Abstract
Limonene is a cyclic terpene found in citrus essential oils and inhibits methamphetamine-induced locomotor activity. Drug dependence is a severe neuropsychiatric condition that depends in part on changes in neurotransmission and neuroadaptation, induced by exposure to recreational drugs such as morphine and methamphetamine. In this study, we investigated the effects of limonene on the psychological dependence induced by drug abuse. The development of sensitization, dopamine receptor supersensitivity, and conditioned place preferences in rats was measured following administration of limonene (10 or 20 mg/kg) and methamphetamine (1 mg/kg) for 4 days. Limonene inhibits methamphetamine-induced sensitization to locomotor activity. Expression of dopamine receptor supersensitivity induced by apomorphine, a dopamine receptor agonist, was significantly reduced in limonenepretreated rats. However, there was no significant difference in methamphetamine-induced conditioned place preferences between the limonene and control groups. These results suggest that limonene may ameliorate drug addiction-related behaviors by regulating postsynaptic dopamine receptor supersensitivity.
Keywords: Dopamine receptor supersensitivity, Methamphetamine, Sensitization, Limonene
INTRODUCTION
Limonene is a common terpene found in citrus fruits. This monoterpene is widely used as a flavor and fragrance and is listed to be generally recognized as safe in food by the Food and Drug Administration (Flamm and Lehman-McKeeman, 1991). Limonene has been shown to exert anxiolytic effects, regulatory effects on neurotransmitters, and antinociceptive effects (do Amaral et al., 2007; Zhou et al., 2009; de Almeida et al., 2012; Lima et al., 2013). Recently, we have reported that limonene inhibits an acute single methamphetamine-induced hyperlocomotion in rats by regulating dopamine levels in the nucleus accumbens (Yun, 2014). However, the potential for limonene in the treatment of drug dependence is largely unknown.
Drug dependence is a condition that involves a cluster of behavioral, cognitive, and physiological symptoms that can develop following repeated substance use. Preclinical models have been shown to be useful in identifying many molecular and cellular targets of drug dependence. In rodents, acute administration of stimulants results in hyperactivity, whereas repeated administration results in progressive, enhanced locomotor activity (Shimosato and Ohkuma, 2000; Filip et al., 2006; Fukushima et al., 2007). This phenomenon is also known as context-dependent behavioral sensitization, and this may play a role in the development of compulsive drug-seeking behaviors (Hooks et al., 1993; Mattingly et al., 2000; Shen et al., 2006). It has been suggested that enhanced mesolimbic dopaminergic neuronal transmission is responsible for the development of behavioral sensitization to an abused drug (Pak et al., 2006; Bello et al., 2011); this is a model for studying the psychotoxicity of dependence-liable drugs (Allen and Young, 1978; Robinson and Becker, 1986). It has also been reported that chronic abuse of drugs can cause the development of postsynaptic dopamine receptor supersensitivity in the central nervous system (CNS) (Martin and Takemori, 1986; Ujike et al., 1990; Kim et al., 1999). This increased sensitivity can be detected as a hypersensitivity to direct-acting dopamine agonists and as an increase in the affinity of dopamine receptors (Martin and Takemori, 1986; Woo et al., 2001). Many drugs that are liable to lead to dependence are known to induce CPP, including morphine, heroin (Bozarth and Wise, 1981; Blander et al., 1984; Reid et al., 1989), cocaine (Morency et al., 1987), and amphetamine (Gilbert and Cooper, 1983). These drugs produce a reinforcing effect, which, according to some hypotheses, may be because they commonly facilitate dopaminergic transmissions, either by stimulating the release of dopamine or inhibiting dopamine uptake (Kim et al., 1998).
In this study, we investigated the effect of limonene on methamphetamine-induced behavioral sensitization and CPP in rats. Furthermore, to discover the possible mechanism underlying limonene’s effects in methamphetamine-induced psychological dependence, we examined the effect of limonene on the development of postsynaptic dopamine receptor supersensitivity in methamphetamine-induced sensitized rats.
MATERIALS AND METHODS
Animals and drugs
Male Sprague-Dawley rats (all male, weight range: 180–220g) were obtained from the Daehan Bio Link (DBL, Chungbuk, Korea) and were housed in groups of 2 rats in a temperature-controlled room (22 ± 2°C) with a 12-h light/dark cycle (lights on 08:00 from 20:00). The rats were given a solid diet and tap water, ad libitum. All animals were treated in Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facilities, operating according to the Guide for the Care and Use of Laboratory Animals. All experiments were approved by the Institutional Animal Care and Use Committee of Chungbuk National University. The following agents were used in this study: methamphetamine·HCl, (R)-(+)-limonene, and apomorphine hydrochloride, all obtained from Sigma (St. Louis, MO, USA). Morphine hydrochloride was purchased from Guju Pharmaceutical Co (Seoul, Korea). All drugs were dissolved in distilled water (0.9% NaCl) immediately prior to the experiments, except for the (R)-(+)-limonene, which was dissolved in water containing 4% Tween 80 and for the apomorphine, which was dissolved in water containing 0.1% ascorbic acid and intraperitoneally (i.p.) injected at a volume of 1 ml/kg.
Locomotor activity
To induce sensitization, 1 or 5 mg/kg of methamphetamine was administered once a day, for 4 days. To test the degree of sensitization development, all groups were challenged with methamphetamines on day 5. Each rat was placed in an activity chamber (43.2 cm×43.2 cm×30.5 cm, ENV-515, Med Associates Inc., St. Albans, VT, USA) and, after an adaptation period of 10 min, 1 mg/kg of methamphetamine was i.p. administered. The locomotor activity of the rats was measured using a photo-beam (infrared) activity chamber for 1 h, immediately after the injection of methamphetamine. The development and inhibition of sensitization was evidenced by enhanced and reduced response to methamphetamine, respectively. To study the effects of limonene on methamphetamine-induced loco-motor sensitization, according to our previous reports (Yun, 2014), 10 or 20 mg/kg of limonene was pretreated 40 min before the injection of methamphetamine, for 4 days. Dosages in this study are considered to have no toxicity in human as well as animals, which is accompanied with previous report (Sun, 2007). To test the degree of sensitization development, all groups were challenged with methamphetamines on day 5. Each rat was placed in an activity chamber (43.2 cm×43.2 cm×30.5 cm, ENV-515, Med Associates Inc.) and, after an adaptation period of 10 min, 1 mg/kg of methamphetamine was i.p. administered. The locomotor activity of the rats was measured using a photo-beam (infrared) activity chamber for 1 h, immediately after the injection of methamphetamine. The development and inhibition of sensitization was evidenced by enhanced and reduced response to methamphetamine, respectively.
Postsynaptic dopamine receptor supersensitivity
Postsynaptic dopamine receptor supersensitivity induced by methamphetamine (1 mg/kg) was demonstrated using 0.5 mg/kg of apomorphine (Fig. 1). Additional groups of rats, which underwent the same chronic methamphetamine and limonene treatment, were used to determine the effects of these treatments on the development of postsynaptic Dopamine receptor supersensitivity. Treatment involved methamphetamine administration (1 mg/kg), once daily, for 4 days, and limonene administration (10 or 20 mg/kg), once a day, 40 min before the injection of methamphetamine, for 4 days. The degree of methamphetamine-induced postsynaptic dopamine receptor supersensitivity developed was determined by measuring the enhanced locomotor activity induced by apomorphine on day 5, 24 h after the final injection of methamphetamine. Each rat was placed in an activity chamber and, after an adaptation period of 10 min, received 0.5 mg/kg of apomorphine (i.p.). The locomotor activity of the rats was measured using an infrared photo-beam activity chamber for 1 h, from immediately after the injection of apomorphine.
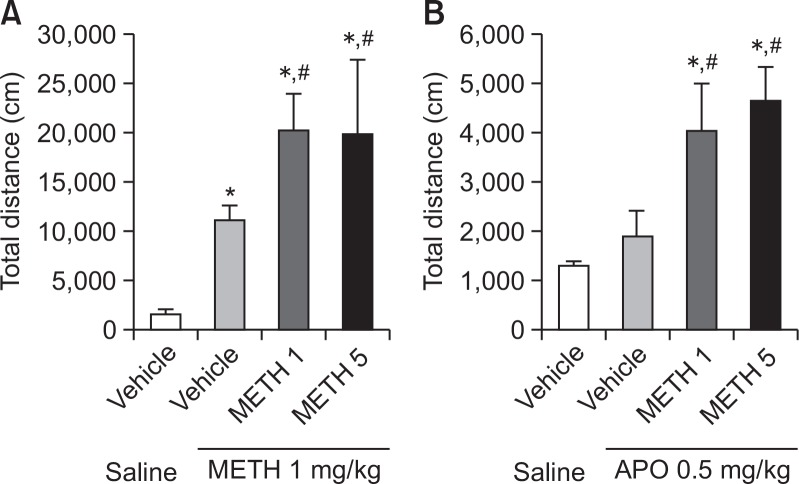
Fig. 1.
Development of methamphetamine-induced sensitization and postsynaptic dopamine receptor supersensitivity. Locomotor activity was measured after (A) methamphetamine (1 mg/kg, i.p.) and (B) apomorphine (0.5 mg/kg, i.p.) injections in vehicle (distilled water)- or methamphetamine-pretreated (1 or 5 mg/kg) rats. Values indicate the mean ± standard error (SE) (n=8). *p<0.05 vs. vehicle/saline, #p<0.05 vs. vehicle/METH 1 mg/kg (one-way ANOVA, followed by Bonferroni’s test). METH, methamphetamine; APO, apomorphine.
CPP
The apparatus and procedure used for the CPP test have been published previously (Kim et al., 1998), but were modified slightly. The CPP test apparatus used in the present study consisted of three compartments, and included the ENV-013 IR Infrared Sensor Package (Med Associates Inc.). The two-side conditioning chambers (interior dimensions: 25.5 cm [L]×21.0 cm [W]×20.9 cm [H]) were separated by a smaller chamber (13.2 cm [L]×21.0 cm [W]×20.9 cm [H]). One conditioning chamber had white walls and a stainless-steel mesh floor (1.3 cm×1.3 cm), and the other had black walls and a floor made of stainless steel rods. Removal of the guillotine doors during the pretesting and the final testing phases allowed the animals to have free access to both compartments, and the time spent by the individual rats in each of the two compartments was recorded using infrared detectors interfaced with an IBM-compatible PC. The time spent by the rats in the center chamber was ignored, since the time spent in the center chamber comprised <5% of the total time measured. All conditioning or test sessions were conducted under ambient light (20–30 lux).
To establish conditioning, we paired methamphetamine with the white compartment. The vehicle rats received an i.p. injection of saline immediately before exposure to the white or black compartment. Methamphetamine was administered immediately before the rats were placed in the white compartment. To test the effect of limonene on the CPP induced by methamphetamine, limonene was administered 40 min prior to the methamphetamine injection.
Pretesting phase: on day 1, the rats were pre-exposed to the test apparatus for 15 min. The guillotine doors were raised and each animal was allowed to move freely between the two compartments. On day 2, baseline preference for either of the two sides was determined for 15 min. Conditioning phase: on days 3, 5, 7, and 9, the rats were injected with methamphetamine prior to confinement in the white compartment, the non-preferred side, for 30 min. On days 4, 6, 8, and 10, the rats were injected with saline before they were confined to the black compartment, the preferred side, for 30 min.
Testing phase: on day 11, the guillotine doors were raised. The rats were placed in the tunnel in the central part of the apparatus and the time spent by the rats in the two compartments was recorded for 15 min. The scores were calculated from the changes noted between the testing phase and the pre-testing phase in the white compartment. An increase in time in the drug-paired compartment indicated that the animal spent more time in the originally less-preferred compartment, presumably due to the reinforcing effects of the morphine that were conditioned to this environment (Kim et al., 1998).
Western blotting
Limonene was administered once a day for 4 days and the striatum was sampled 24 hours after last injection. The tissues were homogenized with 1x lysis buffer (RIPA buffer, Thermo Scientific, MA, USA) including 1x protease inhibitor (Thermo Scientific) and incubated on ice for 30 min, and centrifuged at 13,000 rpm for 150 min at 4°C. An equal amount of total protein (30 μg) was resolved on 12% sodium dodecyl sulfate polyacrylamide gel and then transferred to a PVDF membrane (Immobilon-P; pore size 0.45 μm, EMD Millipore, Burlington, MA, USA). The membranes were blocked for 1 h in 5% skim milk solution and incubated for overnight at 4°C with glutamate decarboxylase 67 (Gad67; 1:1000, Sigma Aldrich, USA) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000, EMD Millipore). The blots were then incubated with secondary antibody; goat anti-mouse IgG-horseradish peroxidase (HRP) (1:5000, Sigma Aldrich, St. Louis, MO, USA). Immunoreactive proteins were detected with an enhanced chemiluminescence Western blotting detection system. The relative density of the protein bands was scanned and quantified by ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Analyses were carried out using one-way analysis of variance (ANOVA), followed by Bonferroni’s test, two-way ANOVA, or repeated-measures ANOVA with Bonferroni’s test, for multi-group comparisons.
RESULTS
Effect of methamphetamine on methamphetamine-induced sensitization and apomorphine-induced postsynaptic dopamine receptor supersensitivity
Locomotor sensitization, induced by repeated administration of a drug of abuse, is associated with the mechanism underlying the development of drug dependence (Bello et al., 2011). We observed that methamphetamine administration (1 or 5 mg/kg, i.p.) for 4 days induced the development of sensitization to locomotor activity in rats (Fig. 1A). Furthermore, apomorphine (0.5 mg/kg) administration triggered the demonstration of postsynaptic dopamine receptor supersensitivity in methamphetamine-pretreated rats (Fig. 1B). In this study, we used 1 mg/kg of methamphetamine as a submaximal dose in locomotor sensitization and 0.5 mg/kg of apomorphine for expression of supersensitivity.
Inhibitory effect of limonene on the development of sensitization to methamphetamine-induced hyperactivity
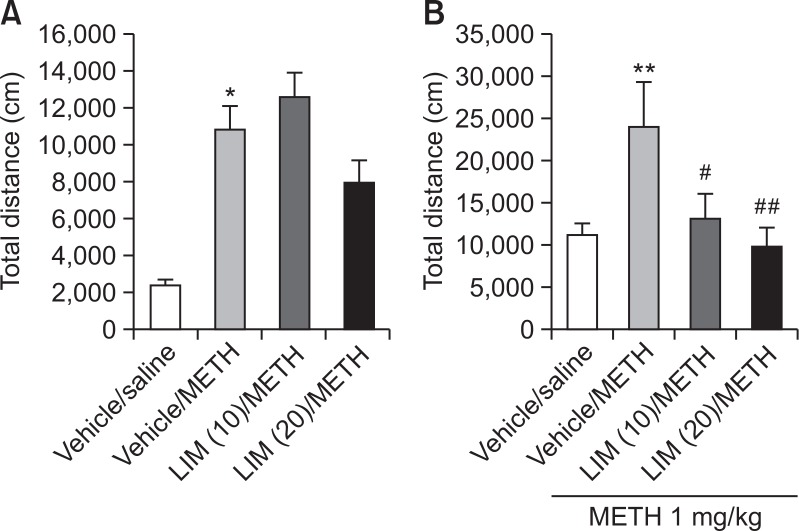
To explore the effect of limonene on methamphetamine-induced sensitization, we pretreated the rats with limonene (10 or 20 mg/kg, i.p.) prior to every methamphetamine injection, for 4 days. Limonene treatment did not inhibit hyperactivity induced by a single methamphetamine injection on day 1 (Fig. 2A). However, the locomotor sensitization induced by meth-amphetamine on day 5 was reduced in the rats pretreated with methamphetamine and limonene (Fig. 2B). These results suggest that limonene inhibits methamphetamine-induced behavioral sensitization.
Fig. 2.
Inhibitory effect of limonene on methamphetamine-induced sensitization of locomotion. The total distance on (A) day 1 and (B) day 5 is shown. On day 5, all groups were administered methamphetamine (1 mg/kg, intraperitoneal [i.p.]). Values indicate the mean ± standard error (SE) (n=8). *p<0.05, **p<0.01 vs. vehicle/saline group and #p<0.05, ##p<0.01 vs. vehicle/METH group (twoway repeated-measures ANOVA followed by Bonferroni’s test). METH, methamphetamine; LIM, limonene.
Inhibitory effect of limonene on the development of postsynaptic dopamine receptor supersensitivity in methamphetamine-induced sensitized rats
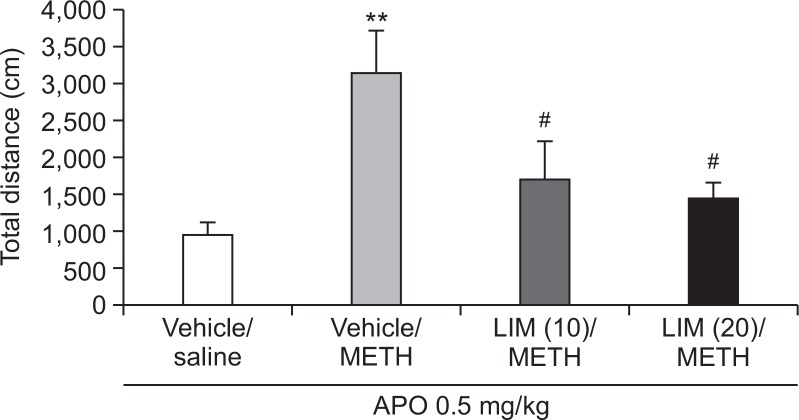
The rats that received the same chronic administration of methamphetamine (1 mg/kg) as used in the sensitization test demonstrated enhanced locomotor activity in response to apomorphine (0.5 mg/kg) treatment, as compared to the vehicle group, suggesting the development of postsynaptic dopamine receptor supersensitivity in methamphetamine-induced sensitized rats (Fig. 3). However, 10 or 20 mg/kg of limonene administered 40 min before the methamphetamine injection was found to reduce the locomotor activity of apomorphine, as compared to the methamphetamine group. These results suggest that limonene inhibits the development of postsynaptic dopamine receptor supersensitivity in methamphetamine-induced sensitized rats.
Fig. 3.
Inhibitory effect of limonene on methamphetamine-induced postsynaptic dopamine receptor supersensitivity. On day 5, all groups were administered apomorphine (0.5 mg/kg, intraperitoneal [i.p.]). Values indicate the mean ± standard error (SE) (n=8). **p<0.01 vs. vehicle/saline group, #p<0.05 vs. vehicle/METH group (one-way ANOVA, followed by Bonferroni’s test). METH, methamphetamine; APO, apomorphine; LIM, limonene.
Effect of limonene on methamphetamine-induced CPP
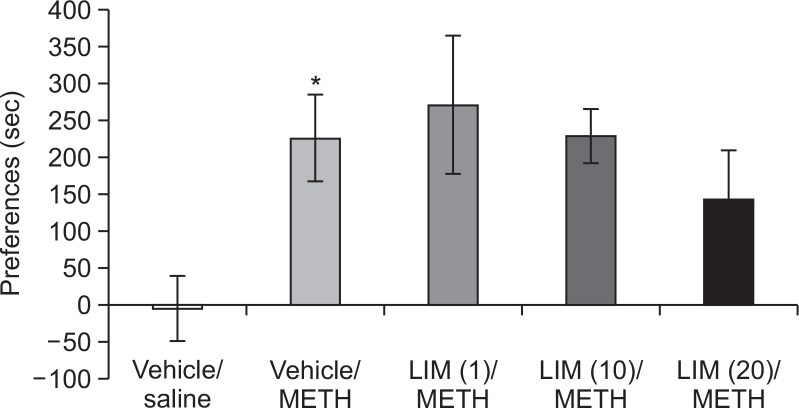
The methamphetamine group showed a significant increase in CPP score, as compared with the vehicle group (Fig. 4). The limonene (20 mg/kg) group showed a decreasing trend in CPP score, but this was not significant (Fig. 4). These results suggest that a dose of 20 mg/kg of limonene inhibits the development of sensitization, although it did not have a significant effect on the CPP induced by methamphetamine.
Fig. 4.
Effect of limonene on CPP induced by methamphetamine. The preferences were calculated from the changes of the testing phase (15 min) and the pre-testing phase (15 min) in the white compartment. Values indicate the mean ± standard error (SE) (n=10–14). *p<0.05 vs. vehicle (one-way ANOVA, followed by Bonferroni’s test). METH, methamphetamine; LIM, limonene.
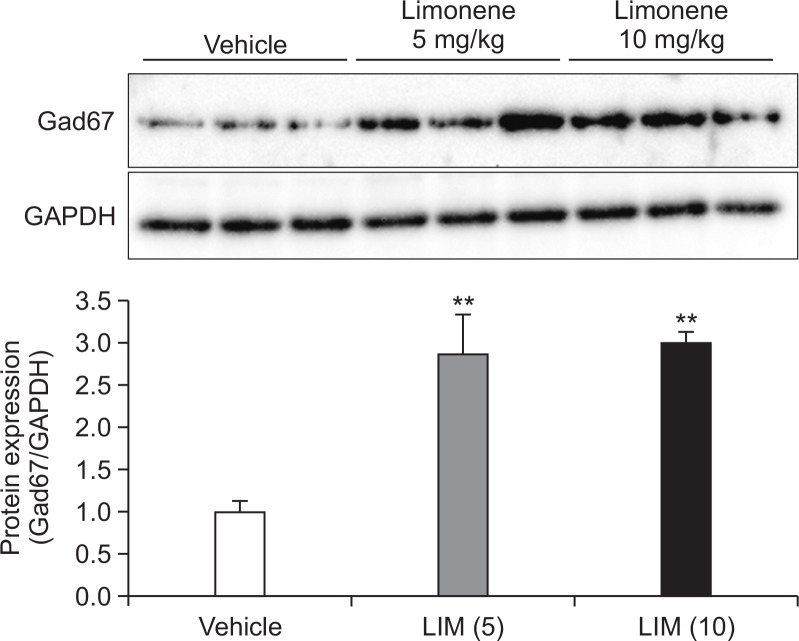
Effect of limonene on protein expression of Gad67 in striatum
Because it has been reported that limonene regulates GABA expression, we measured expression levels of Gad67 protein which is involved in synthesis of GABA in striatum. In this study, limonene (10 or 20 mg/kg) inhibited the development of locomotor sensitization, therefore, we examined whether a lower dose of limonene (10 mg/kg) has effects on the Gad67 expression. Administration of limonene significantly increased the expression levels of Gad67 (Fig. 5). This observation suggest that the increased level of Gad67 protein induced by limonene play a role in the reduced sensitization.
Fig. 5.
Effect of limonene on protein expression of Gad67. The expression of Gad67 was detected by Western blotting using specific antibodies in striatum. Each blot is representative of three experiments. Values indicate the mean ± standard error (SE) (n=8). **p<0.05 vs. vehicle (one-way ANOVA, followed by Bonferroni’s test).
DISCUSSION
We demonstrated that limonene may have therapeutic potential for the treatment of methamphetamine dependence. We have previously reported that limonene inhibits methamphetamineinduced hyperactivity (Yun, 2014); in the present study, limonene administration also reduced the development of sensitization to methamphetamine-induced locomotor activity. Furthermore, the limonene-treated groups showed a decreasing trend in their place preference in the methamphetamine-induced CPP test, even though this was not significant. Sensitization to locomotor activity and CPP are associated with an increase in mesolimbic dopaminergic neurotransmission, which is a mechanism underlying drug dependence (Pak et al., 2006; Bello et al., 2011). We also previously reported that limonene may reverse methamphetamine-induced elevation of dopamine levels in the nucleus accumbens of rats, by regulating GABA levels and activating GABA B receptors (Yun, 2014). Therefore, the results from the present study suggest that the inhibitory effect of limonene on methamphetamine-induced dopamine release may play a role in the reduction of sensitization.
It has previously been reported that an enhanced response to apomorphine, a direct-acting dopamine receptor agonist, results from the development of postsynaptic dopamine receptor supersensitivity after repeated administration of a drug of abuse (Ritzmann et al., 1979; Bhargava, 1980). Overall, evidence indicates that postsynaptic dopamine receptor supersensitivity may be associated with behavioral effects, such as the CPP and sensitization induced by morphine, cocaine, and amphetamines (Wolf et al., 1994; Kim et al., 1995, 1996a, 1996b; Henry et al. 1998). In this study, limonene inhibited the development of postsynaptic dopamine receptor super-sensitivity in the methamphetamine-induced sensitized rats, although the mechanism underlying the limonene-mediated reversal of supersensitivity development remains unclear. Limonene had no effect on the acute behavioral effects of apomorphine (Supplementary Data 1) and the role of dopamine receptors in the development of methamphetamine-induced sensitization remains controversial (Reed et al., 1987; Ujike et al., 1989; Nonaka and Moroji, 1990; Yoo et al., 2010). However, other neurotransmitter systems, including GABAergic, 5-HTergic, and glutamatergic systems, are also associated with methamphetamine-induced locomotor sensitization and postsynaptic dopamine receptor supersensitivity (Ohmori et al., 1994; Kim and Jang, 1997; Yoo et al., 2010). It has been reported that GABAergic (Zhou et al., 2009) and 5-HTergic (Yun, 2014) neuronal systems may mediate the effects of limonene on the CNS. Therefore, we suggest that limonene may inhibit the development of postsynaptic dopamine receptor supersensitivity in methamphetamine-induced sensitized rats via regulation of GABAergic and serotonergic modulation of dopaminergic neuronal transmission. In another study, Zhou et al. (2009) demonstrated that limonene administration significantly increased brain GABA levels in rats, and our present study also showed that protein expression level of Gad67 significantly induced through administration of limonene in striatum. Methamphetamine-induced behavioral sensitization down-regulated Gad67 levels in the nucleus accumbens (Zhang et al., 2006). Furthermore, we observed that a dopamine agonist, apomorphine-induced stereotype behavior was potentiated in Gad67 knock-down animal (data not shown). Pharmacologic increases in brain GABA levels have been reported to block the increase in dopamine levels elicited by morphine or cocaine injection (Klitenick et al., 1992; Morgan and Dewey, 1998). Therefore, limonene may reduce the development of physiological dependence by regulating GABAergic and dopaminergic neuronal transmission.
In conclusion, these results suggest that limonene is a promising candidate in the treatment of drug dependence.
Acknowledgments
This study was supported by the National Research Foundation of Korea [NRF] Grant funded by the Korea government (MSIP) (No. NRF, 2017R1C1B5017929 and MRC, 2017R1A5 A2015541).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Allen RM, Young SJ. Phencyclidine-induced psychosis. Am. J. Psychiatry. 1978;135:1081–1084. doi: 10.1176/ajp.135.9.1081. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN. Cyclo (leucylglycine) inhibits the development of morphine induced analgesic tolerance and dopamine receptor supersensitivity in rats. Life Sci. 1980;27:117–123. doi: 10.1016/0024-3205(80)90452-X. [DOI] [PubMed] [Google Scholar]
- Blander A, Hunt T, Blair R, Amit Z. Conditioned place preference: an evaluation of morphine’s positive reinforcing properties. Psychopharmacology. 1984;84:124–127. doi: 10.1007/BF00432040. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Heroin reward is dependent on a dopaminergic substrate. Life Sci. 1981;29:1881–1886. doi: 10.1016/0024-3205(81)90519-1. [DOI] [PubMed] [Google Scholar]
- de Almeida AA, Costa JP, de Carvalho RB, de Sousa DP, de Freitas RM. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012;1448:56–62. doi: 10.1016/j.brainres.2012.01.070. [DOI] [PubMed] [Google Scholar]
- do Amaral JF, Silva MI, Neto MR, Neto PF, Moura BA, de Melo CT, de Araujo FL, de Sousa DP, de Vasconcelos PF, de Vasconcelos SM, de Sousa FC. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull. 2007;30:1217–1220. doi: 10.1248/bpb.30.1217. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Golda A, Zaniewska M, Vetulani J, Przegalinski E. Various GABA-mimetic drugs differently affect cocaine-evoked hyperlocomotion and sensitization. Eur J Pharmacol. 2006;541:163–170. doi: 10.1016/j.ejphar.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Flamm WG, Lehman-McKeeman LD. The human relevance of the renal tumor-inducing potential of d-limonene in male rats: implications for risk assessment. Regul Toxicol Pharmacol. 1991;13:70–86. doi: 10.1016/0273-2300(91)90042-T. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Shen H, Hata H, Ohara A, Ohmi K, Ikeda K, Numachi Y, Kobayashi H, Hall FS, Uhl GR, Sora I. Methamphetamine-induced locomotor activity and sensitization in dopamine transporter and vesicular monoamine transporter 2 double mutant mice. Psychopharmacology. 2007;193:55–62. doi: 10.1007/s00213-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Cooper SJ. β-phenylethylamine-, d-amphetamine-and l-amphetamine-induced place preference conditioning in rats. Eur J Pharmacol. 1983;95:311–314. doi: 10.1016/0014-2999(83)90653-2. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Hu XT, White FJ. Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology. 1998;140:233–242. doi: 10.1007/s002130050762. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Hemby SE, Justice JB., Jr Environmental and pharmacological sensitization: effects of repeated administration of systemic or intra-nucleus accumbens cocaine. Psychopharmacology. 1993;111:109–116. doi: 10.1007/BF02257416. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jang CG. MK-801 inhibits methamphetamine-induced conditioned place preference and behavioral sensitization to apomorphine in mice. Brain Res Bull. 1997;44:221–227. doi: 10.1016/S0361-9230(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jang CG, Oh KW, Oh S, Rheu HM, Rhee GS, Seong YH, Park WK. Effects of ginseng total saponin on morphine-induced hyperactivity and conditioned place preference in mice. J Ethnopharmacol. 1998;60:33–42. doi: 10.1016/S0378-8741(97)00131-1. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jang CG, Park WK. Inhibition by MK-801 of morphine-induced conditioned place preference and postsynaptic dopamine receptor supersensitivity in mice. Pharmacol Biochem Behav. 1996a;55:11–17. doi: 10.1016/0091-3057(96)00078-0. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kang JG, Seong YH, Nam KY, Oh KW. Blockade by ginseng total saponin of the development of cocaine induced reverse tolerance and dopamine receptor supersensitivity in mice. Pharmacol Biochem Behav. 1995;50:23–27. doi: 10.1016/0091-3057(94)00224-7. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lim HK, Park WK. Antinarcotic effects of the velvet antler water extract on morphine in mice. J Ethnopharmacol. 1999;66:41–49. doi: 10.1016/S0378-8741(98)00193-7. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, Oh S. Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. Brain Res Bull. 1996b;40:201–207. doi: 10.1016/0361-9230(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima NG, De Sousa DP, Pimenta FC, Alves MF, De Souza FS, Macedo RO, Cardoso RB, de Morais LC, Melo Diniz Mde F, de Almeida RN. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol Biochem Behav. 2013;103:450–454. doi: 10.1016/j.pbb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Martin JR, Takemori AE. Chronically administered morphine increases dopamine receptor sensitivity in mice. Eur J Pharmacol. 1986;121:221–229. doi: 10.1016/0014-2999(86)90493-0. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Rice LL, Langfels M, Fields SE. Repeated treatments with 7-OH-DPAT: context-independent behavioral sensitization and conditioned hyperactivity. Pharmacol Biochem Behav. 2000;65:241–246. doi: 10.1016/S0091-3057(99)00204-X. [DOI] [PubMed] [Google Scholar]
- Morency MA, Stewart RJ, Beninger RJ. Circling behavior following unilateral microinjections of cocaine into the medial prefrontal cortex: dopaminergic or local anesthetic effect? J Neurosci. 1987;7:812–818. doi: 10.1523/JNEUROSCI.07-03-00812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AE, Dewey SL. Effects of pharmacologic increases in brain GABA levels on cocaine-induced changes in extracellular dopamine. Synapse. 1998;28:60–65. doi: 10.1002/(SICI)1098-2396(199801)28:1<60::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nonaka R, Moroji T. Effects of chronic methamphetamine treatment on the binding parameters of [3H]SCH 23390, a selective D1-dopamine receptor ligand, in the rat brain. Neurosci Lett. 1990;120:109–112. doi: 10.1016/0304-3940(90)90180-H. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Abekawa T, Muraki A, Koyama T. Competitive and noncompetitive NMDA antagonists block sensitization to methamphetamine. Pharmacol Biochem Behav. 1994;48:587–591. doi: 10.1016/0091-3057(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E, Hardy M, Brensilver J, Lattes C, McCabe R, D’Gati V, Reemtsma K, Suciu-Foca N. Anti-idiotypic antibodies to HLA and their influence on patient sensitization. Transplant Proc. 1987;19:762–763. [PubMed] [Google Scholar]
- Reid LD, Marglin SH, Mattie ME, Hubbell CL. Measuring morphine’s capacity to establish a place preference. Pharmacol Biochem Behav. 1989;33:765–775. doi: 10.1016/0091-3057(89)90468-1. [DOI] [PubMed] [Google Scholar]
- Ritzmann RF, Walter R, Bhargava HN, Flexner LB. Blockage of narcotic-induced dopamine receptor supersensitivity by cyclo(Leu-Gly) Proc Natl Acad Sci USA. 1979;76:5997–5998. doi: 10.1073/pnas.76.11.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/0165-0173(86)90002-0. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–11051. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato K, Ohkuma S. Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav. 2000;66:285–292. doi: 10.1016/S0091-3057(00)00185-4. [DOI] [PubMed] [Google Scholar]
- Sun J. D-Limonene: safety and clinical applications. Altern Med Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after sub-chronic treatment with methamphetamine or cocaine. Psychopharmacology. 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology. 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Kim HS, Yun JS, Lee MK, Oh KW, Seong YH, Oh SK, Jang CG. Inhibition of baclofen on morphine-induced hyperactivity, reverse tolerance and postsynaptic dopamine receptor supersensitivity. Pharmacol Res. 2001;43:335–340. doi: 10.1006/phrs.2000.0789. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Lee HK, Kim HC, Lee SY, Jang CG. GABA(A) receptors mediate the attenuating effects of a 5-HT(3) receptor antagonist on methamphetamine-induced behavioral sensitization in mice. Synapse. 2010;64:274–279. doi: 10.1002/syn.20726. [DOI] [PubMed] [Google Scholar]
- Yun J. Limonene inhibits methamphetamine-induced locomotor activity via regulation of 5-HT neuronal function and dopamine release. Phytomedicine. 2014;21:883–887. doi: 10.1016/j.phymed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Xiong X, Chen Q, Davidson C, Wetsel WC, Ellinwood EH. Methamphetamine induces long-term changes in GABAA receptor alpha2 subunit and GAD67 expression. Biochem Biophys Res Commun. 2006;351:300–305. doi: 10.1016/j.bbrc.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Zhou W, Yoshioka M, Yokogoshi H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol (Tokyo) 2009;55:367–373. doi: 10.3177/jnsv.55.367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.