Abstract
Purpurogallin, a natural phenol obtained from oak nutgalls, has been shown to possess antioxidant, anticancer, and anti-inflammatory effects. Recently, in addition to ultraviolet B (UVB) radiation that induces cell apoptosis via oxidative stress, particulate matter 2.5 (PM2.5) was shown to trigger excessive production of reactive oxygen species. In this study, we observed that UVB radiation and PM2.5 severely damaged human HaCaT keratinocytes, disrupting cellular DNA, lipids, and proteins and causing mitochondrial depolarization. Purpurogallin protected HaCaT cells from apoptosis induced by UVB radiation and/or PM2.5. Furthermore, purpurogallin effectively modulates the pro-apoptotic and anti-apoptotic proteins under UVB irradiation via caspase signaling pathways. Additionally, purpurogallin reduced apoptosis via MAPK signaling pathways, as demonstrated using MAPK-p38, ERK, and JNK inhibitors. These results indicate that purpurogallin possesses antioxidant effects and protects cells from damage and apoptosis induced by UVB radiation and PM2.5.
Keywords: Purpurogallin, Ultraviolet B radiation, Particulate matter 2.5, Oxidative stress, Human HaCaT keratinocytes
INTRODUCTION
Solar radiation is necessary for the synthesis of vitamin D in humans; however, ultraviolet (UV) radiation has been implicated in numerous skin disorders, including tumorigenesis (Feehan et al., 2016). UVB irradiation has been shown to cause inflammation and skin cancer (Glady et al., 2018; Hosseini et al., 2018) through cellular DNA damage or immunosuppression (Nohynek and Schaefer, 2001). An abnormal increase in intracellular reactive oxygen species (ROS), including the hydroxyl radical, induces destruction of cytoskeleton and apoptosis (Kong et al., 2016; Zheng et al., 2018). Furthermore, excessive ROS levels strain the antioxidant defense systems, resulting in oxidative stress and skin damage (Zheng et al., 2014).
Recently, alongside UVB, particulate matter 2.5 (PM2.5) has become the focus of public health research, including research on skin hazards. PM2.5 represents outdoor air pollution and mainly consists of metals, allergens, toxic products of combustion of fossil fuels and endotoxins (He et al., 2016). PM was shown to damage the nervous system (Wang et al., 2017), respiratory epithelium (Liu et al., 2017), immune system (Castañeda et al., 2018) and cardiovascular system (Cao et al., 2016). As skin and keratinocytes form the outermost barrier directly facing harmful PM, the combined effects of UVB and PM2.5 on the skin are worth investigating.
Phenolic compounds were shown to possess antioxidant effects in cardiovascular diseases, anticancer activity, anti-platelet aggregation effects and anti-bacterial activity (Faggio et al., 2017). Furthermore, high levels of polyphenols promote collagen synthesis and protect human skin from photo-aging (Kang et al., 2018). Purpurogallin (PG), a natural phenol, suppressed delayed vasospasm, and protected cardiac and kidney cells (Zeng et al., 1992; Wu et al., 1996; Chang et al., 2014). Additionally, PG reduced inflammation in BV2 microglia cells and osteolytic diseases and showed antioxidant effects by scavenging hydroxyl radicals (Prasad and Laxdal, 1994; Park et al., 2013; Kim et al., 2018).
UVB and PM2.5 aggravate the damage to keratinocytes and PG may possess cytoprotective effects. Hence, in this study, we explored the antioxidant and cytoprotective effects of PG against UVB-and/or PM2.5-induced oxidative stress in HaCaT cells and investigated the underlying mechanisms.
MATERIALS AND METHODS
Reagents and chemicals
Purpurogallin (PG), Diesel particulate matter NIST SRM 1650b (PM2.5), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2′,7′-dichlorofluorescein diacetate (DCF-DA), Primary antibodies anti-caspase-3, anti-caspase-9, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), Hoechst 33342, caspase inhibitor (Z-VAD-FMK), and p38 MAPK inhibitor (SB203580) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Diphenyl-1-pyrenylphosphine (DPPP) was purchased from Molecular Probes (Eugene, OR, USA). 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbe nzimidazolylcarbocyanine iodide (JC-1) was provided by Invitrogen (Carlsbad, CA, USA). SP600125 and U0126 were purchased from Tocris (Bristol, UK) and Calbiochem (La Jolla, CA, USA), respectively. Primary antibodies anti-Bax, anti-Bcl-2, anti-p38, and anti-PARP were purchased from Santa Cruz Biotechnology Inc (Dallas, TX, USA). Primary antibodies anti-ERK and anti-JNK were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-IgG secondary antibodies were purchased from Pierce (Rockford, IL, USA). All other chemicals and reagents were of analytical grade.
Cell culture and UVB radiation
Human HaCaT keratinocytes were provided by the Amore-Pacific Corporation (Yongin, Korea). Cells were maintained in Dulbecco’s modified Eagle medium (10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B) at 37°C in humidified atmosphere containing 5% CO2 (Life Technologies Co., Grand Island, NY, USA). The UVB energy spectrum (280–320 nm) was supplied by CL-1000M UV Crosslinker (UVP, Upland, CA, USA). UVB irradiation dose was 30 mJ/cm2.
Cell viability
MTT assay was used to assess the cytotoxicity of PG and UVB. Cells (1.0×105 cells/well) were plated into a 24-well plate. After 16 h incubation, the cells were exposed to 2.5, 5, or 10 μM PG or UVB radiation. MTT stock solution (2 mg/ml) was added and the cells were incubated for 4 h to yield formazan crystals, which were dissolved in dimethyl sulfoxide (DMSO). Finally, the absorbance was detected at 540 nm using a scanning multi-well spectrophotometer.
DPPH radical detection
PG (2.5, 5, or 10 μM) was mixed with 0.1 mM DPPH, shaken gently, and kept in the dark for 3 h. Residual DPPH was determined at 520 nm using a spectrophotometer.
Determination of intracellular ROS
The ability of PG to inhibit production of intracellular ROS induced by H2O2 (1 mM) or UVB radiation (30 mJ/cm2) was examined using DCF-DA. Cells (1×105 cells/well) were seeded in a 96-well plate. After 16 h incubation, 2.5, 5, or 10 μM PG was added, the cells were incubated for 1 h, and treated with H2O2 or exposed to UVB radiation. DCF-DA (25 μM) was added to each well and cells were incubated for 10 min. Finally, the fluorescence of 2′,7′-dichlorofluorescein was assessed using a LS-5B spectrofluorometer (Perkin-Elmer, Waltham, MA, USA).
Additionally, cells (1.5×105 cells/well) were seeded for 16 h, treated with 10 μM PG, and exposed to UVB (30 mJ/cm2) and/or PM2.5 (50 μg/ml) at 37°C. Data were collected after staining the cells with DCF-DA (25 μM) for 30 min at 37°C. Imaging analysis of intracellular ROS was conducted using a confocal microscope (Carl Zeiss, Oberkochen, Germany), whereas stained cells were counted using a flow cytometer (Becton Dickinson, Mountain View, CA, USA).
Hydroxyl radical scavenging
Hydroxyl radical scavenging was assessed after the reaction of DMPO with hydroxyl radicals generated in the Fenton reaction (FeSO4+H2O2). An electron spin resonance (ESR) spectrometer was used to detect the resultant DMPO/·OH adduct (Oh et al., 2016). Briefly, 0.3 M DMPO, 10 mM FeSO4, 10 mM H2O2, and 10 μM PG (20 μl each, in phosphate buffer, pH 7.4) were used and the mixture was analyzed (recorded for 1 min). The ESR spectrometer parameters were as follows: central magnetic field 336.8 mT, power 1.00 mW, frequency 9.4380 GHz, modulation width 0.2 mT, amplitude 600, sweep width 10 mT, sweep time 0.5 min, gain 200, time constant 0.03 s and temperature 25°C.
Single-cell gel electrophoresis
The comet assay was used to measure oxidative DNA damage (Fernando et al., 2016). Cells (5×104 cells/well) were seeded in medium with 10 μM PG in a 1 ml microtube for 30 min and treated with UVB (30 mJ/cm2) and/or PM2.5 (50 μg/ml) for another 30 min. After coating with 110 μl of 0.5% low-melting agarose, the cells were immersed in a lysis buffer (2.5 M NaCl, 100 mM Na-EDTA, 10 mM Tris, 1% Trion X-100, and 10% DMSO, pH 10) for 1 h at 4°C. An electrical field (300 mA, 25 V) was used for electrophoresis. Slides were stained with 50 μl of ethidium bromide (10 μg/ml) and analyzed using the Komet 5.5 image analyzer (Andor Technology, Belfast, UK). Percentage of total fluorescence and tail lengths of 50 cells per slide were recorded.
Lipid peroxidation assay
Cells were plated on a four-well chamber slide in the presence of 10 μM PG exposed to UVB (30 mJ/cm2) and/or PM2.5 (50 μg/ml) for 5 h, and stained with DPPP for 30 min in the dark. Images were analyzed using a confocal microscope.
Detection of DNA fragmentation
DNA fragmentation was quantified using a cytoplasmic his-tone-associated DNA fragmentation kit (Roche Diagnostics, Mannheim, Germany).
Protein carbonylation assay
Cells were incubated with 10 μM PG for 1 h and exposed to UVB radiation (30 mJ/cm2) or PM2.5 (50 μg/ml) for 24 h. Protein oxidation was assessed using an OxiselectTM Protein Carbonyl ELISA kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions.
Mitochondrial membrane potential (Δψm) analysis
After treatment with 10 μM PG, the cells were exposed to UVB (30 mJ/cm2) or PM2.5 (50 μg/ml) for 5 h, stained with JC-1 (5 μM), and analyzed using confocal microscopy and high-performance flow cytometry.
Hoechst 33342 staining
Cells were treated with 10 μM PG for 1 h and exposed to UVB radiation (30 mJ/cm2) and/or PM2.5 (50 μg/ml) for 18 h. Additionally, after treatment with Z-VAD-FMK (30 μM), SB203580 (10 μM), SP600125 (10 μM), or U0126 (50 nM) for 1 h, the cells were treated with PG (10 μM) for 1 h and exposed to UVB radiation (30 mJ/cm2) for 18 h. The cells were stained with Hoechst 33342 (20 μM) and DNA-specific fluorescence was visualized using a fluorescence microscope equipped with a Cool SNAP-Pro color digital camera. Nuclear condensation levels were evaluated and apoptotic cells were quantified.
Western blotting
Protein levels were analyzed as previously described (Cha et al., 2014). Membranes with proteins were sequentially incubated with the appropriate primary and secondary antibodies. Protein bands were detected by the Amersham ECL Plus Western Blotting Detection System (GE Healthcare Life Sciences, Amersham, UK).
Statistical analysis
All experiments were performed in triplicate. Data are represented as the mean ± standard error and were analyzed by the Sigma Stat (v12) software (SPSS, Chicago, IL, USA) using Tukey’s test and analysis of variance (ANOVA). p-values <0.05 were considered statistically significant.
RESULTS
PG attenuates UVB-induced ROS generation
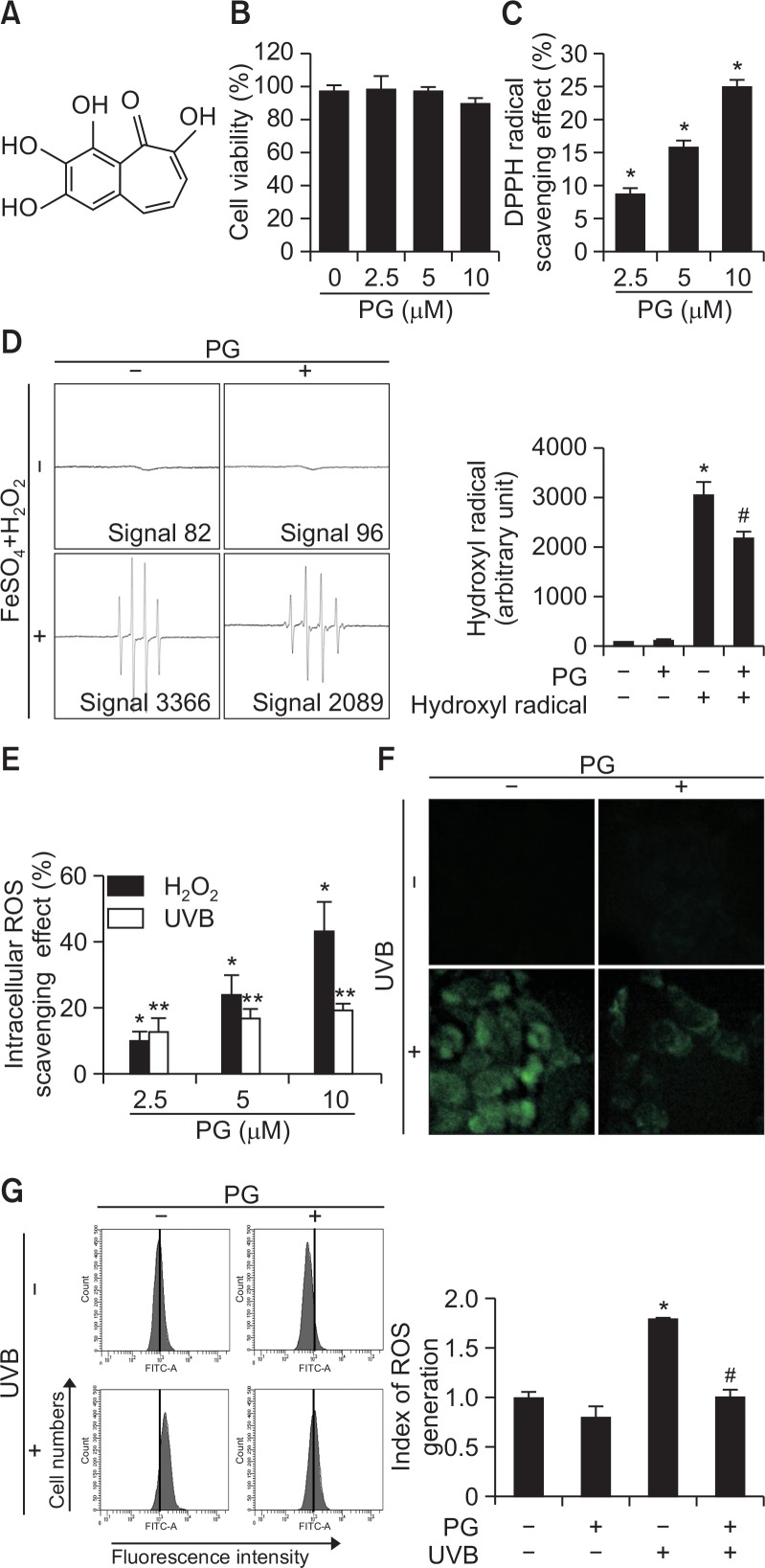
In the MTT assay, PG (Fig. 1A) did not exhibit cytotoxicity to HaCaT cells under various concentrations (Fig. 1B). Cell viability in all treated groups was >96%, similar to control. DPPH radical levels were significantly decreased in a dose-dependent manner in PG-treated groups (Fig. 1C). Hydroxyl radical scavenging potential of PG (10 μM) was evaluated using ESR spectrometry. In FeSO4+H2O2 system, DMPO/·OH adducts signal was reduced from 3366 to 2090 units by PG (Fig. 1D). In addition, DCF-DA assay indicated that H2O2- and UVB-induced intracellular ROS were scavenged by PG (Fig. 1E). Fluorescence spectrometry suggested that PG scavenged intracellular ROS in a concentration-dependent manner in H2O2-and UVB-treated cells, with 10 μM PG scavenging effect up to 42% and 19% ROS in these two cell groups respectively, compared to control. Confocal microscopy images (Fig. 1F) and flow cytometry (Fig. 1G) revealed that PG (10 μM) reduced fluorescence intensity induced by UVB radiation, indicating that PG treatment inhibits ROS generation. Taken together, these data demonstrate that PG possesses ROS-scavenging properties.
Fig. 1.
PG reduced ROS generation. (A) Chemical structure of purpurogallin. (B) The MTT assay was used to determine cell viability after treating HaCaT cells with PG (0, 2.5, 5, 10 μM) for 24 h. (C) DPPH radical scavenging activity of PG (0, 2.5, 5, 10 μM). (D) Hydroxyl radical scavenging potential of PG (10 μM) was estimated using the Fenton reaction. *p<0.05 vs. control cells, #p<0.05 vs. hydroxyl radical. (E) Intracellular ROS scavenging potential of PG (2.5, 5, 10 μM). ROS generated by H2O2 or UVB were detected using the DCF-DA assay. *p<0.05 vs. H2O2-treated cells, **p<0.05 vs. UVB-irradiated cells. (F) Confocal microscopy and (G) flow cytometry were used for detecting intracellular ROS after DCF-DA staining. *p<0.05 vs. control cells, #p<0.05 vs. UVB-irradiated cells.
PG protects cellular macromolecules from UVB-induced damage
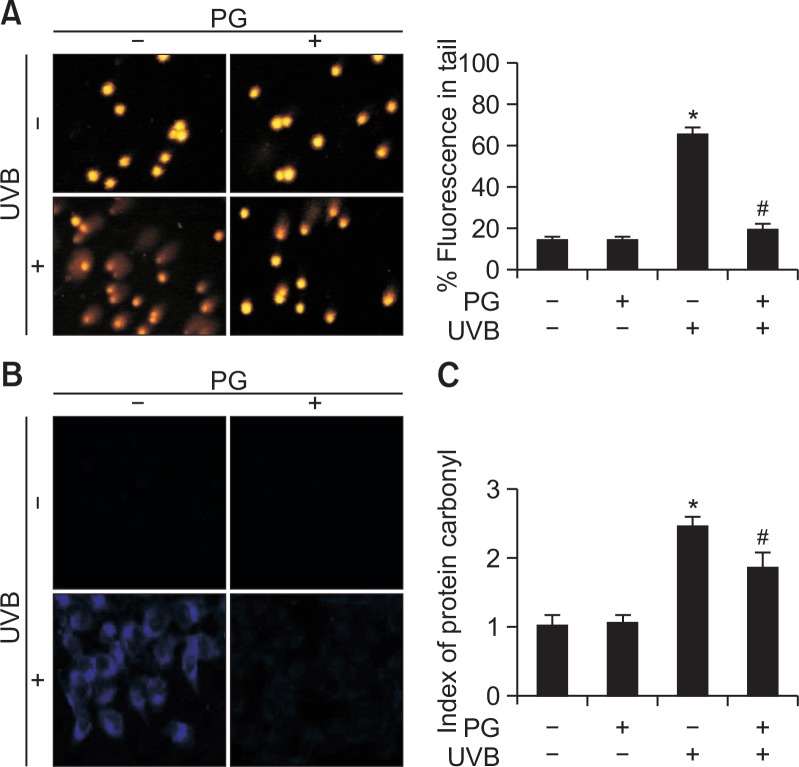
The protective effects of PG on UVB-induced DNA damage were evaluated by using the comet assay (Fig. 2A). The length of comet tails and percentage of tail fluorescence were significantly reduced in cells pretreated with PG compared to cells exposed to UVB (from 65 to 22%). Lipid peroxidation was analyzed using fluorescent DPPP oxide (Fig. 2B). The fluorescence intensity of DPPP oxide was higher in UVB-exposed cells, compared to that in cells treated with PG before UVB exposure. Furthermore, protein oxidation was assessed using the protein carbonylation assay (Fig. 2C). UVB irradiation significantly increased protein carbonylation, this effect ameliorated by PG pretreatment. In general, these data indicate that PG effectively blocked UVB-induced damage to macromolecules, including DNA fragmentation and oxidation of proteins and lipids.
Fig. 2.
PG protected cells from UVB-induced damage to macromolecules. (A) The comet assay was used to detect DNA damage. (B) Confocal microscopy was used for detecting lipid peroxidation after DPPP (blue) staining. (C) Protein carbonylation was determined using a protein carbonyl ELISA kit. *p<0.05 vs. control, #p<0.05 vs. UVB-irradiated cells.
PG suppresses UVB radiation-induced apoptosis
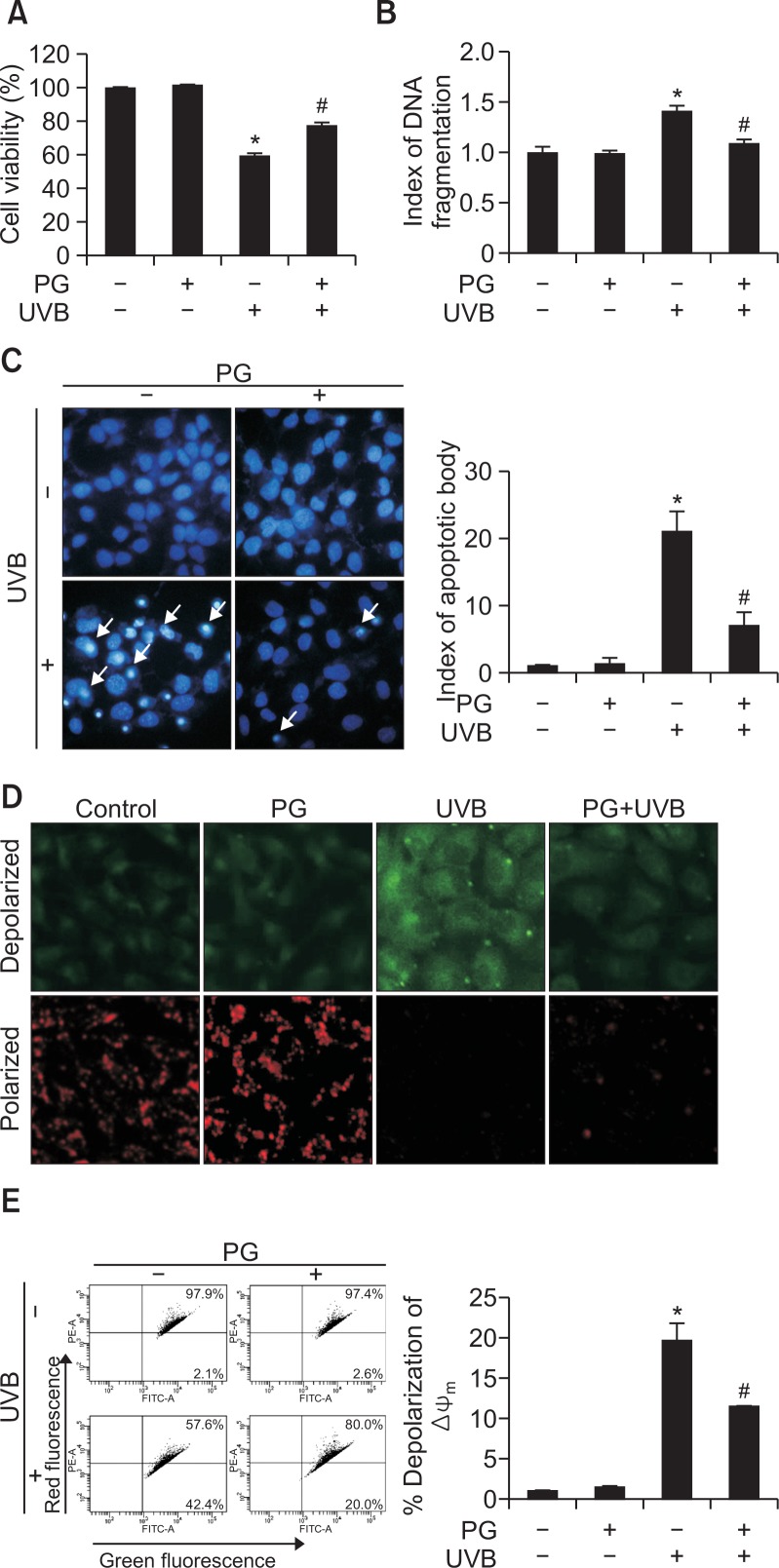
Cell viability after UVB irradiation was also assessed (Fig. 3A). Compared to viability of control cells (100%), cell viability was reduced upon exposure to UVB (59%), an effect ameliorated by PG pretreatment (77%). These findings suggest that PG protected cells from UVB-induced cell death. Similarly, nuclear fragmentation of PG-pretreated cells was significantly reduced, indicating that PG alleviated UVB-induced DNA fragmentation (Fig. 3B). Additionally, Hoechst 33342-stained apoptotic cells in UVB-irradiated group (Fig. 3C) displayed massive nuclear condensation; however, formation of apoptotic bodies was reduced upon PG pretreatment. JC-1 staining was used to explore apoptosis caused by disrupted mitochondrial membrane potential, with red and green fluorescence representing aggregates and monomers of the JC-1 dye, respectively. Mitochondrial depolarization resulted in intense green fluorescence in UVB-exposed cells, which was attenuated by PG treatment (Fig. 3D). Flow cytometry was used to count apoptotic bodies (Fig. 3E). UVB-irradiated cells contained the highest fraction of apoptotic cells among cell groups tested; PG pretreatment decreased the percentage of apoptotic cells from 42 to 20%. These observations suggest that PG protects cells from UVB-induced apoptosis.
Fig. 3.
PG protected cells from UVB-induced apoptosis. (A) HaCaT cell viability after UVB radiation was assessed using the MTT assay. (B) DNA fragmentation was assessed using a cellular DNA fragmentation ELISA kit. (C) Fluorescence microscopy detected apoptotic cells (arrows), stained with Hoechst 33342. The Δψm was evaluated after JC-1 staining by (D) confocal microscopy and (E) flow cytometry. *p<0.05 vs. control, #p<0.05 vs. UVB-irradiated cells.
PG regulates UVB-induced cell death through caspase and MAPK signaling pathways
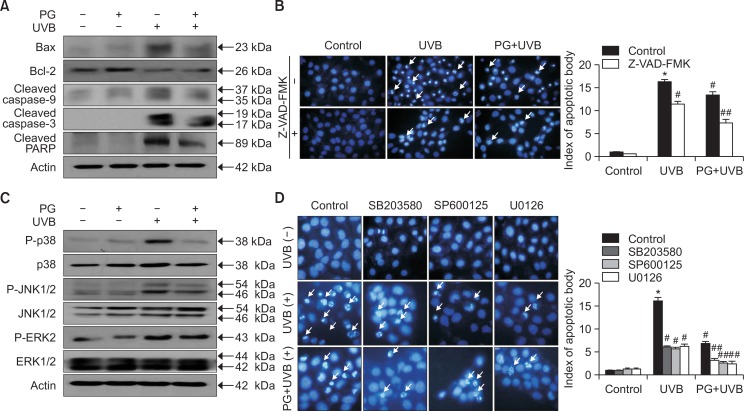
Western blotting was used to analyze the mechanisms of PG cytoprotective effects (Fig. 4A). Levels of pro-apoptotic protein Bax were increased by UVB and decreased by PG treatment. In contrast, the levels of anti-apoptotic protein Bcl-2 were decreased by UVB and increased by PG treatment. Formation of cleaved caspase-9 and caspase-3 was stimulated by UVB exposure but reduced in PG pretreated group. Similarly, UVB irradiation induced cleavage of PARP fragments, a target of caspase-3, whereas in PG-pretreated group, levels of cleaved PARP were notably lower than levels observed in the UVB-exposed group. Apoptotic bodies in cell groups pretreated with an irreversible caspase inhibitor (Z-VAD-FMK) and/or PG decreased considerably, and PG contributing to the effects of the caspase inhibitor (Fig. 4B). To further explore signaling pathways involved in PG-mediated modulation of apoptosis, p38 MAPK, JNK, ERK and their phosphorylated forms were detected by western blot (Fig. 4C). As expected, UVB irradiation increased the levels of phosphorylated p38 MAPK, JNK, and ERK, compared to levels in cells not exposed to UVB; however, this effect was reversed by PG pretreatment. In addition, apoptotic bodies were detected by Hoechst 33342 staining (Fig. 4D). Cells pretreated with p38 MAPK, JNK, and ERK inhibitors SB203580, SP600125, and U0126, respectively, also displayed a decreased number of UVB-induced apoptotic bodies, similar to cells pretreated with PG. Furthermore, PG enhanced the anti-apoptotic effects of these inhibitors. These results demonstrate that PG activates caspase and MAPK signaling pathways and prevents UVB-induced apoptosis by regulating apoptosis-associated proteins.
Fig. 4.
PG inhibited UVB-induced apoptosis by regulation of caspase and MAPK signaling pathways. (A) Protein levels of Bax, Bcl-2, caspase-9, caspase-3, and PARP were assessed using western blotting. Actin was used as loading control. (B) Apoptotic cells pretreated with the caspase inhibitor Z-VAD-FMK and/or PG were stained with Hoechst 33342. (C) PG prevented the UVB-induced phosphorylation of p38 MAPK, JNK, and ERK, as shown by western blot. (D) Analysis of Hoechst 33342-stained apoptotic cells, after treatment with SB203580, SP600125, and U0126, which inhibit p38 MAPK, JNK and ERK, respectively. *p<0.05 vs. control, #p<0.05 vs. UVB-irradiated cells, ##p<0.05 vs. UVB-irradiated and inhibitor-pretreated cells.
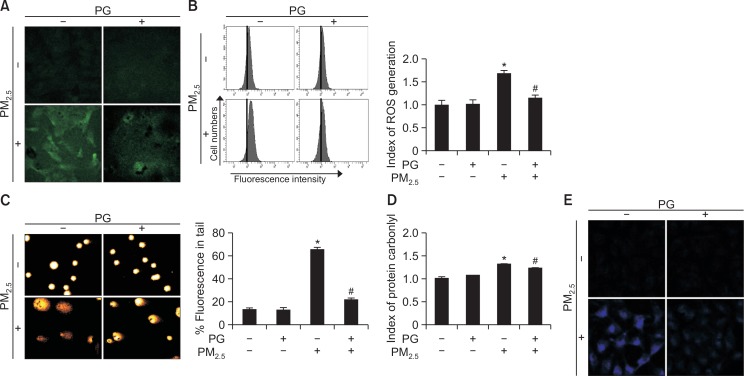
PG attenuates PM2.5-induced oxidative stress and damage to cellular macromolecules
DCF-DA assay (Fig. 5A) and cell numbers (Fig. 5B) revealed that 10 μM PG reduced the levels of PM2.5-generated ROS. As for PM2.5-induced DNA damage, fluorescence and length of tails were significantly reduced in the PG pretreatment group (from 65 to 22%) (Fig. 5C). Additionally, cells pre-treated with PG showed lower levels of protein carbonylation than PM2.5-exposed cells not treated with PG (Fig. 5D). PM2.5 generated higher levels of DPPP oxide in cells not treated with PG, compared to PG-pretreated cells (Fig. 5E). These results suggested that PG suppressed PM2.5-induced ROS generation and protected cellular macromolecules from PM2.5-induced damage.
Fig. 5.
PM2.5 increased ROS generation and caused cellular damage. Intracellular ROS levels were assessed by (A) confocal microscopy and (B) flow cytometry after staining the cells with DCF-DA (green). (C) Comet assay of the DNA damage. (D) Protein carbonylation assay. (E) Lipid peroxidation was assessed after DPPP (blue) staining. *p<0.05 vs. control cells, #p<0.05 vs. PM2.5-exposed cells.
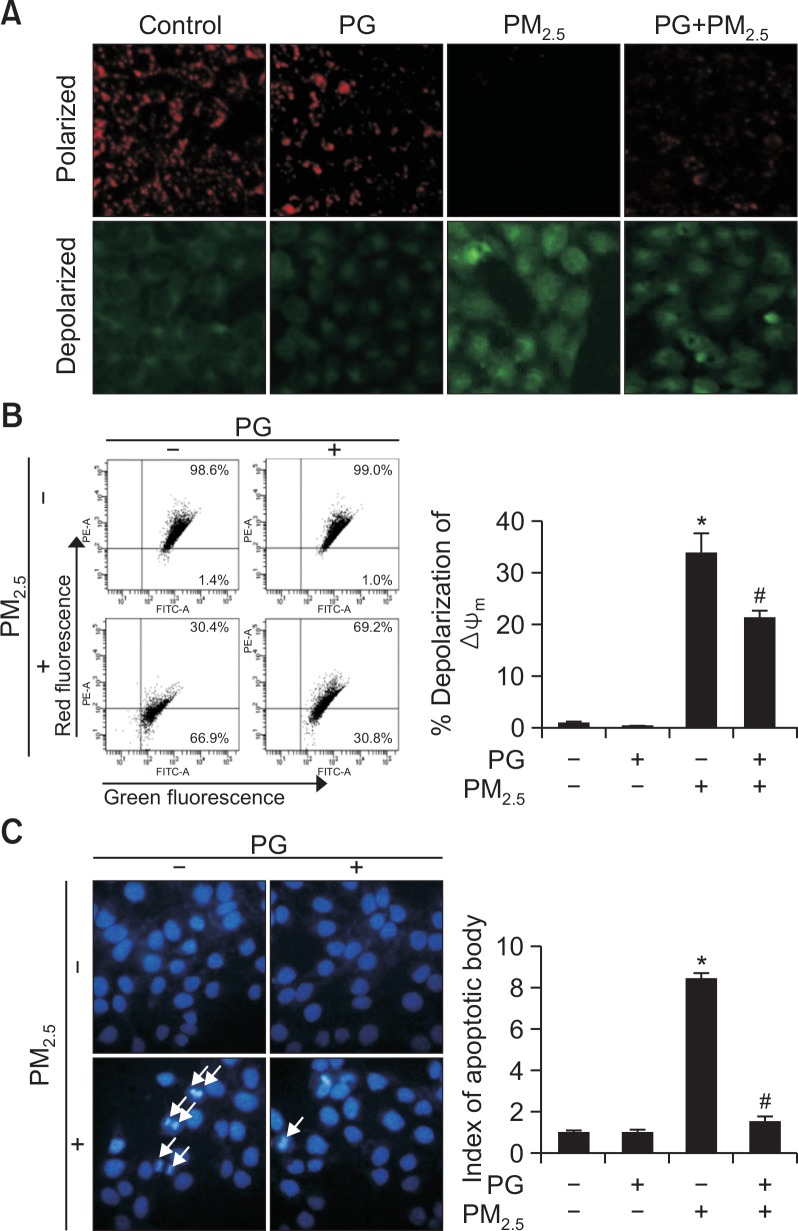
PG blocks PM2.5-induced apoptosis
Using JC-1 staining, normal mitochondrial polarization was observed in PM2.5-free cells, whereas PM2.5-exposed cells showed indications of mitochondrial depolarization (Fig. 6A). Intensity of red and green fluorescence in PG-pretreated cells suggested higher levels of normal mitochondrial polarization and a lower degree of mitochondrial depolarization, compared to mitochondria of the PM2.5-exposed group. Flow cytometry confirmed these observations (Fig. 6B). Additionally, Hoechst 33342 staining of apoptotic bodies indicated that PM2.5-exposed cell group presented the highest number of apoptotic cells, whereas cells pretreated with PG avoided apoptosis to a certain degree (Fig. 6C).
Fig. 6.
PM2.5 causes apoptosis via mitochondrial dysfunction. Cells were stained with JC-1 to detect the mitochondrial membrane potential (Δψm) by (A) confocal microscopy and (B) flow cytometry. (C) Apoptotic bodies (arrows) stained with Hoechst 33342. *p<0.05 vs. control cells, #p<0.05 vs. PM2.5-treated cells.
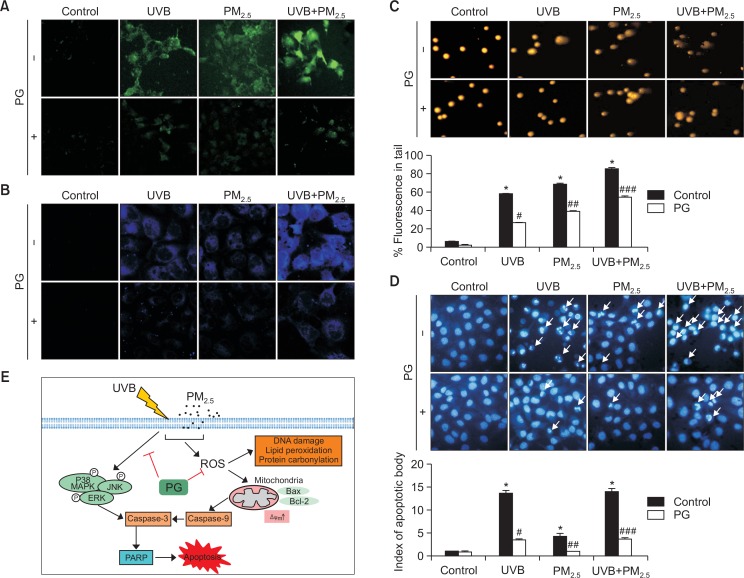
PG protects cells against UVB- and PM2.5-induced apoptosis
In order to confirm that PM2.5 aggravates UVB-induced damage to keratinocytes, intracellular ROS levels were analyzed. Intracellular ROS levels were increased by UVB and/or PM2.5, whereas PG (10 μM) decreased ROS levels induced by UVB and/or PM2.5 (Fig. 7A). Lipid peroxidation was investigated by DPPP staining. PM2.5 combined with UVB induced a high degree of lipid peroxidation, outranking UVB irradiation alone, whereas lipid peroxidation induced by UVB and/or PM2.5 in PG-treated cells was considerably lower (Fig. 7B), indicating that PG ameliorated lipid peroxidation induced by these two factors. UVB- and/or PM2.5-induced DNA damage was analyzed using the comet assay (Fig. 7C). Compared to UVB, PM2.5 prolonged comet tails, whereas PG shortened comet tails in cells treated with UVB and/or PM2.5. Additionally, Hoechst 33342 staining indicated that PM2.5 promoted UVB-induced apoptosis, whereas pretreatment with PG partially protected the cells from UVB- and/or PM2.5-induced apoptosis (Fig. 7D). Taken together, these results suggest that PG possesses cytoprotective effects against UVB- and/or PM2.5-induced oxidative damage and apoptosis.
Fig. 7.
PM2.5 enhanced UVB-induced apoptosis. (A) Cells were stained by DCF-DA (green) for detecting intracellular ROS induced by UVB and/or PM2.5. (B) Lipid peroxidation induced by UVB and/or PM2.5 was detected after DPPP (blue) staining. (C) Comet assay cellular tail lengths induced by UVB and/or PM2.5. (D) Fluorescence microscopy was used to detect Hoechst 33342-stained apoptotic bodies (arrows) induced by UVB and/or PM2.5. *p<0.05 vs. control cells, #p<0.05 vs. UVB-irradiated cells, ##p<0.05 vs. PM2.5-treated cells, ###p<0.05 vs. UVB-irradiated and PM2.5-treated cells. (E) Schematic diagram of the protective mechanism of PG on UVB and PM2.5. PG exerts cytoprotective effects by blocking oxidative-stress-induced damage to cellular components and inhibiting MAPK apoptotic signaling pathway.
DISCUSSION
Skin, the largest human organ, is susceptible to irritation and sunburns through UV exposure. Moreover, epidemiological studies have indicated that UVB suppresses immune reactions, promotes ROS generation, and damages cell membrane proteins and lipids (Boakye et al., 2016). UVB-induced ROS production has also been reported to be a major cause of skin cancer, as it results in the formation of 8-hydroxy-2′-deoxyguanine (Agar et al., 2004). Additionally, PM2.5 (with particle diameter of <2.5 μm) can reach the lungs and stimulate ROS production in the skin, increasing oxidative stress (Piao et al., 2018). High ROS levels disrupt the normal function of endoplasmic reticulum, mitochondria, and lysosomes, leading to apoptosis.
This study evaluated the cytoprotective effects of PG on oxidative stress and apoptosis. Our results show that UVB and PM2.5 increase intracellular ROS production and cause DNA fragmentation, lipid peroxidation, and protein oxidation. In human HaCaT cells, UVB and PM2.5 exposure resulted in dysfunction of mitochondria and a high apoptosis index. Notably, PM2.5 aggravated UVB-induced skin damage (Fig. 7), increasing oxidative stress, damaging DNA, exacerbating lipid peroxidation, and increasing the number of apoptotic bodies. PG pretreatment reduced cellular ROS levels and consequently resulted in fewer apoptotic bodies.
As predominant epidermal cells, keratinocytes protect the organism from external hazards and are inevitably influenced by complex environmental conditions. Apoptotic factors, including caspases-3 and -9 are activated under harmful conditions. Caspase-9 is a necessary factor in initiating apoptosis (Würstle et al., 2012). Furthermore, caspase-9 can directly activate procaspase-3 (Yin et al., 2006), also a key factor of apoptosis (Brentnall et al., 2013). In turn, caspase-3 promotes PARP cleavage (Fulda and Debatin, 2006), preventing PARP from countering DNA damage. As an example, PARP-1 is a nuclear enzyme involved in DNA fragmentation stability and regulates transcription (Rajawat et al., 2017). Anti-apoptotic proteins are also important participants of apoptosis. Bax/Bcl-2 proteins contribute to changes in mitochondrial permeability and Δψm loss (Dlugosz et al., 2006), which protect cells from apoptosis induced by pro-apoptotic Bax/Bak proteins (Reed, 2006). In this study, UVB increased the protein levels of Bax, cleaved caspase-9, cleaved caspase-3, and cleaved PARP and decreased Bcl-2 levels, contributing to apoptosis. In contrast, PG treatment suppressed the increase in protein levels of Bax, cleaved caspase-9, cleaved caspase-3, and cleaved PARP, while increasing Bcl-2 levels and decreasing the number of apoptotic bodies. In order to further explore these mechanisms, we used caspase inhibitor Z-VAD-FMK, which suppressed apoptosis induced by UVB. PG contributed to anti-apoptotic effects of caspase inhibitors, which suggests that PG protects cells from apoptosis through caspase signaling pathways, by regulating the levels of apoptosis-associated proteins.
Additionally, p38 MAPK was previously shown to degrade Bcl-2 (De Chiara et al., 2006) and activate Bax (Kim et al., 2006), resulting in mitochondrial apoptotic cell death (Lee et al., 2008). Therefore, in the current study, we detected the expression of MAPK signaling pathway-associated proteins, including p38, JNK, and ERK. Phosphorylated p38, JNK, and ERK were up-regulated by UVB-irradiation and down-regulated by PG pretreatment. Effects of MAPK signaling pathways were further explored using p38, JNK, and ERK inhibitors. PG pretreatment inhibited phosphorylation of p38, JNK, and ERK, similar to their inhibitors and contributed to reducing the number of apoptotic bodies.
Taken together, these results show that UVB irradiation and PM2.5 contribute to apoptosis and that PG treatment suppresses UVB-induced ROS generation, DNA damage, mitochondrial dysfunction, and protein oxidation. Furthermore, PG pretreatment inhibited UVB-induced cell apoptosis through caspase and MAPK signaling pathways by regulating key proteins participating in these pathways (Fig. 7E). These results suggest that PG could be of potential use in protecting the skin from UVB irradiation and PM2.5.
Acknowledgments
This work was supported by the Basic Research Laboratory Program (NRF-2017R1A4A1014512) using the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP).
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakye CHA, Patel K, Doddapaneni R, Bagde A, Behl G, Chowdhury N, Safe S, Singh M. Ultra-flexible nano-carriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surf. B Biointerfaces. 2016;143:156–167. doi: 10.1016/j.colsurfb.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Qin G, Shi R, Bai F, Yang G, Zhang M, Lv J. Overproduction of reactive oxygen species and activation of MAPKs are involved in apoptosis induced by PM2.5 in rat cardiac H9c2 cells. J Appl Toxicol. 2016;36:609–617. doi: 10.1002/jat.3249. [DOI] [PubMed] [Google Scholar]
- Castañeda AR, Pinkerton KE, Bein KJ, Magaña-Méndez A, Yang HT, Ashwood P, Vogel CFA. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol Lett. 2018;292:85–96. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JW, Piao MJ, Kim KC, Yao CW, Zheng J, Kim SM, Hyun CL, Ahn YS, Hyun JW. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther (Seoul) 2014;22:136–142. doi: 10.4062/biomolther.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CZ, Lin CL, Wu SC, Kwan AL. Purpurogallin, a natural phenol, attenuates high-mobility group box 1 in subarachnoid hemorrhage induced vasospasm in a rat model. Int J Vasc Med. 2014;2014:254270. doi: 10.1155/2014/254270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, Palamara AT, Russo T, Garaci E, Cozzolino F. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, Leber B, Andrews DW. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggio C, Sureda A, Morabito S, Sanches-Silva A, Mocan A, Nabavi SF, Nabavi SM. Flavonoids and platelet aggregation: A brief review. Eur J Pharmacol. 2017;807:91–101. doi: 10.1016/j.ejphar.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Feehan RP, Shantz LM. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem J. 2016;473:2973–2994. doi: 10.1042/BCJ20160471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando PM, Piao MJ, Kang KA, Ryu YS, Hewage SR, Chae SW, Hyun JW. Rosmarinic acid attenuates cell damage against UVB radiation-induced oxidative stress via enhancing antioxidant effects in human HaCaT cells. Biomol. Ther (Seoul) 2016;24:75–84. doi: 10.4062/biomolther.2015.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- Glady A, Tanaka M, Moniaga CS, Yasui M, Hara-Chikuma M. Involvement of NADPH oxidase 1 in UVB-induced cell signaling and cytotoxicity in human keratinocytes. Biochem Biophys Rep. 2018;14:7–15. doi: 10.1016/j.bbrep.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ichinose T, Yoshida S, Shiba F, Arashidani K, Takano H, Sun G, Shibamoto T. Differences in allergic inflammatory responses in murine lungs: comparison of PM2.5 and coarse PM collected during the hazy events in a Chinese city. Inhal Toxicol. 2016;28:706–718. doi: 10.1080/08958378.2016.1260185. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Dousset L, Mahfouf W, Serrano-Sanchez M, Redonnet-Vernhet I, Mesli S, Kasraian Z, Obre E, Bonneu M, Claverol S, Vlaski M, Ivanovic Z, Rachidi W, Douki T, Taieb A, Bouzier-Sore AK, Rossignol R, Rezvani HR. Energy metabolism rewiring precedes UVB-induced primary skin tumor formation. Cell Rep. 2018;23:3621–3634. doi: 10.1016/j.celrep.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Kang CH, Rhie SJ, Kim YC. Antioxidant and skin anti-aging effects of marigold methanol extract. Toxicol Res. 2018;34:31–39. doi: 10.5487/TR.2018.34.1.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim TH, Ihn HJ, Kim JE, Choi JY, Shin HI, Park EK. Inhibitory effect of Purpurogallin on osteoclast differentiation in vitro through the downregulation of c-Fos and NFATc1. Int J Mol Sci. 2018;19:E601. doi: 10.3390/ijms19020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Wang S, Wu X, Zuo F, Qin H, Wu J. Paeoniflorin attenuates ultraviolet B-induced apoptosis in human keratinocytes by inhibiting the ROS-p38-p53 pathway. Mol Med Rep. 2016;13:3553–3558. doi: 10.3892/mmr.2016.4953. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim MS, Park JY, Woo JS, Kim YK. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology. 2008;248:121–129. doi: 10.1016/j.tox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Liu Q, Xu C, Ji GX, Liu H, Shao W, Zhang C, Gu A, Zhao P. Effect of exposure to ambient PM2.5 pollution on the risk of respiratory tract diseases: a meta-analysis of cohort studies. J Biomed Res. 2017;31:130–142. doi: 10.7555/JBR.31.20160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohynek GJ, Schaefer H. Benefit and risk of organic ultraviolet filters. Regul Toxicol Pharmacol. 2001;33:285–299. doi: 10.1006/rtph.2001.1476. [DOI] [PubMed] [Google Scholar]
- Oh MC, Piao MJ, Fernando PM, Han X, Madduma Hewage SR, Park JE, Ko MS, Jung U, Kim IG, Hyun JW. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol. Ther (Seoul) 2016;24:616–622. doi: 10.4062/biomolther.2016.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kim TH, Kim CG, Kim GY, Kim CM, Kim ND, Kim BW, Hwang HJ, Choi YH. Purpurogallin exerts anti-inflammatory effects in lipopolysaccharide-stimulated BV2 microglial cells through the inactivation of the NF-κB and MAPK signaling pathways. Int J Mol Med. 2013;32:1171–1178. doi: 10.3892/ijmm.2013.1478. [DOI] [PubMed] [Google Scholar]
- Piao MJ, Ahn MJ, Kang KA, Ryu YS, Hyun YJ, Shilnikova K, Zhen AX, Jeong JW, Choi YH, Kang HK, Koh YS, Hyun JW. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol. 2018;92:2077–2091. doi: 10.1007/s00204-018-2197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Laxdal VA. Evaluation of hydroxyl radical-scavenging property of purpurogallin using high pressure liquid chromatography. Mol Cell Biochem. 1994;135:153–158. doi: 10.1007/BF00926518. [DOI] [PubMed] [Google Scholar]
- Rajawat J, Shukla N, Mishra DP. Therapeutic targeting of poly(ADP-Ribose) polymerase-1 (PARP1) in cancer: current developments, therapeutic strategies, and future opportunities. Med Res Rev. 2017;37:1461–1491. doi: 10.1002/med.21442. [DOI] [PubMed] [Google Scholar]
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiong L, Tang M. Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol. 2017;37:644–667. doi: 10.1002/jat.3451. [DOI] [PubMed] [Google Scholar]
- Wu TW, Zeng LH, Wu J, Fung KP, Weisel RD, Hempel A, Camerman N. Molecular structure and antioxidant specificity of purpurogallin in three types of human cardiovascular cells. Biochem Pharmacol. 1996;52:1073–1080. doi: 10.1016/0006-2952(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Würstle ML, Laussmann MA, Rehm M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res. 2012;318:1213–1220. doi: 10.1016/j.yexcr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Yin Q, Park HH, Chung JY, Lin SC, Lo YC, da Graca LS, Jiang X, Wu H. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol Cell. 2006;22:259–268. doi: 10.1016/j.molcel.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Wu TW. Purpurogallin is a more powerful protector of kidney cells than Trolox and allopurinol. Biochem Cell Biol. 1992;70:684–690. doi: 10.1139/o92-104. [DOI] [PubMed] [Google Scholar]
- Zheng J, Piao MJ, Kim KC, Yao CW, Cha JW, Shin JH, Yoo SJ, Hyun JW. Photo-protective effect of americanin B against ultraviolet B-induced damage in cultured human keratinocytes. Environ Toxicol Pharmacol. 2014;38:891–900. doi: 10.1016/j.etap.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang B, Si M, Zou H, Song R, Gu J, Yuan Y, Liu X, Zhu G, Bai J, Bian J, Liu Z. Zearalenone altered the cytoskeletal structure via ER stress-autophagy- oxidative stress pathway in mouse TM4 Sertoli cells. Sci Rep. 2018;8:3320. doi: 10.1038/s41598-018-21567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]