Abstract
Sphingosine kinase 1 and its product, sphingosine 1-phosphate (S1P), as well as their receptors, have been implicated in inflammatory responses. The functions of receptors S1P1 and S1P2 on cell motility have been investigated. However, the function of S1P3 has been poorly investigated. In this study, the roles of S1P3 on inflammatory response were investigated in primary perito-neal macrophages. S1P3 receptor was induced along with sphingosine kinase 1 by stimulation of lipopolysaccharide (LPS). LPS treatment induced inflammatory genes, such iNOS, COX-2, IL-1β, IL-6 and TNF-α. TY52156, an antagonist of S1P3 suppressed the induction of inflammatory genes in a concentration dependent manner. Suppression of iNOS and COX-2 induction was further confirmed by western blotting and NO measurement. Suppression of IL-1β induction was also confirmed by western blotting and ELISA. Caspase 1, which is responsible for IL-1β production, was similarly induced by LPS and suppressed by TY52156. Therefore, we have shown S1P3 induction in the inflammatory conditions and its pro-inflammatory roles. Targeting S1P3 might be a strategy for regulating inflammatory diseases.
Keywords: S1P, S1P3, Macrophage, Inflammation, Caspase 1, GPCR
INTRODUCTION
Sphingosine 1-phosphate (S1P) is a specific ligand for five G protein-coupled receptors, S1P1–5 (Park and Im, 2017). In response to inflammatory stimuli, up-regulation or activation of sphingosine kinase 1 (SphK1) and increased generation of its product, S1P, have been observed in many cell types, including RAW264.7 macrophages and microglia (Xia et al., 1998; Pettus et al., 2003; Hammad et al., 2008; Jin et al., 2018). S1P caused pro-inflammatory responses; for examples, S1P increases COX-2, iNOS, PGE2 levels, interleukin-1β (IL-1β) as well as tumor necrosis factor-α (TNF-α) in several cell types, including murine peritoneal macrophages (Lee et al., 2002; Pettus et al., 2003; Hammad et al., 2008; Muller et al., 2017). On the contrary, S1P was shown to block lipopolysaccharide (LPS)-dependent stimulation of NF-κB activation and NO production, implying that S1P exerts an anti-inflammatory role (Hughes et al., 2008).
Murine RAW264.7 macrophages, bone marrow-derived monocytes, human peripheral monocytes, and human alveolar macrophages have been reported to express S1P1 and S1P2 (Lee et al., 2002; Hughes et al., 2008; Ishii et al., 2009). LPS-induced TNF-α, MCP-1, and IL-12 secretion were suppressed in murine peritoneal macrophages pre-incubated with a S1P1 specific agonist, indicating a pivotal role of S1P1 in S1P-dependent activation of anti-inflammatory macrophages (Hughes et al., 2008). S1P1 signaling reciprocally up-regulated IL-6 gene expression in primary mouse macrophages in a JAK2-dependent manner (Zhao et al., 2018). S1P increases arginase I activity and inhibits LPS-induced inducible NO synthase activity in LPS-treated macrophages, again through S1P1 receptor activation on macrophages (Hughes et al., 2008). The inhibitory role of S1P2 in macrophage recruitment during inflammation (Michaud et al., 2010) and the proatherogenic role of S1P2 signaling in the plaque macrophage have been reported (Skoura et al., 2011). Therefore, although anti-inflammatory function of S1P1 has been reported, specific S1P receptors involved in the pro-inflammatory function of S1P have not been fully elucidated. Especially, the function of S1P3 in macrophages has not been studied well. Therefore, we hypothesized that S1P3 is a pro-inflammatory S1P receptor in macrophages and investigated the roles of S1P3 on inflammatory response.
MATERIALS AND METHODS
Materials
LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA). TY52156 was purchased from Tocris Bioscience (cat. 5328, Bristol, UK).
Isolation and culture of mouse peritoneal macrophages
Mouse peritoneal macrophages were isolated from the peritoneal cavity of C57BL/6 mice treated with 3% thioglycol-late at 4 days after treatment and cultured at 37°C in a 5% CO2 humidified incubator. Isolated macrophages were maintained in RPMI1640 medium containing 10% (v/v) heat-inactivated fetal bovine serum, 100 units/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate for 18 h, and then incubated in 0.5% FBS-containing media for 24 h. Samples for RNA or protein analysis were collected at 5 h or 24 h after treatment with LPS (10 ng/ml or 100 ng/ml), respectively. TY52156 was added 1 h before LPS treatment.
Reverse Transcription-PCR
Total RNA was isolated from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration was determined by a Nanodrop ND-1000 spectrophotometer. One microgram of RNA was transcribed by using a ImProm-II Reverse Transicription System (Promega, Madision, WI, USA) in accordance with the manufacturer’s protocol. First-strand cDNA was synthesized from isolated total RNA using Trizol reagent (Invitrogen). Synthesized cDNA products and primers for each gene were used for PCR, which was conducted using Go-Taq DNA polymerase (Promega). Specific primers for IL-6 (sense 5′-CCG GAG AGG AGA CTT CAC AG-3′, antisense 5′-TGG TCT TGG TCC TTA GCC AC-3′), IL-1β (sense 5′-GGA GAA GCT GTG GCA GCT A-3′, antisense 5′-GCT GAT GTA CCA GTT GGG GA-3′) were used to amplify gene fragments. PCR was performed over 27 amplification cycles (denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 30 s) in an Mastcycler gradient PCR machine (Eppendorf, Hamburg, Germany). Specific primers for TNF-α (sense 5′-CAA AGA AAG CCG CCT CAA AC-3′, antisense 5′-TTC ACA GAG AGG GTC ACA GC-3′) were used and annealing was performed at 57°C. iNOS (sense 5′-ACC TAC CAC ACC CGA GAT GGC CAG-3′, antisense 5′-AGG ATG TCC TGA ACA TAG ACC TTG GG-3′), COX-2 (sense 5′-CCG TGG GGA ATG TAT GAG CA-3′, antisense 5′-CCA GGT CCT CGC TTA TGA TCT G-3′), SphK1 (sense 5′-TCC TGG AGG AGG CAG AGA TA-3′, antisense 5′-GCT ACA CAG GGG TTT CTG GA-3′) and GAPDH (sense 5′-TTC ACC ACC ATG GAG AAG GC-3′, antisense 5′-GGC ATG GAC TGT GGT CAT GA-3′) were used and annealing was performed at 60°C and over 30 amplification cycles. For S1P1 (sense 5′-CAC CGG CCC ATG TAC TAT-3′, antisense 5′-GCA GCC CAC ATC TAA CAG-3′), S1P2 (sense 5′-CAT GGG CGG CTT ATA CTC A-3′, antisense 5′-CAC TGC ACG GGA GTT AAG GA-3′), S1P3 (sense 5′-GGG AGG GCA GTA TGT TCG TA-3′, antisense 5′-GGA GCC CGC AAC AGA TAA G-3′), annealing was undertaken at 57°C. Aliquots (5 μl) were electrophoresed in 1.2% agarose gels and stained with ethidium bromide.
Western blotting
Macrophages were harvested and resuspended in RIPA lysis buffer (GenDEPOT, Baker, Carlsbad, CA, USA). Concentration of proteins was determined using a BCA protein assay (ThermoScientific, Rockford, IL, USA). Proteins (50 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis and then electrophoretically transferred to a nitrocellulose paper, which was incubated with specific primary antibodies recognizing β-actin, COX-2, iNOS, IL-1β, and caspase 1, and then incubated HRP-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Signals were developed using an enhanced chemiluminescence system (Pierce Biotechnology Inc., Rockford, IL, USA).
Nitrites measurement
NO production was estimated by measuring the amount of nitrite (a stable metabolite of NO) in medium using Griess re-agent, as previously described (Kang et al., 2018). Cells were pretreated with different concentrations of TY52156 for 1 h and subsequently stimulated with LPS (100 ng/ml) for 48 h. Nitrite concentrations in the medium was determined using a Griess Reagent System (Promega).
ELISA
Ninety-six-well plates (NUNC) were coated with capture antibodies overnight at 4°C (eBioscience, San Diego, CA, USA, IL-1β cat 14-7012-85). The plates were washed, and then blocked with blocking buffer for 1 h at room temperature. Standard dilutions of cytokines were prepared and added to the wells with supernatants. The plates were incubated for 2 h at room temperature with shaking, and then washed five times. Biotinylated detection antibody (eBioscience, IL-1β cat 13-7112-81) was then added to the wells, which were incubated for 1 h at room temperature with shaking. The plates were then washed five times and Avidin-HRP was added for 30 min at room temperature with shaking. The plates were then washed fourteen times and incubated with substrate solution for 15 min at room temperature. Stop solution (eBioscience) was then added and absorbance was read at 450 nM.
Animals
Eight- to ten-week-old male C57BL/6 (19-22 g) mice were purchased from Daehan Biolink (DBL; Seoul, Korea), housed in a Laboratory Animal facility at Pusan National University (Busan, Korea), and provided food and water ad libitum. The animal protocol used in this study was reviewed and approved beforehand by the Pusan National University-Institutional Animal Care Committee (PNU-IACUC) with respect to ethics and scientific care.
Statistics
All results were expressed as mean ± standard deviation. The data were analyzed by one-way ANOVA. A p-value<0.05 was considered statistically significant.
RESULTS
Induction of S1P3 in peritoneal macrophages
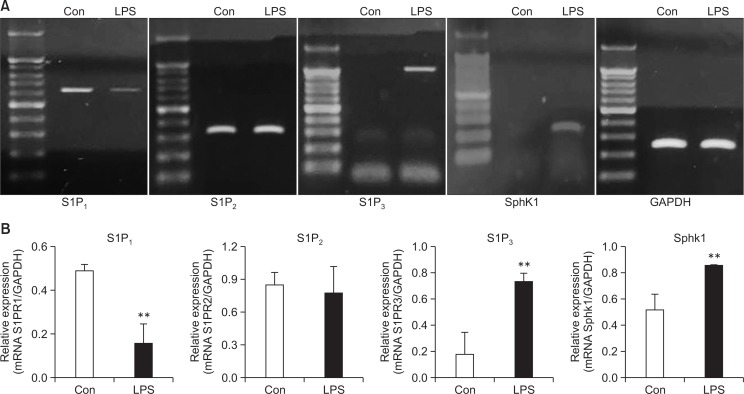
Previously, LPS, a cell wall component of Gram-negative bacteria, was shown to induce the expression and activation of SphK1 in RAW264.7 macrophages. In addition, its product, S1P, has been well studied in relation with the functions of S1P1 and S1P2. We confirmed an LPS-induced increase of SphK1 expression as well as the presence of S1P3 mRNA and its simultaneous induction in mouse peritoneal macrophages after LPS treatment (Fig. 1). Because S1P3 has been poorly investigated in inflammatory responses compared to S1P1 and S1P2, we studied the function of S1P3 by using an S1P3 antagonist, TY52156 (Murakami et al., 2010).
Fig. 1.
Changes in the expression of S1P3 and SphK1 caused by LPS stimulation. (A) Mouse peritoneal macrophages were treated with vehicle or LPS 10 ng/mL for 5 h, and RT-PCR was then performed to detect the expression of the S1P1, S1P2, S1P3, and SphK1 genes. The data shown are representatives of three independent experiments. (B) Histogram of quantitated mRNA expression. Results are the mean ± standard deviation of three independent experiments. Statistically significant at **p<0.01 level vs. the vehicle-treated macrophages.
TY52156 inhibits induction of COX-2 and iNOS in peritoneal macrophages
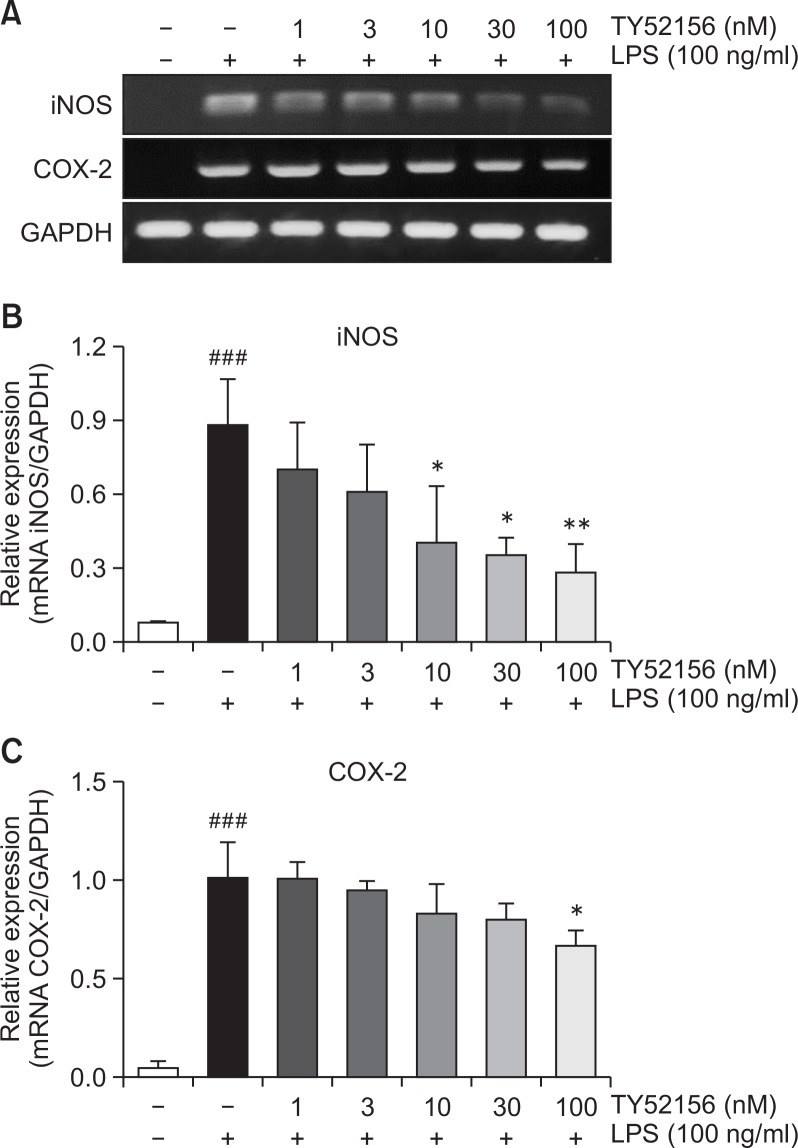
In the presence of 10 ng/ml LPS, expression of pro-inflammatory genes, iNOS and COX-2, was increased, and the induction of iNOS and COX-2 expressions at mRNA level was significantly inhibited by TY52156 in a concentration-dependent manner (Fig. 2).
Fig. 2.
Effect of TY52156 on the mRNA expressions of iNOS and COX-2 in macrophages. (A) Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with vehicle or LPS 10 ng/mL for 5 h, and RT-PCR was performed to detect the expression of the pro-inflammatory genes iNOS and COX-2. The data shown are representatives of three independent experiments. (B, C) Histograms of quantitated mRNA expressions of iNOS and COX-2. Results are the means ± standard deviation of three independent experiments. Statistically significant at ###p<0.001 level vs. the vehicle-treated macrophages, and at *p<0.05 and **p<0.01 vs. the the LPS-treated macrophages.
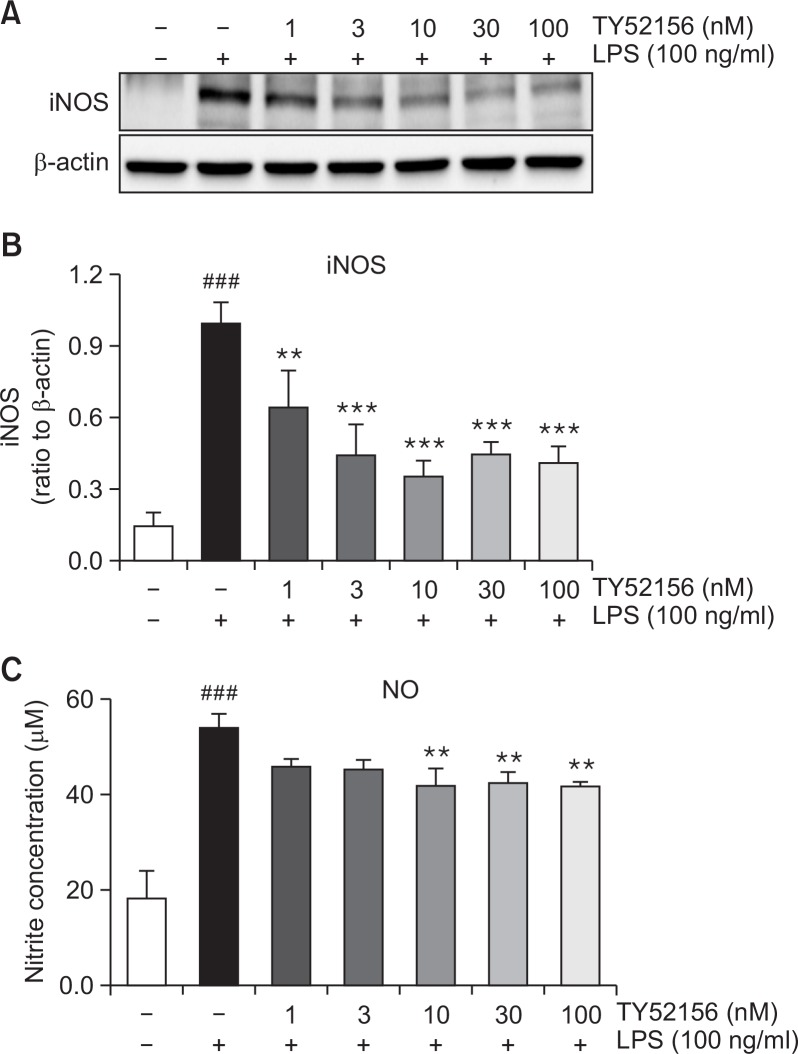
The inhibitory effect of TY52156 on iNOS and COX-2 was studied at protein level. In the presence of 100 ng/ml LPS, western samples were made. The protein expression of iNOS was significantly suppressed by treatment with TY52156 (Fig. 3A, 3B). Also, the level of NO, the product of iNOS, was significantly decreased (Fig. 3C).
Fig. 3.
Effect of TY52156 on iNOS protein expression and NO production in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with LPS 100 ng/mL for 24 or 48 h. Western blotting was conducted on cell lysates. The data shown in (A) are representative of three independent experiments. Relative protein levels of iNOS versus β-actin are presented as histograms (B). LPS-induced productions of nitrite (C) was measured. TY52156 inhibited nitrite production in a concentration-dependent manner (C). Results are the means ± standard deviation of three independent experiments. Statistically significant at ###p<0.001 level vs. the vehicle-treated macrophages, and at **p<0.01 and ***p<0.001 vs. the LPS-treated macrophages.
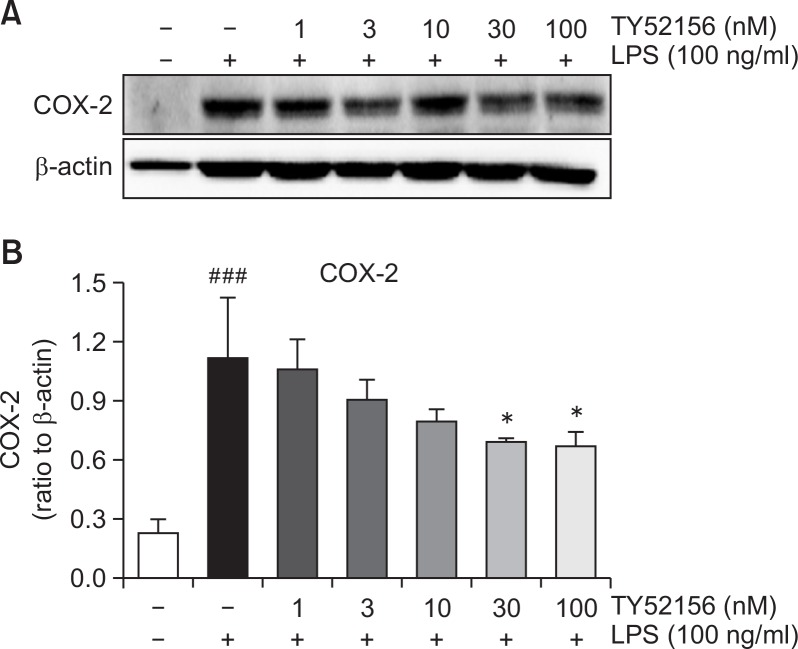
The inhibitory effect of TY52156 on COX-2 mRNA expression was also further studied at protein level. As shown in Fig. 4, TY51256 strongly inhibited the induction of COX-2 at protein level in a concentration-dependent manner (Fig. 4).
Fig. 4.
Effect of TY52156 on the protein expression of COX-2 in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. The data shown in (A) are representative of three independent experiments. Relative protein levels of COX-2 versus β-actin are presented as histograms (B). Results are the means ± standard deviation of three independent experiments. Statistically significant at ###p<0.001 level vs. the vehicle-treated macrophages, and at *p<0.05 vs. the LPS-treated macrophages.
TY52156 inhibits induction of IL-1β in peritoneal macrophages
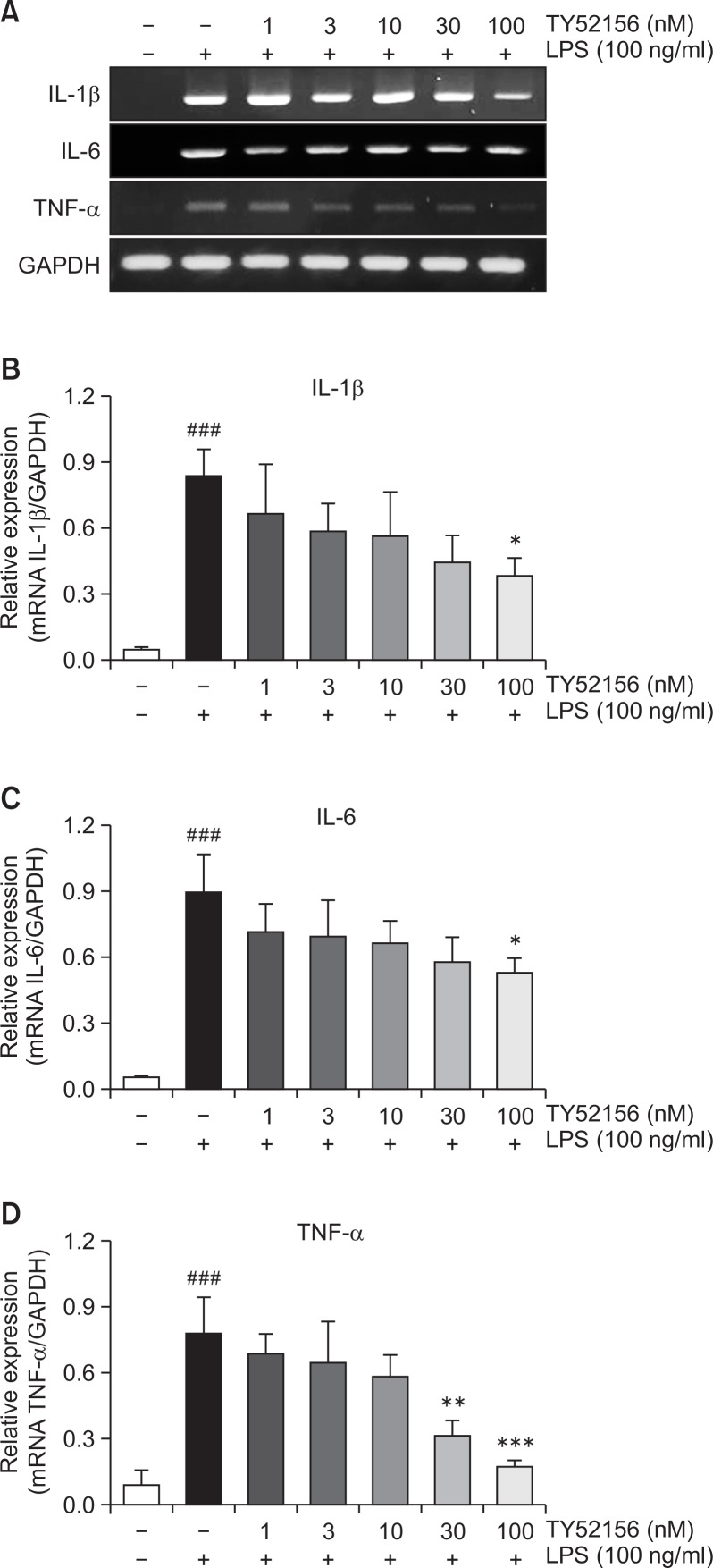
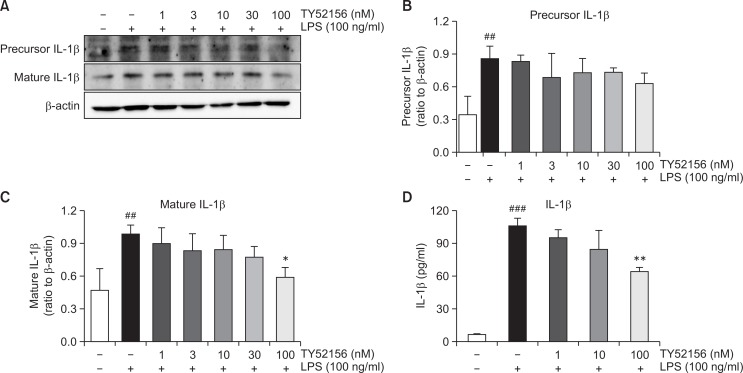
Treatment of TY52156 also inhibited LPS-induced induction of other inflammatory genes of IL-1β, IL-6 and TNF-α (Fig. 5). Because treatment of TY52156 inhibited LPS-induced induction of the IL-1β gene (Fig. 5), the protein level of IL-1β was examined by western blotting and ELISA (Fig. 6). TY52156 inhibited induction of preform and mature form IL-1β. ELISA result showed significant inhibition on IL-1β secretion in the media (Fig. 6D).
Fig. 5.
Effect of TY52156 on the expression of IL-1β, IL-6, and TNF-α in macrophages. (A) Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with vehicle or LPS 10 ng/mL for 5 h, and RT-PCR was performed to detect the expression of the pro-inflammatory genes, IL-1β, IL-6, and TNF-α. The data shown are representatives of three independent experiments. (B–D) Histograms of quantitated mRNA expression of IL-1β, IL-6, and TNF-α. Results are the means ± standard deviation of three independent experiments. Statistically significant at ###p<0.001 level vs. the vehicle-treated macrophages, and at *p<0.05, **p<0.01 and ***p<0.001 vs. the LPS-treated macrophages.
Fig. 6.
Effect of TY52156 on IL-1β protein expression and IL-1β production in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. The data shown in (A) are representatives of three independent experiments. Relative protein levels of IL-1β versus β-actin are presented as histograms (B, C). LPS-induced production of IL-1β in the media was measured by ELISA (D). Results are the means ± standard deviation of three independent experiments. Statistically significant at ##p<0.01 and ###p<0.001 vs. the vehicle-treated macrophages, and at *p<0.05 and **p<0.01 vs. the LPS-treated macrophages.
TY52156 inhibits caspase 1 expression in peritoneal macrophages
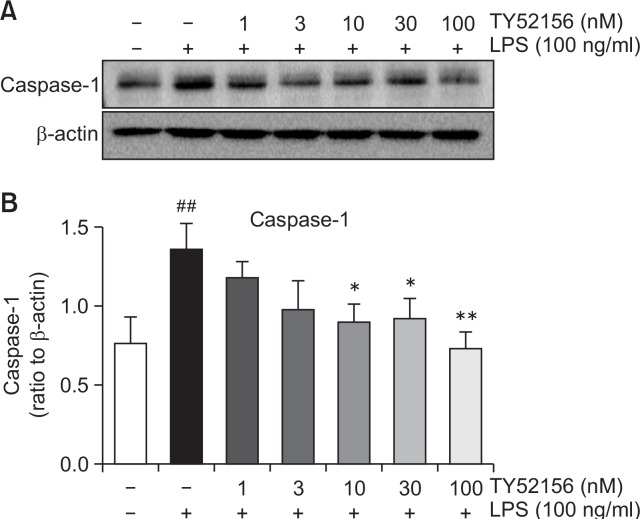
Furthermore, we evaluated the expression of caspase 1, which converts the preform IL-1β to mature IL-1β. Caspase 1 expression was induced by LPS and suppressed by treatment of TY52156 (Fig. 7).
Fig. 7.
Effect of TY52156 on the protein expression of caspase-1 in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. The data shown in (A) are representatives of three independent experiments. Relative protein levels of caspase-1 versus β-actin are presented as histograms (B). Results are the means ± standard deviation of three independent experiments. Statistically significant at ##p<0.01 level vs. the vehicle-treated macrophages, and at *p<0.05 and **p<0.01 vs. the LPS-treated macrophages.
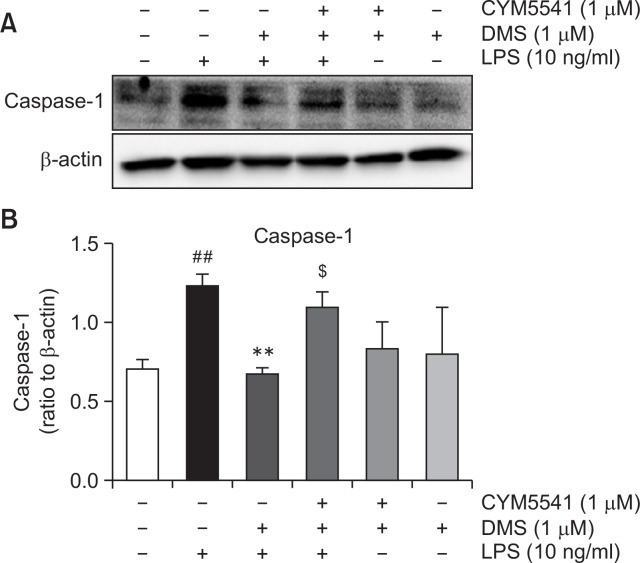
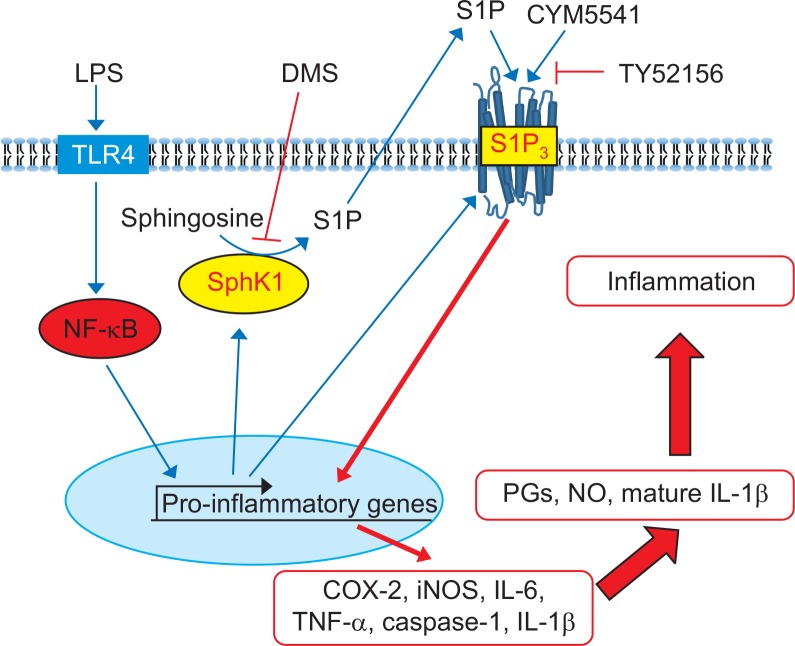
On the basis of these findings, we hypothesized that LPS stimulation may induce not only inflammatory genes, such as, iNOS, caspase 1, and IL-1β, but also SphK1 and S1P3. Induction of SphK1 may increase production of S1P, which activates S1P3, thereby amplifying inflammatory response. In order to confirm this signaling route, we applied dimethylsphingoinse (DMS), a specific inhibitor of SphK1. As shown in Fig. 8, in the presence of DMS, LPS induced less expression of caspase 1. However, treatment with CYM5541, a specific agonist of S1P3, reversed DMS-induced gene suppression (Fig. 8), implying that the induction of SphK1 and its product, S1P, and the action of S1P on S1P3 sequentially mediated the pro-inflammatory response of LPS in macrophages (Fig. 9).
Fig. 8.
Effect of dimethylsphingosine and CYM5541 on the protein expression of caspase-1 in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of TY52156 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. The data shown in (A) are representative of three independent experiments. Relative protein levels of caspase-1 versus β-actin are presented as histograms (B). Results are the means ± standard deviation of three independent experiments. Statistically significant at ##p<0.001 level vs. the vehicle-treated macrophages, at **p<0.01 vs. the LPS-treated macrophages, at $p<0.05 vs. the LPS/DMS-treated macrophages.
Fig. 9.
Proposed signaling of pro-inflammatory S1P and S1P3 in peritoneal macrophages. LPS activates NF-κB and induces induction of SphK1 and S1P3 expression in macrophages. S1P produced by SphK1 activates pro-inflammatory signaling through S1P3, resulting in increased productions of prostaglandins (PGs), nitric oxides, and mature IL-1β.
DISCUSSION
In this study, we found that LPS induced S1P3 expression in murine macrophages. Previously, expression of S1P3 was reported to be very low in macrophages (Lee et al., 2002). However, its expression was confirmed by immunofluorescence, RT-PCR and western blotting in human monocytes/macrophages and murine bone marrow-derived macrophages (Duong et al., 2004; Durafourt et al., 2011; Yang et al., 2015; Muller et al., 2017). In addition, S1P3 was induced during macrophage differentiation from human monocytes (Duong et al., 2004). Moreover, stimulation of S1P3 by FTY-720 reduced monocyte recruitment to atherosclerotic lesions (Theilmeier et al., 2006). Furthermore, TNF-α increased the mRNA of both S1P1 and S1P3 in human astrocytes and LPS upregulated the expression of S1P1 and S1P3 in human gingival epithelial cells (Eskan et al., 2008; Van Doorn et al., 2010). Strong increases in S1P1 and S1P3 expression in reactive astrocytes were detected in active and inactive chronic multiple sclerosis lesions (Van Doorn et al., 2010). Upregulation of S1P3 in astrocytes was also previously reported in a mouse model of Sandhoff disease, a prototypical neuronopathic lysosomal storage disorder (Wu et al., 2008). Therefore, induction of S1P3 has been reported in many cell types.
In our study, SphK1 and S1P3 were concomitantly upregulated, but S1P1 was downregulated. Similarly, concomitant regulation of SphK1 and S1P3 was shown to play a pivotal role in murine cardiac fibrosis and in the transdifferentiation of myoblasts into myofibroblasts (Cencetti et al., 2010; Takuwa et al., 2010). Furthermore, SphK1 and S1P3 were functionally upregulated in astrocytes under pro-inflammatory conditions (Fischer et al., 2011).
In this study, we observed a pro-inflammatory function of S1P3, which was supported by the result of a previous study that bone marrow-derived S1P3-deficient macrophages produced low MCP-1 in response to LPS stimulation (Keul et al., 2011). Previously, the functions of S1P3 have been reported in macrophage migration. S1P exerts a potent migratory action in bone marrow-derived macrophages via S1P2 and S1P3 in cholestatic liver injury (Yang et al., 2015). In vitro, S1P induces a chemotactic action in wild-type peritoneal macrophages, but not in S1P3-deficient peritoneal macrophages (Keul et al., 2011). S1P3 mediates the chemotactic effect of S1P in macrophages in vitro and in vivo, and plays a causal role in atherosclerosis by promoting inflammatory recruitment of monocyte/macrophage (Keul et al., 2011). This was also supported by a finding that S1P, a constituent of HDL, acutely protects the heart against ischemia/reperfusion injury in vivo via an S1P3-mediated and NO-dependent pathway (Theilmeier et al., 2006).
We observed that S1P3 mediated iNOS, COX-2, IL-1β, IL-6, and TNF-α expression under LPS stimulation (Fig. 9). The pro-inflammatory action of S1P has previously been reported in several cell types, including macrophages. For examples, SphK1 activation by LPS or cytokines, including TNF-α and IL-1β, has been observed (Xia et al., 1998; Pettus et al., 2003; Billich et al., 2005; Hammad et al., 2008; Nayak et al., 2010; Snider et al., 2010). Moreover, S1P caused increases in COX-2 and PGE2 levels in several cell types (Pettus et al., 2003; Hammad et al., 2008). S1P increases the expression of iNOS under M2-polarizing conditions (Muller et al., 2017). Furthermore, S1P induces the release of IL-1β and TNF-α from murine peritoneal macrophages (Lee et al., 2002). Therefore, S1P has been reported to play crucial roles in pro-inflammatory responses. However, a specific S1P receptor for the responses has not been elucidated, and for the first time S1P3 was reported as the pro-inflammatory receptor in this study (Fig. 9). On the basis of the results of this study, S1P3 targeting therapeutics may be beneficial as an anti-inflammatory therapy.
Acknowledgments
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (#2016R1D1A1A009917086).
REFERENCES
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol Cell. 2010;21:1111–1124. doi: 10.1091/mbc.e09-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong CQ, Bared SM, Abu-Khader A, Buechler C, Schmitz A, Schmitz G. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim. Biophys Acta. 2004;1682:112–119. doi: 10.1016/j.bbalip.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Durafourt BA, Lambert C, Johnson TA, Blain M, Bar-Or A, Antel JP. Differential responses of human microglia and blood-derived myeloid cells to FTY720. J Neuroimmunol. 2011;230:10–16. doi: 10.1016/j.jneuroim.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Rose BG, Benakanakere MR, Zeng Q, Fujioka D, Martin MH, Lee MJ, Kinane DF. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur J Immunol. 2008;38:1138–1147. doi: 10.1002/eji.200737898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS ONE. 2011;6:e23905. doi: 10.1371/journal.pone.0023905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008;85:107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Lu Z, Li Y, Ru JH, Lopes-Virella MF, Huang Y. LPS and palmitate synergistically stimulate sphingosine kinase 1 and increase sphingosine 1 phosphate in RAW264.7 macrophages. J Leukoc Biol. 2018;104:843–853. doi: 10.1002/JLB.3A0517-188RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Park SJ, Lee AY, Huang J, Chung HY, Im DS. Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization. J Ginseng Res. 2018;42:68–74. doi: 10.1016/j.jgr.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- Lee H, Liao JJ, Graeler M, Huang MC, Goetzl EJ. Lysophospholipid regulation of mononuclear phagocytes. Biochim. Biophys Acta. 2002;1582:175–177. doi: 10.1016/S1388-1981(02)00153-1. [DOI] [PubMed] [Google Scholar]
- Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, von Bernstorff W, Heidecke CD, Schulze T. Differential S1P receptor profiles on M1- and M2-polarized macrophages affect macrophage cytokine production and migration. BioMed Res Int. 2017;2017:7584621. doi: 10.1155/2017/7584621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, Watanabe Y, Mori K, Sato Y, Takahashi A, Mochizuki N, Takakura N. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, Dheen ST. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010;166:132–144. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Park SJ, Im DS. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther (Seoul) 2017;25:80–90. doi: 10.4062/biomolther.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92:707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, Hirano K, Usui S, Wang F, Du W, Yoshioka K, Banno Y, Sasaki M, Ichi I, Okamura M, Sugimoto N, Mizugishi K, Nakanuma Y, Ishii I, Takamura M, Kaneko S, Kojo S, Satouchi K, Mitumori K, Chun J, Takuwa Y. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85:484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, Alewijnse AE, Peters SL, De Vries HE. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Han Z, Tian L, Mai P, Zhang Y, Wang L, Li L. Sphingosine 1-phosphate receptor 2 and 3 mediate bone marrow-derived monocyte/macrophage motility in cholestatic liver injury in mice. Sci Rep. 2015;5:13423. doi: 10.1038/srep13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Adebiyi MG, Zhang Y, Couturier JP, Fan X, Zhang H, Kellems RE, Lewis DE, Xia Y. Sphingosine-1-phosphate receptor 1 mediates elevated IL-6 signaling to promote chronic inflammation and multitissue damage in sickle cell disease. FASEB J. 2018;32:2855–2865. doi: 10.1096/fj.201600788RR. [DOI] [PMC free article] [PubMed] [Google Scholar]