Abstract.
We report the case of a 64-year-old woman found to have urban-acquired Trypanosoma brucei (T.b.) gambiense human African trypanosomiasis (HAT) as the cause of sustained fever starting 9 months after returning to Canada from Democratic Republic of the Congo, in the context of concomitant multiple myeloma and HIV-1 coinfection. Approaches for the management of both clinical stages of T.b. gambiense HAT are well defined for endemic settings using current diagnostics and treatments. However, few data inform the diagnosis and management of patients with bone marrow suppression from active malignancy, recent anticancer therapy, or HIV coinfection. We discuss the implications of immunosuppression for diagnosis and management of T.b. gambiense HAT.
CASE PRESENTATION
A 64-year-old Congolese woman presented to our hospital with a 3-day history of fever without localizing symptoms. Her past medical history included multiple myeloma and HIV infection. Her multiple myeloma had previously responded to chemotherapy with four cycles of cyclophosphamide, bortezomib, and dexamethasone in the 6 months before presentation. Her last cycle finished 4 weeks earlier, but a relapse was diagnosed 1 week before presentation in September 2012. Nine months earlier, the patient returned to Canada after a prolonged stay in Kinshasa, Democratic Republic of the Congo (DRC), from March to December 2011. During this trip, she denied traveling outside Kinshasa’s Ngaliema commune but admitted to caring for a relative with active tuberculosis.
The patient was diagnosed with HIV on her immigration to Canada 6 years earlier. She was found to be homozygous CCR5Δ32 mutation positive and thus remained with an undetectable viral load in the absence of antiretroviral therapy. At presentation, the patient was not receiving antineoplastic medications, and her anti-infective medications included trimethoprim/sulfamethoxazole (160 mg/800 mg per os thrice weekly) for Pneumocystis jirovecii prophylaxis and daily fluconazole (100 mg per os) for Candida prophylaxis.
Physical examination showed a body temperature of 38.6°C, blood pressure of 148/69 mmHg, a regular heart rate of 109/minute, and a normal respiration rate and oxygen saturation on room air. There was mild hepatomegaly without rebound, guarding, abdominal tenderness, or lymphadenopathy. Cardiovascular, respiratory, neurological, and musculoskeletal examinations were unremarkable.
Laboratory testing revealed anemia with a hemoglobin of 8.3 g/dL, and leukocyte and platelet counts within normal limits. After blood cultures were obtained, piperacillin–tazobactam and vancomycin were started empirically. Repeated bacterial cultures of blood, sputum, and urine were negative. Three induced sputa for mycobacterial microscopy and culture were negative. Malaria rapid diagnostic testing and microscopy were negative, as were serology for Strongyloides and human T-lymphotrophic virus-1. HIV-1 serology was positive, but the HIV viral load was undetectable, and the CD4 count was 399/µL. Chest X-ray was unremarkable. Chest, abdominal, and pelvis computed tomography identified three distinct cystic liver lesions of approximately 10 × 30 mm, as well as new osteolytic bone metastases. Pathology of the liver lesions revealed plasmacytomas consistent with the patient’s relapsed multiple myeloma. Although the patient remained febrile, antibiotics were discontinued after 3 days of empiric therapy once routine investigations were found to be negative.
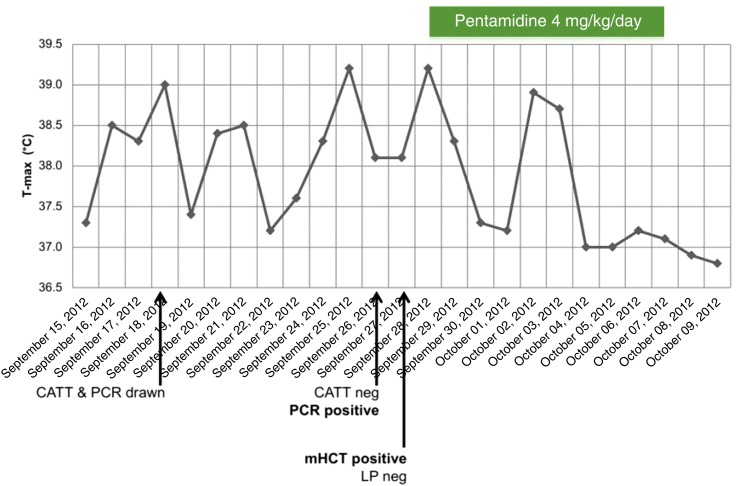
Investigations for human African trypanosomiasis (HAT) were sent in consideration of the patient’s recent residence in DRC, with an unexplained fever. Trypanosoma brucei (T.b.) gambiense polymerase chain reaction (PCR) of the patient’s whole blood was positive, but whole-blood card agglutination test for trypanosomiasis (CATT) was negative. Live trypanosomes were subsequently visualized in seven of 10 tubes of the patient’s blood using the micro-hematic centrifugation technique.1,2 A staging lumbar puncture revealed three leukocytes per microliter in the cerebrospinal fluid (CSF). Cerebrospinal fluid glucose and protein were within normal limits. No trypanosomes were seen on CSF cytocentrifugation, and CSF T.b. gambiense PCR was negative. These findings confirmed the diagnosis of hemolymphatic-stage T.b. gambiense HAT (g-HAT).
The patient received pentamidine isethionate (4 mg/kg intravenous) daily for a total of 10 days. Her fever subsided after 5 days of therapy (Figure 1). Repeated parasitological testing using mini anion exchange centrifugation technique was planned for 3, 6, 12, 18, and 24 months posttreatment to monitor for HAT relapse.1–3 Mini anion exchange centrifugation technique remained negative at 3 and 6 months and our patient denied fever or neurological symptoms at follow-up appointments. Unfortunately, our patient died from complications of relapsed multiple myeloma 9 months after completing her HAT treatment.
Figure 1.
Maximal daily oral temperature reading, with key clinical milestones. CATT = card agglutination test for trypanosomiasis; mHCT = micro-hematic centrifugation technique; LP = lumbar puncture; PCR = polymerase chain reaction.
DISCUSSION
Human African trypanosomiasis, or sleeping sickness, leads to a fatal parasitic encephalitis caused by T.b. gambiense or Trypanosoma brucei rhodesiense and is transmitted by tsetse flies. Its occurrence is geographically restricted to endemic foci in sub-Saharan Africa, with 97% of cases caused by T.b. gambiense (g-HAT), and most of these occurring in the DRC.4 The majority of g-HAT transmission occurs in rural areas. However, the possibility of urban transmission is recognized.5,6 Although the disease is a major threat to public health among affected populations, g-HAT is one of the world’s most neglected diseases and is very rarely encountered outside endemic areas.7,8 In contrast to disease from T.b. rhodesiense, g-HAT may have an indolent presentation with an incubation period of months or years.9,10 The initial hemolymphatic stage of disease can be characterized by prolonged fever without clinical focus, and it eventually leads to invasion of the central nervous system in the second, meningoencephalitic stage.8
Trypanosoma brucei gambiense infections are rare among returning travelers.7,11 No more than five cases were reported by the GeoSentinel network among 324,616 ill returned travelers between 1996 and 2018 (D. Hammer, personal communication). Although more prevalent in endemic areas, there is a lack of data on the relative frequency of stage-1 g-HAT among people presenting with prolonged fever syndromes. Among people presenting with neurological disorders in T.b. gambiense–endemic areas, a recent large prospective study in DRC identified g-HAT in only 10/351 (2.8%) of patients.12 Nonetheless, our case demonstrates that a credible exposure history and lack of alternative diagnosis should prompt appropriate investigations.
Few data inform the care of T.b. gambiense infections in patients with active malignancy or exogenous immunosuppression as g-HAT-endemic areas lack resource-intensive oncology centers. Clinical cure rates of hemolymphatic-stage g-HAT in DRC are reported to be 93–98% with a 7–10-day course of pentamidine, and trials have assessed the comparative efficacy of 3-day regimens.1 We opted for a 10-day course out of concern that concurrent immunosuppression may decrease treatment efficacy. Our patient’s clinical course was notable for a relatively long 9-month incubation period and lack of progression to meningoencephalitic disease. Mini anion exchange centrifugation technique in blood remained negative at 3 and 6 months posttreatment despite ongoing humoral dysfunction from multiple myeloma and repeated chemotherapy.13
The effect of immunosuppression on clinical manifestations of g-HAT is not well understood. Although g-HAT is not generally considered to be an opportunistic infection, a case of meningoencephalitic g-HAT appearing 29 years after exposure has been linked to the administration of azathioprine and prednisolone.14 For our patient, immune dysfunction did not appear to have adversely affected her clinical course. She responded appropriately to a pentamidine regimen commonly used among immunocompetent patients. This may be explained by how T.b. gambiense trypanosomes escape the humoral response via alteration of their variant surface glycoprotein (VSG).9,10,15 This phenomenon, known as antigenic variation, causes periodic waves of parasitemia as the host’s antibodies no longer target the new VSG. It could be hypothesized that a suppressed humoral response negligibly impacts a pathogen that naturally evades antibody-mediated immunity. This concept aligns with the observation that HAT prevalence does not vary according to HIV status.16
Our patient’s clinical response despite ongoing immunosuppression contrasts sharply with the case of other kinetoplastid infections.17,18 Among patients with subclinical Trypanosoma cruzi infection, reactivation of Chagas disease is triggered by various forms of immunosuppression, including corticosteroids, chemotherapy, organ transplantation, and acquired immunodeficiency syndrome.17,19,20 Visceral leishmaniasis is a known opportunistic infection of HIV,18 with relapse posttreatment complicating more than 60% of cases among HIV coinfected patients within 12 months.18
Although immunosuppression does not appear to alter the treatment course of T.b. gambiense, it may decrease the sensitivity of tests required for its diagnosis. In our case, the whole-blood CATT was negative, despite a reported sensitivity between 94 and > 98 percent in DRC.21–23 A false negative remains possible; however, the patient’s multiple myeloma and her immunosuppressive medications may have interfered with the antibody production required for this serological test.24,25
Our patient presented to hospital soon after finishing her first-line multiple myeloma treatment. This temporal association may be related to the known trypanocidal activity of certain anticancer medications.26–28 Bortezomib, a component of our patient’s initial multiple myeloma regimen, has an in vitro trypanocidal effect comparable in magnitude to that of melarsoprol, a medication still used to treat meningoencephalic T.b. rhodesiense.27 Another anticancer medication, the ornithine decarboxylase inhibitor eflornithine, is currently the backbone of recommended regimens for meningoencephalic T.b. gambiense disease.1,26,28
In conclusion, we detail the diagnosis and posttreatment course of hemolymphatic-stage T.b. gambiense infection in the setting of multiple myeloma and antineoplastic chemotherapy. Lack of relapse was documented for 6 months, but the patient died of her malignancy at 9 month follow-up. The relatively long incubation of her disease and chronology of g-HAT presentation four weeks after cessation of anticancer therapy may be related to the known trypanocidal activity of bortezomib. Finally, serological screening was negative in our patient with parasitologically confirmed g-HAT, underscoring the importance of considering how immunosuppression impacts diagnostic accuracy.
REFERENCES
- 1.WHO , 2013. Control and Surveillance of Human African Trypanosomiasis. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 2.Chappuis F, Loutan L, Simarro P, Lejon V, Büscher P, 2005. Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev 18: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büscher P, Mumba Ngoyi D, Kaboré J, Lejon V, Robays J, Jamonneau V, Bebronne N, Van der Veken W, Biéler S, 2009. Improved models of mini anion exchange centrifugation technique (mAECT) and modified single centrifugation (MSC) for sleeping sickness diagnosis and staging. PLoS Negl Trop Dis 3: e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, Simarro PP, Zhao W, Argaw D, 2018. Monitoring the elimination of human African trypanosomiasis: update to 2016. PLoS Negl Trop Dis 12: e0006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robays J, Ebeja Kadima A, Lutumba P, Miaka mia Bilenge C, Kande Betu Ku Mesu V, De Deken R, Makabuza J, Deguerry M, Van der Stuyft P, Boelaert M, 2004. Human African trypanosomiasis amongst urban residents in Kinshasa: a case-control study. Trop Med Int Heal 9: 869–875. [DOI] [PubMed] [Google Scholar]
- 6.Simon F, Mura M, Pagès F, Morand G, Truc P, Louis F, Gautret P, 2012. Urban transmission of human African trypanosomiasis, Gabon. Emerg Infect Dis 18: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leder K, et al. GeoSentinel Surveillance Network , 2013. GEosentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med 158: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simarro PP, Franco JR, Cecchi G, Paone M, Diarra A, Ruiz Postigo JA, Jannin JG, 2012. Human African trypanosomiasis in non-endemic countries (2000–2010). J Travel Med 19: 44–53. [DOI] [PubMed] [Google Scholar]
- 9.Brun R, Blum J, Chappuis F, Burri C, 2010. Human African trypanosomiasis. Lancet 375: 148–159. [DOI] [PubMed] [Google Scholar]
- 10.Büscher P, Cecchi G, Jamonneau V, Priotto G, 2017. Human African trypanosomiasis. Lancet 390: 2397–2409. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS, 2006. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 354: 119–130. [DOI] [PubMed] [Google Scholar]
- 12.Mukendi D, et al. 2017. Clinical spectrum, etiology, and outcome of neurological disorders in the rural hospital of Mosango, the Democratic Republic of Congo. Am J Trop Med Hyg 97: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkumar SV, 2011. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol 87: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudarshi D, et al. 2014. Human African trypanosomiasis presenting at least 29 years after infection—what can this teach us about the pathogenesis and control of this neglected tropical disease? PLoS Negl Trop Dis 8: e3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pays E, 2005. Regulation of antigen gene expression in Trypanosoma brucei. Trends Parasitol 21: 517–520. [DOI] [PubMed] [Google Scholar]
- 16.Meda HA, Doua F, Laveissière C, Miezan TW, Gaens E, Brattegaard K, de Muynck A, De Cock KM, 1995. Human immunodeficiency virus infection and human African trypanosomiasis: a case-control study in Côte d’Ivoire. Trans R Soc Trop Med Hyg 89: 639–643. [DOI] [PubMed] [Google Scholar]
- 17.Perez CJ, Lymbery AJ, Thompson RCA, 2015. Reactivation of Chagas disease: implications for global health. Trends Parasitol 31: 595–603. [DOI] [PubMed] [Google Scholar]
- 18.Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J, 2014. Visceral leishmaniasis and HIV coinfection in east Africa. PLoS Negl Trop Dis 8: e2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartori AMC, Lopes MH, Caramelli B, Duarte MIS, Pinto PL, Neto V, Amato Shikanai-Yasuda M, 1995. Simultaneous occurrence of acute myocarditis and reactivated Chagas’ disease in a patient with AIDS. Clin Infect Dis 21: 1297–1299. [DOI] [PubMed] [Google Scholar]
- 20.Cordova E, Boschi A, Ambrosioni J, Cudos C, Corti M, 2008. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int J Infect Dis 12: 587–592. [DOI] [PubMed] [Google Scholar]
- 21.Truc P, Lejon V, Magnus E, Jamonneau V, Nangouma A, Verloo D, Penchenier L, Büscher P, 2002. Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in west and central Africa. Bull World Health Organ 80: 882–886. [PMC free article] [PubMed] [Google Scholar]
- 22.Simarro PP, Ruiz JA, Franco JR, Josenando T, 2002. Attitude towards CATT-positive individuals without parasitological confirmation in the African trypanosomiasis (T.b. gambiense) focus of Quiçama (Angola). Trop Med Int Health 4: 858–861. [DOI] [PubMed] [Google Scholar]
- 23.Boelaert M, et al. 2017. A phase III diagnostic accuracy study of a rapid diagnostic test for diagnosis of second stage human African trypanosomiasis in the Democratic Republic of the Congo. EBioMedicine 27: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lejon V, Boelaert M, Jannin J, Moore A, Büscher P, 2003. The challenge of Trypanosoma brucei gambiense sleeping sickness diagnosis outside Africa. Lancet Infect Dis 3: 804–808. [DOI] [PubMed] [Google Scholar]
- 25.Garcia A, Jamonneau V, Magnus E, Laveissière C, Lejon V, N’guessan P, N’Dri L, Van Meirvenne N, Büscher P, 2008. Follow-up of card agglutination trypanosomiasis test (CATT) positive but apparently aparasitaemic individuals in Côte d’Ivoire: evidence for a complex and heterogeneous population. Trop Med Int Health 5: 786–793. [DOI] [PubMed] [Google Scholar]
- 26.Nkemgu-Njinkeng J, Rosenkranz V, Wink M, Steverding D, 2002. Antitrypanosomal activities of proteasome inhibitors. Antimicrob Agents Chemother 46: 2038–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steverding D, Wang X, 2009. Trypanocidal activity of the proteasome inhibitor and anti-cancer drug bortezomib. Parasit Vectors 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett SV, Barrett MP, 2000. Anti-sleeping sickness drugs and cancer chemotherapy. Parasitol Today 16: 7–9. [DOI] [PubMed] [Google Scholar]