functional analysis for each participant.
Using the classification criteria outlined earlier, six boys exhibited SIB that was maintained by multiple social functions (i.e. tangible and social/demand/transition escape), one boy exhibited SIB that was maintained by escape from demands, two boys exhibited SIB that was maintained by tangible reinforcement and one boy exhibited SIB that was maintained by attention. SIB appeared to be maintained by automatic reinforcement for two boys and was undifferentiated across conditions for two boys, indicating that the outcome was inconclusive for those boys.
Table 2 shows a summary of these data. Taken together, social functions for SIB were identified for 10 of the 14 boys who showed SIB in the functional analyses. The primary topographies of SIB observed were finger/hand biting (10 of 14 boys) and head/face hitting (6 of 14 boys).
Table 2.
Results of the functional analyses for the 14 boys who showed self-injurious behavior
| Subject | Topography observed | FA outcome |
|---|---|---|
| P2 | Head hitting, hand biting | Attention, demand escape, transition escape |
| P3 | Lip picking, head hitting | Social escape, demand escape |
| P4 | Head hitting, hand biting | Tangible, social escape, demand escape, transition escape |
| P6 | Finger picking, face scratching | Tangible, social escape, demand escape, transition escape |
| P7 | Hand biting | Attention, tangible |
| P8 | Head hitting, finger biting | Automatic reinforcement |
| P9 | Face hitting, chest hitting | Undifferentiated |
| P10 | Face hitting | Demand escape |
| P11 | Chest hitting, chin pressing, lip picking | Automatic reinforcement |
| P12 | Hand biting | Tangible |
| P13 | Finger biting, teeth clicking | Attention |
| P16 | Finger biting | Tangible |
| P21 | Finger biting | Tangible, social escape, transition escape |
| P22 | Finger biting | Undifferentiated |
Aggressive behaviour
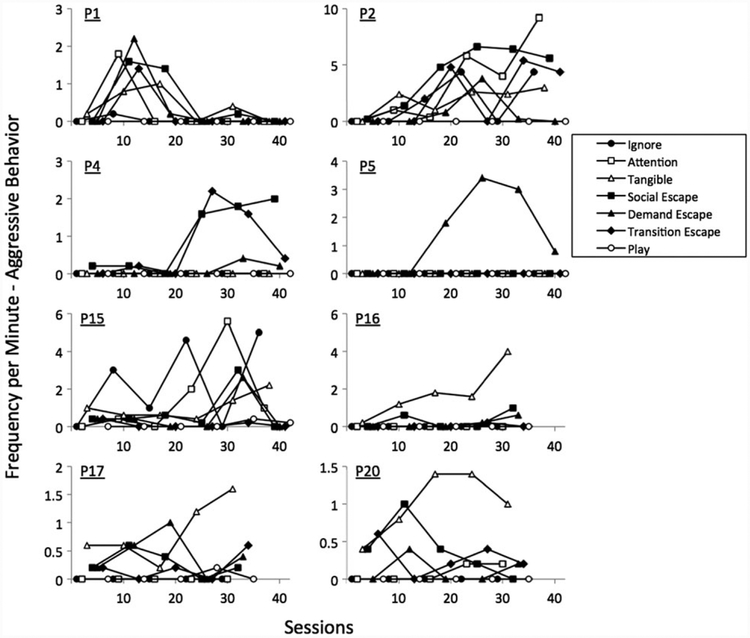
Figure 2 shows the rates of aggressive behaviour observed in each condition for the eight boys who showed aggression in the functional analyses.
Figure 2.
Frequency per minute of aggression observed across sessions in each condition of the functional analysis for each participant.
Two boys exhibited aggression that was maintained by multiple social variables, one boy exhibited aggression that was maintained by escape from demands, three boys exhibited aggression that was maintained by tangible reinforcement and one boy exhibited aggression that was maintained by attention. Aggression was undifferentiated across conditions for one boy, indicating that the outcome was inconclusive for this participant.
Table 3 shows a summary of these data. Taken together, social functions for aggression were identified for 7 of the 8 boys who showed aggression in the functional analyses. The primary topographies of aggression observed were grabbing others (7 of 8 boys) and hitting others (5 of 8 boys).
Table 3.
Results of the functional analyses for the eight boys who showed aggression
| Subject | Topography observed | FA outcome |
|---|---|---|
| P1 | Grabbing, pushing, hitting, cursing | Undifferentiated |
| P2 | Grabbing, pushing, hitting, kicking | Attention, tangible, social escape, transition escape |
| P4 | Grabbing, scratching | Social escape, transition escape |
| P5 | Scratching, hitting | Demand escape |
| P15 | Hitting, biting | Attention |
| P16 | Hitting, kicking, biting, grabbing, spitting | Tangible |
| P17 | Grabbing, pushing | Tangible |
| P20 | Pinching, scratching, grabbing | Tangible |
Discussion
Previous studies examining the influence of social-environmental factors on problem behaviours shown by individuals with FXS have suggested that these behaviours may be maintained by social-environmental variables in the majority of cases. However, in previous studies, the SIB displayed by each participant was combined with other problem behaviours into an aggregated response class in the analyses. Thus, the extent to which SIB may be influenced by social-environmental factors in FXS is unknown. This is also true for aggression in FXS. In the present study, we specifically examined the potential function(s) of SIB (and aggression if it occurred) by conducting separate analyses of the response classes displayed by 22 adolescent boys with FXS. Of the 22 boys who received a functional analysis, 14 (63.6%) boys with FXS displayed SIB in the functional analyses. It is interesting that only eight (36.4%) boys with FXS showed aggressive behaviour in the functional analyses, and only three (13.6%) boys showed aggression and SIB together.
In terms of the functions of SIB identified in the functional analysis, social functions for SIB, primarily in the form of gaining access to tangible items and/or escaping from social/task/transition demands, were identified for 10 (45.5%) boys. Thus, the proportion of participants in which a social function was identified appeared to be lower than that reported in previous studies (e.g. Kurtz et al. 2015). It should be noted that for the three boys who showed both SIB and aggression in the functional analyses (P2, P4 and P16), similar functions for SIB and aggression were identified. For example, P16’s SIB and aggression both appeared to be maintained by access to tangible items.
It is interesting that our results and those of others suggests that in addition to the increased likelihood of escape functions in FXS, tangible reinforcement might be a more common function in FXS than in other populations. Although the reason for this is currently unclear, one potential hypothesis concerns whether individuals with FXS become highly focused on tangible items (e.g. an iPad) given that engaging with a tangible item for a long period of time may be associated with the absence of demands and social interaction.
We refined and extended the FA methodology in a number of ways to ensure that the data were replicable across participants. First, all children were exposed to a social escape condition to systematically evaluate whether problem behaviour was maintained by social escape (Hagopian et al. 2001). Similarly, given that individuals with FXS have been reported to experience difficulty with transitioning from one location to another or between activities (Symonset al. 2003), all children were exposed to a transition escape condition (McCord et al. 2001) to evaluate escape from transitions as a potential function. Interestingly, few boys with FXS showed SIB that was maintained solely by one of those factors. Rather, once problem behaviour occurred, it was likely to continue across the other conditions. It is possible, therefore, that the fixed sequence of condition presentation may not have been optimal. We employed the fixed sequence in order to maximise the motivating operations for problem behaviour (Hammond et al. 2013) and attempted to minimise carryover effects by allowing breaks between sessions and waiting until problem behaviour had ceased before beginning the next session. The fact that problem behaviour rarely occurred in the play condition, even though this condition was always conducted between the transition escape and ignore conditions indicates that participants were sensitive to the environmental manipulations. Future studies need to examine whether children with FXS need longer breaks between conditions or whether a randomised sequence of presentation could have been more appropriate.
Previous studies examining the functions of problem behaviour in individuals with FXS have included very broad categories of problem behaviour (e.g. elopement, screaming and crying) in addition to SIB. To ensure that the potential function(s) of SIB was not obscured by the functions of other problem behaviours in the child’s repertoire, in the present study, we specifically targeted SIB in the functional analysis. We also ensured that all children were exposed to the same number and type of test conditions across participants given that in previous studies, different test conditions were implemented across participants. For example, in the study conducted by Kurtz et al. (2015), there was no test for social escape, and in the study by Machalicek et al. (2014), there was no test for automatic reinforcement. The functional analyses conducted in the present study may therefore have been more comprehensive.
Limitations
There are several potential limitations of the study that should be noted. First, for over a third of participants, SIB did not occur in the functional analyses or occurred at extremely low rates (Kahnget al 2001). For these participants then, the function of SIB was unclear. It should be noted that in clinical studies, including seven conditions per FA is not common practice and may have contributed to a lack of discrimination across conditions in the present study. It is also possible that for the eight individuals who did not show SIB in the functional analyses, higher rates of SIB could have occurred if the functional analyses had been conducted in the child’s natural setting (e.g. home or school) and/or if the child’s primary caregiver had been coached to implement the sessions. Second, we repeated the sequence of conditions at least six to seven times in order to ensure that potential functions could be identified. However, repeating the sequence of conditions in this way may have posed a greater health or safety risk either to the participant and/or the experimenter conducting the functional analyses. We closely monitored behaviour levels during the functional analyses, and although we were ready to discontinue any session that appeared to pose a considerable risk to the participant or experimenter’s health and/or safety, this safety procedure was required in only one case. Third, it is possible that for the three individuals who exhibited both SIB and aggression, providing consequences for aggressive behaviour in each experimental condition may have influenced the occurrence of SIB. One way to examine this possibility would have been to examine whether the behaviours were more likely to co-occur in each condition. Fourth, the sample may not be representative of all boys with FXS who show problem behaviour because we had to exclude some participants who were unable to travel to Stanford University for a two-day visit. Finally, we were not able to offer any direct treatment evaluations for the SIB exhibited by these individuals. For example, treatment evaluations could have been used to further validate the FA results. At the conclusion of their participation, however, all participants were provided with a detailed summary of the assessment results and were encouraged to seek out support from a Board Certified Behaviour Analyst to oversee and carry out treatment recommendations based on the FA results.
A number of behavioural treatment approaches have been conducted to reduce a variety of the symptoms shown by individuals with FXS including problem behaviours (Moskowitz & Jones 2015). Given that the SIB displayed by participants with FXS in the current study were sensitive to social consequences in just under half of cases, it seems critical that individuals with FXS receive environmental support to reduce the likelihood that SIB is inadvertently reinforced. Although care providers may observe an immediate decrease or de-escalation after providing tangible items (toys) following an episode of problem behaviour (suggesting that this strategy is effective), however, repeated exposure to this behaviour-consequence contingency will only serve to strengthen the behaviour and its resistance to extinction. Similarly, SIB in some participants with FXS appeared to be sensitive to negative reinforcement contingencies in the form of escape from nonpreferred tasks. A multitude of function-based treatments for escape-maintained problem behaviour exist, such as functional communication training, differential reinforcement, extinction and noncontingent reinforcement to name a few. However, even when the putative reinforcer is known, significant training and clinical experience may be necessary in order to prescribe specific behavioural interventions (Geiger et al. 2010). In order to optimise treatments for SIB, it is likely that care providers of individuals with FXS will need to receive frequent behavioural support from trained clinical practitioners.
Since the gene for FXS was identified in 1991, there has been a bias toward identifying pharmacological treatments that may be beneficial for decreasing difficult behaviours in this population (Berry-Kravis & Potanos 2004; Hagerman et al. 2009). Several studies have also begun to focus on medications targeted to the ‘downstream’ effects of reduced FMRP, the protein product of the FMR1 gene mutation responsible for causing FXS (Berry-Kravis et al. 2006; Berry-Kravis et al. 2008; Berry-Kravis et al. 2009; Paribello et al. 2010; Jacquemont et al. 2011). Although evidence for the efficacy of these pharmacological treatments, administered in isolation, is extremely limited (Hall 2009), pharmacotherapy remains the most likely treatment modality for individuals with FXS. In the current study, for example, over half of the boys were taking psychoactive medications, primarily in the form of antipsychotics, antidepressants, stimulants and anticonvulsants yet still exhibited significant rates of problem behaviour. The emphasis on conducting pharmacological interventions for FXS is perhaps understandable, particularly given that the potential ‘downstream’ biological pathways that may be involved in FXS are beginning to be targeted with specific pharmacological agents. Indeed, there is limited literature on medication efficacy for SIB in the ID population (Rana et al. 2013). The results of the current study point the way to research examining the impact of social-environmental factors on SIB and function-based treatment for non-responders to medication. It is important, therefore, not to discount the significant role that social-environmental variables may play in the maintenance of these behaviours, even in populations of individuals with known genetic risk for SIB.
Acknowledgements
This research was supported by award number R21 HD072282 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (PI Scott Hall).
References
- Aman MG & Singh NN (1994) Aberrant Behavior Checklist-Community. Slosson Educational Publications Inc. [Google Scholar]
- Beavers GA, Iwata BA & Lerman DC (2013) Thirty years of research on the functional analysis of problem behavior. Journal of Applied Behavior Analysis 46, 1–21. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E & Potanos K (2004) Psychopharmacology in fragile X syndrome–present and future. Mental Retardation and Developmental Disabilities Research Reviews 10, 42–8. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Krause SE, Block SS, Guter S, Wuu J,Leurgans S et al. (2006) Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. Journal of Child and Adolescent Psychopharmacology 16, 525–40. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N et al. (2008) Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. Journal of Developmental and Behavioral Pediatrics 29, 293–302. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J et al. (2009) A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. Journal of Medical Genetics 46, 266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch GS (2001) Evaluating data from behavioural analysis: visual analysis or statistical models? Behavioural Processes 54, 137–54. [DOI] [PubMed] [Google Scholar]
- Geiger KB, Carr JE & Leblanc LA (2010) Function-based treatments for escape-maintained problem behavior: a treatment-selection model for practicing behavior analysts. Behavior Analysis in Practice 3, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A et al. (2009) Advances in the treatment of fragile X syndrome. Pediatrics 123, 378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian LP, Wilson DM & Wilder DA (2001) Assessment and treatment of problem behavior maintained by escape from attention and access to tangible items. Journal of Applied Behavior Analysis 34, 229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS (2009) Treatments for fragile X syndrome: a closer look at the data. Developmental Disabilities Research Reviews 15, 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Debernardis GM & Reiss AL (2006) Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders 36, 935–47. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA & Reiss AL (2008)Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal of Mental Retardation 113, 44–53. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC& Reiss AL (2009) Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry 48, 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Barnett RP & Huffman KM (2016) Problem behavior in adolescent boys with fragile X syndrome: Relative prevalence, frequency and severity. Journal of Intellectual Disability Research 60, 1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JL, Iwata BA, Rooker GW, Fritz JN & Bloom SE (2013) Effects of fixed versus random sequencing during multielement functional analyses. Journal of Applied Behavior Analysis 46, 22–30. [DOI] [PubMed] [Google Scholar]
- Hanley GP, Iwata BA & McCord BE (2003)Functional analysis of problem behavior: a review. Journal of Applied Behavior Analysis 36, 147–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman RL & McGill P (2017) The topographies and operant functions of challenging behaviours in fragile X syndrome: A systematic review and analysis of existing data. Journal of Intellectual & Developmental Disability 42, 190–203. [Google Scholar]
- Hartmann DP (1977) Considerations in the choice of interobserver reliability estimates. Journal of Applied Behavior Analysis 10, 103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M et al. (2002) Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology 27, 855–72. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM & Reiss AL (2004) The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews 10, 17–24. 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Cordeiro L, Koldewyn K,McCormick C, Green C et al. (2008) Brief report: Aggression and stereotypic behavior in males with fragile X syndrome--moderating secondary genes in a “single gene” disorder. Journal of Autism and Developmental Disorders 38, 184–9. [DOI] [PubMed] [Google Scholar]
- Hogg RV, Tanis E & Zimmerman D (2015) Probability and Statistical Inference, 9th edn Pearson Education Inc, Upper Saddle River, NJ. [Google Scholar]
- Iwata BA, Dorsey MF, Slifer KJ, Bauman KE & Richman GS (1994a) Toward a functional analysis of self-injury. Journal of Applied Behavior Analysis 27, 197–209. 10.1901/jaba.1994.27-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata BA, Pace GM, Cowdery GE & Miltenberger RG (1994b) What makes extinction work: an analysis of procedural form and function. Journal of Applied Behavior Analysis 27, 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata BA, Pace GM, Dorsey MF, Zarcone JR,Vollmer TR, Smith RG et al. (1994c) The functions of self-injurious behavior: an experimental-epidemiological analysis. Journal of Applied Behavior Analysis 27, 215–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ et al. (2011) Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Science Translational Medicine 3, 64ra61. [DOI] [PubMed] [Google Scholar]
- Kahng SW, Abt KA & Schonbachler HE (2001)Assessment and treatment of low-rate high-intensity problem behavior. Journal of Applied Behavior Analysis 34, 225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JE & Losh M (2015) Cardiac autonomic regulation in autism and fragile X syndrome: a review. Psychological Bulletin 141, 141–75. 10.1037/a0038237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz PF, Chin MD, Robinson AN, O’Connor JT & Hagopian LP (2015) Functional analysis and treatment of problem behavior exhibited by children with fragile X syndrome. Research in Developmental Disabilities 43–44, 150–66. 10.1016/j.ridd.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Langthorne P & McGill P (2012) An indirect examination of the function of problem behavior associated with fragile X syndrome and Smith-Magenis syndrome. Journal of Autism and Developmental Disorders 42, 201–9. [DOI] [PubMed] [Google Scholar]
- Langthorne P, McGill P, O’Reilly MF, Lang R,Machalicek W, Chan JM et al. (2011) Examining the function of problem behavior in fragile X syndrome: preliminary experimental analysis. American Journal on Intellectual and Developmental Disabilities 116, 65–80. 10.1352/1944-7558-116.1.65. [DOI] [PubMed] [Google Scholar]
- Machalicek W, McDuffie A, Oakes A, Ma M, Thurman AJ, Rispoli MJ et al. (2014) Examining the operant function of challenging behavior in young males with fragile X syndrome: a summary of 12 cases. Research in Developmental Disabilities 35, 1694–704. 10.1016/j.ridd.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Martin N, Oliver C & Hall S (1998) ObsWin: Software for the Collection and Analysis of Observational Data. University of Birmingham, Birmingham. [Google Scholar]
- McCord BE, Thomson RJ & Iwata BA (2001)Functional analysis and treatment of self-injury associated with transitions. Journal of Applied Behavior Analysis 34, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz LJ & Jones EA (2015) Uncovering the evidence for behavioral interventions with individuals with fragile X syndrome. Research in Developmental Disabilities 38, 223–41. [DOI] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM et al. (2010) Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurology 10, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana F, Gormez A & Varghese S (2013) Pharmacological interventions for self-injurious behaviour in adults with intellectual disabilities. Cochrane Database of Systematic Reviews 30, CD009084 10.1002/14651858.CD009084.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roane HS, Fisher WW, Kelley ME, Mevers JL & Bouxsein KJ (2013) Using modified visual-inspection criteria to interpret functional analysis outcomes. Journal of Applied Behavior Analysis 46, 130–46. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB Jr., Hatton DD& Skinner M. (2001) Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology 39, 107–23. [DOI] [PubMed] [Google Scholar]
- Shirley MJ, Iwata BA & Kahng S (1999) False-positive maintenance of self-injurious behavior by access to tangible reinforcers. Journal of Applied Behavior Analysis 32, 201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV & Ball DA (2005) Vineland Adaptive Behavior Scales (2nd ed.). Circles Pines, MN: American Guidance Service. [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M & Bailey DB Jr. (2003) Self-injurious behavior in young boys with fragile X syndrome. American Journal of Medical Genetics. Part A 118, 115–21. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A et al. (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–14. [DOI] [PubMed] [Google Scholar]
- Vollmer TR & Iwata BA (1992) Differential reinforcement as treatment for behavior disorders: procedural and functional variations. Research in Developmental Disabilities 13, 393–417. [DOI] [PubMed] [Google Scholar]
- Vollmer TR, Iwata BA, Zarcone JR, Smith RG & Mazaleski JL (1993) The role of attention in the treatment of attention-maintained self-injurious behavior: noncontingent reinforcement and differential reinforcement of other behavior. Journal of Applied Behavior Analysis 26, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Hazlett HC, Lightbody AA, Reiss AL & Piven J (2013) Repetitive and self-injurious behaviors: associations with caudate volume in autism and fragile X syndrome. Journal of Neurodevelopmental Disorders 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]