Abstract
Two new 1,2-azaborine building blocks that enable the broad diversification of previously not readily accessible C4 and C5 ring positions of the 1,2-azaborine heterocycle are developed. 1,2-Azaborine’s distinct electronic structure allowed the resolution of a mixture of C4- and C5-borylated 1,2-azaborines. The connection between the electronic structure of C4 and C5 positions of 1,2-azaborine and their distinct reactivity patterns is revealed by a combination of reactivity studies and kinetic measurements that are supported by DFT calculations. Specifically, we show that oxidation by N-methylmorpholine N-oxide (NMO) is selective for the C4-borylated 1,2-azaborine, and the Ir-catalyzed deborylation is selective for the C5-borylated 1,2-azaborine via kinetically controlled processes. On the other hand, ligand exchange with diethanolamine takes place selectively with the C4-borylated isomer via a thermodynamically controlled process. These results represent the first examples for chemically distinguishing a mixture of two aryl mono-Bpin-substituted isomers.
Graphical Abstract

INTRODUCTION
BN/CC isosterism has emerged as a viable strategy to expand the chemical space of carbon-based compounds.1 Benzene is a versatile, ubiquitous, carbon-based structural motif, and as a consequence, our group has been focusing on the development of its BN isosteres,2,3 specifically the 1,2-dihydro-1,2-azaborine isomer, or simply 1,2-azaborine.4 Applications of BN-containing arenes are emerging, including advances in materials chemistry,5,6 in biomedical research,7 ligand design in homogeneous catalysis,8 and, more recently, in organic synthesis.9As an emerging field, progress in azaborine chemistry however is still limited by the synthetic access to new derivatives for further exploration. The N-TBS-B-Cl-1,2-azaborine (Scheme 1, TBS = t-butyldimethylsilyl) is now commercially available.10
Scheme 1.
Late-Stage Building-Block Functionalization Approach
Thus, the development of late-stage functionalization technologies from the assembled 1,2-azaborine heterocycle will enable ready synthetic access to new substituted derivatives and inspire further development in azaborine chemistry. To this end, the “building block” functionalization approach is an attractive strategy, where a versatile functional group (e.g., Bpin) can variably control the fate of a specific ring position, allowing for broad diversification at a “late stage” of the synthesis.11
The late-stage building block functionalization strategy has been successfully developed for the C3 and C6 positions of 1,2-azaborine. For example, bromination with Br2 has been determined to occur selectively at the C3 position of the 1,2-azaborine, and subsequent cross coupling with the C3–Br-substituted 1,2-azaborine has been accomplished.12 We have also demonstrated that Ir-catalyzed C−H borylation occurs selectively at the C6 position, which bears the most acidic C– H proton.13 The C6-Bpin-substituted 1,2-azaborine also readily engages in cross-coupling chemistry. Furthermore, we were able to access BN polyparaphenylene (BN-PPP) and discover the NH···arene interaction as a new conformational orientation feature as a direct consequence of the synthetic availability of the difunctionalized C3–Br–C6-Bpin-1,2-azaborine monomer building block (Scheme 2).14
Scheme 2.
New 1,2-Azaborine Building Blocks via Resolution Chemistry
To date, however, no general functionalization strategies have been developed for the C4 and C515 positions of the 1,2-azaborine heterocycle, which is a significant limitation for its envisioned application as a pharmacophore in medicinal chemistry or as a synthon in organic synthesis. In this work, we take advantage of the distinct electronic properties of the C4 and C5 positions of the 1,2-azaborine heterocycle to access C4- and C5-borylated 1,2-azaborine building blocks via resolution chemistry. Specifically, we show that oxidation by N-methylmorpholine N-oxide (NMO) is selective for the C4-borylated 1,2-azaborine, and the Ir-catalyzed deborylation is selective for the C5-borylated 1,2-azaborine via kinetically controlled processes. On the other hand, ligand exchange with diethanolamine takes place selectively with the C4-borylated isomer via a thermodynamically controlled process.
RESULTS AND DISCUSSION
While C–H borylation is selective for functionalization of the C6 position of a B-monosubstituted 1,2-azaborine,13 we determined that borylation of N,B-disubstituted-1,2-azaborines such as 1 (regioselectivity is primarily influenced by steric, not electronic factors in this case)16 occurs only at an elevated temperature and in a nonregioselective fashion, affording a 1:1.2–1.4 mixture of C4- and C5-borylated regioisomers (eq 1).
 |
(1) |
Physical methods to separate the mixture, such as chromatography or recrystallization, were unsuccessful. There- fore, we turned to reaction-based methods. Certain bis-Bpin-substituted alkenes/arenes can exhibit differential reactivity at each of their pinacol boronate esters.17 However, to the best of our knowledge, there are no examples for chemically distinguishing a mixture of monoaryl-Bpin-substituted isomers.18 Because each position of the 1,2-azaborine ring is electronically distinct,19 we envisioned that the asymmetry of the electronic structure of 1,2-azaborine would impart differential reactivity to the two borylated regioisomers 4Bpin and 5Bpin. Our hypothesis is as follows: because of partial localization of the nitrogen lone pair on nitrogen, the C5 carbon of 5Bpin should be more electron rich than the C4 carbon of the 4Bpin, and the boron in the Bpin of the 5Bpin isomer should be less Lewis acidic than the Bpin boron in 4Bpin (Figure 1). The extra resonance contribution provided by the Bpin in 5Bpin should also result in added stability of 5Bpin relative to the 4Bpin isomer.
Figure 1.
Electronic structure calculations (M062X/6–31G(d,p), computed using polarizable continuum model (PCM) simulating CH2Cl2 solvent): select Löwdin charges in parentheses (au), LUMO energies, and orbital illustrations.
DFT electronic structure calculations20 indeed indicate significant differences between 4Bpin and 5Bpin. (1) 5Bpin is thermodynamically more stable than 4Bpin by 3.8 kcal/mol (see Figure 2). (2) The pz-atomic orbital on the boron atom in the Bpin of 4Bpin significantly contributes to the LUMO of the molecule, in stark contrast to the corresponding orbital on the Bpin boron atom of 5Bpin (Figure 1). (3) The calculated LUMO energy of 4Bpin (−0.00923 eV) is significantly lower than the LUMO energy of 5Bpin.21 (4) The calculated Löwdin partial charge is more positive on the boron atom of the 4Bpin than the one on the boron atom of 5Bpin. These calculated trends are consistent with our originally stated hypothesis based on simple Lewis dot structure analysis; i.e., 5Bpin should be thermodynamically more stable than 4Bpin, and the Bpin boron atom in 4Bpin should be significantly more reactive toward Lewis bases.
Figure 2.
Calculated Gibbs free energy reaction coordinate profile of the NMO oxidation of 4Bpin and 5Bpin, computed at 298 K and 1 atm using the M062X/6–31G(d,p) method. The polarizable continuum model (PCM) was used to model the effects on the reaction by the CH2Cl2 solvent. Energies are in kcal/mol. All H atoms as well as the methyl on the pinacol, mesityl, and t-butyl substituents have been omitted for clarity.
OXIDATION WITH N-METHYLMORPHOLINE-N-OXIDE (NMO)
In our initial attempt to kinetically resolve the mixture of 4Bpin and 5Bpin with a Lewis basic reagent, we discovered that NMO indeed selectively oxidizes the 4Bpin isomer over the 5Bpin isomer.22 Thus, under our optimized conditions (4 equiv of NMO at room temperature for 24 h in CH2Cl2), compound 4OH can be isolated in 30% yield, while 53% of the unreacted 5Bpin (based on 55% available starting material) was recovered (Scheme 3).
Scheme 3.
Resolution of 5Bpin and 4Bpin via Selective Oxidation of 4Bpin
We independently measured the rate of the oxidation of the 4Bpin isomer and the rate of decomposition of the 5Bpin isomer23 by 1H NMR against an internal standard (vide infra for the isolation of the 4Bpin compound) and determined that the rate of the oxidation is approximately 29 times faster than the rate of decomposition, resulting in an effective kinetic resolution of the two borylated compounds. As a reference, we determined the rate of NMO oxidation of phenyl boronic acid pinacol ester (PhBpin) to be 20-fold slower than the corresponding rate for 4Bpin (Scheme 3).
The experimentally observed relative rates are consistent with the calculated free energy reaction coordinate profile (M062X/6–31G(d,p); see Supporting Information for details). As can be seen from Figure 2, 5Bpin is calculated to be more stable than 4Bpin by 3.8 kcal/mol. The oxidation with NMO involves the NMO-Bpin adduct as the intermediate, which then subsequently rearranges to the borate ester with concomitant release of the N-methyl morpholine.22d,e Our DFT calculations predict the second step (C to O migration) to be the rate-determining step for both 4Bpin and 5Bpin. The rate-limiting activation barrier for 4Bpin (23.8 kcal/mol) is smaller than the corresponding barrier for the 5Bpin compound (27.7 kcal/mol), which is consistent with the selective oxidation of the 4Bpin isomer. Overall, it appears that the extra stability of the ground state (due to additional resonance with the lone pair of nitrogen) relative to its rate-limiting transition state exhibited by 5Bpin as compared to 4Bpin is responsible for the kinetic resolution.
IRIDIUM-CATALYZED PROTODEBORYLATION
With a method for accessing the 5Bpin isomer in hand, we pursued resolution chemistry that could be used to access 4Bpin. Thus, we needed a reaction that would preferentially react with the less Lewis acidic and more stable 5Bpin isomer. According to our electronic structure calculations (Figure 3), the C5 carbon in 5Bpin is more electron rich than the C4 carbon in 4Bpin. Although the HOMO orbital energies for 4Bpin and 5Bpin are similar, the HOMO orbital coefficient of the C5 carbon in 5Bpin (0.11) is significantly larger than the one for the C4 carbon in 4Bpin (0.01). Overall, the results indicate stronger nucleophilic character for the C5 carbon in 5Bpin than the C4 carbon in 4Bpin. Thus, we hypothesize that a transmetalation-type reaction24 would favor 5Bpin over 4Bpin, and we chose the Ir-catalyzed protodeborylation for our investigation.25,26
Figure 3.
Electronic structure calculations (M062X/6–31G(d,p), computed using polarizable continuum model (PCM) simulating CH2Cl2 solvent): select Löwdin charges in parentheses (au), HOMO energies, and orbital illustrations.
When a 1:1.4 mixture of 4Bpin and 5Bpin was subjected to protodeborylation catalyzed by [Ir(COD)OMe]2 with methanol as the proton source, 4Bpin was isolated in 25% yield based on 42% available starting material (Scheme 4).
Scheme 4.
Kinetic Resolution via Ir-Catalyzed Protodeborylationa
aIndependent rate measurements performed at 40 °C.
Smith observed that in the case of bisborylated compounds the first Bpin to be installed on the molecule was also the first to be protodeborylated.25b Interestingly, 5Bpin is selectively deborylated despite the fact that both C4 and C5 positions of azaborine undergo C–H borylation with nearly equal propensity. We measured the independent rates of the protodeborylation reaction for 4Bpin and 5Bpin at 40 °C and found that the rate of protodeborylation of 5Bpin is about 23 times faster than that of 4Bpin, consistent with the kinetic resolution that occurs on the preparative scale. We also determined the rate of protodeborylation of the benchmark compound PhBpin to be similar to that of 4Bpin (Scheme 4).
TRANSESTERIFICATION WITH DIETHANOLAMINE
We also investigated the transesterification27 of the boron pinacolate ester with diethanolamine with the aim to convert the mixture of the Bpin isomers into a separable mixture of differentially borylated compounds. Diethanolamine when bound to an organoboron compound typically forms a zwitterionic 4-coordinate borate. When the transesterification from pinacol to diethanolamine is performed in a nonpolar ethereal solvent, the charged diethanolamine adduct tends to precipitate out of the solution.28 We hypothesize that the more Lewis acidic 4Bpin should be the favored reactant in a transesterification with diethanolamine. Thus, when we treated a 1:1 mixture of 4Bpin and 5Bpin with diethanolamine in methyl-tert-butylether (MTBE), we observed a white precipitate that was separated from the reaction mixture. The precipitate contained only the 4BO2N isomer (50% yield, 25% based on available starting material), and the filtrate was enriched in the 5Bpin isomer (Scheme 5).
Scheme 5.
Reaction of Borylated Isomers with Diethanolamine
Addition of more equivalents of diethanolamine and changing the temperature did not drive the reaction forward to the diethanolamine product but instead resulted in an erosion of the selectivity.
We conducted studies to determine whether the transesterification reaction is kinetically or thermodynamically controlled. Here, we used THF as the solvent to ensure that the reaction mixture formed a homogeneous solution. Monitoring the transesterification reaction for each isomer independently by 1H NMR at room temperature revealed that the transesterification had reached equilibrium within 3 h. All four components were observed in the reaction mixture: boron pinacol ester (A), free diethanolamine (B), diethanolamine adduct (C), and free pinacol (D). Initial rates of the transesterification reaction of each isomer were measured using reaction calorimetry and were found to be nearly identical (see Supporting Information for details). Determination of the concentration of each component in the reaction mixture by 1H NMR yielded the equilibrium constant, Keq, for each isomer. We found that the Keq(4Bpin) is larger than the Keq(5Bpin) by a factor of 29 (Scheme 6).
Scheme 6.
Kinetic and Thermodynamic Studies of Transesterification Reaction
The observed relative binding propensity Keq(4Bpin):Keq(5Bpin) is corroborated by DFT calculations. The Gibbs free energy of formation of the 5BO2N adduct is ΔG = −5.1 kcal mol−1, while the formation of 4BO2N is more favorable with ΔG = −6.1 kcal mol−1. Thus, the separation of the mixture of borylated 1,2-azaborine, as illustrated in Scheme 5, is an example of a thermodynamically controlled resolution process.
FUNCTIONALIZATION OF THE 4- AND 5-POSITIONS OF THE 1,2-AZABORINE
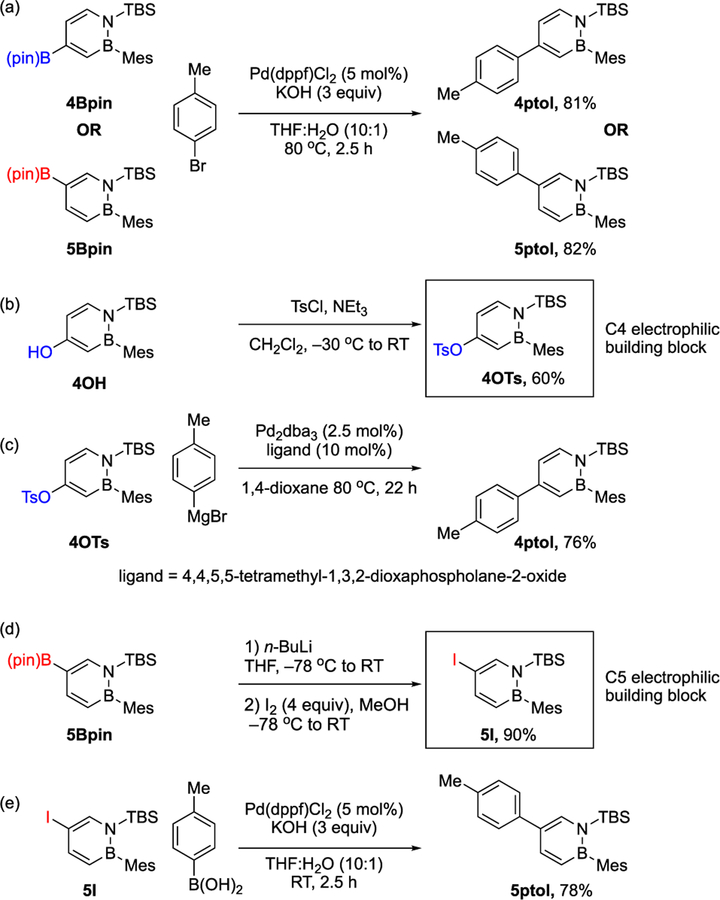
The isolated 4Bpin or 5Bpin building blocks can undergo Suzuki–Miyaura cross-coupling with an aryl halide to afford triaryl 4ptol or 5ptol in good yields (Scheme 7a). It is also possible to generate new electrophilic building blocks at the 4 and 5 positions of the 1,2-azaborine utilizing the versatile transformations of organoboron compounds. For example, compound 4OH, generated via the NMO oxidation of 4Bpin, can be converted into the tosylate 4OTs (Scheme 7b).29 Subsequent Kumada coupling of 4OTs with an aryl Grignard reagent furnishes 4ptol (Scheme 7c).30 An iodine group can be installed at the C5 position using a procedure adapted from the Evans modification of the Zweifel reaction (Scheme 7d).31 5I undergoes facile Suzuki–Miyaura coupling at room temperature with p-tolylboronic acid to afford 5ptol (Scheme 7e).
Scheme 7.
Functionalizations of 4Bpin and 5Bpin
CONCLUSION
We have developed the synthesis of two new 1,2-azaborine building blocks that enables the ready diversification of C4 and C5 ring positions of 1,2-azaborine. We took advantage of 1,2-azaborine’s distinct electronic structure to resolve a mixture of borylated 1,2-azaborines. By means of kinetic and thermodynamic studies and DFT calculations we have been able to rationalize the difference in reactivities of 4Bpin and 5Bpin at their C4 and C5 positions. NMO selectively oxidizes the more Lewis acidic 4Bpin 1,2-azaborine isomer, whereas Ir-catalyzed protodeborylation selectively deborylates the more nucleophilic 5Bpin 1,2-azaborine isomer. Transesterification of the pinacol group with diethanolamine also favors the more Lewis acidic 4Bpin in a thermodynamically controlled process. The aforementioned resolution methods provide access to the previously inaccessible C4-Bpin and C5-Bpin building blocks which could be further functionalized using cross-coupling chemistry. The availability of a general approach to the functionalization of the C4- and C5-positions of the 1,2-azaborine will undoubtedly advance BN/CC isosterism as a versatile strategy for expanding the currently available chemical space and enable emerging applications in the biomedical and materials science fields.
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof. Todd Marder for helpful discussions. This work was supported by NIH NIGMS (R01-GM094541) and Boston College start-up funds.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b03611.
Experimental procedures and compound characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) For an overview, see: Liu Z; Marder TB B-N versus C-C: how similar are they? Angew. Chem., Int. Ed 2008, 47, 242–244. [DOI] [PubMed] [Google Scholar]; (b) Bosdet MJD; Piers WE B-N as a C-C substitute in aromatic systems. Can. J. Chem 2009, 87, 8–29. [Google Scholar]; (c) Campbell PG; Marwitz AJV; Liu S-Y Recent advances in azaborine chemistry. Angew. Chem., Int. Ed 2012, 51, 6074–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Belanger-Chabot G; Braunschweig H; Roy DK Recent Developments in Azaborinine Chemistry. Eur. J. Inorg. Chem 2017, 4353–4368.; (e) Giustra ZX; Liu S-Y The State of the Art in Azaborine Chemistry: New Synthetic Methods and Applications. J. Am. Chem. Soc 2018, 140, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) For 1,3-azaborines, see: Xu S; Zakharov LN; Liu S-YA 1,3-dihydro-1,3-azaborine debuts. J. Am. Chem. Soc 2011, 133, 20152–20155. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu S; Mikulas TC; Zakharov LN; Dixon DA; Liu S-Y Boron-substituted 1,3-dihydro-1,3-azaborines: synthesis, structure, and evaluation of aromaticity. Angew. Chem., Int. Ed 2013, 52, 7527–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) For 1,4-azaborines, see: Braunschweig H; Damme A; Jimenez-Halla JOC; Pfaffinger B; Radacki K; Wolf J Metal-Mediated Synthesis of 1,4-Di-tert-butyl-1,4-azaborine. Angew. Chem., Int. Ed 2012, 51, 10034–10037. [DOI] [PubMed] [Google Scholar]; (b) Schafer M; Beattie NA; Geetharani K; Schafer J; Ewing WC; Krahfuss M; Horl C; Dewhurst RD; Macgregor SA; Lambert C; Braunschweig H Synthesis of Functionalized 1,4-Azaborinines by the Cyclization of Ditert-butyliminoborane and Alkynes. J. Am. Chem. Soc 2016, 138, 8212–8220. [DOI] [PubMed] [Google Scholar]; (c) Liu X; Zhang Y; Li B; Zakharov LN; Vasiliu M; Dixon DA; Liu SY A Modular Synthetic Approach to Monocyclic 1,4-Azaborines. Angew. Chem., Int. Ed 2016, 55, 8333–8337. [DOI] [PubMed] [Google Scholar]
- (4).Marwitz AJV; Matus MH; Zakharov LN; Dixon DA; Liu S-Y A hybrid organic/inorganic benzene. Angew. Chem., Int. Ed 2009, 48, 973–977. [DOI] [PubMed] [Google Scholar]
- (5).(a) For an overview, see: Wang X-Y; Wang J-Y; Pei J BN Heterosuperbenzenes: Synthesis and Properties. Chem. - Eur. J 2015, 21, 3528–3539. [DOI] [PubMed] [Google Scholar]; (b) Morgan MM; Piers WE Efficient synthetic methods for the installation of boron-nitrogen bonds in conjugated organic molecules. Dalton Trans 2016, 45, 5920–5924. [DOI] [PubMed] [Google Scholar]; (c) Huang J; Li Y BN Embedded Polycyclic b-Conjugated Systems: Synthesis, Optoelectronic Properties, and Photovoltaic Applications. Front. Chem 2018, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) For select recent examples, see: Helten HB = N Units as Part of Extended π-Conjugated Oligomers and Polymers. Chem. - Eur. J 2016, 22, 12972–12982. [DOI] [PubMed] [Google Scholar]; (b) Wan W-M; Baggett AW; Cheng F; Lin H; Liu S-Y; Jakle F Synthesis by free radical polymerization and properties of BN-polystyrene and BN-poly(vinylbiphenyl). Chem. Commun 2016, 52, 13616–13619. [DOI] [PubMed] [Google Scholar]; (c) Thiedemann B; Gliese PJ; Hoffmann J; Lawrence PG; Sönnichsen FD; Staubitz A High molecular weight poly(N-methyl-B-vinylazaborine) - a semi-inorganic B-N polystyrene analogue. Chem. Commun 2017, 53, 7258–7261. [DOI] [PubMed] [Google Scholar]; (d) Ishibashi JSA; Dargelos A; Darrigan C; Chrostowska A; Liu S-Y BN Tetracene: Extending the Reach of BN/CC Isosterism in Acenes. Organometallics 2017, 36, 2494–2497. [Google Scholar]; (e) Liu Z; Ishibashi JSA; Darrigan C; Dargelos A; Chrostowska A; Li B; Vasiliu M; Dixon DA; Liu S-Y The Least Stable Isomer of BN Naphthalene: Toward Predictive Trends for the Optoelectronic Properties of BN Acenes. J. Am. Chem. Soc 2017, 139, 6082–6085. [DOI] [PubMed] [Google Scholar]; (f) van de Wouw HL; Awuyah EC; Baris JI; Klausen RS An Organoborane Vinyl Monomer with Styrene-like Radical Reactivity: Reactivity Ratios and Role of Aromaticity. Macromolecules 2018, 51, 6359–6368. [Google Scholar]; (g) Matsui K; Oda S; Yoshiura K; Nakajima K; Yasuda N; Hatakeyama T One-Shot Multiple Borylation toward BN-Doped Nanographenes. J. Am. Chem. Soc 2018, 140, 1195–1198. [DOI] [PubMed] [Google Scholar]; (h) Nakatsuka S; Yasuda N; Hatakeyama T Four-Step Synthesis of B2N2-Embedded Corannulene. J. Am. Chem. Soc 2018, 140, 13562– 13565. [DOI] [PubMed] [Google Scholar]
- (7).(a) Vlasceanu A; Jessing M; Kilburn JP BN/CC isosterism in borazaronaphthalenes towards phosphodiesterase 10A (PDE10A) inhibitors. Bioorg. Med. Chem 2015, 23, 4453–4461. [DOI] [PubMed] [Google Scholar]; (b) Rombouts FJ; Tovar F; Austin N; Tresadern G; Trabanco AA Benzazaborinines as Novel Bioisosteric Replacements of Naphthalene: Propranolol as an Example. J. Med. Chem 2015, 58, 9287–9295. [DOI] [PubMed] [Google Scholar]; (c) Lee H; Fischer M; Shoichet BK; Liu S-Y Hydrogen Bonding of 1,2-Azaborines in the Binding Cavity of T4 Lysozyme Mutants: Structures and Thermodynamics. J. Am. Chem. Soc 2016, 138, 12021–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao P; Nettleton DO; Karki R; Zecri FJ; Liu S-Y Medicinal Chemistry Profiling of Monocyclic 1,2-Azaborines. ChemMedChem 2017, 12, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Bailey JA; Haddow MF; Pringle PG A simple route to azaborinylphosphines: isoelectronic B-N analogues of arylphosphine ligands. Chem. Commun 2014, 50, 1432–1434. [DOI] [PubMed] [Google Scholar]; (b) Sun F; Huang M; Zhou Z; Fang X 4a,8a-Azaboranaphthalene-4-yl phosphine ligands: synthesis and electronic modulation in Suzuki-Miyaura coupling reactions. RSC Adv 2015, 5, 75607–75611. [Google Scholar]; (c) Xu S; Zhang Y; Li B; Liu S-Y Site- and Stereo-selective trans-Hydroboration of 1,3-Enynes Catalyzed by 1,4-Azaborine-Based Phosphine-Pd Complex. J. Am. Chem. Soc 2016, 138, 14566– 14569. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McConnell CR; Campbell PG; Fristoe CR; Memmel P; Zakharov LN; Li B; Darrigan C; Chrostowska A; Liu S-Y Synthesis and Characterization of 1,2-Azaborine-Containing Phosphine Ligands: A Comparative Electronic Structure Analysis. Eur. J. Inorg. Chem 2017, 2017, 2207–2210. [Google Scholar]
- (9).(a) Burford RJ; Li B; Vasiliu M; Dixon DA; Liu S-Y Diels-Alder Reactions of 1,2-Azaborines. Angew. Chem., Int. Ed 2015, 54, 7823–7827. [DOI] [PubMed] [Google Scholar]; (b) Edel K; Yang X; Ishibashi JSA; Lamm AN; Maichle-Mössmer C; Giustra ZX; Liu S-Y; Bettinger HF The Dewar Isomer of 1,2-dihydro-1,2-azaborinines: Isolation, Fragmentation, and Energy Storage. Angew. Chem., Int. Ed 2018, 57, 5296–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).N-(t-Butyldimethylsilyl)-B-chloro-1,2-azaborine is currently available from Strem: http://https://www.strem.com/catalog/v/05-0150/boron_1138164-75-6 (accessed February 22, 2019).
- (11).(a) For an overview, see: Hartwig JF Borylation and silylation of C-H bonds: a platform for diverse C-H bond functionalizations. Acc. Chem. Res 2012, 45, 864–873. [DOI] [PubMed] [Google Scholar]; (b) Larsen MA; Hartwig JF Iridium-Catalyzed C-H Borylation of Heteroarenes: Scope, Regioselectivity, Application to Late-Stage Functionalization, and Mechanism. J. Am. Chem. Soc 2014, 136, 4287–4299. [DOI] [PubMed] [Google Scholar]; (c) Xu L; Zhang S; Li P Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev 2015, 44, 8848–8858. [DOI] [PubMed] [Google Scholar]
- (12).(a) Pan J; Kampf JW; Ashe AJ Electrophilic aromatic substitution reactions of 1,2-dihydro-1,2-azaborines. Org. Lett 2007, 9, 679–681. [DOI] [PubMed] [Google Scholar]; (b) Brown AN; Li B; Liu SY Negishi Cross-Coupling Is Compatible with a Reactive B-Cl Bond: Development of a Versatile Late-Stage Functionalization of 1,2-Azaborines and Its Application to the Synthesis of New BN Isosteres of Naphthalene and Indenyl. J. Am. Chem. Soc 2015, 137, 8932–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brown AN; Li B; Liu SY Expanding the functional group tolerance of cross-coupling in 1,2-dihydro-1,2-azaborines: Installation of alkyl, alkenyl, aryl, and heteroaryl substituents while maintaining a B-H bond. Tetrahedron 2019, 75, 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Baggett AW; Vasiliu M; Li B; Dixon DA; Liu S-Y Late-Stage Functionalization of 1,2-Dihydro-1,2-azaborines via Regioselective Iridium-Catalyzed C-H Borylation: The Development of a New N,N-Bidentate Ligand Scaffold. J. Am. Chem. Soc 2015, 137, 5536–5541. [DOI] [PubMed] [Google Scholar]
- (14).Baggett AW; Guo F; Li B; Liu SY; Jakle F Regioregular Synthesis of Azaborine Oligomers and a Polymer with a syn Conformation Stabilized by NH-pi Interactions. Angew. Chem., Int. Ed 2015, 54, 11191–11195. [DOI] [PubMed] [Google Scholar]
- (15).(a) For available methods of C5 functionalization, see: Zhang Y; Dan W; Fang X Metal Nitrate Mediated Regioselective Nitration of BN-Substituted Arenes. Organometallics 2017, 36, 1677–1680. [Google Scholar]; (b) Zhang Y; Sun F; Dan W; Fang X Friedel-Crafts Acylation Reactions of BN-Substituted Arenes. J. Org. Chem 2017, 82, 12877– 12887. [DOI] [PubMed] [Google Scholar]
- (16).For a mechanistic analysis for Ir-catalyzed C–H borylation, see: Boller TM; Murphy JM; Hapke M; Ishiyama T; Miyaura N; Hartwig JF Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. Intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc 2005, 127, 14263–14278. [DOI] [PubMed] [Google Scholar]
- (17).(a) Shimizu M; Nakamaki C; Shimono K; Schelper M; Kurahashi T; Hiyama T Stereoselective Cross-Coupling Reaction of 1,1-Diboryl-1-alkenes with Electrophiles: A Highly Stereocontrolled Approach to 1,1,2-Triaryl-1-alkenes. J. Am. Chem. Soc 2005, 127, 12506–12507. [DOI] [PubMed] [Google Scholar]; (b) Loach RP; Fenton OS; Amaike K; Siegel DS; Ozkal E; Movassaghi M C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines. J. Org. Chem 2014, 79, 11254–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sridhar T; Berreé F; Sharma GVM; Carboni B Regio- and Stereocontrolled Access to γ-Boronated Unsaturated Amino Esters and Derivatives from (Z)-Alkenyl 1,2-Bis(boronates). J. Org. Chem 2014, 79, 783–789. [DOI] [PubMed] [Google Scholar]; (d) Kallepalli VA; Gore KA; Shi F; Sanchez L; Chotana GA; Miller SL; Maleczka RE; Smith MR Harnessing C-H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization. J. Org. Chem 2015, 80, 8341–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Pareek M; Fallon T; Oestreich M Platinum(0)-Catalyzed Indolyne Insertion into Bis(pinacolato)diboron Followed by Site-Selective Suzuki-Miyaura Cross-Coupling. Org. Lett 2015, 17, 2082–2085. [DOI] [PubMed] [Google Scholar]
- (18).(a) For strategies distinguishing two different boron-based functional groups, see: Wang C; Glorius F Controlled Iterative Cross-Coupling: On the Way to the Automation of Organic Synthesis. Angew. Chem., Int. Ed 2009, 48, 5240–5244. [DOI] [PubMed] [Google Scholar]; (b) Xu L; Zhang S; Li P Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev 2015, 44, 8848–8858. [DOI] [PubMed] [Google Scholar]; (c) For select examples, see: Noguchi H; Hojo K; Suginome M Boron-Masking Strategy for the Selective Synthesis of Oligoarenes via Iterative Suzuki-Miyaura Coupling. J. Am. Chem. Soc 2007, 129, 758–759. [DOI] [PubMed] [Google Scholar]; (d) Fyfe JWB; Fazakerley NJ; Watson AJB Chemoselective Suzuki-Miyaura Cross-Coupling via Kinetic Transmetallation. Angew. Chem., Int. Ed 2017, 56, 1249– 1253. [DOI] [PubMed] [Google Scholar]; (e) Molloy JJ; Clohessy TA; Irving C; Anderson NA; Lloyd-Jones GC; Watson AJB Chemoselective oxidation of aryl organoboron systems enabled by boronic acid-selective phase transfer. Chem. Sci 2017, 8, 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chrostowska A; Xu S; Lamm AN; Mazier̀e A; Weber CD; Dargelos A; Bayler̀e P; Graciaa A; Liu S-Y UV-Photoelectron Spectroscopy of 1,2- and 1,3-Azaborines: A Combined Experimental and Computational Electronic Structure Analysis. J. Am. Chem. Soc 2012, 134, 10279–10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AF; Bloino J; Zheng G; Sonnenberg JL; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Montgomery JA Jr.; Peralta JE; Ogliaro F; Bearpark M; Heyd JJ; Brothers E; Kudin KN; Staroverov VN; Kobayashi R; Normand J; Raghavachari K; Rendell A; Burant JC; Iyengar SS; Tomasi J; Cossi M; Rega N; Millam JM; Klene M; Knox JE; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Martin RL; Morokuma K; Zakrzewski VG; Voth GA; Salvador P; Dannenberg JJ; Dapprich S; Daniels AD; Farkas O; Foresman JB; Ortiz JV; Cioslowski J; Fox DJ Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, 2010. [Google Scholar]
- (21).LUMO energies > 0 eV (for 5Bpin) have no physical meaning but are reported here to allow for direct comparison.
- (22).(a) For examples of oxidation of organoboron compounds with N-oxides, see: Kabalka GW; Hedgecock HC Mild and convenient oxidation procedure for the conversion of organoboranes to the corresponding alcohols. J. Org. Chem 1975, 40, 1776–1779. [Google Scholar]; (b) Soderquist JA; Najafi MR Selective oxidation of organoboranes with anhydrous trimethylamine N-oxide. J. Org. Chem 1986, 51, 1330–1336. [Google Scholar]; (c) Zhu C; Wang R; Falck JR Mild and Rapid Hydroxylation of Aryl/Heteroaryl Boronic Acids and Boronate Esters with N-Oxides. Org. Lett 2012, 14, 3494–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim J; Bertozzi CR A Bioorthogonal Reaction of N-Oxide and Boron Reagents. Angew. Chem., Int. Ed 2015, 54, 15777–15781. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gupta S; Sureshbabu P; Singh AK; Sabiah S; Kandasamy J Deoxygenation of tertiary amine N-oxides under metal-free condition using phenylboronic acid. Tetrahedron Lett 2017, 58, 909–913. [Google Scholar]
- (23).Attempts to convert the 5Bpin to the 5-hydroxy compound were unsuccessful, as elevating the reaction temperature led to decomposition of 5Bpin to unknown products.
- (24).Electron-rich organoboron compounds are known to preferentially undergo a transmetalation reaction. See: Beaumard F; Dauban P; Dodd RH One-Pot Double Suzuki-Miyaura Couplings: Rapid Access to Nonsymmetrical Tri(hetero)aryl Derivatives. Org. Lett 2009, 11, 1801–1804. [DOI] [PubMed] [Google Scholar]
- (25).(a) Movassaghi and Smith have demonstrated that certain bisborylated heterocycles can be selectively monoprotodeborylated under mild conditions. See: Loach RP; Fenton OS; Amaike K; Siegel DS; Ozkal E; Movassaghi M C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines. J. Org. Chem 2014, 79, 11254–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kallepalli VA; Gore KA; Shi F; Sanchez L; Chotana GA; Miller SL; Maleczka RE; Smith MR Harnessing C-H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization. J. Org. Chem 2015, 80, 8341–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).(a) Nishimura T; Yasuhara Y; Hayashi T Highly Selective 1,6-Addition of Aryl Boronic Acids to α,β,γ,δ-Unsaturated Carbonyl Compounds Catalyzed by an Iridium Complex. Angew. Chem., Int. Ed 2006, 45, 5164–5166. [DOI] [PubMed] [Google Scholar]; (b) Nishimura T; Yasuhara Y; Hayashi T Iridium Catalyzed [3 + 2] Annulation of 1,3-Dienes with ortho-Carbonylated Phenylboronic Acids. A Catalytic Process Involving Regioselective 1,2-Addition. J. Am. Chem. Soc 2007, 129, 7506–7507. [DOI] [PubMed] [Google Scholar]
- (27).Roy CD; Brown HC A Comparative Study of the Relative Stability of Representative Chiral and Achiral Boronic Esters Employing Transesterification. Monatsh. Chem 2007, 138, 879–887. [Google Scholar]
- (28).Bonin H; Leuma-Yona R; Marchiori B; Demonchaux P; Gras E Highly practical boronic acid surrogates for the Suzuki-Miyaura cross-coupling. Tetrahedron Lett 2011, 52, 1132–1136. [Google Scholar]
- (29).Lin G; Zhang A Synthesis of Optically Pure Clausenamine-A and its Demethoxylated Analogs. Tetrahedron 2000, 56, 7163–7171. [Google Scholar]
- (30).Ackermann L; Kapdi AR; Fenner S; Kornhaass C; Schulzke C Well-Defined Air-Stable Palladium (HAPSO) Complexes for Efficient Kumada-Corriu Cross-Couplings of (Hetero)Aryl or Alkenyl Tosylates. Chem. - Eur. J 2011, 17, 2965–2971. [DOI] [PubMed] [Google Scholar]
- (31).Evans DA; Crawford TC; Thomas RC; Walker JA Studies Directed toward the Synthesis of Prostaglandins. Useful Boron-Mediated Olefin Syntheses. J. Org. Chem 1976, 41, 3947–3953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.