Abstract
Background:
It is not known whether environmental gamma radiation measured in US cities has detectable adverse health effects. We assessed whether short-term exposure to gamma radiation emitted from ambient air particles [gamma particle activity (PRγ)] is associated with reduced pulmonary function in chronic obstructive pulmonary disease (COPD) patients.
Objective:
We hypothesize that the inhalation of gamma radiation emitted from ambient air particles may be associated with reduced pulmonary function in individuals with COPD.
Methods:
In 125 patients with COPD from Eastern Massachusetts who had up to 4 seasonal one-week assessments of particulate matter ≤ 2.5 pm (PM2.5), black carbon (BC), and sulfur followed by spirometry. The US EPA continuously monitors ambient gamma (γ) radiation including γ released from radionuclides attached to particulate matter that is recorded as 9 γ-energy spectra classes (i = 3–9) in counts per minute (CPMγ) in the Boston area (USA). We analyzed the associations between ambient and indoor PRγi (up to one week) and pre and post-bronchodilator (BD) forced expiratory volume in 1 s (FEV1) and with forced vital capacity (FVC) using mixed-effects regression models. We estimated indoor PRγi using the ratio of the indoor-to-outdoor sulfur in PM2.5 as a proxy for infiltration of ambient radionuclide-associated particles.
Results:
Overall, exposures to ambient and indoor PRγi were associated with a similar decrease in pre- and post- BD FEV1 and FVC. For example, ambient PRγ3 exposure averaged from the day of pulmonary function testing through the previous 3 days [IQR of 55.1 counts per minute (CPMγ)] was associated with a decrease in pre-BD FEV1 of 21.0 ml (95%CI: −38.5 to −3.0 ml; p < 0.01) and pre-BD FVC of 27.5 ml [95% confidence interval (CI): −50.7 to −5.0 ml; p < 0.01] with similar effects adjusting for indoor and outdoor BC and PM2.5.
Conclusion:
Our results show that short-term ambient and indoor exposures to environmental gamma radiation associated with particulate matter are associated with reduced pre- and post- BD pulmonary function in patients with COPD.
Keywords: Ambient and indoor air pollution, Background gamma radiation, Chronic obstructive pulmonary disease, Pulmonary function
1. Introduction
Chronic obstructive pulmonary disease (COPD) affect 15.7 million Americans and in 2016 was the fourth leading cause of death in the USA (Wheaton et al, 2015). According to the World Health Organization, 14% of outdoor air pollution-related premature deaths are associated with COPD. Moreover, indoor air pollution is responsible for over one-third of premature COPD deaths in adults in low and middle-income countries (Bruce et al., 2000).
Our previous studies showed that the effects of indoor pollution are significantly associated with a reduction in pulmonary function, increased inflammatory markers and urinary markers of oxidative stress in individuals with COPD from Eastern Massachusetts where levels of indoor pollutants are low (Hart et al., 2018; Garshick et al., 2018; Grady et al., 2018). These findings suggest that indoor levels of pollutants may have critical impact on the health of individuals with COPD who spend most of their time indoors (Hart et al., 2018; Garshick et al., 2018; Grady et al., 2018). Indoor exposure to radon has been recently linked to COPD mortality (Turner et al., 2012) and to an increased risk for hospital admissions (Barbosa-Lorenzo et al., 2017). The radiation-related risk of non-cancer respiratory diseases also appears to have increased due to ionizing radiation exposures near atomic bomb sites in Japan, although the dose-response curves remain unclear (Ozasa et al., 2016).
Natural terrestrial and extra-terrestrial ionizing radiation are the largest sources of radiation exposure in the world’s population (EPA, 1972). In the United States, the dose equivalent from natural radiation ranges from 80 to 200 mrem/yr. However, dose can vary according to the local altitude, latitude, soil composition, and housing characteristics (EPA, 1972). Sources of natural radiation includes products of galactic cosmic rays and the decay of progeny from the radioactive decay chain of uranium-238 (U-238), thorium-232 (Th-232) and potassium-40 (K-40) located in earth’s crust (EPA, 1972; Ojovan and Lee, 2005; Baskaran, 2016). The U-238 decay chain includes radon-222 (Rn-222), which represents, with its short and long-lived progenies, the major source of internal and external radiation exposure from natural sources (Baskaran, 2016). Excited states of nuclei during the decay of radionuclides from all sources emit gamma-rays of different spectral classes that is monitored daily by US EPA. Gamma-rays are very penetrating radiation that consists of high-energy photons, which are emitted concurrently with beta and alpha radiation during radioactive decay. Alpha radiation causes considerably more biological damage than equivalent beta or gamma radiation, however it cannot penetrate the epidermis. Inhaled alpha-emitting particles have the potential to ionize and excite molecules leading to a cascade of biological effects including inflammation and oxidative stress in vivo and in vitro studies (Narayanan et al., 1999). Air-borne environmental radionuclides have high diffusion coefficients and attach rapidly to ambient particulates, which is referred to as particle-associated radioactivity (PR) (Papastefanou, 2008). Radionuclides emitting radioactive decay products attached to air particulates may be deposited within the respiratory tract by inhalation. Although it is a measure of atmospheric gamma radiation, PRγ may be thought of a surrogate for the whole amount of background ionizing radiation emitted by particle-bound radionuclides, including radiation from alpha and beta - emitting particles since these are being emitted simultaneously with gamma radiation.
Therefore, while the short-term effects of ambient and indoor air particle pollution on lung function in COPD individuals has been described (Hart et al., 2018; Bloemsma et al., 2016), the impact of ionizing radiation associated with ambient particulate matter (PM) has not been previously investigated. In this study, we assessed the effect of short-term exposure (over one week) to ambient and indoor PRγ on pre and post-bronchodilator (BD) forced expiratory volume in 1 s (FEV1) and with forced vital capacity (FVC) in patients with COPD from Eastern Massachusetts, USA. We hypothesize that the inhalation of PRγ may be associated with reduced pulmonary function in individuals with COPD.
2. Material and methods
2.1. Study population
One-hundred twenty-five COPD patients from Eastern Massachusetts were recruited between November 2012 and December 2014 as part of a project on the impact of indoor particulate air pollution exposures on the health of patients with COPD at Veterans Affairs (VA) Boston Healthcare System as previously described (Hart et al., 2018). Since COPD may occur in the absence of a smoking history or in individuals with chronic asthma (Salvi and Barnes, 2009; Miller et al., 2005), individuals were eligible regardless of past smoking history or if under care for chronic asthma. Therefore, individuals were eligible for the study if they were 40 years of age or older, had a FEV1/FVC < 0.70 on post-bronchodilator spirometry or had emphysema reported based on a clinical CT scan and had a clinical diagnosis of COPD (or chronic asthma). Persons with malignancies other than local skin or stable prostate cancer, with a systemic inflammatory disease such as rheumatoid arthritis; with unstable heart disease were excluded. By study design, in order to substantially reduce exposure to indoor sources of PM2.5 and BC, particularly sources of indoor combustion products, we excluded patients who were current smokers or lived with a current smoker, or who had a major source of indoor air pollution (e.g., wood stove or fireplace, frequent burning of incense or candles.
2.1.1. Pulmonary function measures
Spirometry [HDpft, Nspire, Longmont, CO] pre and at least 15 min post 180 pg of albuterol administered by a spacer was conducted using ATS methods (Kang et al., 2010). The highest values of pre- and post-bronchodilator (pre-BD and post-BD) FEV1 and FVC from acceptable efforts were used, and the pre- and post-FEV1/FVC ratio were calculated.
2.2. Exposure assessment
2.2.1. Assessment of indoor and ambient BC and PM2.5
Weekly ambient BC and PM2.5 mass concentration are measured concomitantly at the Harvard supersite located near downtown Boston, MA (EPA, 2005). For weekly indoor BC and PM2.5, a micro-environmental automated particle sampler was installed in the main activity room of the house (excluding the kitchen) for a week prior to each clinic visit as described in Garshick et al., 2018. Details of the pollution assessment are given in Hart et al., 2018 and Grady et al., 2018.
2.2.2. EPA RadNet gamma particle activity measures
Gross gamma count data are collected at a permanent monitoring U.S. EPA station in Boston area (EPA) airborne gamma spectrometry system for environmental program) to detect unusual changes in background radiation levels. Real-time gross gamma data are detected by gamma spectrometry using Thallium-activated sodium iodide [NaI (TI)] detectors located close to the ground surface (~ 6 cm) to increase the counting efficiency. The monitoring station is equipped with a Total Suspended Particle (TSP) high volume air sampler for continuous measurement of gamma radiation emitted by TSP collected on a filter (10 cm diameter synthetic fiber) at an airflow rate of 1 m3/min. The NaI (Tl) detectors are positioned above the filter and connected to a 1024-channel multi-channel analyzer and a local processing unit. The energy of the detected total gross gamma counts accumulates over a period of 10 min (range from 50 to 2000 keV). The full gamma energy spectrum reported hourly is sent to the National Air and Radiation Environmental Laboratory (NAREL).
The data are reported in 9 different channels related to gamma energy (γ) wavelength bands. The energy ranges of these channels are: (γ1) ≤100, (γ2) 101–200, (γ3) 201–400, (γ4) 401–600, (γ5) 601–800, (γ6) 801–1000, (γ7) 1001–1400, (γ8) 1401–1800 and (γ9) 1801–2200 keV. These γ classes serve as surrogates for the most common ambient environmental radionuclides such as for example: Am-241, 1-131, Ra-226, Ra-228, Rn-220, Rn-222, Th-230, Th-232, U-234, U-235, U-238, Rn-220 and Rn-222 and its progenies. Details about the EPA airborne gamma spectrometry system can be found in references (EPA, 2009; Cardarelli et al., 2010).
As these gamma spectrometers are not well-shielded, the daily particle gamma activity measurements are influenced by the background terrestrial and cosmic radiation. So that our measure of gamma radiation does not include background radiation and reflects daily fluctuations attributable to gamma radiation released from particulate matter-bound radionuclides we corrected the measurement values by estimating and subtracting background levels. Since background terrestrial and cosmic radiation does not vary much day-to-day, we estimated the effect of background radiation by the average of gamma activity over a given previous period prior to PM gamma activity measurement. In order to estimate the gamma radiation emitted specifically from inhaled air particulates, we corrected the measurement values by subtracting the mean gamma values for each channel. We assessed different averaging periods such as 7, 21, 28 and 90 days before measurement day. Finally, we selected the 21-day period as it represented the best correlation between ambient PM2.5 mass concentration and PRγ fluctuations (p < 0.05). Note that our approach produces negative and positive values of PRγ because our adjusted measurement it is relative to background levels attributable to cosmic radiation and we are interested in gamma particle activity. In addition, γ1 and γ2 activity data were not included in the analysis because of the large fraction of missing data (~ 25%) or no data available. Therefore, we included data for 7γ channels in our analysis, or wavelength ranges, including γ3 to γ9 activities.
2.2.3. Assessment of indoor PRγi
Our COPD subjects reported spending most of their time indoor (mean 17.1 ± 4.0 h/day indoors). For this reason, we estimated the fraction of outdoor PRγi that penetrates indoors. We used the indoor-to-outdoor sulfur (S) ratio measured in indoor and outdoor PM2.5 filter samples (Sarnat et al., 2002) as a proxy of the infiltration rate for each home to predict indoor PRγi exposure as follows:
| (1) |
where, indoor PRγi is the estimated indoor exposure to particle gamma radioactivity for spectral channel i; PRγi is the corresponding particle radioactivity measured ambient by the RadNet; SI is the indoor sulfur concentration (μg/m3); SO is the outdoor sulfur concentration (μg/m3); the SI/SO ratio represents the fine particle infiltration ratio, dimensionless. This ratio has been used widely to account for the fraction of outdoor particles that penetrate indoors (Tang et al, 2018). Previous study demonstrated that I/O-S ratios are strongly associated with the corresponding I/O-PM2.5 ratios, with a slope of 1.02 and daily values are similar over a week (Kloog et al, 2014). Outdoor S is a constituent of PM2.5 measured at the Boston monitoring supersite, which is highly correlated with indoor S levels (r2 = 0.89) (Kloog et al, 2014). Finally, we calculated indoor and outdoor PRγi moving day averages ranging from 0 to 7 days before pulmonary function testing.
2.3. Model covariates
Model covariates were selected α priori based on their known associations with the outcomes or exposures. Race (white vs. other), sex (male/female), age (data of birth), pulmonary medication use [inhaled steroids, long-acting bronchodilators, and short-acting bronchodilators (administrated ≤6h before the examination)]. Although patients with COPD exacerbation were exclude, we adjusted for self-report of any other respiratory illness in the two weeks before each visit. Body mass index (BMI) was calculated based on height and weight at each visit.
Daily temperature for each participant’s home was estimated using a model based on satellite remote sensing, land use, and ground level temperature data (Hernández-Cadena et al, 2009). Daily relative humidity information was obtained from the Boston Logan International Airport. Seasons were calculated according to the month of the clinical visit into four categories winter (December, January, February), spring (March, April, May), summer (June, July, August), or fall (September, October, November).
2.4. Statistical analysis
We examined whether moving averages of indoor and outdoor PRγi starting on the 0 through up to 7 days prior to the spirometry examination were associated with pre- and post-BD FVC, FEV1, and FEV1/FVC. Repeated linear mixed effects models were performed with a random intercept for each subject. Primary models were performed including either indoor or outdoor PRγi and confounders. Lastly, sensitivity analyses were performed including weekly BC or PM2.5 in the indoor models (secondary models) and corresponding average weekly central site BC or PM2.5 in the ambient analysis. Each of the moving day averages of gamma radiation (0 through up to 7 days before the spirometry) was examined in a separate model to obtain the corresponding effect estimates and 95% CI for each outcome. All models were adjusted for the other model covariates noted above. For each γi we present the effect estimates as the mean change in the pre and post-BD FEV1, FVC and FEV1/FVC-ratio outcomes per interquartile range (IQR) average, adjusted for other covariates in the regression models. We also examined each model residuals to estimate any deviations from linearity. All analyses were performed using SAS 9.3 (SAS Inc. NC, USA).
3. Results
There were 125 patients studied, and the majority were men (98%), with a mean age of 73 years, and with 342 repeated measures of ambient PRγ and up to 334 measurements of indoor PRγ. Approximately, 50% of the individuals had a history of heart disease, 25% had diabetes, and 19% had hypertension. A total of 95% of the participants were former smokers and 5% never smoked. No active smokers were included in the study. Most were receiving treatment with bronchodilators (Table 1). The distribution of ambient (Boston central site) and indoor (residential) PRγi (spectral classes 3 through 9), BC, PM2.5 and meteorological variables are summarized in Table 2.
Table 1.
Study participant characteristics at baseline (N = 125) among COPD patients (2012–2014).
| N = 125 participants (342 assessments) | Mean (SD) or % |
|---|---|
| Patient characteristics at study entry | |
| Age, yr | 73.3 (8.7) |
| Male sex, % | 98.1 |
| Race, % | |
| White | 90.8 |
| Non-White | 9.2 |
| BMI, Kg/m2 | 30.1 (5.8) |
| Height | 171.8 (7.0) |
| Pack-years (Former smokers, n = 119) | 60.5 (41.8) |
| Heart Disease,% | 53.4 |
| Diabetes,% | 25.1 |
| Education, yr | 13.1 (2.2) |
| Patient characteristics from the 342 assessments | |
| Pre-Bronchodilator | |
| FVC (L) | 3.32 (0.80) |
| %-predicted FVC | 87.0 (19.4) |
| FEV1(L) | 1.8 (0.6) |
| %-predicted FEV1 | 66.5 (21.3) |
| FEV1/FVC (L) | 0.5 (0.1) |
| Post-Bronchodilator | |
| FVC (L) | 3.46 (0.81) |
| %-predicted FVC | 90.0 (19.9) |
| FEV1(L) | 1.93 (0.61) |
| %-predicted FEV1 | 69.7 (21.5) |
| FEV1/FVC (L) | 0.5 (0.1) |
| Medications, % | |
| Long-acting bronchodilator | |
| Long-acting muscarinic antagonists | 65.2 |
| Long-acting β2 agonists | 65.4 |
| Theophylline | 1.1 |
| Short-acting bronchodilator | 75.4 |
| Inhaled steroids | 75.2 |
| Cold or other respiratory illness in the past 2 weeks | 14.4 |
| Other environmental characteristics | |
| Season (%) | |
| Winter (Dec - Feb) | 21.7 |
| Spring (Mar - May) | 25.4 |
| Summer (Jun - Aug) | 25.9 |
| Fall (Sep - Nov) | 26.9 |
| Time indoors at home on weekdays (h) | 17.1 (3.9) |
| Time indoors at home on weekends (h) | 17.1 (4.7) |
| Days of indoor home sampling (N) | 7.6 (0.7) |
Table 2.
Distribution of ambient and indoor PRγ, BC, PM2.5, and meteorology.
| N | Mean | Std Dev | Range Min-Max | IQR | |
|---|---|---|---|---|---|
| Indoor 7-day average | |||||
| PRγ 3 | 334 | 0.7 | 19.5 | −81.8– 74.3 | 26.4 |
| PRγ 4 | 333 | 0.3 | 5.0 | −24.2–17.9 | 6.3 |
| PRγ 5 | 333 | 0.5 | 7.0 | −28.5–22.1 | 9.6 |
| PRγ 6 | 334 | 0.07 | 2.3 | −12–7.8 | 2.3 |
| PRγ 7 | 334 | 0.2 | 4.1 | −17.3–12.5 | 4.1 |
| PRγ 8 | 334 | −0.031 | 4.64 | −56.3–22.8 | 6.7 |
| PRγ 9 | 334 | 0.001 | 0.57 | −7.2–1.95 | 0.5 |
| BC | 334 | 0.2 | 0.3 | −0.4–2.8 | 0.2 |
| PM2.5 | 334 | 8.5 | 6.3 | 0.2–45.8 | 5.6 |
| T(°C) | 334 | 11 | 9.6 | −12–28 | 14.5 |
| RH (%) | 334 | 66.4 | 16 | 30–97 | 25.2 |
| Ambient 7-day average | |||||
| PRγ 3 | 342 | 1.3 | 27.1 | −106.7–91.1 | 41.6 |
| PRγ 4 | 342 | 0.5 | 6.9 | − 28.9–23.4 | 9.4 |
| PRγ 5 | 342 | 0.5 | 7.0 | −28.5–22.2 | 9.6 |
| PRγ 6 | 342 | 0.1 | 3.4 | −16.5–8.8 | 3.5 |
| PRγ 7 | 342 | 0.3 | 5.7 | −23.6–12.8 | 6.6 |
| PRγ 8 | 342 | 0.4 | 8.6 | −23.3–18.9 | 10.3 |
| PRγ 9 | 342 | 0.03 | 0.6 | −2.4–1.95 | 0.8 |
| BC | 342 | 0.5 | 0.2 | 0.2–1.4 | 0.3 |
| PM2.5 | 342 | 6.3 | 2.0 | 3.0–13.4 | 2.7 |
| T(°C) | 342 | 11.2 | 9.6 | −12.2–28.0 | 15.4 |
| RH (%) | 342 | 66.4 | 15.7 | 30.7–97.0 | 26.6 |
Definitions of abbreviations: ambient and indoor PRγi (i = γ3 to γ9) on the 7-day moving average (CPMγ); 24hr-weekly BC and 24 hr-weekly PM2.5 (μg/m3); T – temperature in °C; RH – Relative humidity (%)- on the day of examination (0).
3.1. Ambient PRγi exposure
Most moving averages of ambient PRγi from the day of pulmonary function testing (day 0) through 7 days before testing were associated with a decrease in pre- and post-BD FEV1 and FVC across all gamma channels (i = 3 to 9) (Figs. 1 and 2). Exposure to ambient PRγ3 was associated with the strongest decrease in pre-BD FEV1 and FVC. For example, an IQR of 55.1 CPMγ of ambient PRγ3 over 4 days (averaged over day 0 through 3) was associated with a 21.0 ml decrease in FEV1 (95%CI: −38.5 to −3.3m1, p < 0.01) and a 27.5ml decrease in FVC (95%CI: −50.7 to −4.9 ml, p < 0.01). Post-BD, effects of ambient PRγ on FEV1 and FVC were similar to pre-BD effects (supplementary tables). As an example, an IQR of 60 CPMγ of ambient PRγ3 over the 3 days (day 0 through 2) was associated with a 18.0 ml decrease (95%CI: −34.1 to −1.8, p = 0.03) in FEV1 and a 15.5 ml decrease (95%CI: −39.5 to −8.7 ml, p = 0.20) in FVC. After adjustment for weekly average ambient BC and PM2.5 measured at the Boston central site there was no meaningful change on the association of ambient PRγ on FEV1 and FVC pre- or post BD (data not shown). There was also no consistent effect of ambient PRγi on FEV1/FVC pre- or post- BD adjusted or unadjusted for outdoor BC and PM2.5 across all gamma channels (data not shown).
Fig. 1.
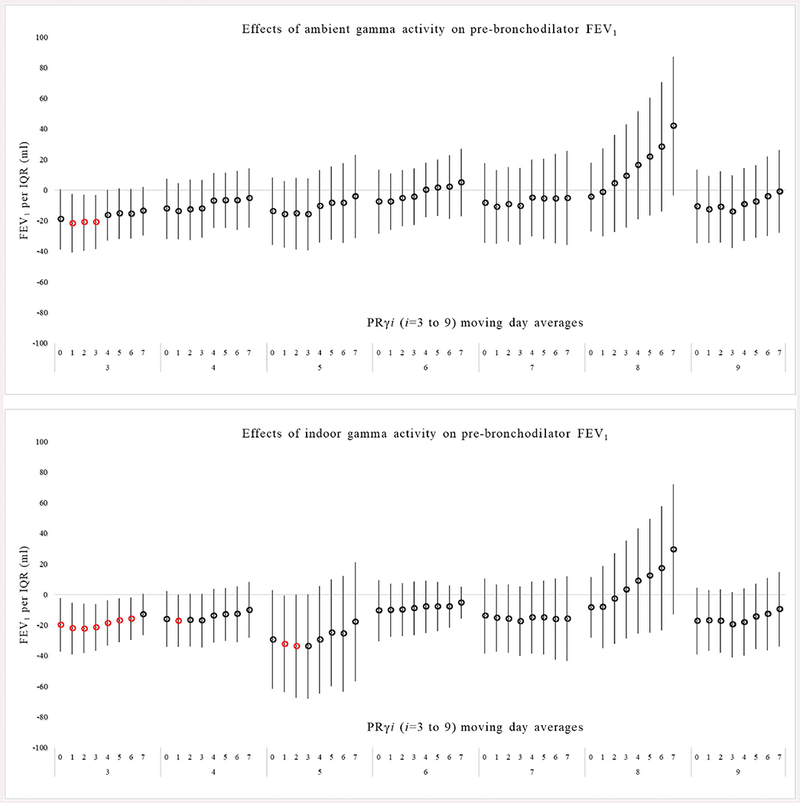
Association between pre-bronchodilator FEV1 in response to ambient and indoor PRγ exposure in multivariable models. Exposure is based on daily gamma radiation moving averages starting with the day of spirometry (day 0) and averaged up to 7 days before spirometry (day 7).
Fig. 2.
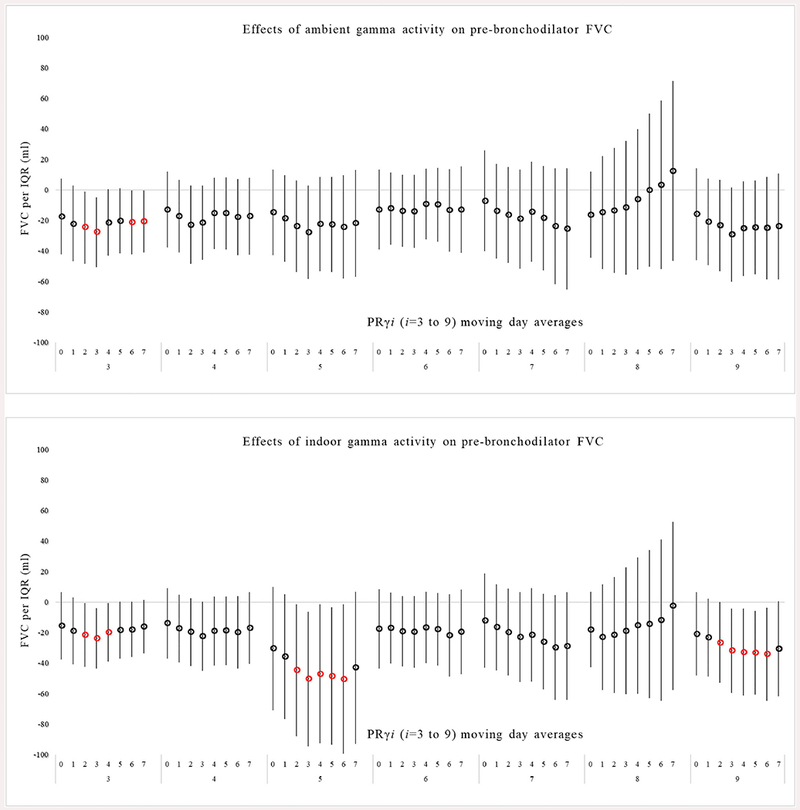
Association between pre-bronchodilator FVC in response to ambient and indoor PRγ exposure in multivariable models. Exposure is based on daily gamma radiation moving averages starting with the day of spirometry (day 0) and averaged up to 7 days before spirometry (day 7).
3.2. Indoor PRγ exposure
Using the ratio of indoor to ambient sulfur measured in the weekly residential and matched central site PM2.5 filter samples, we estimated indoor infiltration of ambient PRγi. (27). Since we excluded samples where the sulfur ratio was > 1.2 (suggestive of an indoor source) there were slightly fewer repeated measures available (n = up to 334, depending on model) (Table 2). Overall, the association between indoor PRγi exposure and a reduction in pre- and post-BD FEV1 and FVC was similar to the ambient associations, including a similar pattern of effect across nearly all gamma channels (Figs. 1 and 2). As an example, there was a decrease in pre-BD FEV1 of 21.5 ml per an IQR of 34.6 CPMγ of indoor PRγ3 exposure averaged over 0–3 days before spirometry (95%CI: −36.7 to −6.2 ml, p < 0.006), and a decrease in pre-BD FVC of 24.0 ml per an IQR of 34.6 CPMγ of indoor PRγ3 (95%CI: −43.6 to −4.1 ml, p < 0.01) averaged over 0–3 days before spirometry.
There were, however, more statistically significant associations with indoor exposures. Statistically significant associations were observed between indoor PRγ3, 4 and 5 and decreases in pre-BD FEV1 for moving averages from 0 to up to 7 days before spirometry (p < 0.05). The effects were similar after adjustment for indoor weekly BC and PM2.5 (data not shown). The effects of indoor PRγ on post-BD FEV1 and FVC were similar to pre-BD effects (supplementary tables). There was no consistent pattern suggestive of an association between indoor PRγi (i = 3 to 9) with FEV1/FVC pre- or post- BD unadjusted or adjusted for indoor BC and PM2.5 (data not shown).
The effects of weekly average ambient and indoor PM2.5 and BC on FEV1 and FVC in multivariable models that included PRγi (i = 3 to 9) were similar to our previous findings described in Hart et al, (2018) that did not include measurements of PRγ. There were significant effects of weekly average indoor BC on a reduction in pre-BD FEV1 that were similar in all models of PRγ moving averages and a non-significant reduction in pre-BD FVC. For example, there was a 16.0 ml (95%CI = −1.5 to −30.5ml, p < 0.01) decrease in pre-BD FEV1 and a 5 ml (95%CI = −24 to −14 ml, p = 0.6) decrease in pre-BD FVC attributable to indoor BC in models with gamma 3 averaged over the 7 days before pulmonary function testing. There were no significant association between pre-bronchodilator FEV1 and FVC with indoor or outdoor PM2.5 or with outdoor BC as we previously reported (Hart et al, (2018)). Similar non-significant associations were observed between post-bronchodilator FEV1 with indoor or outdoor PM2.5 or BC (data not shown).
4. Discussion
To our knowledge, this is the first study to investigate the effects of gamma particle activity on pulmonary function. Our results show that there was a pattern of a reduction in pre- and post-BD FEV1 and FVC associated with nearly all outdoor and indoor PRγi exposures with generally stronger effects in lower gamma channels (primarily gamma 3 through 5). The results were similar when the models were adjusted for BC or PM2.5. In the same cohort, we previously described associations between indoor BC and a reduced FEV1, FVC, and FEV1/FVC assessed pre-bronchodilator, and no associations with ambient BC, or indoor or ambient PM2.5 (Hart et al., 2018). The reasons for differences in the patterns of effects of gamma activity and particulate pollution on pulmonary function in this cohort is uncertain. In addition, the effects of indoor BC were similar to effects we previously described in the same cohort when PRγi exposures were not included. These results suggest that there are independent effects of PM-associated gamma activity and BC measured in PM2.5 on pulmonary function in our COPD cohort.
In general, the exposure to lower energy ambient and indoor PRγi (i = 3, 4 and 5) were found to have a greater effect on decreases in pre- and post-BD FEV1 and FVC than higher energy PRγi (i = 7 and 8) with similar effects over each exposure window studied. Lower energy y spectra (i = 3, 4 and 5) are most related to Rn-222 and its progenies that form as result of decay e.g., Pb-212, Pb-214, Bi-214, and Bi-212. In vitro studies have shown that the relative biological effectiveness of higher-energy γ-radiation is lower than that induced by lower-energy y-radiation (Hunter and Muirhead, 2009), possibly due to total absorption of low-energy gamma radiation (with no scattering) in biological tissues. In addition, the emission of inhaled gamma particle activity occurs concurrently with the emission of inhaled alpha and beta-radiation, which can be considered the main hazard lung function observed in our study. The inhalation and deposition of alpha-particles on lung tissues release heavy ions that have the ability damage cellular division and damage DNA (Narayanan et al., 1999; Zhou et al., 2001).
Gamma rays and alpha/beta particles produce primary and secondary ionization trails that induce electron dislodgement from and disruption of covalent bonds between atoms in tissues, which can also lead to an increase in reactive oxygen species (ROS) production and further damage biological molecules (Mannam et al., 2014). ROS stimulate a cascade of immune response and subsequent migration of inflammatory cells, e.g., neutrophils and macrophages, into the lungs that potentiate even more the local ROS production (Dagle and Sanders, 1984). The effect of ROS production and inflammation may be promoted by PRγi exposure leading to a decrease in lung function. In addition, elderly individuals might be sensitive to the effects of radiation due to a reduced ability to repair its effects that accompanies aging (Hernandez et al., 2015).
The effects of environmental ambient and indoor PRγ in COPD individuals has not been described in the literature, and poorly described in other populations. In the United States, more than one-third of the population lives in regions with high geologic radon potential due mainly to naturally uranium-rich soils. For example, 23% of the Massachusetts population lives in locations with the potential for exposure to more than 4 pCi/L of radon, the threshold for mitigation of household levels as a result of lung cancer risk (Gundersen et al., 1992). In a large prospective study from 50 U.S. states, Turner et al. (2012) found a significant positive linear trend in COPD mortality with increasing categories of indoor radon concentrations (HR 1.13 per 100 Bq/m3, 95%CI: 1.05 to 1.21). However, Kreuzer et al. (2013) found no association between cumulative radon exposure and deaths from a COPD cohort of 58,690 former German uranium miners.
Our study has some limitations. Ambient gamma data from EPA Radnet are recorded in one single Boston area station, and exposures were estimated by adjustment for exposure to background terrestrial and cosmic radiation. We also estimated the indoor PRγi using the ratio of the indoor-to-outdoor sulfur in PM2.5 as a proxy for infiltration of ambient radionuclide-associated particles. All these procedures may have resulted in an imprecise assessment of exposure, reducing our ability to assess the effects of ambient and indoor PRγi exposures on the decreased lung function in our cohort. In addition, most of the individuals with COPD were Caucasian male veterans receiving care at VA Boston, which may not be representative of PRγ effects in all COPD patients.
5. Conclusion
We found that short-term exposures to environmental gamma radiation associated with ambient particles are associated with reduced pre- and post-bronchodilator pulmonary function in patients with COPD. In addition to the radiation effects on lung cancer risk described in the literature, our results suggest that low-dose environmental radiation may promote adverse health effects unrelated to cancer. Since 1979, EPA has been responsible for the regulation of the National Emission Standards for Hazardous Air Pollutants, including the exposure to environmental sources of ionizing radiation, to protect people and the environment from its harmful effects. However, there are no assessments of the effects of low-level environmental ionizing radiation (EPA, 2000), which warrants additional investigation.
Supplementary Material
Acknowledgements
The authors would like to thank the study participants for their dedicated participation. The authors would like to thank Mike Wolfson, Choong-Min Kang, Denise Lee, Anisa Khadraoui, and Daniel Bernard for their assistance in collecting and managing data for this study.
Supported by NIH NIEHS R01 ES019853 and by resources and the use of facilities at the VA Boston Healthcare System. The views expressed in this article are those of the authors and do not reflect the policy of the Department of Veterans Affairs or the United States government.
This publication was made possible by U.S. EPA grant RD-83479801 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflicts of interest
The authors declare that there are no financial conflicts of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.envres.2019.05.032.
References
- Barbosa-Lorenzo R, et al. , 2017. Residential radon and COPD. An ecological study in Galicia, Spain. Int. J. Radiat. Biol. 93 (2), 222–230. [DOI] [PubMed] [Google Scholar]
- Baskaran M, 2016. Radon: A Tracer for Geological, Geophysical and Geochemical Studies Springer Geochemistry (Text book). [Google Scholar]
- Bloemsma LD, Hoek G, Smit LA, 2016. Panel studies of air pollution in patients with copd: Systematic review and meta-analysis. Environ. Res. 151, 458–468. [DOI] [PubMed] [Google Scholar]
- Bruce N, Perez-Padilla R, Albalak R, 2000. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull. World Health Organ. 78, 1078–1092. [PMC free article] [PubMed] [Google Scholar]
- Cardarelli J II, Thomas M, Curry T, 2010. Environmental Protection Agency (EPA) Airborne Gamma Spectrometry System for Environmental and Emergency Response Surveys Imaging Spectrometry XV. International Society for Optics and Photonics, pp. 781205. [Google Scholar]
- Dagle GE, Sanders CL, 1984. Radionuclide injury to the lung. Environ. Health Perspect. 55, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA report, 1972. Natural Radiation Exposure in the United States.
- EPA Report, 2005. Expansion and Upgrade of the Radnet Air Monitoring Network, vols. 1 and 2 (Concept and plan).
- EPA Report, 2009. Radiological Laboratory Sample Analysis Guide for Incidents of National Significance - Radionuclides in Air.
- EPA Report, 2000. Radiation protection at EPA-The first 30 years. Off. Radiat. Indoor Air 1–84. [Google Scholar]
- Garshick E, Grady ST, Hart JE, Coull BA, Schwartz JD, Laden F, et al. , 2018. Indoor black carbon and biomarkers of systemic inflammation and endothelial activation in COPD patients. Environ. Res. 165, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady ST, Koutrakis P, Hart JE, Coull BA, Schwartz J, Laden F, et al. , 2018. Indoor black carbon of outdoor origin and oxidative stress biomarkers in patients with chronic obstructive pulmonary disease. Environ. Int. 115, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen LC, Schumann RR, Otton JK, Dubiel RF, Owen DE, Dickinson KA, 1992. Geology of radon in the United States. Geol. Soc. Am. Spec. Pap. 271, 1–6. [Google Scholar]
- Hart JE, Grady ST, Laden F, Coull BA, Koutrakis P, Schwartz JD, et al. , 2018. Effects of indoor and ambient black carbon and PM2.5 on pulmonary function among individuals with COPD. Environ. Health Perspect. 126 (12), 127008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, et al. , 2015. Aging and radiation: bad companions. Aging Cell 14, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cadena L, et al. , 2009. Increased levels of outdoor air pollutants are associated with reduced bronchodilation in children with asthma. Chest 136 (6), 1529–1536. [DOI] [PubMed] [Google Scholar]
- Hunter N, Muirhead CR, 2009. Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. J. Radiol. Prot. 29 (1), 5. [DOI] [PubMed] [Google Scholar]
- Kang CM, Koutrakis P, Suh HH, 2010. Hourly measurements of fine particulate sulfate and carbon aerosols at the Harvard-US environmental protection agency supersite in Boston. J. Air Waste Manag. Assoc. 60 (11), 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Nordio F, Coull BA, Schwartz J, 2014. Predicting spatiotemporal mean air temperature using modis satellite surface temperature measurements across the northeastern USA. Remote Sens. Environ. 150, 132–139. [Google Scholar]
- Kreuzer M, Sogl M, Brtiske I, Mohner M, Nowak D, Schnelzer M, Walsh L, 2013. Silica dust, radon and death from non-malignant respiratory diseases in German uranium miners. Occup. Environ. Med. 70 (12), 869–875. [DOI] [PubMed] [Google Scholar]
- Mannam P, Srivastava A, Sugunaraj JP, Lee PJ, Sauler M, 2014. Oxidants in acute and chronic lung disease. J. Blood Lymph 4,1000128 10.4172/2165-7831.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson JATS, Brusasco V, Burgos F, Casaburi R, Coates A, et al. , 2005. Standardisation of spirometry. Eur. Respir. J. 26 (2), 319–338. [DOI] [PubMed] [Google Scholar]
- Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE, 1999. Alpha particles induce the production of interleukin-8 by human cells. Radiat. Res. 152, 57–63. [PubMed] [Google Scholar]
- Ojovan MI, Lee WE, 2005. An Introduction to Nuclear Waste Immobilization. (Text book). [Google Scholar]
- Ozasa K, Takahashi I, Grant EJ, 2016. Radiation-related risks of non-cancer outcomes in the atomic bomb survivors. Ann. ICRP 45 (l_Suppl. 1), 253–261. [DOI] [PubMed] [Google Scholar]
- Papastefanou C, 2008. Radioactive Aerosols. Radioactivity in the Environment. Text book; (Chapter 2). [Google Scholar]
- Salvi SS, Barnes PJ, 2009. Chronic obstructive pulmonary disease in non-smokers. The Lancet 374 (9691), 733–743. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, et al. , 2002. Using sulfur as a tracer of outdoor fine particulate matter. Environ. Sci. Technol. 36 (24), 5305–5314. [DOI] [PubMed] [Google Scholar]
- Tang CH, Garshick E, Grady S, Coull B, Schwartz J, Koutrakis P, 2018. Development of a modeling approach to estimate indoor-to-outdoor sulfur ratios and predict indoor PM 2.5 and black carbon concentrations for Eastern Massachusetts households. J. Expo. Sci. Environ. Epidemiol. 28 (2), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, Thun MJ, 2012. Radon and COPD mortality in the American cancer society cohort. Eur. Respir. J. 39 (5), 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB, 2015. Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 64 (11), 290–295. [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, et al. , 2001. Radiation risk to low fluences of a particles may be greater than we thought. Proc. Natl. Acad. Sci. Unit. States Am. 98 (25), 14410–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


