Abstract.
Faced with the reemergence of yellow fever (YF) in the metropolitan region of São Paulo, Brazil, we developed a retrospective study to describe the cases of YF attended at the Institute of Infectology Emilio Ribas from January to March 2018 and analyze the factors associated with death, from the information obtained in the hospital epidemiological investigation. A total of 72 cases of sylvatic YF were confirmed, with 21 deaths (29.2% lethality rate). Cases were concentrated in males (80.6%) and in the age group of 30 to 59 years (56.9%). Two logistic regression models were performed, with continuous variables adjusted for the time between onset of symptoms and hospitalization. The first model indicated age (odds ratiosadjusted [ORadj]: 1.038; CI 95%: 1.008–1.212), aspartate aminotransferase (AST) (ORadj: 1.038; CI 95%: 1.005–1.072), and creatinine (ORadj: 2.343; CI 95%: 1.205–4.553) were independent factors associated with mortality. The second model indicated age (ORadj: 1.136; CI 95%: 1.013–1.275), alanine aminotransferase (ALT) (ORadj: 1.118; CI 95%: 1.018–1.228), and creatinine (ORadj: 2.835; CI 95%: 1.352–5,941). The risk of death in the model with continuous variables was calculated from the increase of 1 year (age), 1 mg/dL (creatinine), and 100 U/L for AST and ALT. Another logistic regression analysis with dichotomous variables indicated AST > 1,841 IU/L (ORadj: 12.92; CI 95%: 1.50–111.37) and creatinine > 1.2 mg/dL (ORadj: 81.47; CI 95%: 11.33–585.71) as independent factors associated with death. These results may contribute to the appropriate clinical management of patients with YF in health-care services and improve the response to outbreaks and public health emergencies.
INTRODUCTION
Yellow fever (YF) is an infectious disease endemic in the tropical forests of America and Africa, transmitted to humans after bites from hematophagous insects of the Culicidae family, especially the genus Aedes and Haemagogus. The YF virus belongs to the Flavivirus genus of the Flaviviridae family, being a single-stranded RNA virus.1 The disease is endemic in 44 countries in Africa and the Americas. In West Africa, the risk of epidemics has been declining following large-scale vaccination campaigns, where millions of people were vaccinated in a short period of time.2
The last record of urban YF in Brazil occurred in 1942, although there have been sporadic reports of human cases of sylvatic YF, especially in the Amazon region in unvaccinated individuals.3 From 1980 to 2016, 797 cases of sylvatic YF occurred in Brazil, with increased circulation of the virus from 1998 to the central west, southeast, and south regions.4
Yellow fever is a mandatory reportable disease in Brazil, and the definition of a suspected case consists of an individual with fever (< 7 days of onset), accompanied by jaundice or hemorrhagic manifestations, who had been in risk areas for YF and not been previously vaccinated.3 In 2009, a study analyzed 28 confirmed cases of sylvatic YF in the state of São Paulo, demonstrating that only 50% met the case definition.5
According to a report from the Ministry of Health, from December 2016 to May 13, 2017, 792 human cases of sylvatic YF and 274 deaths were confirmed in the country (34.5%), with 487 cases in Minas Gerais state (61.4%), 260 cases in Espirito Santo state (32.8%), and 20 cases in São Paulo state (4.1%). In this period, 642 cases were confirmed in nonhuman primates (NHP).6 From July 2017 to May 2018, 1,266 human cases of sylvatic YF were confirmed and 415 deaths (lethality rate 32.8%), and 752 were confirmed in NHP. Most human cases occurred in the southeast region: 520 cases and 177 deaths in Minas Gerais (lethality rate 34.0%), 516 cases and 163 deaths in São Paulo (lethality rate 31.6%), and 223 cases and 73 deaths in Rio de Janeiro (lethality rate 32.7%).7
Yellow fever is an acute viral infectious disease with variable severity and may manifest as oligosymptomatic forms to fulminant presentations (5–10%), with a high lethality. A study analyzing data from the YF epidemic in Gambia showed that for each severe case, there were 12 unapparent cases.8 Another study in Maranhao, Brazil, found a proportion of asymptomatic cases equal to 45.2%.9
The YF vaccine is the main control measure and is presently administered in Brazil as a single dose from 9 months or older, for residents or travelers to areas where vaccination is recommended. In response to the reemergence of the disease in the metropolitan region of São Paulo, the São Paulo State Department of Health defined an algorithm for YF cases, with participation of the Institute of Infectology Emilio Ribas (IIER). Thus, the objective of this study was to describe the clinical, epidemiological, and laboratory characteristics of confirmed YF cases attended in the IIER and to evaluate the factors associated with death from January to March 2018.
MATERIALS AND METHODS
This is a retrospective study with data from the Epidemiological Investigation Files (EIF) of the National Disease Notification System collected in the routine of epidemiological investigation of the suspected cases of YF attended in the IIER. The IIER is a reference hospital for infectious diseases in São Paulo state, with 199 beds, of which 12 are intensive care unit (ICU) beds. The Epidemiology Service performs an active search daily of all patients attended at the hospital to investigate cases requiring a mandatory report. The information was collected by interviews with patients, by evaluation of medical records, and from results of laboratory tests during patient admission.
The case definitions followed Brazil’s epidemiological surveillance standards (3).3 This study included the cases of sylvatic YF attended in the IIER, confirmed by real-time polymerase chain reaction (RT-PCR) and/or positive serology (IgM), carried out in the Adolfo Lutz Institute. Samples of serum or tissue fragments are subjected to RNA extraction by commercial kits according to the manufacturers’ guidelines. Next, the RNA extracted is subjected to the RT-PCR protocol, using primers and probes specific for amplification of RNA segments of the YF virus. Initially, the extracted RNA is submitted to the protocol that amplifies a noncoding region of the 5′ end of the virus, being conserved among the different strains. This protocol can detect both the wild-type virus genome and the vaccine-virus genome.10 The positive samples in the first reaction are submitted to a reaction for differentiation between wild and vaccine viruses. In this second stage, a new RT-PCR is performed, which detects only the vaccine virus.11 Therefore, samples with a positive result in the first reaction and negative result in the second reaction are considered positive for wild virus. Samples positive in both reactions are considered positive for vaccine virus.
The cases were classified according to severity, based on the algorithm of the Department of Health of Minas Gerais state: patients with aspartate aminotransferase (AST) > 2,000 U/L and/or creatinine > 2 mg/dL and/or the international normalized ratio (INR) > 1.5 seconds and/or total bilirubin (TB) > 5 mg/dL.12
Spatial distribution of the cases was presented according to the probable municipalities of infection, in a thematic map created in Quantum Geographic Information System (version 2.16.1; QGIS, Community-Team). The associations between qualitative variables were analyzed by Pearson’s χ2 test (or Fisher’s exact test, if necessary), and for quantitative variables, Student’s t- or Mann–Whitney test was used, according to the data distribution. The linear association between the continuous variables was verified by the Spearman correlation coefficient, and the factors associated with death were assessed by logistic regression, resulting in adjusted and unadjusted odds ratios (OR), with respective confidence intervals (CI 95%). We performed two logistic regressions models to evaluate the risks associated with death. The first model considered the continuous variables with variations of one unit; every 100 units for AST, alanine aminotransferase (ALT), and leukocytes; or every 1,000 units for platelets, and the second model transformed these variables into nominal values with cutoff points. The significance level adopted was 5%, and statistical analyses were performed in Statistical Package for Social Science (version 25; SPSS, Chicago, IL). The study used information obtained from hospital epidemiological surveillance, and the data use was approved by the Ethics Committee of IIER (Protocol 024329/2018).
RESULTS
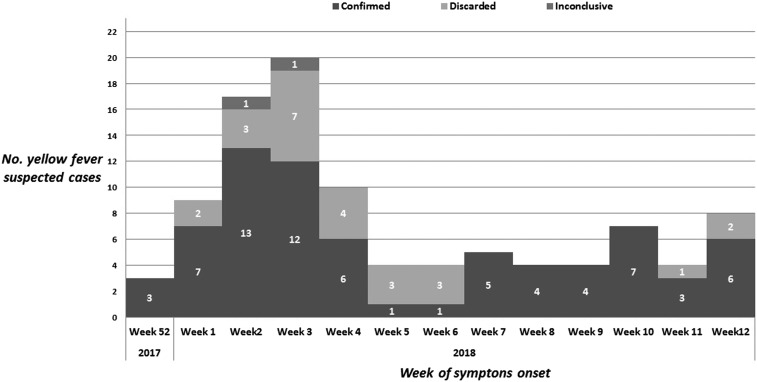
From January to March 2018, 99 suspected cases of YF were attended at IIER and reported by the Epidemiology Service. Seventy-two cases of sylvatic YF (72.7%) were confirmed, 25 cases were discarded (25.3%), and two mild cases were inconclusive (2.0%)—one case without a sample collection and one case with negative IgM serology in a sample collected 48 days after the onset of symptoms. The first confirmed case presented a symptom onset in epidemiological week (EW) 52 on December 26, 2017, and the last included a case in EW 12 on March 23, 2018. Cases were concentrated in January, with a peak in weeks 2 and 3 (January 7–20), according to Figure 1.
Figure 1.
Number of suspected cases of yellow fever, according to laboratory confirmation and epidemiological week of onset of symptoms, Institute of Infectology Emilio Ribas, 2018.
All discarded cases (25) presented negative RT-PCR and/or negative IgM results for YF in samples collected at an appropriate time. There was confirmation of another diagnosis in 10 cases (40.0%): 6 cases of hepatitis A (IgM positive) (24.0%), 2 cases of leptospirosis (8.0%), 1 case of exogenous intoxication (4.0%), and 1 case with toxic shock syndrome, presenting positive blood culture for Streptococcus pyogenes (4.0%).
Among the confirmed cases of YF (72), serum RT-PCR was detectable for 52.8%, positive IgM serology was 9.7%, and the two examinations were positive for 37.5%. One case was confirmed in the puerperal period, which also showed positive RT-PCR in breast milk.
Among all confirmed YF cases (72), 21 died, with a lethality rate of 29.2%. Necropsy was performed in 13 cases (61.9%), with confirmation of the YF virus by the RT-PCR technique in fragments of the liver (100%), brain (75.9%), and spleen (53.8%).
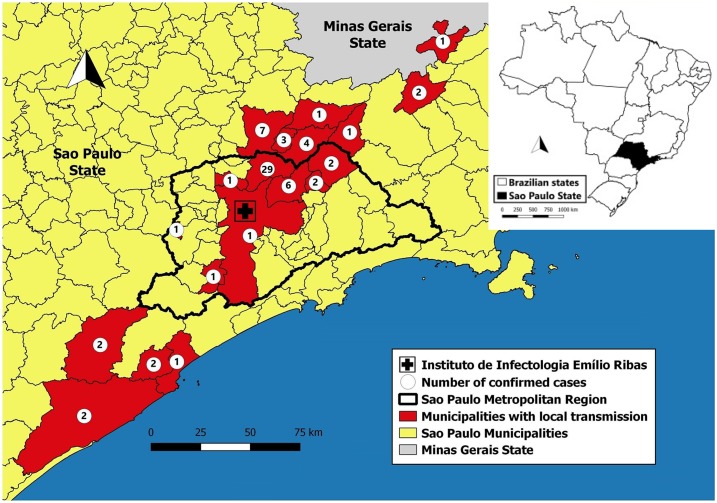
All the patients lived or traveled to sylvatic areas and 72.2% of cases were autochthonous, considering the probable place of infection (PPI). Most PPIs were located in the metropolitan region of São Paulo (59.7%), with the municipality of Mairipora corresponding to the highest proportion of cases (40.3%), followed by Guarulhos (8.3%), Aruja (2.8%), Santa Isabel (2.8%), and São Paulo (1.4%). The Regional Department of Campinas accounted for 20.8% of the PPIs of the confirmed cases, with Atibaia (9.7%), Nazare Paulista (5.6%), and Bom Jesus dos Perdoes (4.2%) being some of the most relevant municipalities. In March, cases were confirmed in the Regional Department of Registro (8.3%) and Santos (1.4%). Three cases traveled to the state of Minas Gerais. The cases with PPI in São Paulo state are shown in the map in Figure 2.
Figure 2.
Distribution of yellow fever cases, according to probable place of infection, Institute of Infectology Emilio Ribas, 2018. This figure appears in color at www.ajtmh.org.
The majority of cases were male (80.6%), in the age group of 30–59 years (56.9%), white race/skin color (65.3%), and had more than 8 years of study (55.9%). Among the occupations, those who performed rural activities (15.3%) and worked in construction (13.9%) were the most relevant. When comparing the 72 cases, there was no statistical significance among the sociodemographic variables, except for age, with a median of 55 years for the deaths and 37 years for the cures, P < 0.001 (Table 1).
Table 1.
Number of confirmed cases of sylvatic yellow fever, according to evolution and sociodemographic variables, Institute of Infectology Emilio Ribas, 2018
| Sociodemographic characteristics | Total | Clinical evolution | P-value | |
|---|---|---|---|---|
| Cure | Death | |||
| No. (%) | No. (%) | No. (%) | ||
| Gender | ||||
| Female | 14 (19.4) | 11 (21.6) | 3 (14.3) | 0.744* |
| Male | 58 (80.6) | 40 (78.4) | 18 (85.7) | |
| Age range (years) | ||||
| 11–29 | 16 (22.2) | 15 (29.4) | 1 (4.8) | 0.004* |
| 30–59 | 41 (56.9) | 30 (58.8) | 11 (52.4) | |
| ≥ 60 | 15 (20.8) | 6 (11.8) | 9 (42.9) | |
| Median age (min†–max‡) (years) | 41.5 (11–86) | 37 (11–68) | 55 (21–86) | < 0.001§ |
| Race/skin color | ||||
| White | 47 (65.3) | 32 (62.7) | 15 (71.4) | 0.482‖ |
| Nonwhite | 25 (34.7) | 19 (37.3) | 6 (28.6) | |
| Years of study¶ | ||||
| Up to 8 | 30 (44.1) | 20 (39.2) | 10 (58.8) | 0.159‖ |
| More than 8 | 38 (55.9) | 31 (60.8) | 7 (41.2) | |
* Fisher’s exact test.
† Minimum value.
‡ Maximum value.
§ Student’s t-test.
‖ Pearson’s χ2 test.
¶ Four cases without information.
The most common symptoms among sylvatic YF cases were as follows: fever (98.6%), myalgia (93.1%), nausea (84.6%), and vomiting (88.9%). There were no statistical differences in symptoms and signs in cases that evolved to cure and death, except for jaundice considering TB levels ≥ 2.0 mg/dL (90.5% deaths × 28.0% cure), which presented P < 0.001. Twenty-two cases reported comorbidities (31.4%): 50.5% of deaths and 24.0% of cured patients (P = 0.047). Among the elderly ≥ 60 years, the prevalence of comorbidities was 66.7% (33.3% of those who cured and who died; P = 0.580), and in the age range of < 60 years, the percentage was 20.3% (12.7% cured × 9.1% death; P = 0.048). A history of alcohol and/or drug abuse was reported in 34.7% of the cases (52.4% who died × 27.5% who were cured; P = 0.058).
Considering the 72 confirmed cases and the severity classification, 41 cases were considered as severe at admission (56.9%). Of the total number of patients, 65 were hospitalized (90.3%) and, of these, 61.5% were in the ICU, 26.2% in the emergency room, and 12.3% in inpatient units. The nonhospitalized cases were attended in the emergency room and remained for less than 24 hours in the hospital. Nine (12.5%) of the cases attended, reported, and investigated in the IIER were later transferred to a hospital specialized in fulminant hepatitis. All these cases evolved to death and are included in the analysis of this study. The total proportion of patients who received hemodialysis was 15.3%; among those who died, this proportion was 47.6% and among those who recovered, the proportion was 2.0%. The median time from symptom onset to hospitalization was 4 days (range 0–11 days) among the deaths and 5 days (range 1–18 days) in the cases that evolved to cure (P = 0.272). Regarding the time of hospitalization until discharge, the median was 4 days (range 1–57 days) for the deaths and 6 days for the cures (range 1–36 days) (P = 0.072).
Considering the 69 cases who reported YF vaccine status (95.8%), nine cases were vaccinated before the onset of symptoms (13.0%), with one case in a 66-year-old woman vaccinated 30 days before the onset of symptoms, one in a 24-year-old woman vaccinated 100 days before disease onset, and three cases (4.3%) vaccinated 1 to 3 days, three cases (4.3%) 4 to 10 days, and one case (1.4%) 11 days before presenting symptoms. The majority of these cases evolved to cure (98.5%), except for one case vaccinated 3 days before symptom onset. Information about the clinical and epidemiological characteristics of cases is presented in Table 2.
Table 2.
Number of cases of sylvatic YF, according to evolution and clinical and epidemiological variables, Institute of Infectology Emilio Ribas, 2018
| Clinical and epidemiological characteristics | Total | Clinical evolution | P-value | ||||
|---|---|---|---|---|---|---|---|
| Cure | Death | ||||||
| No. of available information (%) | No. positive (%) | No. positive (%) | |||||
| Symptoms and signs | |||||||
| Fever | 71 | 98.6 | 51 | 100.0 | 20 | 100.0 | 1.000* |
| Myalgia | 67 | 93.1 | 42 | 82.4 | 15 | 93.8 | 0.430* |
| Nausea | 65 | 84.6 | 37 | 83.3 | 14 | 88.2 | 1.000* |
| Headache | 67 | 93.1 | 37 | 72.5 | 14 | 87.5 | 0.320* |
| Vomiting | 64 | 88.9 | 34 | 70.8 | 12 | 75.0 | 1.000* |
| Abdominal pain | 70 | 97.2 | 25 | 50.0 | 16 | 80.0 | 0.031* |
| Jaundice (total bilirubin ≥2.0 mg/dL) | 71 | 98.6 | 14 | 28.0 | 19 | 90.5 | < 0.001* |
| Chills | 57 | 79.1 | 23 | 52.3 | 05 | 38.5 | 0.530* |
| Back pain | 29 | 40.3 | 20 | 95.2 | 07 | 87.5 | 0.482* |
| Diarrhea | 63 | 87.5 | 10 | 20.8 | 04 | 26.6 | 0.725* |
| Hemorrhagic signs | 72 | 100.0 | 04 | 7.8 | 04 | 23.5 | 0.219* |
| Presence of comorbidities† | 70 | 97.2 | 12 | 24.0 | 10 | 50.5 | 0.047* |
| < 60 years | 55 | 96.5 | 07 | 12.7 | 05 | 9.1 | 0.048* |
| ≥ 60 years | 15 | 100.0 | 05 | 33.3 | 05 | 33.3 | 0.580* |
| History of alcoholism and/or drug use† | 72 | 100.0 | 14 | 27.5 | 11 | 52.4 | 0.058* |
| Previous YF vaccination | 69 | 95.8 | 08 | 15.7 | 01 | 5.5 | 0.428* |
| Median number of days from disease onset to hospital admission (min‡–max§) | 5 (0–18) | 5 (1–18) | 4 (0–11) | 0.272‖ | |||
| Median number of hospitalization days (min‡–max§) | 6 (0–57) | 6 (1–36) | 4 (1–57) | 0.072‖ | |||
YF = yellow fever.
* Fisher’s exact test.
† Information recorded in the medical chart.
‡ Minimum value.
§ Maximum value.
‖ Mann–Whitney test.
Comparing cases who evolved to cure and death, the findings from the laboratory tests at the time of hospital admission indicate that patients who died presented lower median platelet count and higher median levels of white blood cell count, AST, ALT, TB, direct bilirubin (DB), INR, urea, creatinine, and creatine phosphokinase (CPK), with statistical significance (Table 3).
Table 3.
Number of cases of sylvatic yellow fever, according to evolution and laboratory results, Institute of Infectology Emilio Ribas, 2018
| Laboratory results | No. of available laboratory results (%) | Clinical evolution | P-value* | |
|---|---|---|---|---|
| Cure | Death | |||
| Median (min†–max‡) | Median (min†–max‡) | |||
| Leukocytes (cells/mm) | 71 (98.6) | 3,050 (1,000–8,300) | 6,000 (1,500–27,300) | 0.001 |
| Platelets (cells/mm3) | 71 (98.6) | 110,000 (30,000–237,000) | 75,000 (21,000–179,000) | 0.022 |
| Total bilirubin (mg/dL) | 71 (98.6) | 1.1 (0.3–11.8) | 6.2 (0.5–14.6) | < 0.001 |
| Direct bilirubin (mg/dL) | 69 (95.8) | 0.7 (0.2–9.5) | 5.2 (0.3–10) | < 0.001 |
| Aspartate aminotransferase (U/L) | 71 (98.6) | 1,357 (28–12,010) | 8,966 (839–36,520) | < 0.001 |
| Alanine aminotransferase (U/L) | 71 (98.6) | 1,420.5 (22–6,249) | 3,320 (908–10,100) | < 0.001 |
| International normalized ratio (seconds) | 71 (98.6) | 1.2 (0.9–13.3) | 2.4 (1–15) | < 0.001 |
| Urea (mg/dL) | 70 (97.2) | 28 (14–155) | 146 (31–409) | < 0.001 |
| Creatinine (mg/dL) | 71 (98.6) | 0.9 (0.6–5.6) | 5.8 (0.7–14.5) | < 0.001 |
| Creatine phosphokinase (U/L) | 67 (93.0) | 193 (22–6,151) | 796 (64–15,262) | < 0.001 |
* Mann–Whitney test.
† Minimum value.
‡ Maximum value.
The correlation of laboratory tests, evaluated by the Spearman coefficient, indicated a strong to moderate correlation between TB and DB; AST and ALT; AST, ALT, and INR; and urea and creatinine. Logistic regression was used to identify factors associated with death. Unadjusted and adjusted OR were calculated and are presented in Table 4.
Table 4.
Factors related to mortality in patients with yellow fever, Institute of Infectology Emilio Ribas, 2018
| Laboratory results | ORunadjusted (CI 95%) | P-value | Model 1* | Model 2† | ||
|---|---|---|---|---|---|---|
| ORadjusted (CI 95%) | P-value | ORadjusted (CI 95%) | P-value | |||
| Age (one year) | 1.078 (1.034–1.123) | 0.001 | 1.105 (1.008–1.212) | 0.033 | 1,136 (1,013–1,275) | 0.030 |
| Leukocytes (cells/mm3) (100 U/L) | 1.044 (1.018–1.071) | 0.001 | ||||
| Platelets (cells/mm3) (1,000 U/L) | 0.996 (0.997–0.999) | 0.030 | ||||
| Total bilirubin (mg/dL) | 1.455 (1.193–1.773) | < 0.001 | ||||
| Direct bilirubin (mg/dL) | 1.521 (1.216–1.902) | < 0.001 | ||||
| Aspartate aminotransferase (U/L) (100 U/L) | 1.043 (1.021–1.066) | < 0.001 | 1.038 (1.005–1.072) | 0.023 | ||
| Alanine aminotransferase (U/L) (100 U/L) | 1.086 (1.039–1.136) | < 0.001 | 1.118 (1.018–1.228) | 0.020 | ||
| International normalized ratio (seconds) | 1.873 (1.108–3.165) | 0.019 | ||||
| Urea (mg/dL) | 1.040 (1.021–1.058) | < 0.001 | ||||
| Creatinine (mg/dL) | 2.833 (1.570–5.111) | 0.001 | 2.343 (1.205–4.553) | 0.012 | 2.835 (1.352–5.941) | 0.006 |
| Creatine phosphokinase (U/L) | 1.001 (1.00–1.001) | 0.113 | ||||
| Time between onset of symptoms and hospital admission (days) | 0.903 (0.767–1.062) | 0.216 | 0.885 (0.546–1.276) | 0.404 | 0.779 (0.449–1,218) | 0.273 |
OR = odds ratio.
* Hosmer and Lemeshow test = 0.907.
† Hosmer and Lemeshow test = 0.998.
Excluding CPK, all other measures of laboratory tests were associated with mortality in the study group. Considering the collinearity between some of the variables, we constructed two regression models. In model 1, age, AST, and creatinine values were independent factors associated with mortality (both with P < 0.05). The models were adjusted for the time between onset of symptoms and hospitalization (TOSH). The risk of death increases by 10% for each 1 year increase in age. In relation to AST, the increase is 3.8% for each growth of 100 units, whereas the creatinine for each unit increases the risk of death by 134%, according to model 1 (Table 4). Age and values of ALT and creatinine were independent factors associated with mortality in model 2. The risk of death increases by 14% for each one year increase in age. In relation to ALT, the increase is 11.8% for each growth of 100 units, whereas the creatinine for each unit increases the risk of death by 183%, according to model 2 (Table 4).
From the models described, it was possible to calculate the probability (P) of death, given age; the values of AST, creatinine, and TOSH; or age, ALT, creatinine at hospital admission, and TOSH. The equations from the logistic regression models for quantitative variations in one unit of the variables are shown as follows:
Model 2: Age, ALT, creatinine, and TOSH
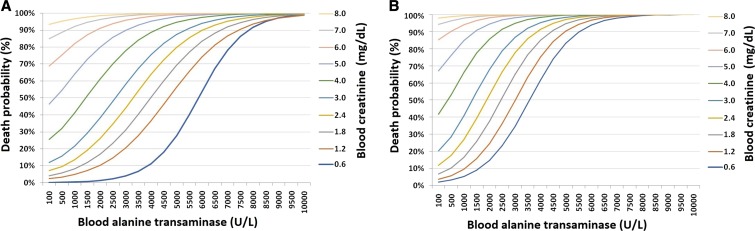
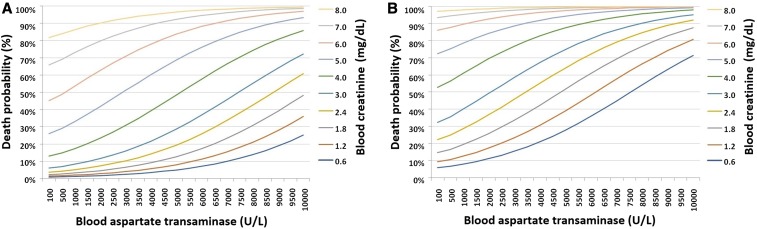
Based on the equations (models 1 and 2), it was possible to calculate the probability of death due to YF (in percentages), fixing the age at 40 years or 60 years and also the TOSH at 5 days, presented in Figures 3 and 4 for specific values of creatinine and AST, and creatinine and ALT, respectively.
Figure 3.
Death probability for age 40 (A) and 60 (B) years according to blood alanine transaminase values (U/L) and creatinine (mg/dL) at hospital admission. Institute of Infectology Emilio Ribas, 2018.
Figure 4.
Death probability for age 40 (A) and 60 (B) years according to blood aspartate transaminase values (U/L) and creatinine (mg/dL) at hospital admission. Institute of Infectology Emilio Ribas, 2018. This figure appears in color at www.ajtmh.org.
The graphs presented in Figures 3 and 4 indicate a remarkable increase in the probability of death due to YF at age 60 years when compared with subjects aged 40 years, and also a progressive increase related to ALT, AST, and creatinine blood values. For example, a 40-year-old individual with creatinine = 2.4 mg/dL and ALT = 3,000 U/L at hospital admission would have a 21.1% probability of death, whereas an individual aged 60 years and with the same laboratory results at admission would have a probability of dying equal to 77.5%. When analyzing the probability with the same creatinine value (2.4 mg/dL), but considering an AST value equal to 3,000 U/L, a 40-year-old patient would have a 10.2% chance of death, whereas a 60-year-old individual would have a 45.7% probability of death.
The quantitative variables were also categorized as dichotomous according to the median of general values for the construction of another logistic regression model, which indicated AST > 1,841 U/L (odds ratiosadjusted[ORadj]: 12.92; CI 95%: 1.50–111.37) and creatinine > 1.2 mg/dL (ORadj: 81.47; CI 95%: 11.33–585.71) as factors independently associated with death (Table 5).
Table 5.
Factors associated with death due to yellow fever, Institute of Infectology Emilio Ribas, 2018
| Factors associated | ORunadjusted (CI 95%) | P-value | ORadjusted (CI 95%) | P-value |
|---|---|---|---|---|
| Age (ref: < 42 years) | 4.96 (1.57–15.68) | 0.006 | ||
| Platelets (ref: ≥ 98,000 cells/mm3) | 4.07 (1.35–12.32) | 0.012 | ||
| Total bilirubin (ref: ≤ 1.72 mg/dL) | 20.19 (4.19–97.38) | < 0.001 | ||
| Direct bilirubin (ref: ≤ 1.19 mg/dL) | 12.00 (3.08–46.83) | < 0.001 | ||
| Aspartate aminotransferase (ref: ≤ 1,841 U/L) | 20.19 (4.19–97.38) | < 0.001 | 12.92 (1.50–111.37) | 0.020 |
| Alanine aminotransferase (ref: ≤ 1,924 U/L) | 5.22 (1.65–16.58) | 0.005 | ||
| International normalized ratio (ref: ≤ 1.32 seconds) | 20.19 (4.19–97.38) | < 0.001 | ||
| Urea (ref: ≤ 40 mg/dL) | 21.53 (4.44–104.40) | < 0.001 | ||
| Creatinine (ref: ≤ 1.2 mg/dL) | 109.25 (18.43–647.54) | < 0.001 | 81.47 (11.33–585.71) | < 0.001 |
| Creatine phosphokinase (ref: ≤ 304 U/L) | 7.97 (2.29–27.72) | < 0.001 |
OR = odds ratio.
DISCUSSION
The present study described the cases of sylvatic YF treated at a reference hospital for infectious diseases in the state of São Paulo. The confirmed cases had a PPI in sylvatic areas, especially in the metropolitan region of São Paulo, with particular emphasis on the municipality of Mairipora, whose rural area corresponds to 62.9% of the territory and 80.1% of the total area is within the Law of Protection of Watersheds,13 with abundant vegetation and environmental conditions favorable to the existence of vectors and nonhuman hosts. In 1999 and 2000, during the sylvatic YF epidemic which occurred in the central-western region of the country, there were also cases in other areas, such as the southeast, where there had been no record of virus circulation for more than four decades. In the following year, the virus was also identified in the southern region. Factors related to this expansion include increased rainfall, wild vector proliferation, vector longevity, virulence of the virus, and large numbers of the population being susceptible. Another important factor would be contact of unvaccinated humans in environments with NHP, motivated by hunting, thus increasing the probability of transmission of the sylvatic cycle.14 A study was conducted in the state of Rio Grande do Sul, Brazil, in 2008 and 2009, based on information from YF epizootic in NHP, to evaluate the distribution of NHP (Alouatta spp.) and the vector (Haemagogus leucocelaenus) to define risk areas for YF and environmental predictors. The results showed the following variables with the greatest influence on suitability for YF: water vapor pressure (36%), distribution of NHP (32%), maximum wind speed (11%), annual mean rainfall (7%), and maximum temperature in the warmest month (5%).15 A study was carried out with 683 confirmed cases of YF in humans and 331 cases in NHP, from December 2016 to June 2017, in the southeastern region of Brazil, combining epidemiological, spatial, and genomic data to characterize the transmission of the disease. The analysis showed a positive correlation between the number of cases in humans and in NHP (R = 0.62, P < 0.0001). Phylogenetic analysis of the virus from 62 confirmed cases of YF (33 in humans and 29 in NHP), the majority coming from the state of Minas Gerais, showed a unique clade, South American I, probably from endemic areas of the country (north and center-west regions) and no reemergence of the lineage that circulated in 2003 in the state of Minas Gerais.16
The cases were predominately attended in the month of January, and were mainly male, construction professionals, white, and with low schooling. The observed lethality rate was 27.8%. The analysis of cases of YF in Brazil from 1999 to 2009 demonstrated significant seasonality, with 93% of the cases concentrated from November to May, with 30.1% in January. The lethality rate in the country ranged from 25% to 47%.14 In a sylvatic YF outbreak in the state of São Paulo in 2009, 64.3% of the cases were male and the mean age was 29 years.5 An analysis of sylvatic YF cases in Brazil from 1998 to 2002 showed that 81.3% were male and the median age was 26 years.17
The higher concentration in males reflects greater displacement and exposure to the virus, especially for those working in sylvatic regions. The median age in the present study was higher among patients who died, probably related to a greater probability of disease severity. Most cases presented symptoms of fever, myalgia, nausea, and vomiting. A Brazilian study with YF cases from 1998 to 2002 showed similar results: fever (94.4%), headache (83.3%), vomiting (75.8%), and jaundice (69.1%).17 In the present study, the proportion of jaundice was lower (46.5%), and this can be justified by our validation of the jaundice sign by laboratory tests (TB ≥ 2.0 mg/dL), and also by most of the mild and moderate cases of our sample. Sixty-nine cases reported their YF vaccination status (95.8%), and 3 (4.3%) of those cases were vaccinated at least 10 days before the onset of symptoms. The Brazilian study showed a lower proportion (2.0%) of vaccinated individuals; however, it presented an important portion of ignored information regarding YF vaccination (19.5%). These cases may reflect primary vaccine failure. The attenuated virus YF vaccine, the 17D strain, was developed in 1936. A multicenter clinical trial conducted to evaluate two vaccines derived from strain 17-DD, ARILVAX and YF-VAX, showed an efficacy of 98.6% and 99.3%, respectively, when seroconversion was evaluated 30 days after vaccination.18 The 17-DD vaccine is produced at Biomanguinhos-Fundação Oswaldo Cruz-RJ, Brazil, and supplies the country and other countries of South and Central America, Africa, and Asia. A randomized clinical trial with 1,087 adults in Rio de Janeiro showed equivalence between the different substrains, 17D and 17-DD. The seroconversion rate was 98% among the seronegative and 90% among the seropositive subjects before the vaccine.19 In 2013, the WHO concluded that a single dose of the vaccine provided prolonged immunity without the need for a booster dose. Studies were conducted to evaluate the immunity of the vaccine after 10 years, and the results showed that 88% of the vaccinated individuals remained seropositive after this period. However, when stratified by area, 97.6% of vaccinees in endemic areas maintained detectable levels of nourishing antibodies in endemic areas, whereas only 83.7% in non-endemic areas. The change in the WHO’s recommendation for YF vaccine has led to intense debates among several groups.20 It is important to emphasize that the effectiveness of the vaccine in mass campaigns is not the same as that in randomized control studies, and may increase primary vaccine failure.
Yellow fever is a disease with a variable clinical spectrum, from asymptomatic cases to critical manifestations. Studies in Africa showed that for 7–12 asymptomatic cases, there was one symptomatic case.21 This study evaluated the incidence of YF from data from 11 studies conducted in Africa and South America and estimated the likelihood of clinical manifestations among those infected. The results showed the following: 55% (37–74%) asymptomatic, 33% (13–52%) with moderate clinical manifestations, and 12% (5–26%) critical.22 Considering our cases classified as severe YF (56.9%), there was probably an important proportion of asymptomatic, mild, and moderate cases among the population who were not diagnosed and reported, reflecting a possible lack of information about the real burden of the disease during this public health emergency.
The initial clinical presentation is similar to a nonspecific viral disease, with sudden onset of fever, headache, myalgia, nausea, and vomiting. Following this framework, most patients recover. However, approximately 15% of patients evolve after a short period of remission (hours to 1 day) to a severe condition, with jaundice, hemorrhagic symptoms, and multiple organ failure. The lethality rate for cases with renal and hepatic insufficiency ranges from 20% to 50%,23 a similar result to our findings (27.8%).
The regression models demonstrated an association between age, ALT, and AST, and creatinine. Hepatic impairment is classically described as a determinant of outcome in YF. Age > 60 years was a significant factor related to death in this series. The higher case-fatality rate for older adults has been previously reported in both dengue24 and YF,17,25 and is probably associated with a higher prevalence of comorbidities in this age group, as observed in the case analyzed in the study. A study conducted in Brazil identified AST (> 1,200 IU/L) and jaundice as independent factors associated with higher mortality.17 A study evaluated 23 cases of YF during the Senegal epidemic in 1965, and when analyzing serum AST levels in patients who died, the mean was at least three times higher than the cases that survived, and twice or greater than the mean of ALT.26 An experimental study evaluated the pathogenicity of YF virus in an animal model, with intraperitoneal inoculation in hamsters, using strains of the sylvatic YF virus of humans. The results indicated that the mortality rate was related to the viral load and age of the animals. Alanine aminotransferase, a more specific marker of hepatic necrosis, showed significantly elevated levels between day 4 and day 6.27 A report of a death from YF of a resident of China who traveled to Angola presented an extensive multilobular necrosis (critical) and hepatitis with panlobular characteristics and confluent hepatic necrosis in a liver biopsy.28
Another study evaluated the histopathological findings of the liver of 53 patients who evolved to death during the occurrence of sylvatic YF, and all cases were confirmed with laboratory tests. There was a predominance of CD4+ T lymphocytes, accompanied by CD8+ T lymphocytes, natural killer cells (CD57), macrophages, and antigen-presenting cells (S100). The disproportion between the inflammatory response and the degree of liver damage is probably related to intense apoptosis. Despite this, the immune cellular response plays an important role in the pathogenesis of liver damage observed in YF.29
The impact of renal impairment on the clinical outcome of YF was evaluated in a study conducted in Brazil. In the univariate analysis of risk factors related to death in patients with YF, the value of urea > 100 mg/dL presented a relative risk of 5.77 (1.43–23.22, P < 0.01). However, in the multivariate analysis, this variable did not remain significant. It should be noted that the creatinine value was not significant, although it presented lower values among the survivors (1.3 × 4.7 P = 0.16), probably due to the small number of tests available (n = 20).17 In our analysis, creatinine was an independent factor related to death. The value of creatinine is more specific to renal impairment than urea, especially in this analyzed context, where the underlying disease is associated with bleeding. The upper digestive bleeding is a cause of increased urea and could influence this result, regardless of renal function. Another aspect that compromises the value of urea is the influence of liver function on the levels detected, as it is synthesized in the liver and could be decreased by hepatic failure, independently of renal function. In 2018, five cases of YF were reported in international travelers who were infected in Brazil: in São Paulo (1), in Minas Gerais (1), and in Rio de Janeiro (3). Two cases evolved to death, both presenting renal and hepatic insufficiency. Among the survivors, only 1 presented these complications.30
The limitations of the study include the sample size, which made it impossible to analyze a greater number of variables in the regression models. Underreporting of cases can also be a limitation, although the Epidemiology Service of IIER develops an active search daily in the emergency room, inpatient units, and in the laboratory, thus maintaining high sensitivity for the detection of suspected cases of YF. The quality of information may be compromised, especially in cases of hospitalization of critical patients who quickly died. However, the information was collected on a standardized form (EIF) with an interview performed on the patient’s admission to the emergency room, and with the case relatives when possible, thus minimizing this bias. The hospital is specialized in infectious diseases, maintaining qualified and trained clinical staff to identify suspected cases of YF.
In conclusion, the reemergence of YF in the state of São Paulo, especially in the metropolitan region of São Paulo, impacted on the organization of patient care in the IIER. The study identified the association between age, hepatic, and renal impairment, evaluated through elevated levels of AST, ALT, and creatinine, as independent factors for death in individuals with YF, which may contribute to the appropriate clinical management of patients with YF in other contexts. The timely identification, reporting, and epidemiological investigation of suspected cases enable pertinent implementation of control measures. It is important to emphasize the need to maintain high vaccine coverage in recommended areas, as well as vaccines for travelers.
Acknowledgments:
We thank the staff of Epidemiology Service of IIER for their cooperation on collecting the surveillance data. We also thank the Yellow Fever Group of the IIER, the Adolfo Lutz Institute, and those who contributed to this study.
REFERENCES
- 1.Vasconcelos PFC, 2003. Febre amarela. Rev Soc Bras Med Trop 36: 275–293. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization , 2016. Situation Report—Yellow Fever, 28 October 2016. Geneva, Switzerland: WHO; Available at: http://apps.who.int/iris/bitstream/10665/250661/1/yellowfeversitrep28Oct16-eng.pdf?ua=1. Accessed July 15, 2018. [Google Scholar]
- 3.Secretariat of Health Surveillance, Ministry of Health, Brazil , 2017. General Coordination of Epidemiology Development in Services. Health Surveillance Guide: Single Volume, 2nd edition Brasília, Brazil. Available at: http://portalarquivos.saude.gov.br/images/pdf/2017/outubro/06/Volume-Unico-2017.pdf. Accessed July 20, 2018. [Google Scholar]
- 4.Secretariat of Health Surveillance, Ministry of Health, Brazil , 2017. Epidemiological Situation of Yellow Fever. Brasília, Brazil. Available at: http://portalms.saude.gov.br/saude-de-a-z/febre-amarela-sintomas-transmissao-e-prevencao/situacao-epidemiologica-dados. Accessed July 22, 2018. [Google Scholar]
- 5.Mascheretti M, et al. 2013. Yellow fever: reemerging in the state of Sao Paulo, Brazil, 2009. Rev Saúde Pública 47: 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Secretariat of Health Surveillance, Ministry of Health, Brazil , 2017. Emergency Operations Center in Public Health on Yellow Fever—Report 43. Brasília, Brazil. Available at: http://portalarquivos.saude.gov.br/images/pdf/2017/junho/02/COES-FEBRE-AMARELA---INFORME-43---Atualiza----o-em-31maio2017.pdf. Accessed July 25, 2018. [Google Scholar]
- 7.Secretariat of Health Surveillance, Ministry of Health, Brazil , 2018. Emergency Operations Center in Public Health on Yellow Fever—Report 26. Brasília, Brazil. Available at: http://portalarquivos2.saude.gov.br/images/pdf/2018/maio/18/Informe-FA-26.pdf. Accessed July 26, 2018. [Google Scholar]
- 8.Monath TP, et al. 1980. Yellow fever in the Gambia, 1978–1979: epidemiologic aspects with observations on the occurrence of orungo virus infections. Am J Trop Med Hyg 29: 912–928. [DOI] [PubMed] [Google Scholar]
- 9.Vasconcelos PF, Rodrigues SG, Degallier N, Moraes MA, da Rosa JF, da Rosa ES, Mondet B, Barros VL, da Rosa AP, 1997. An epidemic of sylvatic yellow fever in the southeast region of Maranhao State, Brazil, 1993–1994: epidemiologic and entomologic findings. Am J Trop Med Hyg 57: 132–137. [DOI] [PubMed] [Google Scholar]
- 10.Domingo C, Patel P, Yillah J, Weidmann M, Méndez JA, Nakouné ER, Niedriga M, 2012. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol 50: 4054–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M, 2003. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Methods 110: 185–191. [DOI] [PubMed] [Google Scholar]
- 12.Secretary of State for Health of Minas Gerais , 2018. Flowchart for Yellow Fever Care. Minas Gerais, Brazil. Available at: http://www.saude.mg.gov.br/images/documentos/Fluxograma%20de%20Atendimento%20-%20Febre%20Amarela%20V02_03_2018.pdf. Accessed July 29, 2018. [Google Scholar]
- 13.Sanchez OS, 2003. O processo de ocupação em áreas de proteção aos mananciais: conflito com a lei e realidade social na Região Metroplitana de São Paulo. Martins RC, Valencio NFLS, eds. Uso e Gestão dos Recursos Hídricos no Brasil. São Carlos, Brazil: RiMa, 293 Available at: http://www.teses.usp.br/teses/disponiveis/18/18139/tde-17112016-120909/publico/Dissert_Sanchez_PatriciaS_corrigido.pdf. Accessed July 20, 2018. [Google Scholar]
- 14.Costa ZGA, Romano APM, Elkoury ANM, Flannery B, 2011. Evolução histórica da vigilância epidemiológica e do controle da febre amarela no Brasil. Rev Pan-Amaz Saude 2: 11–26. [Google Scholar]
- 15.de Almeida MAB, dos Santos E, Cardoso JC, Silva LG, Rabelo RM, Bicca-Marques JC, 2018. Predicting yellow fever through species distribution modeling of virus, vector, and monkeys. EcoHealth 16: 95–108. [DOI] [PubMed] [Google Scholar]
- 16.Faria NR, et al. 2018. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 361: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuboi SH, Costa ZGA, Vasconcelos PFC, Hatch D, 2007. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans R Soc Trop Med Hyg 101: 169–175. [DOI] [PubMed] [Google Scholar]
- 18.Monath TP, et al. 2002. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 66: 533–541. [DOI] [PubMed] [Google Scholar]
- 19.Camacho LAB, Freire MS, Leal MLF, de Aguiar SG, do Nascimento JP, Iguchi T, Lozana JA, Farias RHG; Collaborative Group for the Study of Yellow Fever Vaccines , 2004. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saúde Pública 38: 671–678. [DOI] [PubMed] [Google Scholar]
- 20.Amanna IJ, Slifka MK, 2016. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev Vaccines 15: 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monath TP, Vasconcelos PFC, 2015. Yellow fever. J Clin Virol 64: 160–173. [DOI] [PubMed] [Google Scholar]
- 22.Monath TP, Gershman M, Staples JE, Barrett ADT, 2013. Yellow fever vaccine. Plotkin SA, Orenstein WA, Offitt PA, eds. Vaccines, 6th edition Edinburgh, Scotland: Elsevier/Saunders, 870–968. [Google Scholar]
- 23.Tesh RB, Guzman H, da Rosa AP, Vasconcelos PFC, Dias LB, Bunnel JE, Zhang H, Xiao SY, 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). I. Virologic, biochemical and immunologic studies. J Infect Dis 183: 1431–1436. [DOI] [PubMed] [Google Scholar]
- 24.Moraes GH, Duarte EF, Duarte EC, 2013. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. Am J Trop Med Hyg 88: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson H, 1929. Observations on the age and sex incidence of deaths and recoveries in the yellow fever epidemic in the department of Lambayeque, Peru, in 1921. Am J Trop Med 9: 233–239. [Google Scholar]
- 26.Oudart JL, Rey M, 1970. Proteinuria, proteinaemia, and serumtransaminase activity in 23 confirmed cases of yellow fever [article in French]. Bull World Health Organ. 42: 95–102. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, et al. 2016. A fatal yellow fever virus infection in China: descriptions and lessons. Emerg Microbes Infect 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quaresma JAS, Barros VLRS, Pagliari C, Fernandes ER, Jr., Andrade HF, Vasconcelos PFC, Duarte MI, 2007. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans R Soc Trop Med Hyg 101: 161–168. [DOI] [PubMed] [Google Scholar]
- 29.Hamer DH, et al. 2018. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep 67: 340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson MA, Vasconcelos PFC, Staples JE, 2014. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg 108: 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]