Abstract.
The CYD-TDV vaccine is licensed in multiple endemic countries based on vaccine efficacy (VE) against symptomatic, virologically confirmed dengue demonstrated in two phase 3 trials (CYD14, 2- to 14-year-olds, Asia; CYD15, 9- to 16-year-olds, Latin America). 50% plaque reduction neutralization test (PRNT50) titers at baseline and month 13 (post-vaccination) were associated with VE and may enable bridging VE to adults. Two phase 2 trials of CYD-TDV measured baseline and month 13 PRNT50 titers: CYD22 (9- to 45-year-olds, Vietnam) and CYD47 (18- to 45-year-olds, India). 50% plaque reduction neutralization test distributions were compared between age cohorts, and four versions of an epidemiological bridging method were used to estimate VE against any serotype (dengue virus [DENV]-Any) and against each serotype over 25 months post first vaccination in a hypothetical CYD14 + CYD15 18- to 45-year-old cohort (bridging population 1) and in the actual CYD47 18- to 45-year-old cohort (bridging population 2). Baseline and month 13 geometric mean PRNT50 titers to each serotype were significantly greater in 18- to 45-year-olds than 9- to 16-year-olds for all comparisons. The four methods estimated VE against DENV-Any at 75.3–86.0% (95% CIs spanning 52.5–100%) for bridging population 1 and 68.4–77.5% (95% CIs spanning 42.3–88.5%) for bridging population 2. The vaccine efficacy against serotype 1, 2, 3, and 4 was estimated at 56.9–76.9%, 68.3–85.8%, 91.4–95.0%, and 93.2–100% (bridging population 1) and 44.5–66.9%, 53.2–69.2%, 79.8–92.0%, and 90.6–95.0% (bridging population 2), respectively; thus, CYD-TDV would likely confer improved efficacy in adults than 9- to 16-year-olds. Using the same methods, we predicted VE against hospitalized DENV-Any over 72 months of follow-up, with estimates 59.1–73.5% (95% CIs spanning 40.9–92.2%) for bridging population 1 and 50.9–65.9% (95% CIs spanning 38.1–82.1%) for bridging population 2.
INTRODUCTION
The four serotypes of dengue virus (DENV-1, DENV-2, DENV-3, and DENV-4) are transmitted by Aedes mosquitoes and have been estimated to cause nearly 400 million infections (100 million symptomatic) annually.1–3 The spectrum of dengue disease ranges from mild febrile illness to dengue hemorrhagic fever (DHF) and potentially fatal dengue shock syndrome (DSS). Dengue occurs in more than 100 countries worldwide, predominantly in tropical and subtropical regions, and up to 4 billion people worldwide are at the risk of infection.4 Epidemiological studies have shown that the seroprevalence of dengue neutralizing antibodies elicited by natural infection increases with age in endemic regions, indicating the ongoing and repeated exposure to DENV in these regions.5–8 There is clearly a need for wide geographic deployment of an effective dengue vaccine in endemic regions, including one indicated for adults.9–13
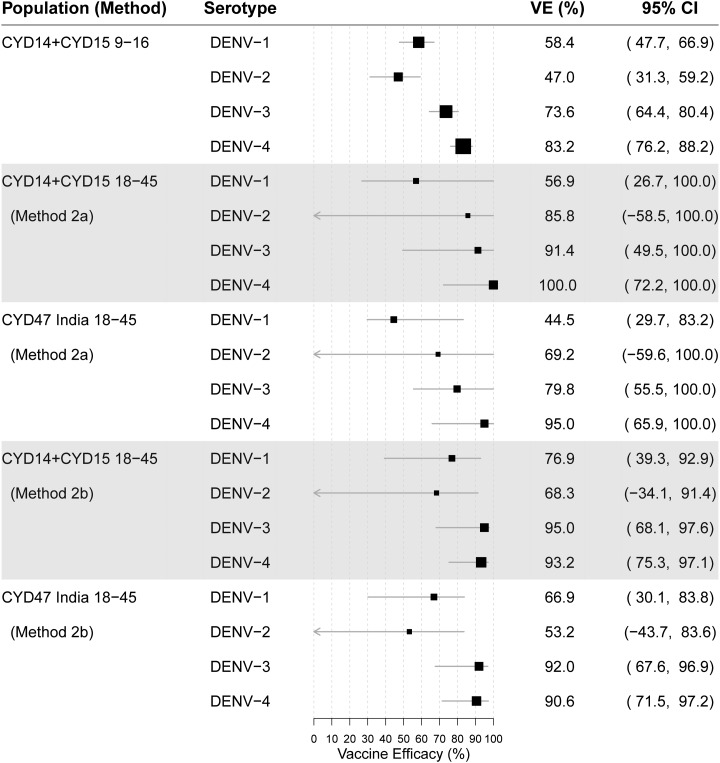
The only dengue vaccine licensed to date is the recombinant live attenuated tetravalent CYD-TDV vaccine (Dengvaxia®), developed and manufactured by Sanofi Pasteur (Marcy-l’Etoile, France).14 Estimated vaccine efficacy (VE) was 56.5% (95% CI: 43.8–66.4%) and 60.8% (52.0–68.0%) to prevent symptomatic virologically confirmed dengue (VCD) of any serotype in the active phase of two phase 3 trials (CYD14 in 2- to 14-year-olds in Asia and CYD15 in 9- to 16-year-olds in Latin America, respectively).15,16 Vaccine efficacy also varied by serotype, with the lowest VE observed against DENV-2 in both trials.17 In 9- to 16-year-olds pooled across the two trials (active phase), overall VE was 65.6% and the serotype-specific VEs were 58.4% (DENV-1), 47.0% (DENV-2), 73.6% (DENV-3), and 83.2% (DENV-4). Exploratory analyses in both trials indicated that VE increased with age and VE was higher in individuals who were dengue seropositive at the baseline.15,16,18 Vaccination of individuals aged at least 9 years is currently approved in several dengue-endemic regions, including in more than a dozen countries, with most countries up to 45 years and a few with other age caps (age 16 for Indonesia and age 60 for Paraguay).
We previously analyzed data from CYD14 and CYD15 to determine how VE varied with dengue neutralizing antibody titers measured by the 50% plaque reduction neutralization test (PRNT50), both at the baseline (pre-vaccination) and at 1 month after the final vaccination (month 13).18 We found that, in 9- to 16-year-olds pooled across the two trials, both baseline and month 13 PRNT50 titers were significantly associated with CYD-TDV VE to prevent VCD of any serotype, with month 13 PRNT50 titers being stronger predictors of VE than baseline titers. Serotype-specific analyses revealed that, in 9- to 16-year-olds pooled across the two trials, estimated VE against VCD of a given serotype increased significantly with homologous titer for DENV-1, DENV-2, and DENV-4. Consistent with previous reports,5–8 we found that baseline seroresponse rates and baseline PRNT50 titers increased with age.18
Given that CYD-TDV had lower VE against VCD through 25 months in baseline dengue seronegative individuals, and initial exploratory analyses suggested that VE against hospitalization for VCD and against severe VCD over longer term follow-up could be negative in this subgroup,19 Sridhar et al.20 conducted additional analyses of VE by baseline serostatus, considering the three endpoints of VCD through 25 months, hospitalized VCD through 72 months, and severe VCD through 72 months. Sridhar et al. found that VE against hospitalized and severe VCD was positive in baseline seropositive individuals uniformly over 72 months of follow-up (about 70% risk reduction) and was negative in baseline seronegative individuals, with increased risk by vaccination (about doubled risk) evident starting at about 2–3 years post first vaccination.
As the risk of severe dengue (DHF/DSS) is known to be higher during secondary infection than during primary infection,21–23 it has been hypothesized that CYD-TDV vaccination of a dengue seronegative individual acts as a primary infection, placing she or he at an increased risk of severe dengue after natural infection post-vaccination.24 Morover, anti-dengue antibody titers at the time of exposure appear to be important factors in influencing the risk of severe dengue with a relationship that is not monotonic.25 For example, Salje and others26 dynamic transmission model supported associations of antibody titers with an instantaneous risk of dengue infection, VCD, hospitalized VCD, and DHF, where dengue seronegative individuals had lower risk than individuals with low-level titers (until around log2 titer 3). These findings are also consistent with the hypothesis described previously and further discussed by others.27
In April of 2018, the WHO published updated recommendations of the Strategic Advisory Group of Experts on Immunization (SAGE) on the use of CYD-TDV: “For countries considering vaccination as part of their dengue control program, a ‘pre-vaccination screening strategy’ would be the preferred option, in which only dengue-seropositive persons are vaccinated.”28 Although the updated SAGE recommendations imply that individual pre-vaccination screening based on a reliable and rapid test to determine previous dengue exposure would be ideal, no such test has been widely registered for this indication, such that vaccination policies are forged without such a test. In highly endemic settings where most vaccinated individuals are seropositive, extrapolation of the vaccine versus placebo risk difference estimates of Sridhar et al.20 suggests that the use of CYD-TDV without screening confers large overall reductions in hospitalized and severe VCD,20 although at the cost of causing some cases for seronegative individuals.
Given that CYD14 and CYD15 focused on a population with high VCD attack rates, but did not include adults, an important consideration is to now develop an evidence-based approach to support the decisions of regulatory agencies about whether the indication for the CYD-TDV vaccine may be extended to adults. In general, where a treatment has been licensed in a population studied in a phase 3 trial and it is deemed unethical to conduct a new randomized placebo-controlled phase 3 trial of the treatment in a new population of interest not represented in the phase 3 trial cohort, a surrogate endpoint can sometimes be used for the provisional approval of the treatment for the new population. For example, the U.S. Food and Drug Administration’s Accelerated Approval process may be used when all four of the following criteria apply: 1) a treatment in a population would fill an unmet medical need; 2) there exists a surrogate end point that has not yet been validated but has been demonstrated to be “reasonably likely” to predict the real clinical benefit; 3) the treatment is shown to have a beneficial effect on the surrogate endpoint in the new population; and 4) a commitment is made to directly study the treatment’s effect on the true clinical endpoint in the new population in a phase 4 post-approval study (Food and Drug Administration Safety Innovations Act of 2012). The CYD-TDV vaccine with VCD, hospitalized VCD, and/or severe VCD as the clinical endpoint and the PRNT50 titer as the surrogate endpoint could fit this scenario, given that its use in adults may be expected to significantly reduce morbidity and mortality in adults. Specifically, for the endpoint VCD over 25 months, month 13 PRNT50 titers were a strong effect modifier of VE in the CYD14 and CYD15 trials, overall and in baseline seropositive and baseline seronegative subgroups,18 with high PRNT50 titers associated with high VE in all age and baseline serostatus subgroups. In addition, two phase 2 trials of CYD-TDV have been conducted to evaluate the PRNT50 titer responses in 18- to 45-year-old adults in highly endemic countries: CYD22 and CYD47. The CYD22 study enrolled healthy individuals in Vietnam aged 2 to 45 years,29 whereas the CYD47 trial enrolled healthy adults in India aged 18–45 years.30
If PRNT50 titers were judged to meet the “reasonably likely” criterion noted previously, then comparing vaccine-induced titers between 9- to 16-year-olds and 18- to 45-year-olds could help support the use of the vaccine in adults in terms of the reduction in the overall dengue incidence. We first conducted a standard bridging analysis that fits an Accelerated Approval–type paradigm, by comparing PRNT50 titers between 18- to 45-year-olds and 9- to 16-year-olds based on the CYD22, CYD47, CYD14, and CYD15 data. Demonstrating non-inferiority of PRNT50 titers in the older cohort would constitute the typical evidence provided to regulatory agencies to justify the bridging clinical efficacy based on a surrogate end point. In addition, we then estimated VE against VCD over the first 25 months post first vaccination in 18- to 45-year-olds based on an epidemiological method we developed for bridging efficacy based on baseline covariates and intermediate response end points and accounting for multiple serotypes of the pathogen.31
MATERIALS AND METHODS
CYD14 and CYD15 trials.
The trials had the same study design and protocol, which randomly assigned healthy children in 2:1 allocation to vaccine or placebo administered at months 0, 6, and 12 (further details are given in refs. 15 and 16). Active surveillance was used to identify primary endpoint cases (VCD) of any of the 4 serotypes (DENV-Any) over 25 months since first vaccination. A case–cohort design was used to measure neutralization responses in CYD14 and CYD15, whereby enrollees in the first 2–4 months of the trials were randomly assigned to an immunogenicity subset for measuring the neutralization response at months 0 (baseline before first vaccination), 7, 13, and 25.18 Neutralization responses were also measured at month 13 from all subsequent DENV-Any cases. The correlates analyses in Moodie et al.18 were performed in participants who had not previously experienced the DENV-Any endpoint by month 13.
CYD22 and CYD47 trials.
Sanofi Pasteur conducted the CYD22 study (ClinicalTrials.gov ID NCT0087552429), a phase 2 trial of the CYD-TDV vaccine that enrolled 180 healthy individuals in Vietnam aged 2–45 years. Within four age strata 2–5, 6–11, 12–17, and 18–45, participants were randomized in 2:1 allocation to receive either the CYD-TDV vaccine or a control (placebo or control vaccine), administered at months 0, 6, and 12 (the identical schedule as for CYD14 and CYD15). Relevant for our analyses, n = 52 9- to 16-year-olds and n = 30 18- to 45-year-olds (vaccine and placebo combined) had baseline PRNT50 measurements. In addition, n = 32 9- to 16-year-old and n = 18 18- to 45-year-old vaccine recipients received all three doses of the CYD-TDV vaccine and had month 13 PRNT50 measurements. A primary aim of CYD22 was to measure dengue neutralizing antibodies at baseline and at 28 days after each CYD-TDV vaccination (including at month 13). The study began enrollment in March 2009 and was completed in December 2014.
Another phase 2 trial, CYD47 (ClinicalTrials.gov ID NCT0155028930), evaluated the immunogenicity and safety of the CYD-TDV vaccine in healthy adults aged 18–45 years in India.30 Participants were randomized 2:1 to receive CYD-TDV (n = 128) or placebo (n = 61) at months 0, 6, and 12; n = 187 of these had baseline PRNT50 measured and n = 115 CYD-TDV recipients received all three vaccinations and had month 13 PRNT50 measured. A main objective was to measure dengue neutralizing antibodies at the baseline and at 28 days after each CYD-TDV vaccination (including at month 13). The study began enrollment in March 2012 and was completed in February 2014.
50% plaque reduction neutralization test assay.
In this study, 50% plaque reduction neutralization test titers were measured using an optimized and validated assay as previously described.32 The assay was conducted in a centralized Sanofi Pasteur facility for all studies. The assay measures the ability of antibodies present in serum samples to neutralize the infectivity to Vero cells of each of the four parental DENVs from which the CYD-TDV recombinant vaccine viruses were derived. Titers were expressed as the reciprocal of the highest serum dilution at which the number of plaques in infected wells was ≥ 50% lower than that in negative control wells. The lower limit of quantitation was 10; values below this were set to 5.
Statistical analysis.
Non-inferiority of month 13 neutralizing responses in 18- to 45-year-olds versus 9- to 16-year-olds.
For each analyzed cohort and time point (baseline or month 13), the geometric mean (GM) PRNT50 was estimated, with CIs calculated as transformed t-distribution CIs of log base 10 PRNT50 values. Point and CI estimates about GM ratios comparing cohorts were calculated similarly. Titers to each serotype and the average titer were assessed, where a participant’s average titer was the GM of the four serotype-specific antibody titers.18
Estimating VE in 18- to- 45-year-olds.
The full statistical analysis plan (SAP) that was prespecified for the bridging analysis is provided in the supplementary material (“SAP for bridging VE from the CYD14 + CYD15 9- to 16-year-old cohort to a new setting (18- to 45-year-old adults) via the Gilbert and Huang (2016) bridging method”; April 5, 2017) and describes all of the used methods in detail, including the assumptions needed for valid bridging. Here, we summarize the used methods, which address two objectives. Objective 1 estimated VE against DENV-Any occurring between months 0 (first vaccination) and 25 (13 months post last vaccination at month 12) in a population of 18- to 45-year-olds; this VE parameter was one minus the ratio (vaccine versus placebo) of the cumulative probabilities of VCD occurrence by month 25. Objective 2 was the same, except VE against VCD is estimated for each serotype v separately, VE(v) for v = 1, 2, 3, and 4, where VE(v) was one minus the ratio (vaccine versus placebo) of the cumulative probabilities of VCD occurrence by month 25 and the first VCD occurrence was of serotype v. Objectives 1 and 2 were repeated for additive–difference versions of the VE parameters—referred to as VEd and VEd(v)—which were (vaccine minus placebo) cumulative probabilities. Under an assumption that vaccination did not increase the risk of VCD in 18- to 45-year-olds, each of these parameters VEd and VEd(v) had an attributable risk interpretation as the probability a randomized participant had VCD by month 25 averted by vaccination.
The VE parameters were estimated for two populations of 18- to 45-year-olds. Bridging population 1 was 18- to 45-year-olds for the hypothetical scenario, where the entire CYD14 + CYD15 study had included an 18- to 45-year-old cohort under identical follow-up/ecological conditions and surveillance for VCD as for the 9- to 16-year-olds. Bridging population 2 was 18- to 45-year-olds in India (CYD47) for the hypothetical scenario that the background/unvaccinated risk of VCD over the first 25 months post first vaccination was the same as that for CYD14 + CYD15 9- to 16-year-olds.
Estimation for bridging population 1 was performed using the CYD22 study as a “calibration study” because it measured PRNT50 titers for both 9- to 16-year-olds and 18- to 45-year-olds, allowing estimation of age–cohort differences in titer distributions. Assuming that these age–cohort differences in CYD22 would be the same in CYD14 + CYD15 allowed using the PRNT50 titer data from CYD14 + CYD15 9- to 16-year-olds—plus a calibration factor estimated from CYD22—to estimate these titer distributions in the hypothetical CYD14 + CYD15 18- to 45-year-old study. (See the SAP Section 2.2 Assumption 2 for details on how the calibration was performed.) Estimation for bridging population 2 used the CYD47 study titer data. Because CYD47 did not include a 9- to 16-year-old cohort, the calibration technique could not be used, such that we defined bridging population 2 as the CYD47 18- to 45-year-old population itself, for which the direct estimates of the CYD47 18- to 45-year-old titer distributions could be used.
Different versions of the Gilbert and Huang31 transport formula were used for both objectives, each with multiple steps. The first step of every method estimated from CYD14 + CYD15 data how VE varied over vaccinated subgroups defined by month 13 PRNT50 titers and/or how VE varied over subgroups defined by baseline PRNT50 titers, as performed by ref. 18. For this step, we always used the cohort of 9- to 16-year-olds in CYD14 + CYD15 pooled, based on the facts that the CYD14 and CYD15 protocols were the same, the pooling increased precision for estimation of the VE curves, and the PRNT50 titer distributions and estimated vaccine efficacies were similar in CYD14 9- to 14-year-olds and CYD15 9- to 16-year-olds.18 The first set of methods address bridging objective 1 to estimate the overall VE, with four variants 1a, 1b, 1c, and 1d that differ by being based on (a) month 0 and 13 serotype titers, (b) month 0 serotype titers, (c) month 0 and 13 average titers, and (d) month 0 average titers. The second set of methods address bridging objective 2 to estimate serotype-specific VE, with two variants, where method 2a uses both month 0 and month 13 serotype titers and method 2b only uses month 0 serotype titers. We summarize the methods addressing objective 2 first because the methods for addressing objective 1 use results from the objective 2 analyses.
Method 2a: Serotype-specific VE objective based on month 0 and 13 serotype titers.
Based on the CYD14 + CYD15 9- to 16-year-old cohort, step 1 estimated each of the four serotype-specific VE curves VE14.15.9–16(t = 25, v|Sv = s) for DENV-v through 25 months as functions of month 13 homologous serotype v titers Sv using the Juraska et al. method33 as implemented in Moodie et al.18 (Supplemental Figure 1); these VE curves express how VE varied over vaccinated subgroups defined by month 13 neutralization titers, using the VE curve effect modification framework.34–36 Step 2 estimated each of the four serotype-specific VE curves VE14.15.9–16(t = 13, v|Xv = s) for DENV-v through 13 months as functions of baseline homologous titers Xv using the Huang et al.37 logistic regression method as also implemented in ref. 18 (Supplemental Figure 2). Step 3 estimated VEd(v) in the 18- to 45-year-old cohort by combining two averages. The first averaged the estimate of VE14.15.9–16(t = 25, v|Sv = s) over the estimated distribution of Sv in the 18- to 45-year-old cohort, with weighting by 1) a specified ratio of the VE curve VE18–45(t = 25, v|Sv = s) in the 18- to 45-year-old cohort compared with VE14.15.9–16(t = 25, v|Sv = s) and 2) an estimate of the background/unvaccinated DENV-v risk from month 13 to 25 conditional on Sv in the 18- to 45-year-old cohort. The second averaged the estimate of VE14.15.9–16(t = 13, v|Xv = s) over the estimated distribution of Xv in the 18- to 45-year-old cohort, with weighting by 1) a specified ratio of the true curve VE18–45(t = 13, v|Xv = s) in the 18- to 45-year-old cohort compared with VE14.15.9–16(t = 13, v|Xv = s) and 2) an estimate of the background/unvaccinated DENV-v risk from month 0 to 13 conditional on Xv in the 18- to 45-year-old cohort. This formula was published as equations (8)–(11) in Gilbert and Huang31 and is listed in the April 5, 2017, SAP as equations (8) and (13)–(15) (with τ = 13 and t = 25). Last, VE(v) was estimated by plugging the estimate of VEd(v) into equation (16) in the April 5, 2017, SAP.
Method 2b: Serotype-specific VE objective based on month 0 serotype titers.
This approach was based on baseline titers Xv, not accounting for month 13 titers Sv. Step 1 was the same as step 2 mentioned previously, except for DENV-v end points from month 0 to 25. Step 2 was the same as the second average computed in step 3 mentioned previously, except for DENV-v end points from month 0 to 25; specifically, it averaged the estimate of VE14.15.9–16(t = 25, v|Xv = s) over the estimated distribution of Xv in the 18- to 45-year-old cohort, with weighting by 1) a specified ratio of the true curve VE18–45(t = 25, v|Xv = s) in the 18- to 45-year-old compared with VE14.15.9–16(t = 25, v|Xv = s) and 2) an estimate of the background/unvaccinated DENV-v risk from month 0 to 25 conditional on Xv in the 18- to 45-year-old cohort. This formula was published as equation (11) in Gilbert and Huang31 and is listed in the April 5, 2017, SAP as equations (15) and (16) (with τ = 25).
Method 1a: Overall VE objective based on month 0 and 13 serotype titers.
The estimate of VEd against DENV-Any was the sum of the estimates of VEd(v) for v = 1, 2, 3, and 4 obtained from analysis 2a. Then, VE was estimated by plugging the estimate of VEd into equation (16) of the April 5, 2017, SAP.
Method 1b: Overall VE objective based on month 0 serotype titers.
Similarly, the estimate of VEd against DENV-Any was the sum of the estimates of VEd(v) for v = 1, 2, 3, and 4 obtained from analysis 2b, and then, VE was estimated using equation (16) of the April 5, 2017, SAP.
Method 1c: Overall VE objective based on month 0 and 13 average titers.
This approach was identical to method 2a, except the DENV-v end point was replaced with DENV-Any and homologous serotype v titers Sv and Xv were replaced with average titers Savg and Xavg, respectively.
Method 1d: Overall VE objective based on month 0 average titers.
Similarly, this approach was identical to method 2b, with DENV-v replaced with DENV-Any and the homologous serotype v titer Xv was replaced with the average titer Xavg.
The month 0 and 13 methods made use of baseline and month 13 titers, whereas the month 0 methods used baseline titers only. An advantage of the former methods is that month 13 titers were shown to be more predictive of VE than baseline titers (Figure 4 of ref. 18). By contrast, it is logistically simpler to base bridging on baseline titers because future phase 1 bridging studies would not require 13 months of follow-up for measuring postvaccination titers. Moreover, by not requiring estimation of VE curves VE14.15.9–16(t = 25, v|Sv = s) that condition on month 13 titers if assigned vaccine, Sv, these methods avoid making assumptions to accommodate the missing month 13 responses of placebo recipients. However, the challenge posed to the month 0 methods is that, because of a lack of available samples, baseline titers could only be measured in 13.4% of VCD cases in CYD14 and CYD15, limiting precision. We included both approaches to assess the consistency of results obtained with methods that make different assumptions, where consistent results may strengthen the evidence base for bridging.
Figure 4.
Estimated additive–difference vaccine efficacy against dengue virus-v from month 0 to 25. Estimates are shown for CYD14 + CYD15 9- to 16-year-olds (top row panel, for reference); for hypothetical CYD14 + CYD15 18- to 45-year-olds (bridging population 1) under method 2a (second row panel); for India 18- to 45-year-olds (bridging population 2) under method 2a (third row panel), and repeating the latter two analyses under method 2b (fourth and fifth row panels).
Key assumptions of the bridging methods.
We summarize and briefly discuss the three key assumptions for the methods (Supplemental Table 1, complete details in the April 5, 2017, SAP Section 2). For methods 1a and 2a, assumption 1 specified that the VE(v) curve by the month 13 serotype v titer is the same in the 18- to 45-year-old and 9–16-year-old cohorts, and similarly for the VE(v) curve by the baseline serotype v titer. This posited that the homologous serotype v titer was the key predictor of VE(v) and that conditional on its value, VE against DENV-v was the same in the two age cohorts. Assumption 2 was only needed for bridging to population 1. It posited that the cumulative distribution functions (cdfs) of each titer variable (Sv and Xv for v = 1, 2, 3, and 4) for the two CYD22 age cohorts (18–45 versus 9–16 years) were linked by a mixed binary and continuous location shift model, that the odds ratio of positive response (i.e., titer > 10) for the two CYD14 + CYD15 age cohorts was the same as that for the two CYD22 age cohorts, and that the location-shift model in positive responders was the same for CYD14 + CYD15 and CYD22. Assumption 2 was used to obtain estimates of the distributions of Sv and Xv for CYD14 + CYD15 18- to 45-year-olds, using Hodges–Lehmann estimators of the shift parameters based on the CYD22 data (for each v = 1, 2, 3, and 4). Assumption 3 posited that after accounting for Sv, the age cohort did not affect the background/unvaccinated dengue-v risk from month 13 to 25, and that after accounting for Xv, the age cohort did not affect the background/unvaccinated dengue-v risk from month 0 to 13 and from month 0 to 25. For the objective 1 methods based on average titers, the parallel assumptions were made for DENV-Any and average titers. The month 0 and 13 method 1c made the same assumptions, except in terms of the average titer instead of serotype-specific titers. Moreover, the month 0 methods (1b and 2b) made the same assumptions, except only for baseline titers and for dengue VCD follow-up from month 0 to 25 instead of from month 0 to 13 (see Supplemental Table 1).
Assumption 1 could be violated if there was another immune response marker with a different distribution in CYD14 + CYD15 9- to 16-year-olds and 18- to 45-year-olds that modified VE(v) after accounting for the homologous serotype PRNT50 titer. For assumption 2, the location-shift model in CYD22 could be directly checked from the observed data, and the bridging of this model to CYD14 + CYD15 is reasonable if we expect the age cohort to have a similar effect on titers in Vietnam and in the other CYD14 + CYD15 countries. Assumption 3 posited that the age cohort adds no more information about the dengue risk of unvaccinated persons after accounting for neutralizing antibody titers, and so essentially is about the sufficiency of this marker for capturing the risk. There seems to be limited epidemiological data available for verifying this assumption. It is reasonable under a premise/model that the degree of prior dengue exposure and infection is the fundamental underlying dengue risk factor in 9- to 45-year-olds and the neutralization titer is a better proxy for that factor than age. Some support for this premise derives from the four multivariable logistic regression models of DENV-v risk for CYD14 and CYD15 9- to 16-year-old placebo recipients conditional on both serotype v titer and age (9–11 versus 12–16 years) for each v = 1, 2, 3, and 4, which showed strong inverse associations of serotype v titers with DENV-v risk and no evidence of age associations.18
CIs about VE in the 18- to 45-year-old cohorts.
Bootstrap percentile 95% CIs were computed about the VE parameters that accounted for the multiple sources of sampling variability, namely, the estimated VE curves from CYD14 + CYD15 9- to 16-year-olds; the estimated distributions of Sv, Xv, Savg, and Xavg in the 18- to 45-year-old cohorts (which accounted for sampling variability in the CYD22 calibration study for bridging population 1 and for the sampling variability in CYD47 for bridging population 2); and the estimated background dengue risks as a function of month 0 titers in CYD14 + CYD15 9- to 16-year-olds. In addition, bootstrap percentile 95% estimated uncertainty intervals (EUIs) were computed about the VE parameters that accounted for uncertainty both due to sampling variability and to possible violations of assumptions 1 and 3 (see the April 5, 2017, SAP).
Sensitivity analysis to possible violations of assumptions 1 and 3.
Two sensitivity analyses were conducted for all of the methods, which we describe for method 2a; parallel approaches were used for the other five methods. The first sensitivity analysis specified the ratio of the Denv-v VE curves in the two age cohorts, VE18–45(t = 25, v|Sv = s)/VE14.15.9–16(t = 25, v|Sv = s), to be a fixed constant ϕ that varied between 0.8 and 1.2, and studied how the results varied with ϕ. In the second sensitivity analysis, ϕ was again varied from 0.8 to 1.2, and a second sensitivity parameter ρ, defined as the ratio of the background/unvaccinated incidence of DENV-v from month 0 to month 13 and from month 0 to 25 conditional on Xv in the two age cohorts (18–45/9–16 years), was set equal to 0.8. This allowed the background DENV-v risk to be lower in the older age cohort, where ρ above 1 was not considered because it is thought to be unlikely based on the epidemiological literature.
RESULTS
Non-inferiority of month 13 neutralizing responses in 18- to 45-year-olds versus 9–16-year-olds.
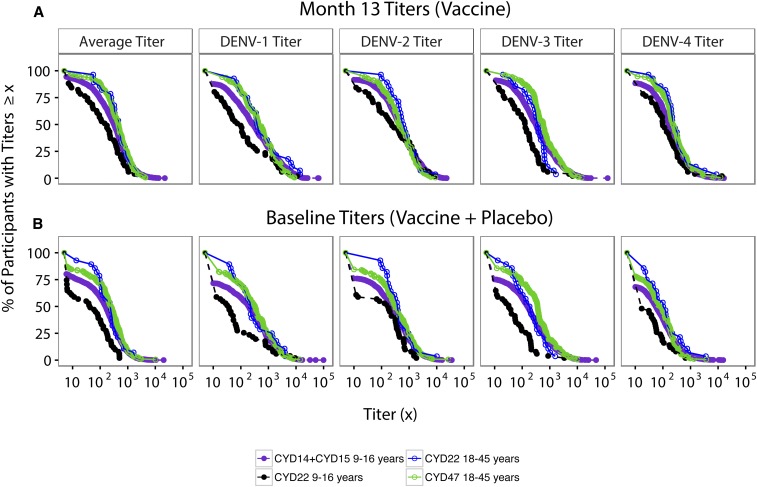
We first assessed whether month 13 neutralizing responses in 18- to 45-year-olds were non-inferior to those in 9- to 16-year-olds, by comparing month 13 PRNT50 titers 1) between the two age cohorts within CYD22 (CYD22 18- to 45-year-olds versus CYD22 9–16-year-olds) and 2) between the two age cohorts across trials (CYD47 18- to 45-year-olds versus CYD14 + CYD15 9- to 16-year-olds). Within CYD22, for all four serotypes, the month 13 GMs of vaccine recipients were significantly greater for 18- to 45-year-olds than 9–16-year-olds, with GM ratios 2.09–3.94 (Table 1). Moreover, the month 13 GMs were significantly greater for CYD47 18- to 45-year-olds than CYD14 + CYD15 9- to 16-year-olds for serotypes 3 and 4 (GM ratios 1.64 and 1.46, respectively), without a significant difference for serotypes 1 and 2 (GM ratios 1.31 and 0.86, respectively) (Table 1). For a more complete comparison of month 13 neutralizing responses in 18- to 45-year-olds versus those in 9- to 16-year-olds, we also examined empirical estimates of reverse cdfs (rcdfs) in the two age cohorts. Within CYD22, clear separation of the average titer curves and of all four serotype-specific curves between the two age cohorts was observed at all titer levels below 1,000 (Figure 1A). Similar results were observed for the comparison in CYD14 + CYD15 9- to 16-year-olds versus CYD47 18- to 45-year-olds, except for DENV-2, where little or potentially no separation was observed (Figure 1A). The similar rcdf curves for the CYD14 and CYD15 trials (Supplemental Figure 3) also help justify basing the bridging analysis on the pooled CYD14 + CYD15 data.
Table 1.
Comparison of month 13 neutralizing antibody titers (50% plaque reduction neutralization test) in CYD-TDV vaccine recipients receiving all three vaccinations between age cohorts (18–45 vs. 9–16 years) in the CYD22, CYD47, and CYD14 + CYD15 studies
| Study | CYD22 | CYD47 | CYD14 + CYD15 | ||
|---|---|---|---|---|---|
| Age (years) | 18–45 | 9–16 | 18–45 | 9–16 | |
| Sample size | 18 | 32 | 115 | 1870 | |
| Average titer | Response rate (95% CI) | 100.0% (82.4, 100.0) | 100.0% (89.3, 100.0) | 100.0% (96.8, 100.0) | 99.8% (99.5, 99.9) |
| GM (95% CI) | 549.6 (392.6, 769.3) | 204.5 (125.6, 333.0) | 480.5 (387.8, 595.3) | 374.8 (352.5, 398.5) | |
| GM ratio (95% CI) | 2.69 (1.49, 4.84) | Ref | 1.28 (1.03, 1.60) | Ref | |
| 1.47 (1.04, 2.07) | – | – | Ref | ||
| DENV-1 | Response rate (95% CI) | 100.0% (82.4, 100.0) | 96.9% (84.3, 99.4) | 97.4% (92.6, 99.1) | 94.8% (93.7, 95.7) |
| GM (95% CI) | 695.5 (335.3, 1,442.7) | 176.7 (87.3, 357.7) | 461.2 (340.2, 625.3) | 352.0 (321.1, 385.8) | |
| GM ratio (95% CI) | 3.94 (1.43, 10.85) | Ref | 1.31 (0.95, 1.80) | Ref | |
| 1.98 (0.95, 4.13) | – | – | Ref | ||
| DENV-2 | Response rate (95% CI) | 100.0% (82.4, 100.0) | 100.0% (89.3, 100.0) | 97.4% (92.6, 99.1) | 98.6% (97.9, 99.0) |
| GM (95% CI) | 825.2 (492.5, 1,382.6) | 350.6 (187.1, 656.9) | 484.5 (370.3, 633.9) | 565.3 (527.2, 606.1) | |
| GM ratio (95% CI) | 2.35 (1.05, 5.29) | Ref | 0.86 (0.65, 1.13) | Ref | |
| 1.46 (0.87, 2.46) | – | – | Ref | ||
| DENV-3 | Response rate (95% CI) | 100.0% (82.4, 100.0) | 96.9% (84.3, 99.4) | 99.1% (95.2, 99.8) | 97.9% (97.2, 98.5) |
| GM (95% CI) | 423.8 (286.3, 627.4) | 157.1 (94.9, 260.1) | 709.0 (551.9, 911.0) | 431.6 (400.4, 465.2) | |
| GM ratio (95% CI) | 2.70 (1.43, 5.10) | Ref | 1.64 (1.26, 2.13) | Ref | |
| 0.98 (0.66, 1.47) | – | – | Ref | ||
| DENV-4 | Response rate (95% CI) | 100.0% (82.4, 100.0) | 96.9% (84.3, 99.4) | 100.0% (96.8, 100.0) | 97.8% (97.0, 98.3) |
| GM (95% CI) | 375.0 (250.7, 561.0) | 179.7 (106.8, 302.4) | 336.3 (271.4, 416.8) | 229.8 (216.9, 243.5) | |
| GM ratio (95% CI) | 2.09 (1.08, 4.02) | Ref | 1.46 (1.17, 1.83) | Ref | |
| 1.63 (1.09, 2.45) | – | – | Ref | ||
DENV = dengue virus; GM = geometric mean; Ref = reference group in the denominator.
Figure 1.
Reverse cumulative distribution functions of baseline and month 13 neutralizing antibodies by serotype. (A) Month 13 50% plaque reduction neutralization test (PRNT50) titers in CYD-TDV vaccine recipients receiving all three vaccinations by age cohort in CYD22, CYD47, and CYD14 + CYD15. (B) Baseline PRNT50 titers in the CYD-TDV vaccine and placebo recipients pooled. This figure appears in color at www.ajtmh.org.
Non-inferiority of month 0 neutralizing responses in 18- to 45-year-olds versus 9- to 16-year-olds.
Because month 0 titers were positively associated with VE, non-inferiority of month 0 titers for the older age cohort would also suggest non-inferior VE. Within CYD22, for all four serotypes, the month 0 GMs of vaccine recipients were significantly greater for 18- to 45-year-olds than 9- to 16-year-olds, with GM ratios 3.29–4.80 (Table 2). Moreover, the month 0 GMs were significantly greater for CYD47 18- to 45-year-olds than CYD14 + CYD15 9- to 16-year-olds for all four serotypes (GM ratios 1.27–2.01) (Table 2). Similar to the aforementioned results, the rcdfs of baseline PRNT50 titers, pooled across vaccine and placebo recipients, in the two age cohorts were compared. Within CYD22, clear separation (potentially even greater than that seen for the month 13 curves) of the average titer curves and of all four serotype-specific curves between the two age cohorts was observed at all titer levels below 1,000 (Figure 1B). Results for the CYD14 + CYD15 9- to 16-year-old versus CYD47 18- to 45-year-old comparison were similar (Figure 1B).
Table 2.
Comparison of month 0 neutralizing antibody titers (50% plaque reduction neutralization test) in the CYD-TDV vaccine and placebo recipients (pooled) between age cohorts (18–45 vs. 9–16 years) in the CYD22, CYD47, and CYD14 + CYD15 studies
| Study | CYD22 | CYD47 | CYD14 + CYD15 | ||
|---|---|---|---|---|---|
| Age (years) | 18–45 | 9–16 | 18–45 | 9–16 | |
| Sample size | 30 | 52 | 187 | 2,728 | |
| Average titer | Response rate (95% CI) | 93.3% (78.7, 98.2) | 75.0% (61.8, 84.8) | 87.2% (81.6, 91.2) | 80.1% (78.5, 81.5) |
| GM (95% CI) | 156.2 (93.2, 262.0) | 39.0 (23.7, 64.1) | 152.3 (117.8, 196.9) | 94.1 (87.6, 101.1) | |
| GM ratio (95% CI) | 4.00 (1.96, 8.20) | Ref | 1.62 (1.24, 2.11) | Ref | |
| 1.66 (0.99, 2.80) | – | – | Ref | ||
| DENV-1 | Response rate (95% CI) | 90.0% (74.4, 96.5) | 61.5% (48.0, 73.5) | 82.4% (76.3, 87.1) | 71.5% (69.8, 73.2) |
| GM (95% CI) | 215.5 (107.1, 433.6) | 44.9 (24.0, 83.9) | 199.2 (146.4, 271.1) | 116.1 (106.3, 126.9) | |
| GM ratio (95% CI) | 4.80 (1.88, 12.29) | Ref | 1.72 (1.24, 2.36) | Ref | |
| 1.86 (0.92, 3.76) | – | – | Ref | ||
| DENV-2 | Response rate (95% CI) | 93.3% (78.7, 98.2) | 63.5% (49.9, 75.2) | 84.5% (78.6, 89.0) | 76.0% (74.3, 77.5) |
| GM (95% CI) | 290.3 (163.6, 515.0) | 70.6 (37.3, 133.8) | 215.8 (160.8, 289.8) | 137.6 (126.6, 149.5) | |
| GM ratio (95% CI) | 4.11 (1.74, 9.69) | Ref | 1.57 (1.15, 2.13) | Ref | |
| 2.11 (1.18, 3.77) | – | – | Ref | ||
| DENV-3 | Response rate (95% CI) | 93.3% (78.7, 98.2) | 65.4% (51.8, 76.8) | 85.6% (79.8, 89.9) | 75.0% (73.4, 76.6) |
| GM (95% CI) | 132.2 (76.7, 227.7) | 33.4 (20.4, 54.8) | 217.7 (163.5, 289.9) | 108.3 (99.8, 117.6) | |
| GM ratio (95% CI) | 3.95 (1.89, 8.26) | Ref | 2.01 (1.49, 2.71) | Ref | |
| 1.22 (0.70, 2.12) | – | – | Ref | ||
| DENV-4 | Response rate (95% CI) | 83.3% (66.4, 92.7) | 50.0% (36.9, 63.1) | 78.1% (71.6, 83.4) | 68.0% (66.3, 69.8) |
| GM (95% CI) | 72.1 (38.4, 135.3) | 21.9 (13.9, 34.6) | 57.5 (45.6, 72.5) | 45.3 (42.3, 48.5) | |
| GM ratio (95% CI) | 3.29 (1.51, 7.18) | Ref | 1.27 (1.00, 1.62) | Ref | |
| 1.59 (0.84, 3.00) | – | – | Ref | ||
DENV = dengue virus; GM = geometric mean; Ref = reference group in the denominator.
Estimated VE against VCD 0–25 months in 18- to 45-year-olds.
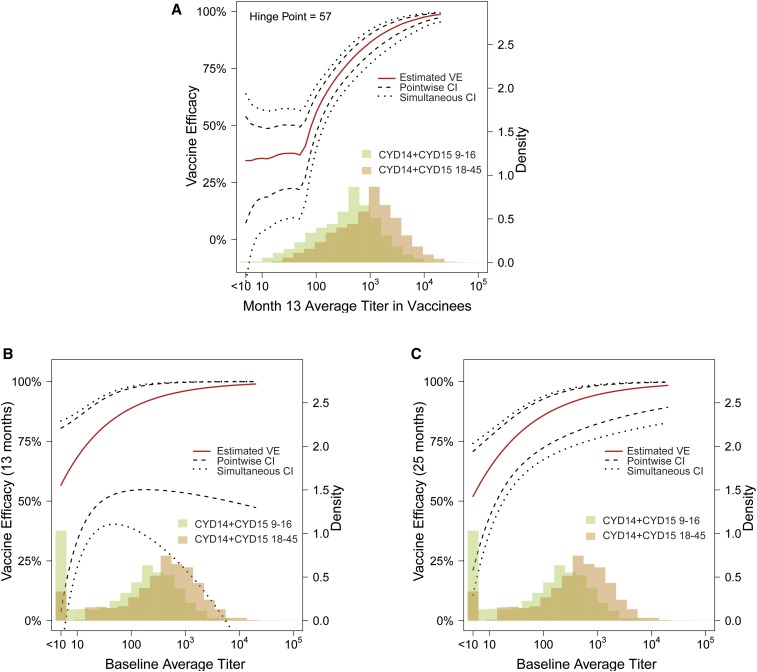
Toward the objectives to estimate VE in 18- to 45-year-olds, we first consider the estimated VE against DENV-Any by neutralization titer curves in CYD14 + CYD15 9- to 16-year-olds (Figure 2). The first curve (panel A) shows that estimated VE against DENV-Any from month 13 to 25 remains around 33% in individuals without seroresponse and in individuals with low month 13 average titer until reaching the hinge point titer of 57, after which estimated VE rises steeply with month 13 average titer. The second curve (panel B) shows that estimated VE against DENV-Any from month 0 to 13 is just over 50% for baseline seronegative individuals and rises gradually with baseline average titer. The third curve (panel C) shows a similar pattern for estimated VE against DENV-Any from month 0 to 25 by baseline average titer as that shown in panel B. These estimated curves are key elements of the bridging formula, where the curves in (A) and (B) were used by method 1c and the curve in (C) was used by method 1d.
Figure 2.
Estimated vaccine efficacy against dengue virus-Any for CYD14 + CYD15 9- to 16-year-olds. (A) From month 13 to 25 by month 13 average titer; (B) from month 0 to 13 by the baseline average titer; and (C) from month 0 to 25 by the baseline average titer. This figure appears in color at www.ajtmh.org.
We next used all four methods, 1a–1d, to estimate VE against DENV-Any for bridging populations 1 and 2 (objective 1). For population 1, estimated VE was greater than 75% for all of the methods, with the widest 95% CI extending from 52.5% to 100.0% (Table 3). For population 2, estimated VE was greater than 68% for all of the methods, with the widest 95% CI extending from 42.3% to 88.5% (Table 3). All of the VE estimates were greater than the observed estimate in CYD14 + CYD15 9- to 16-year-olds (65.6%, 95% CI: 60.6–69.9%). This is explained by the monotone increasing VE curves in CYD14 + CYD15 9- to 16-year-olds (Figure 2, Supplemental Figures 1 and 2) combined with the fact that baseline and month 13 titers are consistently higher in 18- to 45-year-olds than 9- to 16-year-olds (Tables 1 and 2, Figure 1 based on Vietnam data and India data versus the 10 endemic countries in CYD14 and CYD15).
Table 3.
Estimated VE against dengue virus-Any from month 0 to 25 for hypothetical CYD14 + CYD15 18- to 45-year-olds (bridging population 1; as if an 18- to 45-year-old cohort had been included in CYD14 + CYD15) and for India 18- to 45-year-olds (bridging population 2) (methods 1a–1d), with results from CYD14 + CYD15 9- to 16-year-olds for comparison
| Bridging population | Estimated VE (95% CI) against VCD from month 0 to 25 | |||
|---|---|---|---|---|
| Method 1a | Method 1b | Method 1c | Method 1d | |
| CYD14 + CYD15 18- to 45-year-olds | 86.0 (52.5, 100.0) | 84.7 (66.6, 90.9) | 75.3 (59.3, 100.0) | 84.0 (68.6, 92.7) |
| India 18- to 45-year-olds | 70.7 (42.3, 88.5) | 77.4 (61.5, 84.5) | 68.4 (58.2, 82.3) | 77.5 (65.3, 86.2) |
| CYD14 + CYD15 9- to 16-year-olds | 65.6 (60.6, 69.9) (reported in ref. 18) | |||
VE = vaccine efficacy.
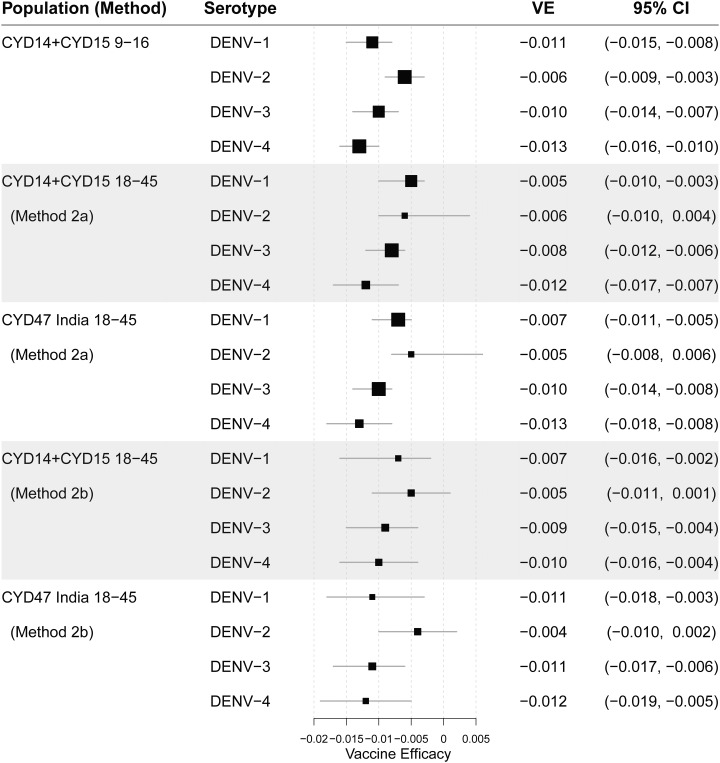
Figure 3 shows the parallel results of Table 3 for estimating the serotype-specific parameters VE(v) by methods 2a and 2b for bridging populations 1 and 2, for each v = 1, 2, 3, and 4 (objective 2). For both populations, the estimates for serotypes 3 and 4 were high (79.8–100.0%)—higher than the corresponding estimates observed for CYD14 + CYD15 9- to 16-year-olds of 73.6% (95% CI: 64.4, 80.4%) and 83.2% (95% CI: 76.2, 88.2%). For serotype 1, the estimates of VE were 56.9% and 76.9% by methods 2a and 2b for population 1, respectively, comparable with or higher than the CYD14 + CYD15 9- to 16-year-old estimate of 58.4% (95% CI: 47.7, 66.9%), whereas for population 2, methods 2a and 2b gave different VE estimates of 44.5% and 66.9%, respectively. Whereas the 44.5% estimate might be surprising, given it is less than the estimate observed in CYD14 + CYD15 9- to 16-year-olds (58.4%), it is larger than the estimate of 39.6% in CYD14 + CYD15 9- to 16-year-olds if method 2a were applied to CYD14 + CYD15 9- to 16-year-olds; it may be explained by the random difference in the estimated unvaccinated dengue risk and the estimated VE from month 0 to month 13 and from month 0 to month 25 between the sub-cohort with the baseline titer measured and the full cohort: the former was used in method 2a. For both populations, the estimates for serotype 2 were all higher than the estimate from CYD14 + CYD15 9- to 16-year-olds of 47.0% (95% CI: 31.3, 59.2%), although with wide CIs. In methods-validation analyses that applied VE curves to estimate the overall VE for CYD14 + CYD15 9- to 16-year-olds, we found that application of the month 13 VE curves (used in methods 1a, 1c, and 2a) tended to yield estimates of overall VE that were smaller than the direct empirical estimates, whereas application of the month 0 VE curves (used in methods 1b, 1d, and 2b) tended to yield similar estimates, suggesting that the bridging estimates 56.9% and 44.5% may be conservative lower bounds.
Figure 3.
Estimated vaccine efficacy against dengue virus-v for each serotype v = 1, 2, 3, and 4 from month 0 to 25. Estimates are shown for CYD14 + CYD15 9- to 16-year-olds (top row panel, for reference); for hypothetical CYD14 + CYD15 18- to 45-year-olds (bridging population 1) under method 2a (second row panel); for India 18- to 45-year-olds (bridging population 2) under method 2a (third row panel), and repeating the latter two analyses under method 2b (fourth and fifth row panels).
The CIs in Figure 3 show reasonable precision about the beneficial efficacy in 18- to 45-year-olds against serotypes 3 and 4 (lower 95% confidence limits [LCLs] varying between 49.5% and 75.3% depending on the bridging method), and reasonable but less precision about the beneficial efficacy against serotype 1 (LCLs from 26.7% to 39.3%). By contrast, for serotype 2, there is lower precision, with all LCLs well below 0%. The CIs support a conclusion that there is fairly high confidence that the CYD-TDV vaccine would protect well against serotypes 3 and 4 in both bridging populations, and at least moderately well against serotype 1, whereas for serotype 2, much uncertainty remains as to the protection level.
Supplemental Table 2 and Figure 4 show the parallel results as Table 3 and Figure 3, for the additive–difference parameters VEd and VEd(v), respectively. The results in Supplemental Table 2 estimate that for every 100,000 18- to 45-year-olds vaccinated in bridging population 1, between 3,100 and 3,700 DENV-Any VCD cases are predicted to be averted by vaccination, with 95% CIs spanning from 1,900 to 5,100 averted for the four overall VE bridging methods 1a–1d.
Estimated VE against hospitalized VCD 0–72 months in 18- to 45-year-olds.
Supplemental Figure 4 shows estimated VE against hospitalized DENV-Any by neutralization titer curves in CYD14 + CYD15 9- to 16-year-olds. The first curve (panel A) shows that estimated VE against hospitalized DENV-Any from month 13 to 72 is near zero in individuals without month 13 seroresponse and individuals with low month 13 average titer until reaching the hinge point titer of 31, after which estimated VE rises steeply with month 13 average titer (estimated VE is 0% for vaccine recipients with no month 13 seroresponse and reaches about 60% and 90% at titers 100 and 1,000, respectively). The second curve (panel B) shows a similar monotone pattern for estimated VE against DENV-Any from month 0 to 72 by baseline average titer, but less steep. These estimated curves are key elements of the bridging formula, where the curve in (A) was used by method 1c and the curve in (B) was used by method 1d. Vaccine efficacy curves for hospitalized DENV-Any by serotype were not computed because of limited serotype-specific end point counts. Table 4 shows the results of methods 1c and 1d applied to the two bridging populations. For bridging population 1, estimated VE is 59.1% (95% CI: 40.9–92.2%) and 73.5% (95% CI: 44.7–89.0%) by method 1c and 1d, respectively. For bridging population 2, estimated VE is 50.9% (95% CI: 38.1–70.1%) and 65.9% (95% CI: 39.2–82.1%), respectively. These estimates for adults are comparable or slightly less than the estimates from Sridhar et al.20 for CYD14 + CYD15 + CYD23 9- to 16-year-olds: estimated VE = 66% (95% CI: 27–84%) (Table 4).
Table 4.
Estimated VE against hospitalized dengue virus-Any from month 0 to 72 for hypothetical CYD14 + CYD15 18–45-year-olds (bridging population 1; as if an 18- to 45-year-old cohort had been included in CYD14 + CYD15) and for India 18- to 45-year-olds (bridging population 2) (methods 1c and 1d), with results from CYD14 + CYD15 9- to 16-year-olds for comparison
| Bridging population | Estimated VE (95% CI) against hospitalized VCD from month 0 to 72 | |
|---|---|---|
| Method 1c | Method 1d | |
| CYD14 + CYD15 18- to 45-year-olds | 59.1% (40.9%, 92.2%) | 73.5% (44.7%, 89.0%) |
| India 18- to 45-year-olds | 50.9% (38.1%, 70.1%) | 65.9% (39.2%, 82.1%) |
| CYD14 + CYD15 + CYD57 9- to 16-year-olds | 66 (27, 84)* | |
TMLE = targeted maximum likelihood estimation; VE = vaccine efficacy.
* Estimates from Sridhar et al.20 The log cumulative relative risk (vaccine/placebo) for all CYD14 + CYD15 + CYD57 9- to 16-year-olds was estimated as the weighted average of the log cumulative relative risk estimates for baseline seropositive and seronegative participants reported in Figure 1 of Sridhar et al.20 (the “TMLE, month 0 onward” entries). CYD57 contributed less than 5% of the total sample size. The weights include inverse variance estimates of the log cumulative relative risks reported in Figure 1 (1/0.164 and 1/0.136 for seropositive and seronegative participants) and the reported estimates (by Superlearner) of the fractions of participants who are baseline seropositive and seronegative (76.0% and 24.0%). Cumulative VE is estimated as 100% multiplied by one minus the exponentiated log cumulative relative risk estimate with 95% CI computed by transforming symmetric Wald confidence limits for the log cumulative relative risk.
Supplemental Table 3 shows the results for VEd for the hospitalized DENV-Any VCD endpoint from 0 to 72 months. The results estimate that for every 100,000 18- to 45-year-olds vaccinated in bridging population 1, between 1,000 and 1,200 hospitalized DENV-Any VCD cases are predicted to be averted by vaccination, with 95% CIs spanning from 600 to 1,900 averted for the two overall VE bridging methods 1c and 1d.
Sensitivity analysis for estimated VE against VCD 0–25 months in 18- to 45-year-olds.
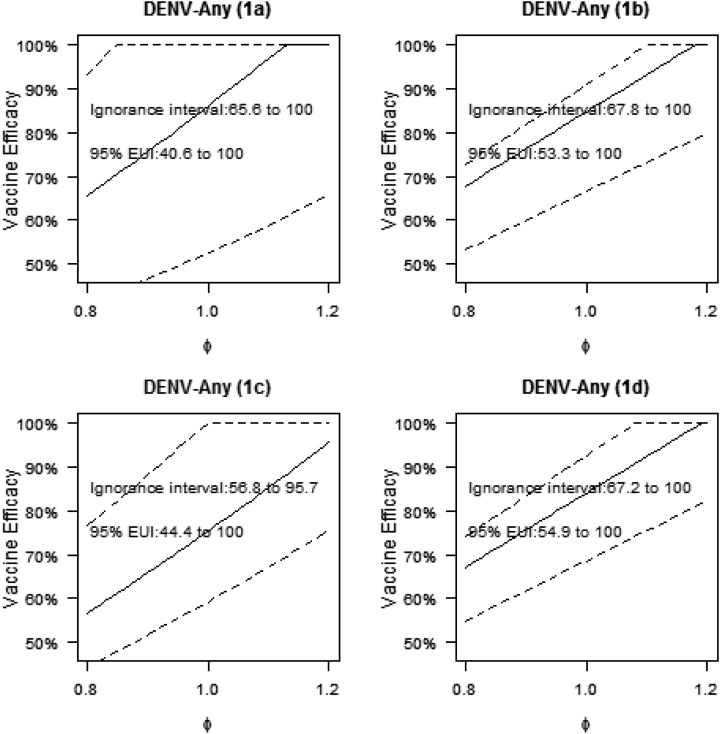
Figure 5 shows the estimates of VE against DENV-Any for bridging population 1 in the first sensitivity analysis, which varied the sensitivity parameter ϕ from 0.8 to 1.2 and fixed the sensitivity parameter ρ to value 1.0. For ϕ above one, the VE estimates were greater than the original estimates at ϕ = 1, and we focus on the estimates for ϕ < 1, as there is particular interest in understanding VE if the original assumptions were incorrect in giving optimistically high VE. At the boundary point ϕ = 0.8, the estimates of VE across methods 1a–1d were 65.6%, 67.8%, 56.8%, and 67.2%, decreases of 23.7%, 20.0%, 24.6%, and 20.0%, respectively, compared with the original estimates at ϕ = 1. These results show that if assumption 1 is violated such that the VE curve by month 13 titers is 20% lower in 18- to 45-year-olds than 9- to 16-year-olds, then VE in CYD14 + CYD15 18- to 45-year-olds is predicted to be about the same as that observed in CYD14 + CYD15 9- to 16-year-olds (VE = 65.6%). Supplemental Figure 5 shows the estimates of VE against DENV-Any for bridging population 1 in the second sensitivity analysis (with ρ = 0.8): the results at the most conservative point in ϕ (ϕ = 0.8, ρ = 0.8) were the same as those at (ϕ = 0.8, ρ = 1.0).These results show that this particular violation of assumption 3 does not affect the results. Supplemental Figures 6 and 7 show the parallel results for bridging population 2, where at (ϕ = 0.8, ρ = 1.0), the VE estimates are 53.3%, 61.9%, 51.3%, and 62.0% and at (ϕ = 0.8, ρ = 0.8), are 53.4%, 61.9%, 51.5%, and 62.0% under the four methods, respectively. Thus, estimated VE in India 18- to 45-year-olds ranges from 51.3% to 62%, depending on the method and bounds of the sensitivity analysis, which is slightly less than the estimated VE observed in CYD14 + CYD15 9- to 16-year-olds. Vaccine efficacy is projected to be less in 18- to 45-year-olds in India than in CYD14 + CYD15, given the higher estimated titers in hypothetical CYD14 + CYD15 18- to 45-year-olds than for CYD47 18- to 45-year-olds (Supplemental Figure 8). The sensitivity analyses for the serotype-specific VE parameters show a similar amount of sensitivity of the results to violations of assumptions 1 and 3 (results not shown).
Figure 5.
First sensitivity analysis showing estimated vaccine efficacy (VE) against dengue virus-Any for hypothetical CYD14 + CYD15 18- to 45-year-olds. Estimated VE against DENV-Any was calculated from month 0 to 25 with 95% CIs for the sensitivity parameter ϕ varying between 0.8 and 1.2 and sensitivity parameter ρ = 1.0 for hypothetical CYD14 + CYD15 18- to 45-year-olds (bridging population 1). The union of the point estimates is the estimated ignorance interval, and the union of the 95% CIs is the EUI. The analysis is performed using each method 1a, 1b, 1c, and 1d.
Sensitivity analysis for estimated VE against hospitalized VCD 0–72 months in 18- to 45-year-olds.
Supplemental Figures 4 and 5 list ignorance intervals and 95% EUIs for VE and VEd against hospitalized dengue over 0–72 months in each bridging population for ϕ varying from 0.8 to 1.2 and ρ = 0.8 (results are almost identical for ρ = 1.0). Based on the 95% EUIs, the vaccine effect is consistently estimated to be beneficial, for example, 95% EUIs 31.2–100% and 28.9–88.2% for bridging populations 1 and 2 using method 1c, respectively.
DISCUSSION
Neutralizing antibody titers measured by PRNT50 at baseline and at month 13 (1 month post dose 3) have been shown to be strongly associated with CYD-TDV VE to prevent VCD from month 13 to 25 in the CYD14 and CYD15 phase 3 trials, with VE increasing with titers, and consistent result that high titers are associated with high VE across both trials, both baseline serostatus subgroups, all age groups, and for all four dengue serotypes. Although there was some variation in the estimated VE curves between the two efficacy trials in the lower titer range, indicating that other factors may influence VE, especially at lower titers post third vaccination, the VE curves were similar when focusing on the 9- to 16-year-old cohort. These statistical results, together with the assumption that neutralizing antibodies are a mechanism of protection for the CYD-TDV vaccine, provide a ground for extrapolating VE to 18- to 45-year-old adults from the CYD14- and CYD15-combined 9- to 16-year-old cohort based on baseline and month 13 PRNT50 titers, in the absence of direct efficacy data in 18- to 45-year-old adults. To enable bridging analyses, phase 2 studies were conducted in Vietnam (CYD22) and India (CYD47) that measured baseline and month 13 PRNT50 titers in CYD-TDV vaccine recipients in 18- to 45-year-olds.
In the Vietnam study that compared PRNT50 titers in 18- to 45-year-olds versus 9- to 16-year-olds, baseline and month 13 titers were consistently higher in the older age group across the serotypes (GM ratio of vaccine recipient month 0 and 13 average titers of 4.00 and 2.69, respectively). To a lesser degree, in the India study, titers in 18- to 45-year-olds were consistently higher than those in 9- to 16-year-olds combining across CYD14 and CYD15 (GM ratio of vaccine recipient month 0 and 13 average titers of 1.62 and 1.28, respectively), as well as being consistently higher than each of the 10 countries included in CYD14 and CYD15. These results imply that if PRNT50 titers are a reliable correlate of VE that operates similarly in the two age cohorts, then VE in the adult cohort is expected to be at least as high as that in the younger cohort, for VCD of each serotype and for VCD of any of the four serotypes (DENV-Any). The result of higher titers in the older age cohort may be generalized to other highly endemic settings, given that the cumulative amount of dengue exposure increases with age and this cumulative exposure increases PRNT50 titers.16,18,38,39 Therefore, it is reasonable to expect that in any given highly endemic setting, VE of CYD-TDV would be equal to or greater in 18- to 45-year-olds than in 9- to 16-year-olds. Although differences in prior exposure to dengue and in the distribution of circulating serotypes across highly endemic settings would impact the absolute level of VE across the settings, the fact that titers would generally be higher in the older population within any such setting implies that VE would also be expected to be at least as high in the older than younger population within each setting.
Our formal estimation and inference of VE against DENV-Any from 0 to 25 months in a hypothetical cohort of 18- to 45-year-olds (had it been included in CYD14 and CYD15—bridging population 1) support this conclusion, with VE estimates against DENV-Any across the four statistical methods of 75.3–86.0%, compared with the 65.6% estimate in CYD14 + CYD15 9- to 16-year-olds. These VE estimates decrease to 56.8–67.8% under a sensitivity analysis that allows the VE by titer curves to be lower in the older age cohort than the younger age cohort.
For making inference for a hypothetical cohort of 18- to 45-year-olds in India that would have the same background/unvaccinated dengue incidence as the CYD14 + CYD15 9- to 16-year-old study, our methods yielded estimates of VE against DENV-Any from 0 to 25 months of 68.4–77.5%, which decrease to 51.3–62.0% under the sensitivity analysis allowing departures from assumptions. Because there was not an internal 9- to 16-year-old comparison group within CYD47, and India was not included in CYD14 or CYD15, it is more difficult to interpret these VE estimates relative to a 9- to 16-year-old cohort in India. The bridging results for India should be interpreted as “what if” results in a hypothetical scenario that an 18- to 45-year-old cohort in India experienced the same incidence of symptomatic VCD incidence in the absence of vaccination as the CYD14- and CYD15-combined 9- to 16-year-old cohort. By contrast, the first set of bridging results is less hypothetical because of the internal age cohort comparison within CYD22 and because the bridging is from the CYD14 + CYD15 efficacy trial to a hypothetical older age cohort in the same CYD14 + CYD15 context (same geographies and time periods of exposure to dengue). In general, the bridging estimation methodology may also be applied to any assumed/what if scenario about background/unvaccinated DENV-Any VCD risk and/or serotype frequencies in a new population, where these scenarios may be informed by epidemiological data in the new population.
For estimating serotype-specific VE in CYD14 + CYD15 18- to 45-year-olds, estimated VE is higher than that in CYD14 + CYD15 9- to 16-year-olds for all four serotypes. The result for DENV-2 is of particular interest, given that VE was lowest against this serotype in CYD14 and CYD15 (as well as in the earlier efficacy trial CYD2340), despite DENV-2 titers being as high or higher than titers to the other three serotypes, and the estimated DENV-2 VE curve by the month 13 DENV-2 titer was the steepest of the four curves for each of these three trials. Presumably, the higher levels of antibody compensate for the quality differences across serotypes in the antibody response, with high DENV-2 titers protecting against DENV-2; some heterotypic cross-response from any recent DENV exposure might also account for this. For estimating serotype-specific VE in CYD47 18- to 45-year-olds, estimated VE is higher than that in CYD14 + CYD15 9- to 16-year-olds for the four serotypes yet by one method was less for serotype 1. Although this latter result is surprising, given that titers were higher in CYD47 18- to 45-year-olds than in CYD14 + CYD15 9- to 16-year-olds, it can be explained as an anomaly because the bridging method used data from the relatively small sub-cohort with baseline titers measured, and sampling variability led to a too-low estimate.
We also applied methods 1c and 1d used for bridging to VE against DENV-Any over 0–25 months for 18- to 45-year-olds in an identical way for bridging to VE against hospitalized DENV-Any over 0–72 months for 18- to 45 year-olds, with VE estimates 59.1–73.5% for bridging population 1 and VE estimates 50.9–65.9% for bridging population 2, which are comparable with or slightly less than the 64% estimate in CYD14 + CYD15 + CYD57 9- to 16-year-olds from Sridhar et al.20 Method 1c requires an estimate of how VE against hospitalized DENV-Any through month 13 varies with baseline average titer. Of all participants who experienced the hospitalized DENV-Any endpoint by month 13, there were only 4 participants in the placebo group and 0 participants in the vaccine group for whom baseline average titer was measured. Thus, we modeled this VE curve by a logistic regression model with two independent variables (treatment assignment and baseline average titer), without an interaction term. This modeling choice is conservative, tending to result in lower estimated VE in adults. We discuss in the following paragraphs further ways in which the methods were implemented in a manner that aims for conservative lower bounds; thus, the overall conclusion is that the multiplicative VE in preventing hospitalized dengue over 6 years of follow-up is expected to be as high in adults as children.
Extrapolation of efficacy results to a new population without directly measuring efficacy in the new population is generally a difficult task, given the inherent inability to empirically check the results, at least until results are available from phase 4 post-licensure trials. A limitation of the bridging estimation methods that use both month 0 and month 13 titers is that the key assumption 1 would fail if there exists another immune response to vaccination besides the month 13 neutralization titer measured by PRNT50, which modifies VE after accounting for month 13 PRNT50, and which has a different distribution in 18- to 45-year-olds than 9–16-year-olds. This could occur, for example, if the CD4 and/or CD8 T-cell response is also important for VE and increases with age (albeit antibody alone has been validated as a correlate of protection for Japanese encephalitis virus vaccine and yellow fever vaccine),41 and there is no validated T-cell correlate of protection, which is being evaluated in an ongoing ancillary study of CYD14. For example, even though the CYD-TDV chimeric vaccine contains yellow fever virus nonstructural (NS) proteins rather than DENV NS proteins, CYD-TDV vaccination appears to result in higher DENV-2/-3 NS3–specific CD8+ T-cell responses in adults than adolescents at multiple time points postvaccination.42 This selective boosting of CD8+ T-cell responses (i.e., from prior DENV infection) in vaccinated adults compared with adolescents could contribute to increased VE in adults because both serotype-specific and cross-serotype–reactive CD8+ T-cell responses against DENV NS proteins (including NS3) can contribute to protection from PCR-confirmed DENV infection, at least in a transgenic mouse model.43 We note that CD4+/CD8+ T-cell responses specific to DENV structural proteins, which can contribute to natural protection,44,45 are also elicited in CYD-TDV vaccinees.24,42,46 Although it is not known whether these responses differ significantly between 18- to 45-year-old versus 9- to 16-year-old CYD-TDV vaccinees, which would impact the validity of assumption 1, CD4+ T-cell responses to staphylococcal enterotoxin B have been shown to generally increase with age until adulthood.47 This finding raises the possibility that similar DENV-specific CD4+ T-cell response boosting could occur in adult CYD-TDV vaccinees.
Assumption 1 would also be invalidated if baseline PRNT50 titer modifies VE after accounting for month 13 PRNT50 titer, given that baseline titers tend to be higher in 18- to 45-year-olds than 9- to 16-year-olds. Had baseline titers been measured from most VCD cases in CYD14 and CYD15, then it would have been possible to base the bridging analysis on the bivariate VE surface that expresses how VE varies jointly over subgroups defined by both baseline and month 13 titers; the fact that baseline titers were only available from 13.4% of VCD cases precluded this analysis. Given this limitation, the sensitivity analysis is important, which showed how the estimate of VE changes if assumption 1 is allowed to have up to a 20% violation, and quantifies uncertainty via an EUI that accounts for uncertainty both due to sampling variability and due to possible violations of assumption 1 up to 20%. Moreover, if there is an unmeasured immune response that modifies VE after accounting for the month 13 PRNT50 titer that tends to be higher in 18- to 45-year-olds than 9–16-year-olds, then not accounting for this immune response in the analysis would likely lead to biased estimates of VE in the new population that are too low; this would occur if VE does not decrease with increasing levels of the unmeasured immune response after fixing the month 13 PRNT50 titer. Under the premise that the relevant immune responses do not decrease as age increases from 9–16 to 18–45 years, this implies that the estimated VEs may be interpreted as conservative lower bounds; moreover, the estimates under the sensitivity analysis may be interpreted as more conservative lower bounds.
For the bridging methods that only use month 0 titers (not month 13 titers), the same arguments apply swapping the roles of month 13 and baseline titers. Given that month 13 titers were a stronger modifier of VE than baseline titers in CYD14 and CYD15 and are higher in 18- to 45-year-olds than 9–16-year-olds, it seems likely that VE depends on the month 13 titer after accounting for the baseline titer, thus biasing the VE estimates. However, similar arguments as mentioned previously support that the direction of bias would render the obtained estimates of VE as conservative lower bounds.
The challenges in assuring that assumptions are true for bridging estimation led us to apply several versions of the bridging methods, that either use both month 0 and 13 titers, only month 0 titers, and that are based on serotype-specific VE curves or on average titer DENV-Any VE curves. The fairly consistent results obtained across the methods provide some support for the reliability of the results. The main advantage of the methods using both month 0 and 13 titers is that month 13 titers modify VE more strongly than baseline titers, such that not including month 13 titers likely excludes an effect modifier. By contrast, it is logistically simpler to base bridging on month 0 baseline titers because future phase 1 bridging studies would not require 13 months of follow-up for measuring postvaccination titers, and moreover, the methods based on baseline titers do not make any assumptions about counterfactual causal parameters whose estimation is made more complicated by missing data.
We next discuss some additional limitations of this work. It would be of considerable interest to apply our method to estimate VE against symptomatic VCD in 18- to 45-year-olds over a longer period of follow-up than 25 months, such as out to 72 months. This question is important given the concern of waning VE. However, we restricted our analysis of the symptomatic VCD end point to the protocol-specified 25-month follow-up period because analysis of symptomatic VCD through 72 months is hindered by the gap (median 27.1 months) between the 25-month active surveillance phase of follow-up for symptomatic VCD and the resumption of active surveillance. The intermediate passive surveillance phase tended to only capture highly symptomatic cases, and it is difficult to conduct valid and interpretable analyses with multiple case-ascertainment systems in the same study. Given this limitation, we acknowledge that the analysis in this study does not directly support the extrapolation of VE against the symptomatic VCD end point for time frames longer than 25 months.
It is also of considerable interest to apply our method to estimate VE against hospitalized or severe VCD in 18- to 45-year-olds over the entire follow-up period of 72 months. For these dengue endpoints, the surveillance system was consistent during the 72-month follow-up period. However, precision of estimates would be relatively low because of the relatively small number of hospitalized or severe VCD end points than symptomatic VCD endpoints (in CYD14 and CYD15 9- to 16-year-olds pooled, there were an estimated 886 total symptomatic VCD end points over 25 months compared with 242 hospitalized and 62 severe VCD end points over 72 months). Although we were able to estimate VE against hospitalized VCD of any serotype through 72 months, given the 242 endpoints, this number was too small to produce reliable estimates by serotype, and the total number of severe VCD end points was too small to venture any estimates of VE against severe VCD.
Moreover, whereas it would also be of interest to bridge VE for baseline seronegative and baseline seropositive subgroups separately, because baseline serostatus was only measurable in 13.4% of symptomatic VCD cases we determined that the resulting precision would be too low to warrant these analyses. However, because most 18- to 45-year-olds in endemic regions are seropositive (93.3% in CYD22 and 84% in CYD47), the bridging estimates of multiplicative VE reported here may be interpreted as reasonably on target or lower bounds for multiplicative VE in seropositive 18- to 45-year-olds. Because our bridging analyses were based on VE curves including all CYD14 + CYD15 9- to 16-year-olds and the estimated VE curves were higher for baseline seropositive participants than baseline seronegative participants, the multiplicative VE estimates in 18- to 45-year-olds are probably lower bounds. Another limitation of the analyses is that estimation of the VE curves is challenging, given the missing counterfactual immune responses if assigned vaccine in placebo recipients, and to tackle this challenge, our analyses relied on parametric hinge linear logistic models. These parametric assumptions cause the CIs about the VE curves to be narrower than if these assumptions were avoided, especially in the tails, where there are small numbers of participants with marker measurements. As a consequence, our reported confidence and uncertainty intervals about VE parameters do not account for uncertainty in whether these parametric assumptions hold, and, thus, are likely too narrow in a sense. In addition, although our methods for calculating CIs and EUIs account for the finite-sampling variability of the CYD22 and CYD47 trials, the limited sample sizes of these trials that provided critical data for immuno-bridging constitutes another limitation.
Although our focus has been on bridging estimation of multiplicative and additive–difference VE for populations aged 18–45 years as a group, we observe that within this age range, based on our modeling approach, we expect multiplicative VE to increase moving from 18-year-olds to 45-year-olds, and additive-difference VE may potentially decrease moving from 18-year-olds to 45-year-olds. The former statement is supported by two findings, the first being that baseline seropositivity frequency increases with age in CYD22 and CYD47 (e.g., in CYD47 an estimated 69%, 73%, 77%, and 63% of 18-year-olds are seropositive for serotypes 1, 2, 3, and 4, respectively, compared with 94%, 95%, 94%, and 93% of 45-year-olds), and the mean baseline serotype titer increases with age (e.g., in CYD47, the estimated mean titer is 126, 138, 120, and 32 for serotypes 1, 2, 3, and 4 for 18-year-olds compared with 389, 417, 513, and 132 for 45-year-olds). The second finding is that the estimated VE curves from CYD14 + CYD15 9- to 16-year-olds increase with baseline and month 13 titers (Figure 2, Supplemental Figures 1 and 2). Together, these two findings and our modeling approach imply higher predicted VE for higher ages within 18–45 years. The latter statement on additive–difference VE is supported by the finding that baseline and month 13 neutralization titers are inverse correlates of VCD risk in both the vaccine and placebo groups,18 such that the VCD risks in both groups are expected to decrease closer to zero as age increases from 18 to 45 years.
An important question is whether the increased risk for hospitalized and severe VCD seen in vaccinated seronegative (versus placebo seronegative) children and adolescents20 would also be seen in adults, as such a scenario could limit the public health impact of CYD-TDV vaccination of adults in endemic areas where seropositivity rates of adults are insufficiently high (we recently found that seroposivity rates of 18- to 45-year-olds in endemic areas range from 48.7% [Singapore] to 94.5% [Vietnam]48). Given the extensive evolution of the human immune system from infancy through old age (reviewed in ref. 49) and the fact that the contributions of age to infection outcomes and disease severity remain poorly quantified, this remains an open question. In the context of the previously discussed hypothesis that vaccination of a baseline seronegative individual acts as a primary infection, this question could be examined by comparing the risk of developing severe disease in secondary infection in adults versus children. Unfortunately, few such analyses have been performed. However, there is hospital and seroepidemiological evidence to support that children have much higher risk of developing DHF/DSS in secondary infection than adults, in addition to much higher risk of dying from DHF/DSS in secondary infection than adults.50 Whereas it is unknown whether this finding holds true in other geographic regions, with different circulating viral variants, the available evidence suggests that we would not expect the increased risk for hospitalized and severe VCD of vaccinated seropositive (compared with seronegative) adults to be as high as that for their corresponding child/adolescent counterparts. Another modeling consideration of interest is whether widespread vaccination with CYD-TDV could lead over time to a delay in the age at which natural DENV-Any seropositivity occurs, which could theoretically increase the proportion of 18- to 45-year-olds who are dengue seronegative.
In the future, the epidemiological bridging methods applied here to dengue vaccination may be useful for other vaccines, especially vaccines against antigenically heterogeneous pathogens such as influenza, HIV, and malaria.
Supplemental tables and figures
Acknowledgments:
We thank all participants in the CYD14, CYD15, CYD22, and CYD47 trials, and administrative and clinical research staff who helped ensure successful completion of the trials.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Murray NE, Quam MB, Wilder-Smith A, 2013. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 5: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , 2012. World Health Organization (WHO) Global Strategy for Dengue Prevention and Control, 2012–2020. Geneva, Switzerland: WHO Press. [Google Scholar]
- 3.Bhatt S, et al. 2013. The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI, 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6: e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thai KT, Binh TQ, Giao PT, Phuong HL, Hung le Q, Van Nam N, Nga TT, Groen J, Nagelkerke N, de Vries PJ, 2005. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health 10: 379–386. [DOI] [PubMed] [Google Scholar]
- 6.Low SL, Lam S, Wong WY, Teo D, Ng LC, Tan LK, 2015. Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg 93: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew CH, et al. 2016. Rural-urban comparisons of dengue seroprevalence in Malaysia. BMC Public Health 16: 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Barraquer I, Buathong R, Iamsirithaworn S, Nisalak A, Lessler J, Jarman RG, Gibbons RV, Cummings DA, 2014. Revisiting Rayong: shifting seroprofiles of dengue in Thailand and their implications for transmission and control. Am J Epidemiol 179: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carabali M, Hernandez LM, Arauz MJ, Villar LA, Ridde V, 2015. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect Dis 15: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sam SS, Omar SF, Teoh BT, Abd-Jamil J, AbuBakar S, 2013. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl Trop Dis 7: e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moraes GH, de Fatima Duarte E, Duarte EC, 2013. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. Am J Trop Med Hyg 88: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger JR, Coleman PG, 2007. Age and clinical dengue illness. Emerg Infect Dis 13: 924–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thai KT, Nishiura H, Hoang PL, Tran NT, Phan GT, Le HQ, Tran BQ, Nguyen NV, de Vries PJ, 2011. Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl Trop Dis 5: e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy B, Lang J, Saville M, Jackson N, 2016. Vaccination against dengue: challenges and current developments. Annu Rev Med 67: 387–404. [DOI] [PubMed] [Google Scholar]
- 15.Villar L, et al. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372: 113–123. [DOI] [PubMed] [Google Scholar]
- 16.Capeding MR, et al. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 17.Juraska M, et al. 2018. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci USA 115: E8378–E8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moodie Z, et al. 2018. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis 217: 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadinegoro SR, et al. 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373: 1195–1206. [DOI] [PubMed] [Google Scholar]
- 20.Sridhar S, et al. 2018. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 379: 327–340. [DOI] [PubMed] [Google Scholar]
- 21.Mizumoto K, Ejima K, Yamamoto T, Nishiura H, 2014. On the risk of severe dengue during secondary infection: a systematic review coupled with mathematical modeling. J Vector Borne Dis 51: 153–164. [PubMed] [Google Scholar]
- 22.Pancharoen C, Mekmullica J, Thisyakorn U, 2001. Primary dengue infection: what are the clinical distinctions from secondary infection? Southeast Asian J Trop Med Public Health 32: 476–480. [PubMed] [Google Scholar]
- 23.Endy TP, Yoon IK, Mammen MP, 2010. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol 338: 1–13. [DOI] [PubMed] [Google Scholar]
- 24.Guy B, Jackson N, 2016. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 14: 45–54. [DOI] [PubMed] [Google Scholar]
- 25.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E, 2017. Antibody-dependent enhancement of severe dengue disease in humans. Science 358: 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salje H, et al. 2018. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 557: 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson NM, Rodriguez-Barraquer I, Dorigatti I, Mier-Y-Teran-Romero L, Laydon DJ, Cummings DA, 2016. Benefits and risks of the Sanofi-Pasteur dengue vaccine: modeling optimal deployment. Science 353: 1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization , 2018. Revised SAGE Recommendation on Use of Dengue Vaccine. Available at: http://www.who.int/immunization/diseases/dengue/revised_SAGE_recommendations_dengue_vaccines_apr2018/en/. Accessed April 19, 2018. [Google Scholar]
- 29.Tran N, Luong C, Vu T, Forrat R, Lang J, Vu Q, Bouckenooghe A, Wartel T, 2012. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD-TDV) in healthy Vietnamese adults and children. J Vaccines Vaccin 3: 162. [Google Scholar]
- 30.Dubey AP, Agarkhedkar S, Chhatwal J, Narayan A, Ganguly S, Wartel TA, Bouckenooghe A, Menezes J, 2016. Immunogenicity and safety of a tetravalent dengue vaccine in healthy adults in India: a randomized, observer-blind, placebo-controlled phase II trial. Hum Vaccin Immunother 12: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert PB, Huang Y, 2016. Predicting overall vaccine efficacy in a new setting by re-calibrating baseline covariate and intermediate response endpoint effect modifiers of type-specific vaccine efficacy. Epidemiol Methods 5: 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW, 2013. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg 88: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juraska M, Huang Y, Gilbert PB, 2018. Inference on treatment effect modification by biomarker response in a three-phase sampling design. Biostatistics. Available at: 10.1093/biostatistics/kxy074 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plotkin SA, Gilbert PB, 2012. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 54: 1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG, 2007. A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis 196: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert PB, Gabriel EE, Miao X, Li X, Su SC, Parrino J, Chan IS, 2014. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis 210: 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Gilbert PB, Janes H, 2012. Assessing treatment-selection markers using a potential outcomes framework. Biometrics 68: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clapham HE, et al. 2016. Dengue virus (DENV) neutralizing antibody kinetics in children after symptomatic primary and postprimary DENV infection. J Infect Dis 213: 1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E, 2016. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci USA 113: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabchareon A, et al. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 41.Plotkin SA, 2010. Correlates of protection induced by vaccination. Clin Vaccine Immunol 17: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harenberg A, et al. 2013. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum Vaccin Immunother 9: 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elong Ngono A, Chen HW, Tang WW, Joo Y, King K, Weiskopf D, Sidney J, Sette A, Shresta S, 2016. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine 13: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A, 2015. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci USA 112: E4256–E4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew A, Townsley E, Ennis FA, 2014. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol 9: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dayan GH, Galan-Herrera JF, Forrat R, Zambrano B, Bouckenooghe A, Harenberg A, Guy B, Lang J, 2014. Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naive adults in Mexico. Hum Vaccin Immunother 10: 2853–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna-Wakim R, Yasukawa LL, Sung P, Fang M, Sullivan B, Rinki M, DeHovitz R, Arvin AM, Gans HA, 2009. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J Infect Dis 200: 1921–1927. [DOI] [PubMed] [Google Scholar]
- 48.L’Azou M, et al. 2018. Dengue seroprevalence: data from the clinical development of a tetravalent dengue vaccine in 14 countries (2005–2014). Trans R Soc Trop Med Hyg 112: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon AK, Hollander GA, McMichael A, 2015. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282: 20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB, 2002. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 6: 118–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.