Abstract
Primary cilia and autophagy are two distinct nutrient-sensing machineries required for maintaining intracellular energy homeostasis, either via signal transduction or recycling of macromolecules from cargo breakdown, respectively. Potential correlations between primary cilia and autophagy have been recently suggested and their relationship may increase our understanding of the pathogenesis of human diseases, including ciliopathies and cancer. In this review, we cover the current issues concerning the bidirectional interaction between primary cilia and autophagy and discuss its role in cancer with cilia defect.
Keywords: Cilia, Autophagy, Ciliopathy, Cancer
INTRODUCTION
Primary cilia are nonmotile, antenna-like organelles derived from the centrioles after cell division, and are therefore closely related to cell cycle control. These structure sense extracellular stimuli, including growth factors and mechanical stress, via multiple receptors clustered along the ciliary membrane. They are also known to contribute to metabolic regulation (Delaine-Smith et al., 2014). Autophagy is an intracellular process required for maintaining energy homeostasis, whereby damaged proteins are removed and recycled. This process is generally inhibited by the energy-sensing mTORC1 (mammalian target of rapamycin complex 1) under fed conditions, and is triggered by cellular stresses, including serum deprivation (Glick et al., 2010).
Potential correlations between primary cilia and autophagy have been recently suggested and an increasing number of studies are attempting to identify its specific molecular mechanisms (Cloonan et al., 2014; Pampliega and Cuervo, 2016; Avalos et al., 2017). Herein, we summarize the current issues regarding the bidirectional interplay between ciliogenesis and autophagy, and discuss its pathophysiological implications.
PRIMARY CILIA
Primary cilia are finger-like organelles protruding from the apical membrane of many mammalian cells. Non-motile primary cilia were considered to be ancient cellular organelles that lack a specific biological function. However, recent studies have reported that various signaling proteins and channels are localized to ciliary membranes and respond to diverse stimuli such as mechanical stress (Delaine-Smith et al., 2014; Battle et al., 2015) and various signaling molecules from the extracellular environment (Malicki and Johnson, 2017; Song et al., 2018). The biological significance of primary cilia is exemplified by the fact that functional or structural defects in the primary cilia in mice results in pathological phenotypes known as ciliopathies, such as cystic disease (Jonassen et al., 2008, 2012), cancer (Menzl et al., 2014; Jenks et al., 2018; Higgins et al., 2019), obesity (Volta and Gerdes, 2017; Ritter et al., 2018), blindness (Servattalab et al., 2012; Wheway et al., 2014), polydactyly (Taylor et al., 2015; Agbu et al., 2018), left-right asymmetry defects (Okada et al., 1999; Dasgupta and Amack, 2016), skeletal abnormalities (Xiao and Quarles, 2010), and neurological impairment (Youn and Han, 2018). Based on this evidence, primary cilia have emerged as signaling hubs involved in the regulation of diverse cell signaling.
Primary cilia consist of dynamic microtubule-based axoneme regulated by a precise mechanism called ciliogenesis (Taschner et al., 2012). Key proteins during ciliogenesis are the intraflagellar transport (IFT) particles, which are protein complexes that move bidirectionally along the ciliary axoneme (Taschner et al., 2012; Lechtreck, 2015). In addition to IFT complexes, cell cycle regulators are involved in ciliogenesis (Pugacheva et al., 2007; Plotnikova et al., 2012), with primary cilia typically assembling in response to quiescence (G1/G0 phase) and disassembling upon cell cycle re-entry (Plotnikova et al., 2009; Basten and Giles, 2013). Interestingly, multiple lines of research have revealed that autophagy (Tang et al., 2013), the ubiquitin-proteasome system (Kasahara et al., 2014), actin remodeling factors such as LIM kinase 2 (LIMK2), testicular protein kinase (TESK1) (Kim et al., 2015b), and serine/threonine kinases such as intestinal cell kinase (ICK) (Chaya et al., 2014; Moon et al., 2014) are critical for the maintenance and function of primary cilia. More recently, among these various regulators of ciliogenesis or ciliary function, interest in the bidirectional interaction between primary cilia and autophagy is increasing (Pampliega and Cuervo, 2016; Wiegering et al., 2019). Indeed, it has been reported that cells with defective cilia show reduced autophagy (Pampliega et al., 2013) and dysfunction of ICK known as ciliary protein leads to perturbation of ciliary signaling and autophagy (Tong et al., 2017), which suggests that there is a close relationship between primary cilia and autophagy.
AUTOPHAGY
Autophagy is a highly conserved intracellular process in which misfolded or damaged proteins are sequestered into double membrane-bound vesicles named autophagosomes. More than 30 autophagy-related (Atg) genes are sequentially involved in autophagosome formation, and once formed, these vesicles fuse with lysosomes so that their engulfed cargo can be degraded by hydrolytic enzymes. Autophagy eliminates harmful intracellular proteins and recycles the functional macromolecular components, helping to ensure the maintenance of cellular homeostasis (Glick et al., 2010).
The stepwise process of autophagy is divided into four stages: initiation, vesicle nucleation, elongation of autophago-some, and fusion with a lysosome (Mizushima et al., 2011; Stanley et al., 2014). It initially starts with the accumulation of the ULK1/2 (Unc-51 like autophagy activating kinase 1/2) complex (ULK1/2-ATG13-FIP200), which is normally inhibited by the energy-sensing mTORC1 under fed conditions. mTORC1 inactivation, under autophagy-related stimuli including nutrient deprivation, in turn de-phosphorylates ULK1/2 and ATG13 and enhances the interaction between them. The active ULK1/2 complex translocates to cytosolic membrane structures where the phagophore membrane is possibly derived (Alers et al., 2012). Beclin 1, which is phosphorylated by ULK1, is one of the core proteins that initiate vesicle nucleation. It forms a complex with VPS34 (phosphatidylinositol 3-kinase catalytic subunit type 3) and subsequently interacts with co-activators (i.e., Vps15, UVRAG (UV radiation resistance associated gene) and Bif-1 (SH3 domain containing GRB2 like, endophilin B1)) to generate phosphatidylinositol-3-phosphate (Ptdlns3P), which recruits other ATG proteins to grow the autophagosomal membrane from the phagophore (Kihara et al., 2001). During vesicle elongation, the microtubule-associated protein 1A/1B-light chain 3 (LC3) is processed to an active lipid-conjugated form, which allows for its incorporation into the autophagosomal membrane. Specific ATG proteins including ATG3, ATG4, the ATG5/ATG12/ATG16L complex, and ATG7 are sequentially involved in this stage (Nakatogawa et al., 2007). As the autophagosome becomes enclosed and completely matured, it fuses with lysosomes, which then allows for the proteolytic degradation of engulfed cargo proteins (Zhao and Zhang, 2019).
INTERPLAY BETWEEN AUTOPHAGY AND PRIMARY CILIA
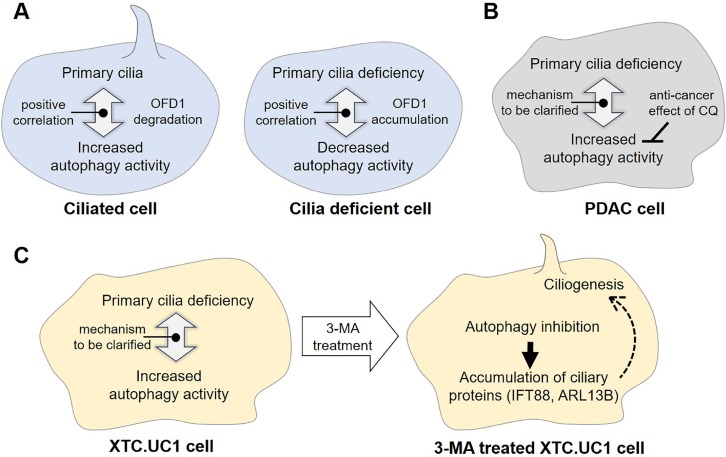
Autophagy and ciliogenesis occur concurrently under serum deprivation and both are involved in maintaining intracellular energy balance, which suggests that the two might be linked. The first two studies identifying interplay between autophagy and ciliogenesis were published in 2013. One study found that autophagy regulated a cilia-related protein Oral-facial-digital syndrome 1 protein (OFD1, centriole and centriolar satellite protein). The authors identified that a centriolar satellite protein OFD1 negatively regulates ciliogenesis, and under fasted conditions, autophagy eliminates it (Tang et al., 2013) (Fig. 1A). The second study showed that impaired autophagic flux as well as reduced ciliogenesis occurred following serum withdrawal in cilia-defect models. In addition, a large number of ATG proteins turned out to be localized either to the basal body, where a cilium is primarily nucleated, or along the ciliary axoneme, suggesting a potential cilia-mediated autophagy initiation mechanism (Pampliega et al., 2013). Studies to further identify the functional relationship between autophagy and ciliogenesis are currently underway.
Fig. 1.
Controversial interplay between primary cilia and autophagy and effect of autophagy inhibitors in cancer cells. (A) Proposed model for the positive correlation between primary cilia and autophagy involving OFD1 protein in normal cells (B) Pancreatic ductal adenocarcinoma (PDAC) cells with increased autophagy activity despite absence of primary cilia. In this cancer cell, the autophagy inhibitor, chloroquine (CQ), shows therapeutic effect. (C) XTC.UC1 cells derived from thyroid Hürthle cell carcinoma with increased autophagy activity despite decreased frequency of ciliated cells. In this cancer cell, the autophagy inhibitor, 3-MA, increases frequency of ciliated XTC.UC1 cells via accumulation of ciliary proteins, such as IFT88 and ARL13B.
Early approaches attempted to determine whether the two processes were concurrently triggered following a common stimulus, and whether they influenced each other. Bidirectional regulation between autophagy and cilia was indeed observed, with impaired autophagic flux in cells with cilia defects as well as shorter cilia led by autophagy inhibition (Wang et al., 2015). Several studies also demonstrated either genetic or chemical inhibition of autophagy attenuated cilia growth that was stimulated by chemicals including Sertraline, BIX01294, and Mefloquine in retinal pigment epithelium (RPE) cells (Kim et al., 2015a; Shin et al., 2015a, 2015b). These studies demonstrated positive correlations between autophagy and cilia growth; however, other studies have made contradictory findings. Using mouse embryo fibroblast 3T3-L1 cells, cilia were shortened by histone deacetylase 6 (HDAC6)-mediated autophagy via decreasing the expression of ciliary proteins such as IFTs and KIF3a (kinesin family member 3A) (Xu et al., 2016). Similarly, downregulation of the HDAC6-autophagy pathway was involved in cilia growth promoted by type I collagen, which provides mechanical strength to modulate cellular morphology or shape (Xu et al., 2018). More recently, studies to identify specific mediators regulating the autophagy-ciliogenesis axis were attempted. PPARA (peroxisome proliferator activated receptor alpha) was identified as one of these potential meditators, as it was found to positively regulate ciliogenesis, which was changed by drugs or genetic manipulations that targeted autophagy. In vivo data showing impaired autophagy as well as kidney damage, commonly observed in ciliopathies, in Ppara−/− mice was further evidence for the involvement of autophagy in ciliogenesis (Liu et al., 2018). In addition, another group suggested Gli2 as a link between primary cilia-dependent cell cycle control and autophagy, in which Gli2 repressed Ofd1-eliminating autophagy under serum deprivation (Hsiao et al., 2018).
Taken together, these studies indicate that there is a substantial link between autophagy and ciliogenesis, in which impaired autophagy leads to a ciliary defect and vice versa. However, further studies are still required to identify the specific molecular mechanisms.
CONTROVERSIAL INTERPLAY BETWEEN PRIMARY CILIA AND AUTOPHAGY IN CANCER
Multiple human cancers, including melanoma (Zingg et al., 2018), renal cell carcinoma (Basten et al., 2013), pancreatic cancer (Seeley et al., 2009a; Kobayashi and Itoh, 2017), and breast cancer (Menzl et al., 2014; Nobutani et al., 2014), are accompanied by primary ciliary defects (Yuan et al., 2010) and dysregulated autophagy (Wang et al., 2018). The relationship between primary cilia and autophagy still requires further studies; however, primary cilia are generally known to have a positive effect on autophagy regulation (Pampliega et al., 2013; Wang et al., 2015) (Fig. 1A). If this is true, how is autophagy regulated in cilia-deficient cancer models and what are the effects of autophagy regulators in cilia-defective cancer models?
It can be speculated that most cancer cells that do not have primary cilia have lower autophagic activity. Indeed, autophagy was suppressed in renal cell carcinoma (RCC) cell lines (Wang et al., 2018) with decreased ciliated frequency (Basten et al., 2013). However, many research groups have reported that autophagy has a dual function as both a tumor suppressor and tumor promoter, depending on the cancer subtype and development/progression stage (White, 2015; Zhi and Zhong, 2015). In this context, it is interesting that many cancer cells do not have primary cilia and yet they display differences in their autophagic activities, which indicates that the correlation between primary cilia and autophagy is still unclear in these cancer models. An example of this comes from a study where the effect of autophagy repression on cancer cells was investigated. In that study chloroquine (CQ), an inhibitor of late stage of autophagy (Mauthe et al., 2018), was used in various human cancer models. Among these cancers, the pancreatic ductal adenocarcinoma (PDAC) cell line and its primary tumor display increased autophagic activities (Yang et al., 2011) despite the absence of primary cilia (Fig. 1B) (Seeley et al., 2009b; Kobayashi and Itoh, 2017). Thus, even though primary cilia are suspected to have a positive effect on autophagy, the PDAC model indicates that there must be a cilia-independent mechanism for autophagy regulation (Fig. 1B). In the study with the PDAC cell lines, CQ treatment reduced growth and tumorigenesis (Yang et al., 2011), suggesting that even if cancer cells do not have primary cilia, autophagy inhibition can show some anti-cancer effects (Fig. 1B). In addition to study of PDAC model, there is another research showing this controversial interplay in thyroid cancer (Lee et al., 2016). XTC. UC1 cells derived from thyroid Hürthle cell carcinoma show higher activity of autophagy even though these cells display decreased frequency of ciliated cells compared with that in controls (Fig. 1C) (Lee et al., 2016). Interestingly, pharmacological inhibition of autophagosome formation of XTC.UC1 cells using 3-MA treatment increases ciliogenesis via restoring expression of ciliary proteins, IFT88 and ARL13B (Fig. 1C) (Lee et al., 2016). Likewise, cilia and autophagy seem to be related to each other in cancer, but may not be applied to cancer models with positive correlation observed in normal cells. Therefore, further studies are needed to reveal underlying regulatory mechanism between primary cilia and autophagy in various cancer models.
CONCLUSIONS
In the last few years, the interplay between primary cilia and autophagy has been an active area of research (Hsiao et al., 2018; Struchtrup et al., 2018; Takahashi et al., 2018), and several studies have indicated that there is biological interaction between these two entities. Because primary cilia and autophagy have various cellular functions (Cao and Zhong, 2015), the interaction of these two regulatory mechanism will provide critical evidences to help understand disease pathogenesis. However, the functional significance of primary cilia on autophagy and vice versa remains controversial. There is a bidirectional interplay between primary cilia and autophagy, but more studies are needed to explain this complicated connection. This is especially true in more complex diseases models such as cancer, where the interplay between primary cilia and autophagy is not as clear. Further studies are needed to investigate the regulatory mechanism between primary cilia and autophagy in disease models, as they may provide new therapeutic approaches of ciliopathies, including cancer.
Acknowledgments
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (2016R1A6A3A11932842, 2016R1A2A1A050 05295, 2016R1A5A1011974).
REFERENCES
- Agbu SO, Liang Y, Liu A, Anderson KV. The small GTPase RSG1 controls a final step in primary cilia initiation. J Cell Biol. 2018;217:413–427. doi: 10.1083/jcb.201604048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos Y, Pena-Oyarzun D, Budini M, Morselli E, Criollo A. New roles of the primary cilium in autophagy. Biomed Res Int. 2017;2017:4367019. doi: 10.1155/2017/4367019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten SG, Giles RH. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia. 2013;2:6. doi: 10.1186/2046-2530-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten SG, Willekers S, Vermaat JS, Slaats GG, Voest EE, van Diest PJ, Giles RH. Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia. 2013;2:2. doi: 10.1186/2046-2530-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle C, Ott CM, Burnette DT, Lippincott-Schwartz J, Schmidt CF. Intracellular and extracellular forces drive primary cilia movement. Proc Natl Acad Sci USA. 2015;112:1410–1415. doi: 10.1073/pnas.1421845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Zhong Q. Cilia in autophagy and cancer. Cilia. 2015;5:4. doi: 10.1186/s13630-016-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaya T, Omori Y, Kuwahara R, Furukawa T. ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J. 2014;33:1227–1242. doi: 10.1002/embj.201488175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan SM, Lam HC, Ryter SW, Choi AM. “Ciliophagy”: the consumption of cilia components by autophagy. Autophagy. 2014;10:532–534. doi: 10.4161/auto.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Amack JD. Cilia in vertebrate left-right patterning. Philos. Trans. R. Soc. Lond., B, Biol Sci. 2016;371:20150410. doi: 10.1098/rstb.2015.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014;28:430–439. doi: 10.1096/fj.13-231894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M, Obaidi I, McMorrow T. Primary cilia and their role in cancer. Oncol Lett. 2019;17:3041–3047. doi: 10.3892/ol.2019.9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CJ, Chang CH, Ibrahim RB, Lin IH, Wang CH, Wang WJ, Tsai JW. Gli2 modulates cell cycle re-entry through autophagy-mediated regulation of the length of primary cilia. J. Cell Sci. 2018;131:jcs221218. doi: 10.1242/jcs.221218. [DOI] [PubMed] [Google Scholar]
- Jenks AD, Vyse S, Wong JP, Kostaras E, Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M, Michaelis M, Cinatl J, Jr, Huang PH, Tanos BE. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep. 2018;23:3042–3055. doi: 10.1016/j.celrep.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183:377–384. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen JA, SanAgustin J, Baker SP, Pazour GJ. Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol. 2012;23:641–651. doi: 10.1681/ASN.2011080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Kawakami Y, Kiyono T, Yonemura S, Kawamura Y, Era S, Matsuzaki F, Goshima N, Inagaki M. Ubiquitin-proteasome system controls ciliogenesis at the initial step of axoneme extension. Nat Commun. 2014;5:5081. doi: 10.1038/ncomms6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclinphosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Shin JH, Park SJ, Jo YK, Kim JS, Kang IH, Nam JB, Chung DY, Cho Y, Lee EH, Chang JW, Cho DH. Inhibition of autophagy suppresses sertraline-mediated primary ciliogenesis in retinal pigment epithelium cells. PLoS ONE. 2015a;10:e0118190. doi: 10.1371/journal.pone.0118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee JK, Heo WD, Kim J. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun. 2015b;6:6781. doi: 10.1038/ncomms7781. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Itoh H. Loss of a primary cilium in PDAC. Cell Cycle. 2017;16:817–818. doi: 10.1080/15384101.2017.1304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF. IFT-cargo interactions and protein transport in cilia. Trends Biochem Sci. 2015;40:765–778. doi: 10.1016/j.tibs.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yi S, Kang YE, Chang JY, Kim JT, Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, Kim KS, Shong M. Defective ciliogenesis in thyroid hurthle cell tumors is associated with increased autophagy. Oncotarget. 2016;7:79117–79130. doi: 10.18632/oncotarget.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZQ, Lee JN, Son M, Lim JY, Dutta RK, Maharjan Y, Kwak S, Oh GT, Byun K, Choe SK, Park R. Ciliogenesis is reciprocally regulated by PPARA and NR1H4/FXR through controlling autophagy in vitro and in vivo. Autophagy. 2018;14:1011–1027. doi: 10.1080/15548627.2018.1448326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki JJ, Johnson CA. The cilium: cellular antenna and central processing unit. Trends Cell Biol. 2017;27:126–140. doi: 10.1016/j.tcb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, Coppes RP, Engedal N, Mari M, Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosomelysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzl I, Lebeau L, Pandey R, Hassounah NB, Li FW, Nagle R, Weihs K, McDermott KM. Loss of primary cilia occurs early in breast cancer development. Cilia. 2014;3:7. doi: 10.1186/2046-2530-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Moon H, Song J, Shin JO, Lee H, Kim HK, Eggenschwiller JT, Bok J, Ko HW. Intestinal cell kinase, a protein associated with endocrine-cerebro-osteodysplasia syndrome, is a key regulator of cilia length and Hedgehog signaling. Proc Natl Acad Sci USA. 2014;111:8541–8546. doi: 10.1073/pnas.1323161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Nobutani K, Shimono Y, Yoshida M, Mizutani K, Minami A, Kono S, Mukohara T, Yamasaki T, Itoh T, Takao S, Minami H, Azuma T, Takai Y. Absence of primary cilia in cell cycle-arrested human breast cancer cells. Genes Cells. 2014;19:141–152. doi: 10.1111/gtc.12122. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell. 1999;4:459–468. doi: 10.1016/S1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Pampliega O, Cuervo AM. Autophagy and primary cilia: dual interplay. Curr Opin Cell Biol. 2016;39:1–7. doi: 10.1016/j.ceb.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol. Biol. Cell. 2012;23:2658–2670. doi: 10.1091/mbc.e11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Pugacheva EN, Golemis EA. Primary cilia and the cell cycle. Methods Cell Biol. 2009;94:137–160. doi: 10.1016/S0091-679X(08)94007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A, Friemel A, Kreis NN, Hoock SC, Roth S, Kielland-Kaisen U, Bruggmann D, Solbach C, Louwen F, Yuan J. Primary cilia are dysfunctional in obese adipose-derived mesenchymal stem cells. Stem Cell Reports. 2018;10:583–599. doi: 10.1016/j.stemcr.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009a;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009b;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servattalab S, Yildiz O, Khanna H. Tackling primary cilia dysfunction in photoreceptor degenerative diseases of the eye. Int. J. Ophthalmic. Pathol. 2012;1:e101. doi: 10.4172/2324-8599.1000e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Bae DJ, Kim ES, Kim HB, Park SJ, Jo YK, Jo DS, Jo DG, Kim SY, Cho DH. Autophagy regulates formation of primary cilia in mefloquine-treated cells. Biomol Ther (Seoul) 2015a;23:327–232. doi: 10.4062/biomolther.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Kim PS, Kim ES, Park SJ, Jo YK, Hwang JJ, Park TJ, Chang JW, Seo JH, Cho DH. BIX-01294-induced autophagy regulates elongation of primary cilia. Biochem Biophys Res Commun. 2015b;460:428–433. doi: 10.1016/j.bbrc.2015.03.050. [DOI] [PubMed] [Google Scholar]
- Song DK, Choi JH, Kim MS. Primary cilia as a signaling platform for control of energy metabolism. Diabetes Metab J. 2018;42:117–127. doi: 10.4093/dmj.2018.42.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley RE, Ragusa MJ, Hurley JH. The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends Cell Biol. 2014;24:73–81. doi: 10.1016/j.tcb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struchtrup A, Wiegering A, Stork B, Ruther U, Gerhardt C. The ciliary protein RPGRIP1L governs autophagy independently of its proteasome-regulating function at the ciliary base in mouse embryonic fibroblasts. Autophagy. 2018;14:567–583. doi: 10.1080/15548627.2018.1429874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nagai T, Chiba S, Nakayama K, Mizuno K. Glucose deprivation induces primary cilium formation through mTORC1 inactivation. J. Cell Sci. 2018;131:jcs208769. doi: 10.1242/jcs.208769. [DOI] [PubMed] [Google Scholar]
- Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, Zhong Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502:254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83:S12–S22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SP, Dantas TJ, Duran I, Wu S, Lachman RS, University of Washington Center for Mendelian Genomics Consortium. Nelson SF, Cohn DH, Vallee RB, Krakow D. Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nat Commun. 2015;6:7092. doi: 10.1038/ncomms8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Park SH, Wu D, Xu W, Guillot SJ, Jin L, Li X, Wang Y, Lin CS, Fu Z. An essential role of intestinal cell kinase in lung development is linked to the perinatal lethality of human ECO syndrome. FEBS Lett. 2017;591:1247–1257. doi: 10.1002/1873-3468.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta F, Gerdes JM. The role of primary cilia in obesity and diabetes. Ann N Y Acad Sci. 2017;1391:71–84. doi: 10.1111/nyas.13216. [DOI] [PubMed] [Google Scholar]
- Wang S, Livingston MJ, Su Y, Dong Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy. 2015;11:607–616. doi: 10.1080/15548627.2015.1023983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZL, Deng Q, Chong T, Wang ZM. Autophagy suppresses the proliferation of renal carcinoma cell. Eur Rev Med Pharmacol Sci. 2018;22:343–350. doi: 10.26355/eurrev_201801_14178. [DOI] [PubMed] [Google Scholar]
- Wheway G, Parry DA, Johnson CA. The role of primary cilia in the development and disease of the retina. Organogenesis. 2014;10:69–85. doi: 10.4161/org.26710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegering A, Ruther U, Gerhardt C. The role of primary cilia in the crosstalk between the ubiquitin-proteasome system and autophagy. Cells. 2019;8:241. doi: 10.3390/cells8030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann N Y Acad Sci. 2010;1192:410–421. doi: 10.1111/j.1749-6632.2009.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Liu W, Liu X, Liu W, Wang H, Yao G, Zang L, Hayashi T, Tashiro S, Onodera S, Ikejima T. Silibinin negatively contributes to primary cilia length via autophagy regulated by histone deacetylase 6 in confluent mouse embryo fibroblast 3T3-L1 cells. Mol Cell Biochem. 2016;420:53–63. doi: 10.1007/s11010-016-2766-2. [DOI] [PubMed] [Google Scholar]
- Xu Q, Liu W, Liu X, Otkur W, Hayashi T, Yamato M, Fujisaki H, Hattori S, Tashiro SI, Ikejima T. Type I collagen promotes primary cilia growth through down-regulating HDAC6-mediated autophagy in confluent mouse embryo fibroblast 3T3-L1 cells. J Biosci Bioeng. 2018;125:8–14. doi: 10.1016/j.jbiosc.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Yang SH, Wang XX, Contino G, Liesa M, Sahin E, Ying HQ, Bause A, Li YH, Stommel JM, Dell’Antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YH, Han YG. Primary cilia in brain development and diseases. Am J Pathol. 2018;188:11–22. doi: 10.1016/j.ajpath.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ, Steg AD, Serra R, Frost AR. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Zhang H. Autophagosome maturation: an epic journey from the ER to lysosomes. J Cell Biol. 2019;218:757–770. doi: 10.1083/jcb.201810099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X, Zhong Q. Autophagy in cancer. F1000Prime Rep. 2015;7:18. doi: 10.12703/P7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg D, Debbache J, Pena-Hernandez R, Antunes AT, Schaefer SM, Cheng PF, Zimmerli D, Haeusel J, Calcada RR, Tuncer E, Zhang Y, Bossart R, Wong KK, Basler K, Dummer R, Santoro R, Levesque MP, Sommer L. EZH2-mediated primary cilium deconstruction drives metastatic melanoma formation. Cancer Cell. 2018;34:69–84.e14. doi: 10.1016/j.ccell.2018.06.001. [DOI] [PubMed] [Google Scholar]