Abstract
For more than 20 years, the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Center (EPC) Program has been identifying and synthesizing evidence to inform evidence-based healthcare. Recognizing that many healthcare settings continue to face challenges in disseminating and implementing evidence into practice, AHRQ’s EPC program has also embarked on initiatives to facilitate the translation of evidence into practice and to measure and monitor how practice changes impact health outcomes. The program has structured its efforts around the three phases of the Learning Healthcare System cycle: knowledge, practice, and data. Here, we use a topic relevant to the field of hospital medicine—Clostridium difficile colitis prevention and treatment—as an exemplar of how the EPC program has used this framework to move evidence into practice and develop systems to facilitate continuous learning in healthcare systems.
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure 1).1-3
FIG 1.
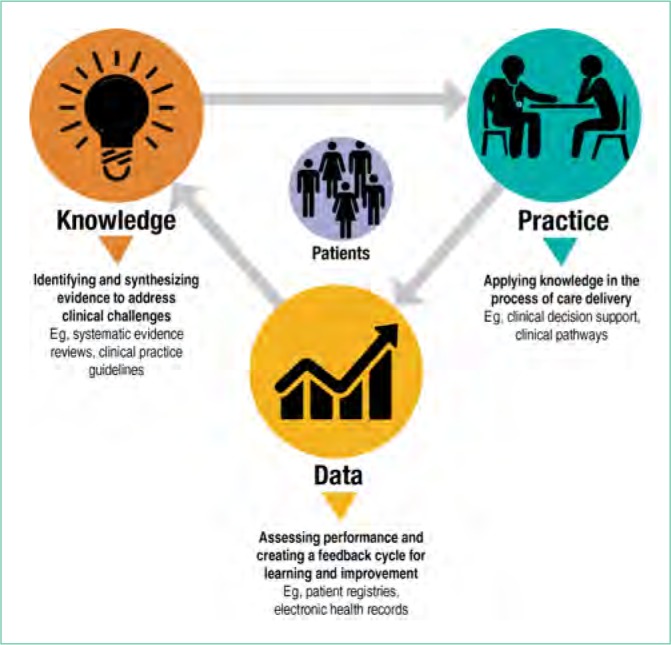
Knowledge to Practice to Data Cycle to Strengthen the Value of Patient Care
Knowledge: Identifying and synthesizing evidence to address clinical challenges
Practice: Applying knowledge in the process of care delivery
Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
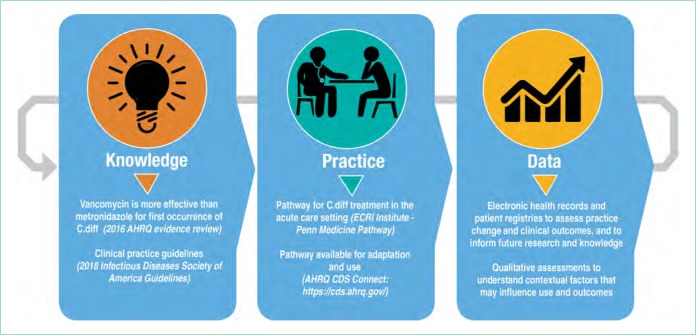
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
FIG 2.
Case Example of Knowledge to Practice to Data Cycle: Clostridium difficile colitis
Adapted from: https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
Rapid Evidence Products. AHRQ understands that health-care systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Footnotes
Disclosures: Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer: The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
References
- 1.Smith M, Saunders R, Stuckhardt L, McGinnis JM, editors. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. Committee on the Learning Health Care System in A, Institute of M. [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality [Accessed September 26, 2018];Learning Health Systems. 2017 https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html.
- 3.Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354–355. doi: 10.1001/jamainternmed.2014.5293. [DOI] [PubMed] [Google Scholar]
- 4.Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012. [Google Scholar]
- 5.Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745–754. doi: 10.18553/jmcp.2013.19.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12):2237–2239. doi: 10.1007/s11606-018-4633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal–a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi: 10.1186/s13012-016-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 10.Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012;9:Cd009401. doi: 10.1002/14651858.CD009401.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 13.Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. [Accessed September 28, 2018];Prevention Policy Matters Blog. 2018 https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/
- 14.Eden J, Levit L, Berg A, Morton S, editors. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. [Accessed January 17, 2019]. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. [PubMed] [Google Scholar]
- 15.Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. [Accessed January 17, 2019];Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update Rockville (MD): Agency for Healthcare Research and Quality (US) 2016 https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. [PubMed]
- 16.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for health-care epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores EJ, Mull NK, Lavenberg JG, et al. BMJ Qual Saf. 2018. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. bmjqs-2018. [DOI] [PubMed] [Google Scholar]
- 19.Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. (Agency for Healthcare Research and Quality). [Google Scholar]
- 20.Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. AHRQ methods for effective health care. [PubMed] [Google Scholar]
- 21.Hartling L, Guise JM, Kato E, et al. EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. AHRQ comparative effectiveness reviews. [PubMed] [Google Scholar]
- 22.Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451–1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Hartling L, Guise JM, Hempel S, et al. EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. AHRQ methods for effective health care. [PubMed] [Google Scholar]
- 24.Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality [Accessed September 26, 2018];Synthesizing Evidence for Quality Improvement. 2018 https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement.