Abstract
Bone metastases are a frequent complication of solid tumors, leading to significant skeletal sequelae that negatively impact quality of life and survival. Prevention and management of skeletal-related complications are critical treatment goals in oncology. Endpoints used in clinical trials to evaluate skeletal-related complications have evolved. In contrast to single measures of bone health, contemporary clinical trial endpoints reflect composite measures of skeletal-related complications, and increasingly also survival. In addition, key symptomatic components, which are more reflective of quality of life and the patient experience, are being incorporated. Given the evolution and resulting diversity of the endpoints being used in pivotal trials, it is becoming increasingly relevant to clarify the utility and the potential clinical impact of these measures not only within the context of trials but also in the real-world setting. Here, we describe the development and evolution of skeletal endpoints used in trials, and discuss their clinical relevance.
Keywords: bone, bone metastases, endpoint, skeletal-related event, symptomatic skeletal event
1. Introduction
The bone is the most common site of metastasis across a range of solid tumor types. Bone metastases occur in over 80% of patients with metastatic prostate cancer [1–3] and 65–80% of patients with metastatic breast cancer [4, 5]. Other advanced-stage cancers with a notable incidence of skeletal metastases include lung cancer (30–40% of patients) [6], invasive bladder cancer (32% of patients) [7], and renal cell carcinoma (32% of patients) [8]. In addition, over 80% of patients with multiple myeloma suffer from bone lesions, termed myeloma bone disease [9, 10].
Bone metastases lead to skeletal complications, such as bone pain and pathologic fracture [11–13], which are associated with a decline in quality of life (QoL) and increased cost for patients and health systems [14, 15]. If left untreated, patients with bone metastases will, on average, experience a skeletal complication every 3–6 months, with events typically clustering together and increasing in frequency as a result of disease progression [13, 16]. Bone metastases-related pain and other skeletal complications are associated with reduced overall survival (OS) in various bone-metastatic cancers, including prostate, breast, and non-small cell lung cancer [17–22].
Cancers that have metastasized to the bone are frequently characterized by osteoblastic or osteolytic skeletal lesions that result in bone formation, driven by osteoblasts, or bone resorption, driven by osteoclasts, respectively (Fig. 1) [23, 24]. Signaling crosstalk between tumor cells, osteoblasts, and osteoclasts, leads to a cycle of bone turnover and tumor growth. Bone metastases typically form either osteolytic or osteoblastic lesions, depending upon tumor type. For example, prostate cancer bone metastases are typically osteoblastic, whereas breast cancer bone metastases are mainly osteolytic [24, 25]. Myeloma bone disease differs from solid tumor bone metastases in that it is essentially a purely osteolytic process in which bone growth plays a minimal role [9, 10].
Fig 1. Vicious cycle of bone metastases.
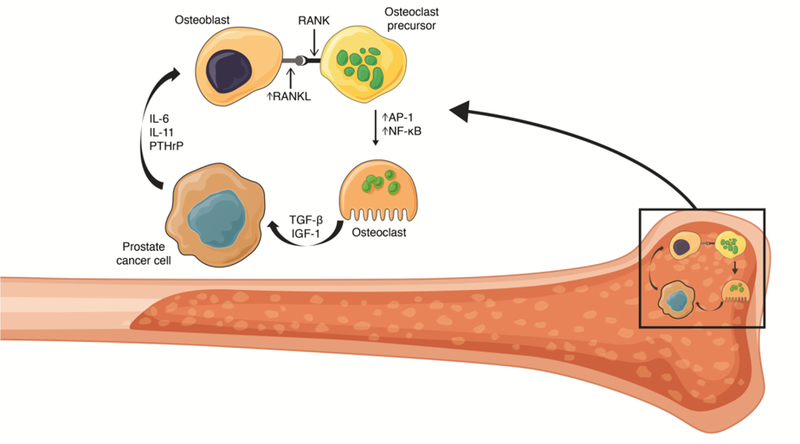
Metastatic tumor cells in bone have been shown to secrete a range of proteins including IL-6, IL-11, and PTHrP [26, 27]. This results in increased expression of RANKL on osteoblasts, RANKL can then bind RANK receptors on osteoclasts and osteoclast precursor cells resulting in activation of AP-1 and NF-κB transcription factors [26, 28]. AP-1 and NF-κB activation results in osteoclast differentiation, maturation, fusion, and ultimately increased bone resorption, which releases previously sequestered growth factors such as TGF-β and IGF-1 that stimulate tumor cell proliferation, in turn increasing PTHrP expression, resulting in reinforcement of the ‘vicious cycle’ of bone metastases [23, 26, 28].
AP-1, activator protein 1; IGF-1, insulin-like growth factor 1; IL, interleukin; PTHrP, parathyroid hormone-related protein; RANK, receptor activator of NF-κB; RANKL, receptor activator of NF-κB ligand; TGF-β, transforming growth factor β.
While OS remains the key benchmark in phase III trials of advanced cancer, endpoints that can be reported earlier may provide added value in terms of patient management. Furthermore, incremental gains in survival may not be the priority for some subsets of patients, such as the elderly; rather, for some patients, maintaining QoL and reducing pain may be of greater importance [6]. To this end, clinical trials in bone-metastatic disease should include endpoints that assess clinical benefit through symptomatic improvement (prevention or delay in symptomatic decline) and not just based on estimates of survival or detection of asymptomatic metastases. This approach has been recommended by the United States Food and Drug Administration (FDA), in addition to the Prostate Cancer Clinical Trials Working Group (PCWG) [29, 30].
Various measures of disease burden and morbidity related to bone metastases have been reported in clinical trials, including evaluation of bone with imaging (e.g. bone scintigraphy, computed tomography [CT], magnetic resonance imaging [MRI], among others) and assessment of bone pain with patient-reported outcomes (e.g. the brief pain inventory). As our understanding of the clinical impact of bone metastases has advanced, skeletal-related trial endpoints have evolved from separate single measures to composite measures of events (pathologic fractures, spinal cord compression) and interventions (bone surgery, radiation to bone), collectively termed skeletal-related events (SREs). The various SRE-based endpoints that have been assessed in clinical trials include ‘time to first SRE’, ‘proportion of SREs’ at landmark timepoints, and ‘number of SREs over time’ (skeletal morbidity rate). Other clinically relevant endpoints include radiographic progression-free survival (PFS), metastasis-free survival (MFS), and bone MFS. However, there is inconsistency in when and how these endpoints are being used. Furthermore, the SRE-based endpoint has continued to evolve in an effort to better assess and understand the clinical relevance of the bone metastasis problem. Given the range and inconsistency in defining these endpoints, the interpretation of each of the endpoints in terms of meaningful clinical benefit to patients in the real-world setting may be unclear. Here, we describe the evolution of skeletal event-specific endpoints used in clinical trials and discuss their utility and clinical relevance.
2. Assessment of bone-metastatic disease
2.1. Evaluation of bone health
Bone loss, as measured by decreases in bone mineral density (BMD), has been used as an endpoint in clinical trials of bone-metastatic disease [31–33]. The most common way of quantifying BMD is to assess bone calcium content through dual energy X-ray absorptiometry (DEXA) scans [34].
Hormonal therapies are common in the treatment of both breast and prostate cancer; however, use of hormonal therapies is associated with higher incidence of osteopenia and osteoporosis, termed cancer therapy-induced bone loss, and consequent fragility fractures [35]. BMD determination (e.g. DEXA scan) and use of bone-targeted therapies (e.g. denosumab and bisphosphonates) to improve BMD are recommended by the National Comprehensive Cancer Network for patients with breast or prostate cancer receiving hormonal therapy (Table 1) [36, 37]. At a minimum, each patient should receive vitamin D and calcium, as these can reduce bone loss and are necessary for bone-targeted agents to function. Serum 25-hydroxy vitamin D should be maintained at or above 30 ng/mL.
Table 1.
National Comprehensive Cancer Network guidelines for screening for and treating cancer treatment-induced osteoporosis and risk of fragility fractures in patients with breast or prostate cancer receiving hormonal therapy.
| Breast cancer [36] | Prostate cancer [37] |
|---|---|
| • Postmenopausal patients with breast cancer receiving adjuvant endocrine therapy should be monitored for bone health with a BMD determination (e.g. DEXA scan) at baseline and periodically thereafter | • In men with prostate cancer receiving ADT, screening and treatment for osteoporosis are
recommended: • Calcium 1000–1200 mg daily from food and supplements • Vitamin D3 400–1000 IU daily • Fracture risk can be assessed by FRAX® algorithm |
| • The bone-targeted therapies denosumab and bisphosphonates are the recommended intervention to improve BMD in these patients, and should be supplemented with calcium and vitamin D | • ADT can cause secondary osteoporosis as assessed by this
algorithm • For men ≥50 years old with low bone mass (T-score –1.0 to –2.5, osteopenia) at the femoral neck, total hip, or lumbar spine by DEXA and a 10-year probability of either hip fracture ≥3% or major osteoporotic fracture ≥20%: • Denosumab 60 mg every 6 months, zoledronic acid 5 mg annually, or alendronate 70 mg weekly is recommended to increase BMD • A follow-up DEXA scan after one year of therapy is recommended |
ADT, androgen deprivation therapy; BMD, bone mineral density; DEXA, dual energy X-ray absorptiometry.
Despite these guidelines, in three recent trials of patients with non-metastatic castration-resistant prostate cancer receiving androgen deprivation therapy (ADT) at study entry, only 8% were also receiving bone-targeted therapies to prevent or treat osteoporosis [38–40]. However, it should be noted that the baseline BMD and overall fracture risk of these patients has not been reported. Furthermore, it is unknown if any of these patients were contraindicated for bone-targeted therapies.
To what extent cancer therapy-induced bone loss contributes to overall skeletal morbidity compared with bone metastases per se, particularly among advanced breast and prostate cancer patients, remains an open question.
2.2. Measurement of pain related to bone metastases
Bone pain is one of the most debilitating symptoms associated with bone metastases. Treatment effects of various interventions have been evaluated with instruments that assess the extent of pain palliation [41–43]. External beam radiation therapy is commonly used for palliation of pain from bone metastases [44–46], and patient-reported pain measures (e.g. Brief Pain Inventory score, Visual Analogue Scale) have been used as endpoints in randomized radiation therapy-based clinical trials [47–50]. Pain measures are also among some of the endpoints in trials of bone-targeted therapies, including anti-resorptive agents and radiopharmaceuticals, as well as trials of systemic chemotherapy [42, 43, 51–56]. Regarding the latter, for instance, the US FDA approved several agents based on pain as a primary endpoint in the 1990s, including strontium-89, samarium-153, and mitoxantrone, for palliation of pain due to metastatic castration-resistant prostate cancer (mCRPC) [42, 53–56]. While patient-reported pain assessments are undeniably useful, they remain subjective and open to potential bias due to missing data, particularly regarding analgesic use [41, 57].
2.3. Imaging tools used to assess bone metastases
Objective assessments of bone metastases in clinical trials have historically included serial imaging with standard techniques such as CT and bone scintigraphy; however, the clinical significance of changes in size or intensity of bone metastases, particularly on a bone scan, is difficult to interpret [57–59].
For prostate cancer, the PCWG3 recommends 99mTc-methylene diphosphonate bone scintigraphy as the standard for bone imaging, and it defines progression as at least two new lesions on a bone scan, with a confirmatory scan showing at least two new lesions 6 or more weeks later [30, 58, 60, 61]. In bone-metastatic breast cancer, 99mTc-methylene diphosphonate bone scintigraphy remains widely used, and historical issues regarding specificity have been improved through the introduction of single-photon emission computed tomography (SPECT) and SPECT-CT [62]. In some cases, confirmation by MRI or fine-cut CT can be useful, although criteria for progression of disease using these techniques remain ill-defined [30, 60]. Other imaging techniques, such as positron emission tomography based on 18F-fluorodeoxyglucose, 18F-fluciclovine, or prostate-specific membrane antigen, have shown greater sensitivity than more traditional bone scintigraphy methods [62–65]; however, these are not used routinely in clinical trials yet.
Efforts to improve bone scintigraphy from a traditional, non-quantitative modality into a more useful imaging biomarker through the use of quantifiable measures have led to the development of certain indices, such as the bone scan index (BSI), which can be used to quantify metastatic tumor burden [66–68]. While this parameter has been validated to assess disease burden in metastatic prostate cancer [69], there are issues with the applicability of such methods to breast cancer and other bone-metastatic cancers with a higher predisposition to lytic bone lesions, which are less well visualized by bone scintigraphy techniques [26, 67].
3. Clinical trial endpoints for evaluating skeletal metastasis
Two key endpoints in phase III trials for evaluating skeletal metastasis are radiographic PFS and MFS (Table 2). Radiographic PFS, defined as time to first occurrence of progression on bone scan, CT, or MRI, or death from any cause, combines disease progression and death into a single composite endpoint. Radiographic PFS was first validated in the phase III COU-AA-302 study of abiraterone in men with mCRPC, where it was found to be strongly associated with OS [70]. Radiographic PFS was also shown to be a robust and clinically meaningful endpoint that correlated with OS in the PREVAIL study of enzalutamide in mCRPC [71]. MFS, defined as time to first detection of distant metastasis on imaging or death from any cause, has been used in several clinical trials across tumor types. A variation of MFS – bone MFS – was the primary endpoint in a phase III trial of denosumab in men with non-metastatic castration-resistant prostate cancer at high risk of bone metastases [72]. Bone MFS was defined as time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause [72]. More recently, MFS (including metastasis to bone and other sites) was the primary endpoint in three phase III trials of androgen receptor antagonists in men with non-metastatic castration-resistant prostate cancer: the ARAMIS trial of darolutamide [40], the SPARTAN trial of apalutamide [38], and the PROSPER trial of enzalutamide [39]. The ARAMIS, SPARTAN, and PROSPER studies have demonstrated significant improvements in MFS with darolutamide, apalutamide, and enzalutamide, respectively, versus placebo [38, 39]. While OS was not mature at the time of analysis in these studies, a systematic review of 28 randomized controlled trials in localized prostate cancer has demonstrated a strong correlation between MFS and OS [73], validating MFS as a surrogate endpoint for OS. Moreover, the FDA has issued draft guidance related to the use of MFS as a clinical trial endpoint [74]. MFS is a secondary endpoint in several ongoing phase III clinical trials in breast cancer (e.g. NCT02311959 and NCT02992574).
Table 2.
Definitions and comparisons of endpoints designed to measure clinically meaningful skeletal outcomes in trials of bone-metastatic tumors.
| Endpoint components: | Endpoints | |||||
|---|---|---|---|---|---|---|
| SRE [75–78] | SSE [79] | SRE-FS [80, 81] | SSE-FS [82] | Radiographic PFS [70, 71] | Bone MFS [72] | |
| EBRT | For any cause | For skeletal symptoms | For any cause | For skeletal symptoms | Progression by bone scan, CT, or MRI by RECIST Imaging every 8 weeks during the first 24 weeks and every 12 weeks thereafter, independent of any symptoms |
N/A |
| Pathologic bone fracture | Asymptomatic or symptomatic | Symptomatic only | Asymptomatic or symptomatic | Symptomatic only | ||
| Spinal cord compression | Yes | Yes | Yes | Yes | ||
| Bone surgery | For any cause | Tumor-related | For any cause | Tumor-related | ||
| Metastasis | No | No | No | No | No | Bone metastases |
| Survival | No | No | Death from any cause [81] / death due to cancer [80] | Death from any cause | Death from any cause | Death from any cause |
CT, computed tomography; EBRT, external beam radiation therapy; MFS, metastasis-free survival; MRI, magnetic resonance imaging; PFS, progression-free survival; RECIST, Response Evaluation Criteria In Solid Tumors; SRE, skeletal-related event; SRE-FS, skeletal-related event-free survival; SSE, symptomatic skeletal event; SSE-FS, symptomatic skeletal event-free survival.
4. Composite endpoints related to complications of bone metastasis: Skeletal-related events
Complications of bone metastasis, such as pain, pathologic fracture, and spinal cord compression, are typically managed with radiotherapy and/or surgery. Radiation therapy has been shown to reduce pain in patients with bone metastases [45] and external beam radiation therapy has been shown to delay the onset of skeletal complications in patients with solid tumors and asymptomatic bone metastases [83]. Surgery is used to manage pathologic fractures and spinal cord compression in patients with bone metastases and improves outcomes [84–87]. Such adverse events related to the skeleton have been routinely captured in clinical trials of bone-metastatic cancer; however, a composite endpoint approach to include both events (pathologic fractures, spinal cord compression) and interventions (radiation to bone, bone surgery), termed SREs, has emerged to assess the efficacy of treatments to delay or reduce skeletal complications. This represents a pivotal shift in trial design for bone-metastatic tumor types. The FDA defines SREs as pathologic fractures (both vertebral and non-vertebral), spinal cord compression, bone surgery, or radiation therapy to bone (Table 2) [29]. Composite endpoints in clinical trials are now commonly used as the greater event rate achieved by combining several outcomes may reduce sample size requirements for the trial. However, complete ascertainment of each component of the composite endpoint is required, and this is often missing in clinical trials.
The FDA recognizes delay to onset of SREs as a clinically relevant indicator of preserving patient functionality and health-related QoL [88–90]. The link between SREs and health-related QoL is supported by several post-hoc analyses of large clinical trials [88–90]. Regulatory approval has been granted to several systemic bone-targeted therapies across a range of tumor types based on clinical trials that assessed reduction or delay in SREs. The first drug to be approved based on the use of an SRE-based endpoint was the bisphosphonate pamidronate in bone-metastatic breast cancer and advanced multiple myeloma [91–94]. Following the approval of pamidronate, other bone-targeted therapies were approved based on trials that assessed SREs as the primary endpoint, including zoledronic acid (another bisphosphonate) and the receptor activator of NF-κB ligand (RANKL) inhibitor denosumab, for the treatment of bone-metastatic cancer and myeloma bone disease [95]. Various SRE analyses have been performed in phase III clinical trials (Table 3), including time to first SRE, number of SREs, proportion of patients experiencing an SRE, and SRE rate ratio. This potentially leads to issues of comparability between trials and confusion over what each endpoint means. Furthermore, the methods used to detect SREs also differed between some of the trials (Table 3).
Table 3.
Primary skeletal-related event-based endpoints used in phase III trials in bone-metastatic cancers.
| Primary endpoint | Study | Tumor type | Design | Intervention | Endpoint definition | Fracture assessment methods* |
|---|---|---|---|---|---|---|
| Proportion of patients with ≥1 SRE | Rosen et al. 2001 [77] | Breast cancer and multiple myeloma | R | Zoledronic acid (versus pamidronate) | Pathologic fractures, spinal cord compression, radiation therapy to bone, or surgery to bone | N/A |
| Saad et al. 2002 [96] | mCRPC | R, DB, PC | Zoledronic acid | Pathologic bone fractures (vertebral or non-vertebral), spinal cord compression, surgery to bone, radiation therapy to bone (including the use of radioisotopes), or a change of antineoplastic therapy to treat bone pain | Bone surveys at baseline and every 3 months, including films of the lateral skull, cervical spine, thoracic spine, lumbar spine, chest, pelvis, and upper and lower extremities | |
| Rosen et al. 2003, 2004 [99, 100] | Lung cancer | R, DB, PC | Zoledronic acid | Pathologic fractures, spinal cord compression, radiation therapy to bone, or surgery to bone | Bone surveys at baseline and 3, 6, and 9 months | |
| Berenson et al. 1996 [92] | Multiple myeloma | R, DB, PC | Pamidronate | Pathologic fractures, irradiation of or surgery on bone, and spinal cord compression | Complete survey of bones, including long bones, before treatment and after 6 and 9 cycles | |
| Time to first SRE | Hortobagyi et al. 1996 [93] | Breast cancer | R, DB, PC | Pamidronate | Pathologic fractures, the need for radiation to bone or bone surgery, spinal cord compression, and hypercalcemia | Radiographic skeletal surveys at baseline and after 3, 6, and 12 cycles |
| Stopeck et al. 2010 ( NCT00321464 [78]) | Breast cancer | R, DB | Denosumab (versus zoledronic acid) | Pathologic fractures (excluding major trauma), radiation therapy to bone, surgery to bone, or spinal cord compression | Skeletal surveys (X-ray) every 12 weeks or radiographic assessments (X-ray, CT, or MRI) during the course of standard care | |
| Fizazi et al. 2011 ( NCT00321620 [97]) | mCRPC | R, DB, PC | Denosumab (versus zoledronic acid) | Pathologic fractures (excluding fractures from severe trauma), radiation therapy to bone (including use of radioisotopes), surgery to bone, or spinal cord compression | Skeletal surveys at baseline and every 12 weeks, including radiographs of the skull, spine, chest, pelvis, arm from shoulder to elbow, and leg from hip to knee | |
| Henry et al. 2011, 2014 ( NCT00330759 [51, 75]) | Lung cancer | R, DB, PC | Denosumab (versus zoledronic acid) | Pathologic fractures, spinal cord compression, or radiation or surgery to bone | Skeletal surveys (X-ray) every 12 weeks or unscheduled radiographic assessments (X-ray, CT, or MRI) during routine care | |
| Raje et al. 2018 ( NCT01345019 [76]) | Multiple myeloma | R, DB, PC | Denosumab (versus zoledronic acid) | Pathologic fractures (vertebral or non-vertebral), radiation therapy to bone (including the use of radioisotopes), surgery to bone, or spinal cord compression | Skeletal surveys using conventional radiography at baseline and then every 12 weeks | |
| Skeletal-related event-free survival† | Smith et al. 2014 ( NCT00079001, CALGB 90202 [80]) | mCSPC | R,DB, PC | Zoledronic acid | Radiation therapy to bone (including use of bone-targeted radiopharmaceuticals), clinical fractures, spinal cord compression, surgery to bone, death due to prostate cancer | No required radiographic assessments on study |
| Symptomatic skeletal event-free survival | Smith et al. 2019 ( NCT02043678, ERA 223 [82]) | mCRPC | R, DB, PC | Abiraterone plus radium-223 | Symptomatic pathologic fractures, external beam radiation therapy to relieve skeletal symptoms, spinal cord compression or tumor-related orthopedic surgical intervention, death from any cause | Assessed by investigator based on symptoms |
Methods used to assess bone fractures per available information
Endpoint referred to as ‘time to first SRE’ in publication.
CT, computed tomography; DB, double-blind; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; MRI, magnetic resonance imaging; PC, placebo controlled; R, randomized; SRE, skeletal-related events.
It is important to understand that delaying the development of SREs will also reduce the number of SREs at a specific timepoint; however, this does not necessarily mean that there will be an absolute reduction in total SREs. If the patient is to be monitored over a longer period of time, there may or may not be a correlation between delay and absolute reduction in SREs. This may underpin the lack of clarity in interpreting this type of endpoint.
In mCRPC, zoledronic acid was approved based on a phase III clinical trial in which the primary endpoint was the proportion of patients with at least one SRE, which included changes of antineoplastic therapy to treat bone pain as part of the composite SRE definition [96]. In contrast, the primary endpoint in the phase III trial leading to approval of denosumab in mCRPC was time to first on-study SRE, which excluded changes in antineoplastic therapy [97]. Denosumab resulted in a longer time to first SRE than zoledronic acid in this study.
In the pivotal phase III trial of zoledronic acid for the treatment of bone-metastatic breast cancer and multiple myeloma with osteolytic lesions, the primary endpoint was the proportion of patients with at least one SRE over a 13-month period [77]. This study demonstrated that zoledronic acid was non-inferior to pamidronate in these patients [77]. The phase III Zoledronate versus Ibandronate Comparative Evaluation (ZICE) trial in metastatic breast cancer had a primary endpoint of frequency and timing of SREs over a 96-week period, and demonstrated that zoledronic acid was superior to ibandronic acid in this setting [98]. Denosumab was approved in advanced breast cancer following the results of a phase III trial with a primary endpoint of time to first on-study SRE [78]. In this study denosumab delayed the time to first on-study SRE by 18% compared with zoledronic acid [78].
Much like the trial of zoledronic acid in breast cancer and multiple myeloma, the pivotal phase III trial of zoledronic acid in metastatic lung cancer used the proportion of patients with at least one SRE at a landmark timepoint as the primary endpoint (Table 3) [99, 100]. The pivotal phase III trial of denosumab in metastatic lung cancer assessed the time to first on-study SRE as the primary endpoint, demonstrating that denosumab was non-inferior, and there was a trend towards superiority, when compared with zoledronic acid in delaying SREs in a cohort of patients with non-small cell lung cancer [51, 75].
Recently, denosumab has been approved for the prevention of SREs in multiple myeloma following the largest trial in this setting [76]. In total, 1718 patients with multiple myeloma and at least one lytic bone lesion were enrolled in this study of denosumab versus zoledronic acid. The primary endpoint was time to first SRE [76]. Thus, in multiple myeloma, as observed in other tumor types, proportion of patients with at least one SRE has been superseded by time to first on-study SRE as the primary outcome measure in clinical trials.
5. Symptomatic skeletal events
In recent years, several trials in mCRPC and breast cancer have excluded asymptomatic events from the SRE composite measure and utilized the composite endpoint of symptomatic skeletal events (SSEs) (Table 2). The SSE endpoint reflects only the symptomatic events that patients experience, making it more relevant to patients’ health-related QoL. This is of particular importance for patients with advanced disease and/or advanced age for whom QoL may be a greater priority than survival alone. A delay or reduction in SSEs represents an immediate clinical benefit as it will delay or reduce bone pain, functional impairment, and the need for surgical intervention. SSEs have been shown to be associated with health-related QoL decline and increased healthcare resource utilization [101]. Clinically meaningful differences in functional well-being and pain and significantly higher rates of emergency room, outpatient, and inpatient visits have been reported for patients with mCRPC who experienced SSEs compared with those who did not [101]. Importantly, SSEs are more clinically relevant than SREs because they do not include asymptomatic fractures. The latter are only identified by systematic radiographic assessment and are not considered to be relevant by many physicians, who do not typically carry out routine radiographic assessments in clinical practice, instead only assessing for fractures if a patient has symptoms.
The first prospective study to include an SSE endpoint in prostate cancer was the phase III ALSYMPCA trial of radium-223 in men with symptomatic bone mCRPC [79]. Radium-223 is a targeted alpha-emitting radiopharmaceutical that selectively binds to areas of increased bone turnover (such as bone metastases), emitting high-energy alpha particles of short range [102] that have a localized cytotoxic effect [103]. This study assessed time to first SSE as a secondary endpoint, where SSEs were defined as occurrence of a new symptomatic pathologic fracture, use of external beam radiation therapy for bone pain, tumor-related orthopedic surgery, or spinal cord compression [79, 104]. The ALSYMPCA trial reported a significant improvement in OS for radium-223 versus placebo (hazard ratio [HR] 0.70, 95% confidence interval [CI] 0.58–0.83; P < 0.001) and a significant improvement in time to first SSE (HR 0.66, 95% CI 0.52–0.83; P < 0.001) [79].
Altering the definition of a composite endpoint will impact the findings generated when applied to a dataset. For example, in an exploratory analysis of the extension phase of the pivotal zoledronic acid trial in mCRPC, the proportion of patients reporting a skeletal event was reduced from 38% to 30% when SSEs were considered rather than SREs [105]. Furthermore, in a retrospective analysis of the mCRPC denosumab trial, there were fewer first on-study SSEs than SREs (641 versus 937, respectively), with a similar treatment benefit observed with denosumab versus zoledronic acid on SSEs (22% risk reduction) and SREs (18% risk reduction) [106]. Since asymptomatic fractures are not captured, fractures contribute less to the SSE endpoint compared with the SRE endpoint, resulting in an outcome largely driven by radiation events. Use of external beam radiation therapy and symptomatic pathological fractures were the most common first SSEs in 32% and 6% of patients in the ALSYMPCA study, respectively [104]. In comparison, radiation to bone and pathological fractures were the first SREs in 20% and 15% of patients in the denosumab trial, respectively [97]. Therefore, it is essential to document radiation use specifically for palliation of bone pain when using the SSE endpoint in clinical trials to remove any potential for bias.
Since the ALSYMPCA trial was reported, the PCWG3 has recommended that prostate cancer clinical trials should include clinically relevant time-to-event endpoints, such as time to SSE, marking another step in the evolution of these clinical endpoints [60]. Subsequent phase III trials, such as the LATITUDE and STAMPEDE studies of abiraterone in metastatic castration-sensitive prostate cancer (mCSPC), have assessed time to first SSE, indicating the increased use of clinically relevant SSE-based endpoints [107, 108]. The adoption of SSEs as clinical trial endpoints is not limited to prostate cancer. For example, time to first SSE is the primary endpoint in an ongoing phase III clinical trial comparing denosumab dosing regimens in both prostate and breast cancer patients ( NCT02051218). In addition, the number of patients with an SSE is the primary endpoint in a phase II trial of patients with non-small cell lung cancer and bone metastases receiving radium-223 [109] and time to first SSE is a secondary endpoint in a phase I trial of patients with renal cell carcinoma and bone metastases receiving radium-223 in combination with either sorafenib or pazopanib [110].
6. Combining skeletal event composites with survival
The latest iteration in skeletal complication clinical trial endpoints is the incorporation of survival into the SRE endpoint, resulting in SRE-free survival (SRE-FS). SRE-FS includes death as an event, in addition to standard SREs, thus combining survival and SREs due to disease progression into a single endpoint (Table 2). This may provide an objective variable to measure clinically meaningful benefit while also avoiding the under-reporting of symptomatic bone progression, which is likely to precede death [111]. The first completed clinical trial to use SRE-FS as an endpoint was a single-arm phase II study of zoledronic acid combined with ADT for the treatment of metastatic treatment-naïve prostate cancer; this study reported a 24-month SRE-FS rate of 84% [81]. Subsequently, a randomized phase III study of zoledronic acid versus placebo in men with mCSPC used SRE-FS as the primary endpoint, where the SRE-FS definition included death due to prostate cancer (Table 3). No statistical difference in median SRE-FS was observed between the zoledronic acid and placebo treatment groups [80].
Given the increased clinical relevance of SSEs compared with SREs, several ongoing trials have combined SSEs with survival to give the composite endpoint of SSE-free survival (SSE-FS), defined as time from randomization to the first occurrence of a symptomatic pathologic fractures, external beam radiation therapy to relieve skeletal symptoms, spinal cord compression, or tumor-related orthopedic surgical intervention, or death from any cause [82]. The phase III ERA 223 trial of abiraterone in combination with either radium-223 or placebo in asymptomatic or mildly symptomatic patients with mCRPC employed SSE-FS as the primary endpoint (Table 3). This trial was designed as a registrational study and endorsed by the FDA under Special Protocol Assessment, recognizing SSE-FS as a clinically meaningful endpoint ( NCT02043678). The results of the trial showed no improvement in SSE-FS with abiraterone in combination with radium-223 compared with abiraterone in combination with placebo. Furthermore, fractures occurred more frequently with abiraterone plus radium-223 [82]. Initiation of bone-targeted anti-resorptive therapies was not allowed during the study to prevent confounding effects on study outcomes. Such bone-targeted therapies were only permitted for patients already receiving them at baseline (approximately 40% of the study population). A subgroup analysis highlighted that baseline use of bone-targeted therapies mitigated the risk of fractures [82]. It has been hypothesized that use of bone-targeted therapies, which target the host bone, may have prevented damage to normal bone from radium-223, which targets the host–tumor interface [112]. The US prescribing information for radium-223 has been updated to carry a warning about combining radium-223 with abiraterone [113].
SSE-FS is also being used as an endpoint in three ongoing clinical trials: a phase III study of the androgen receptor antagonist darolutamide in combination with docetaxel and ADT in mCSPC ( NCT02799602); a phase II study of radium-223 in combination with exemestane and everolimus in human epidermal growth factor receptor 2-negative, hormone receptor-positive, bone predominant metastatic breast cancer ( NCT02258451); and a phase II study of radium-223 in combination with background hormonal therapy in human epidermal growth factor receptor 2-negative, hormone receptor-positive, bone predominant metastatic breast cancer ( NCT02258464).
7. Summary and clinical perspectives
It can be challenging to understand, compare, and put in proper clinical context the various skeletal endpoints used in clinical trials given that different variations of these endpoints have been used to gauge the outcome of the trials. Nevertheless, the fact that these endpoints have continued to evolve reflects improvements in technology and increased understanding of not only the clinical relevance of skeletal metastasis on patient morbidity but also the impact of interventions on metastasis-related morbidity.
The evolution of skeletal endpoints from scintigraphy- and pain-based assessments into more clinically relevant composite measures focused on improvement of symptoms and morbidity is reflected by the increased adoption of SSE-based endpoints across clinical trials for different tumor types. A substantial proportion of patients with advanced cancer have bone metastases and are at risk of subsequent skeletal complications leading to symptomatic decline. Therefore, evaluation of the treatment effect of novel therapies on SSEs is of value for these patients. Furthermore, the SSE endpoint may be more relevant to daily clinical practice since it does not depend on systematic radiographic assessment. As results from ongoing and future clinical trials become available, the incorporation of survival metrics, such as SSE-FS, into these composite endpoints may prove even more meaningful both to the patient and the treating oncologist in the real-world setting.
Acknowledgements
Part of AH’s time was supported by a Merit Review Award (I01 BX000545), Medical Research Service, Department of Veterans Affairs. Medical writing assistance was provided by Karl Kemp-O’Brien, PhD, and Michael Sheldon, PhD, of Scion, London, UK, according to Good Publication Practice guidelines (Link), funded by Bayer. Responsibility for opinions, conclusions, and interpretation of data lies with the authors.
Funding
The authors did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
AH has received compensation as a consultant from Novartis, Bristol-Myers-Squibb, AstraZeneca and Bayer, research funding from Bayer, Merck Sharp & Dohme, Clovis, Sotio, Agensys, Constellation and Roche, and honoraria from Pfizer; RJL has received compensation as a consultant from Janssen and research funding from Janssen; JNG has received research funding from Merck, Bristol-Myers Squibb, Astellas Pharma Global, Medivation, and Sanofi and honoraria from Astellas Pharma Global;
Footnotes
Conflict of interest
SH has no conflicts of interest to disclose.
References
- 1.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Human Pathology 2000; 31: 578–583. [DOI] [PubMed] [Google Scholar]
- 2.Pezaro C, Omlin A, Lorente D, Rodrigues DN, Ferraldeschi R, Bianchini D et al. Visceral disease in castration-resistant prostate cancer. Eur Urol 2014; 65: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol 2016; 34: 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Body JJ, Quinn G, Talbot S, Booth E, Demonty G, Taylor A et al. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol 2017; 115: 67–80. [DOI] [PubMed] [Google Scholar]
- 5.Yanae M, Fujimoto S, Tane K, Tanioka M, Fujiwara K, Tsubaki M et al. Increased risk of SSEs in bone-only metastatic breast cancer patients treated with zoledronic acid. J Bone Oncol 2017; 8: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Husaini H, Wheatley-Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol 2009; 4: 251–259. [DOI] [PubMed] [Google Scholar]
- 7.Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2–4): An autopsy study on 367 patients. Urol Int 1999; 62: 69–75. [DOI] [PubMed] [Google Scholar]
- 8.Woodward E, Jagdev S, McParland L, Clark K, Gregory W, Newsham A et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011; 48: 160–166. [DOI] [PubMed] [Google Scholar]
- 9.Hameed A, Brady JJ, Dowling P, Clynes M, O’Gorman P. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 2014; 7: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristinsson SY, Minter AR, Korde N, Tan E, Landgren O. Bone disease in multiple myeloma and precursor disease: novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn 2011; 11: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berruti A, Dogliotti L, Bitossi R, Fasolis G, Gorzegno G, Bellina M et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J Urol 2000; 164: 1248–1253. [PubMed] [Google Scholar]
- 12.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer 2000; 88: 2989–2994. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 14.Roghmann F, Antczak C, McKay RR, Choueiri T, Hu JC, Kibel AS et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol 2015; 33: 17.e19–17.e18. [DOI] [PubMed] [Google Scholar]
- 15.Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer Curr Opin Oncol 2014; 26: 274–283. [DOI] [PubMed] [Google Scholar]
- 16.Brodowicz T, Hadji P, Niepel D, Diel I. Early identification and intervention matters: A comprehensive review of current evidence and recommendations for the monitoring of bone health in patients with cancer. Cancer Treat Rev 2017; 61: 23–34. [DOI] [PubMed] [Google Scholar]
- 17.Howard LE, De Hoedt AM, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis 2016; 19: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santini D, Barni S, Intagliata S, Falcone A, Ferrau F, Galetta D et al. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci Rep 2015; 5: 18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007; 57: 229–232. [DOI] [PubMed] [Google Scholar]
- 20.Yong M, Jensen AO, Jacobsen JB, Norgaard M, Fryzek JP, Sorensen HT. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011; 129: 495–503. [DOI] [PubMed] [Google Scholar]
- 21.Hussain A, Aly A, Daniel Mullins C, Qian Y, Arellano J, Onukwugha E. Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med 2016; 5: 3300–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain A, Yong C, Tkaczuk KHR, Qian Y, Arellano J, Mullins CD et al. Prevalence and risk of skeletal complications and use of radiation therapy in elderly women diagnosed with metastatic breast cancer. PLoS One 2018; 13: e0193661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol 2011; 7: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol 2008; 180: 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3: 537–549. [DOI] [PubMed] [Google Scholar]
- 28.Owen KL, Parker BS. Beyond the vicious cycle: The role of innate osteoimmunity, automimicry and tumor-inherent changes in dictating bone metastasis. Mol Immunol 2017; doi: 10.1016/j.molimm.2017.1011.1023. [DOI] [PubMed]
- 29.Food and Drug Administration. Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics 2007. Available at: https://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf. Accessed September 29, 2017
- 30.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol 2011; 29: 3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 2012; 118: 1192–1201. [DOI] [PubMed] [Google Scholar]
- 32.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26: 4875–4882. [DOI] [PubMed] [Google Scholar]
- 33.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syed Z, Khan A. Bone densitometry: applications and limitations. J Obstet Gynaecol Can 2002; 24: 476–484. [DOI] [PubMed] [Google Scholar]
- 35.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol 2008; 26: 5465–5476. [DOI] [PubMed] [Google Scholar]
- 36.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018; 16: 310–320. [DOI] [PubMed] [Google Scholar]
- 37.Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D’Amico AV et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012; 10: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 38.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018; 378: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 39.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018; 378: 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2019; 380: 1235–1246. [DOI] [PubMed] [Google Scholar]
- 41.Gomella LG, Sartor OA. The current role and limitations of surrogate endpoints in advanced prostate cancer. Urol Oncol 2014; 32: 28 e21–29. [DOI] [PubMed] [Google Scholar]
- 42.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14: 1756–1764. [DOI] [PubMed] [Google Scholar]
- 43.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 44.McQuay HJ, Collins SL, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev 2000; CD001793. [DOI] [PubMed]
- 45.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007; 25: 1423–1436. [DOI] [PubMed] [Google Scholar]
- 46.Thanos L, Mylona S, Galani P, Tzavoulis D, Kalioras V, Tanteles S et al. Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol 2008; 37: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Velden JM, Verkooijen HM, Seravalli E, Hes J, Gerlich AS, Kasperts N et al. Comparing conVEntional RadioTherapy with stereotactIC body radiotherapy in patients with spinAL metastases: study protocol for an randomized controlled trial following the cohort multiple randomized controlled trial design. BMC Cancer 2016; 16: 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol 1998; 47: 233–240. [DOI] [PubMed] [Google Scholar]
- 49.Foro Arnalot P, Fontanals AV, Galceran JC, Lynd F, Latiesas XS, de Dios NR et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008; 89: 150–155. [DOI] [PubMed] [Google Scholar]
- 50.Chi MS, Yang KL, Chang YC, Ko HL, Lin YH, Huang SC et al. Comparing the Effectiveness of Combined External Beam Radiation and Hyperthermia Versus External Beam Radiation Alone in Treating Patients With Painful Bony Metastases: A Phase 3 Prospective, Randomized, Controlled Trial. Int J Radiat Oncol Biol Phys 2018; 100: 78–87. [DOI] [PubMed] [Google Scholar]
- 51.Henry D, Vadhan-Raj S, Hirsh V, von Moos R, Hungria V, Costa L et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer 2014; 22: 679–687. [DOI] [PubMed] [Google Scholar]
- 52.Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. J Am Med Assoc 2017; 317: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewington VJ, McEwan AJ, Ackery DM, Bayly RJ, Keeling DH, Macleod PM et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 1991; 27: 954–958. [DOI] [PubMed] [Google Scholar]
- 54.Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993; 25: 805–813. [DOI] [PubMed] [Google Scholar]
- 55.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004; 63: 940–945. [DOI] [PubMed] [Google Scholar]
- 56.Tripathi M, Singhal T, Chandrasekhar N, Kumar P, Bal C, Jhulka PK et al. Samarium-153 ethylenediamine tetramethylene phosphonate therapy for bone pain palliation in skeletal metastases. Indian J Cancer 2006; 43: 86–92. [DOI] [PubMed] [Google Scholar]
- 57.Morris MJ, Autio KA, Basch EM, Danila DC, Larson S, Scher HI. Monitoring the clinical outcomes in advanced prostate cancer: what imaging modalities and other markers are reliable? Semin Oncol 2013; 40: 375–392. [DOI] [PubMed] [Google Scholar]
- 58.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmid S, Omlin A, Blum D, Strasser F, Gillessen S, Rothermundt C. Assessment of anticancer-treatment outcome in patients with metastatic castration-resistant prostate cancer-going beyond PSA and imaging, a systematic literature review. Ann Oncol 2015; 26: 2221–2247. [DOI] [PubMed] [Google Scholar]
- 60.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016; 34: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018; 73: 178–211. [DOI] [PubMed] [Google Scholar]
- 62.Cook GJ, Azad GK, Goh V. Imaging Bone Metastases in Breast Cancer: Staging and Response Assessment. J Nucl Med 2016; 57 27s-33s. [DOI] [PubMed]
- 63.Montilla-Soler JL, Makanji R. Skeletal Scintigraphy. Cancer Control 2017; 24: 137–146. [DOI] [PubMed] [Google Scholar]
- 64.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M et al. Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 2114–2121. [DOI] [PubMed] [Google Scholar]
- 65.Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol 2017; 197: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dennis ER, Jia X, Mezheritskiy IS, Stephenson RD, Schoder H, Fox JJ et al. Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J Clin Oncol 2012; 30: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Idota A, Sawaki M, Yoshimura A, Hattori M, Inaba Y, Oze I et al. Bone Scan Index predicts skeletal-related events in patients with metastatic breast cancer. Springerplus 2016; 5: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imbriaco M, Larson SM, Yeung HW, Mawlawi OR, Erdi Y, Venkatraman ES et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res 1998; 4: 1765–1772. [PubMed] [Google Scholar]
- 69.Anand A, Morris MJ, Kaboteh R, Bath L, Sadik M, Gjertsson P et al. Analytic Validation of the Automated Bone Scan Index as an Imaging Biomarker to Standardize Quantitative Changes in Bone Scans of Patients with Metastatic Prostate Cancer. J Nucl Med 2016; 57: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris MJ, Molina A, Small EJ, de Bono JS, Logothetis CJ, Fizazi K et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 2015; 33: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rathkopf DE, Beer TM, Loriot Y, Higano CS, Armstrong AJ, Sternberg CN et al. Radiographic Progression-Free Survival as a Clinically Meaningful End Point in Metastatic Castration-Resistant Prostate Cancer: The PREVAIL Randomized Clinical Trial. JAMA Oncol 2018; 4: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012; 379: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie W, Regan MM, Buyse M, Halabi S, Kantoff PW, Sartor O et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol 2017; 35: 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Food and Drug Administration. Nonmetastatic, castration-resistant prostate pancer: considerations for metastasis-free survival endpoint in clinical trials Guidance for industry; 2018. Available at: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm625703.pdf. Accessed November 26, 2018 [Google Scholar]
- 75.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 76.Raje N, Terpos E, Willenbacher W, Shimizu K, Garcia-Sanz R, Durie B et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol 2018; 19: 370–381. [DOI] [PubMed] [Google Scholar]
- 77.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 2001; 7: 377–387. [PubMed] [Google Scholar]
- 78.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- 79.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 80.Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 2014; 32: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nozawa M, Inagaki T, Nagao K, Nishioka T, Komura T, Esa A et al. Phase II trial of zoledronic acid combined with androgen-deprivation therapy for treatment-naive prostate cancer with bone metastasis. Int J Clin Oncol 2014; 19: 693–701. [DOI] [PubMed] [Google Scholar]
- 82.Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 408–419. [DOI] [PubMed] [Google Scholar]
- 83.Shulman RM, Meyer JE, Li T, Howell KJ. External beam radiation therapy (EBRT) for asymptomatic bone metastases in patients with solid tumors reduces the risk of skeletal-related events (SREs). Ann Palliat Med 2018. [DOI] [PubMed]
- 84.Ristevski B, Jenkinson RJ, Stephen DJ, Finkelstein J, Schemitsch EH, McKee MD et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg 2009; 52: 302–308. [PMC free article] [PubMed] [Google Scholar]
- 85.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005; 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 86.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res 2003; S230–244. [DOI] [PubMed]
- 87.Johnson SK, Knobf MT. Surgical interventions for cancer patients with impending or actual pathologic fractures. Orthop Nurs 2008; 27: 160–171; quiz 172–163. [DOI] [PubMed] [Google Scholar]
- 88.Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005; 16: 579–584. [DOI] [PubMed] [Google Scholar]
- 89.Biskup E, Cai F, Vetter M. Bone targeted therapies in advanced breast cancer. Swiss Med Wkly 2017; 100: w14440. [DOI] [PubMed] [Google Scholar]
- 90.Saad F, Ivanescu C, Phung D, Loriot Y, Abhyankar S, Beer TM et al. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis 2017; 20: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 1998; 16: 593–602. [DOI] [PubMed] [Google Scholar]
- 92.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med 1996; 334: 488–493. [DOI] [PubMed] [Google Scholar]
- 93.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 94.Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998; 16: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 95.Polascik TJ. Bisphosphonates in oncology: evidence for the prevention of skeletal events in patients with bone metastases. Drug Des Devel Ther 2009; 3: 27–40. [PMC free article] [PubMed] [Google Scholar]
- 96.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 97.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011; 377: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barrett-Lee P, Casbard A, Abraham J, Hood K, Coleman R, Simmonds P et al. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial. Lancet Oncol 2014; 15: 114–122. [DOI] [PubMed] [Google Scholar]
- 99.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003; 21: 3150–3157. [DOI] [PubMed] [Google Scholar]
- 100.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 2004; 100: 2613–2621. [DOI] [PubMed] [Google Scholar]
- 101.McKay R, Haider B, Duh MS, Valderrama A, Nakabayashi M, Fiorillo M et al. Impact of symptomatic skeletal events on health-care resource utilization and quality of life among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis 2017; 20: 276–282. [DOI] [PubMed] [Google Scholar]
- 102.Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006; 12: 6250s–6257s. [DOI] [PubMed] [Google Scholar]
- 103.Henriksen G, Breistol K, Bruland OS, Fodstad O, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002; 62: 3120–3125. [PubMed] [Google Scholar]
- 104.Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15: 738–746. [DOI] [PubMed] [Google Scholar]
- 105.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–882. [DOI] [PubMed] [Google Scholar]
- 106.Smith MR, Coleman RE, Klotz L, Pittman K, Milecki P, Ng S et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 2015; 26: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017; 377: 352–360. [DOI] [PubMed] [Google Scholar]
- 108.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017; 377: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taber AM. Radium-223 following front-line chemotherapy for patients with non-small cell lung cancer and bone metastases. In ASCO 2018; Abst e21211.
- 110.McKay RR, Bosse D, Gray KP, Michaelson MD, Krajewski K, Jacene HA et al. Radium-223 Dichloride in Combination with Vascular Endothelial Growth Factor-Targeting Therapy in Advanced Renal Cell Carcinoma with Bone Metastases. Clin Cancer Res 2018; 24: 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 2003; 95: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 112.Ryan C. Apollo 14 and the lessons from fractures 2018. Available at: https://www.urotoday.com/center-of-excellence/mcrpc-treatment/from-the-editor/108224-apollo-14-and-the-lessons-from-fractures.html. Accessed November 23, 2018
- 113.Bayer HealthCare Pharmaceuticals Inc. Xofigo (radium-223) [prescribing information] 2018. Available at: Accessed.


