Abstract
The aim of the present study was to analyze the acquisition of the differentiated phenotype in the human gastric signet ring cell adenoma cancer KATO-III cell line in vitro. The morphology of KATO-III cells was explored by microcinematography. Different cytokines secreted by both adherent and non-adherent KATO-III cells into medium were observed. The cancer stem cell phenotypes were identified by reverse transcription-quantitative polymerase chain reaction using primers (E-Cad, Slug, Snail, vimentin, NANOG, NESTIN, OCT3/4 and C-X-C motif chemokine receptor 4) or antibodies [cluster of differentiation (CD)90 and CD117] by flow cytometry (FACS). The influence of the induction media for the differentiation of mesenchymal cells was studied through viability and proliferation assays, by evaluating gene expression and the expression of markers via FACS. Cell viability and cell cycle distribution were evaluated following the treatment of KATO-III with acetyl salicylic acid and using the induction media as an inhibitor of epithelial-mesenchymal transition (EMT) and heparanase. A total of 3 phenotypes of KATO-III were observed (adherent, non-adherent and cell cluster), which have internal potential for cell transition into one of the other phenotypes. KATO-III was differentiated into adipocyte-, chondrocyte-, osteocyte- and neurocyte-like cells by the induction media. Identification of the induced cells was conducted using cell dyes. Reduced mRNA expression of EMT-associated molecules, stem cell markers and heparanase was observed with acetyl salicylic acid and induction media. An inhibitory effect of acetyl salicylic acid and the induction media was also noted in regard to cell proliferation. In addition, acetyl salicylic acid induced G0/G1 phase cell cycle arrest in KATO-III cells. In conclusion, the induction of the differentiation of cancer stem cells into non-proliferating cells offers the possibility for novel drug design to overcome the issues associated with metastasis, drug resistance and systemic toxicity with improved therapeutic efficacy.
Keywords: acetyl salicylic acid, differentiation, epithelial-mesenchymal transition, heparanase, KATO-III cell line, Signet ring cell adenocarcinoma
Introduction
Gastric signet ring cell adenocarcinoma (SRCA) is characterized by the presence of isolated or small groups of malignant non-cohesive cells (1). The characteristics of SRCA are its resistance to chemotherapy (2), tissues fibrosis and peritoneal invasion (3). Recently we reported the upregulated expression of heparanase in SRCA, which is involved in the acquisition of the mesenchymal phenotype and tumor cell malignancy (4). In the present study, it was also revealed that the epithelial-mesenchymal transition (EMT) process could be inhibited by suramin, an heparanase inhibitor. A recent study revealed that non-steroid anti-inflammatory drugs such as acetyl salicylic acid inhibit proliferation and induce cell cycle arrest as well as apoptosis in different cancer cells (5). Previously, we reported that an ovarian cancer cell line, OVCAR-3 NIH, expressed both cluster of differentiation (CD)-133 and CD-117 stem cell markers and secreted cytokines implicated in tumor growth and cell differentiation (6). In another previous study, it was indicated that poorly differentiated ovarian cancer cells with a high proliferative index could be transported to other tissues with no proliferation potential (7).
The differentiation of cancer cells to non-proliferated cells is well known. Previously, ~35 years ago, Flynn et al (8) described the use of 13-cis-retinoic acid for the treatment of acute promyelocytic leukemia (APL). Today, recent clinical trials have demonstrated that the majority of patients with APL could be definitively cured by the combination of 2 targeted therapies: Retinoic acid and arsenic (9). APL cells have immature characteristics and can be differentiated to other non-proliferative myelocyte cell lines (10). This example justifies the investigation of differentiation-inducing factors in solid tumor therapy. In a variety of solid tumors, a subpopulation of tumorigenic cells was identified as cancer stem cells (CSCs) (11). These CSCs have the ability to initiate tumor growth in immunocompromised mice (12). CSCs, by giving rise to a large population of differentiated progeny, make up the bulk of the tumor but they lack tumorigenic potential (13). An association between CSC and epithelial-mesenchymal transition (EMT) has been attributed (14) and these CSCs could acquire the differentiated phenotype following cytotoxic chemotherapy (15).
On the basis of the pluripotency of CSC, the aim of the present study was to demonstrate that the gastric cancer cell line, KATO-III, with a high proliferative tendency could be inhibited and differentiated into other cells in vitro using cell-differentiating inductors.
Materials and methods
Cell lines and reagents
The human SRCA cell line used KATO-III was obtained from the American Type Culture Collection (ATCC). The primary drug used, acetyl salicylic acid (Aspegic), was purchased from Sanofi-Aventis.
Cell culture
Cells were cultured in Iscove's modified Dulbecco's medium (IMDM) containing 10% heat-inactivated fetal bovine serum (FBS), 50 µg/ml of streptomycin, 50 IU/ml of penicillin and 2 nM of L-glutamine (Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Cytokine array
The present study examined the supernatant of KATO-III cells grown in serum-free IMDM using a protein cytokine array (RayBio® Human Cytokine Antibody; RayBiotech Life); this technique is based on the principle of the sandwich immunoassay (16). It consists of screening, in duplicate, 174 different membrane-coupled anti-cytokines along with the appropriate controls (experiments were repeated 3 times).
KATO-III cells were incubated in IMDM at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Non-adherent cells (106 cells/ml) from the culture flask were recovered by centrifugation (130 × g), washed with PBS (1X) and then re-suspended in serum-free IMDM. Concurrently, adherent cells from the same flask were washed with PBS (1X) and then incubated in the same conditions as those applied for non-adherent cells.
Following 24 h, the supernatants containing cytokines from adherent and non-adherent cells were retrieved and cytokines were allowed to couple with their specific antibodies previously immobilized on the nitrocellulose membranes. The membranes were saturated for 2 h at room temperature with bovine serum albumin (BSA). Incubation of the array membranes with supernatants was conducted overnight at 4°C using the corresponding antibodies. Following several successive washes, the membranes were incubated in the presence of a mixture of antibodies and anti-cytokines biotinylated antibodies at 4°C overnight. Streptavidin, coupled with horseradish peroxidase (HRP), was added to the membranes for 2 h at room temperature. The presence of the antibody-coupled proteins was evaluated by applying enhanced chemiluminescence (RayBio®) to the membranes, according to the recommendations of the manufacturer. Membranes were then exposed to photosensitive film (Kodak X-OMAT; Kodak).
The intensity of chemiluminescence captured on the photosensitive film was measured and recorded. Once the background noise was removed, the results were expressed as a ratio of chemiluminescence intensity of the experimental vs. control spots. The positive control was considered to be 1; a ratio value <-5 indicated a reduction of the cytokine and a value >+5 indicated an increase in cytokine expression.
RNA isolation, reverse transcription (RT) and quantitative polymerase chain reaction (qPCR)
RNA isolation, RT and qPCR. Total RNA from the cells was extracted using a Qiagen RNeasy Mini kit (Qiagen GmbH) according to the manufacturer's instructions. RNA samples (70 ng/µl) were transcribed to cDNA in a 20-µl volume, using the QuantiTect Reverse Transcription kit (Qiagen GmbH). The mRNA expression levels of the different markers were detected by qPCR with β-actin as the internal reference, using Mesa Blue qPCR Master Mix Plus for SYBR® assay (Eurogentec Ltd.) on the Mastercycler® Realplex2 (Eppendorf). The thermocycling conditions for RT-qPCR were as follows: 95°C for 5 min, followed by 40 cycles of denaturation for 15 sec at 95°C, annealing for 20 sec at 60°C and extension for 20 sec at 72°C. The primer sequences and PCR product size for the target and reference genes are listed in Table SI.
Relative quantification was performed using the comparative quantitative cycle (Cq) method with Realplex software. The mean Cq of triplicate measurements was used to calculate ΔCq as the difference in Cq for the target and internal reference (β-actin) genes. The difference between the ΔCq of the control experiment (KATO-III) and the ΔCq of each sample were calculated to produce ΔΔCq. The fold increase in mRNA was calculated using the 2−ΔΔCq method (17). The PCR products of the cell lines following RT-qPCR were electrophoresed by E-Gel Precast Agarose Electrophoresis System (Invitrogen).
Fluorescence-activated cell sorting (FACS) analysis
Confluent KATO-III cells (0.1×106) were seeded in a 25-cm2 culture flask, followed by 24 h in either control or inductor media (StemPro®Adipogenesis, Chondrogenesis, Osteogenesis Differentiation kit and Neurobasal® medium) for 14 days or with 4.5 mM acetyl salicylic acid for 6 days.
The cells and tumor spheres were dissociated as a single cell suspension, washed with PBS and then labeled with antibodies (10 µl/1×106 cells), including mouse anti-human CD90 (2:100 dilution; cat. no. 559869; BD Biosciences) and CD117 (7:100 dilution; cat. no. 550412; BD Biosciences) at 4°C in the dark for 30 min. The samples (minimum 10,000 cells) were analyzed by flow cytometry (FACSAria II; BD Biosciences).
Cell cycle distribution analysis
Cell cycle distribution was analyzed using the Click-iT™ Plus EdUAlexa Fluor 647™ Flow Cytometry Assay Kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The KATO-III cell line was incubated with IMDM and 5% FBS for 24 h. The following day, the cells were treated with or without 4.5 mM acetyl salicylic acid. Following 4 days of treatment, the cells were trypsinized, harvested and incubated in culture medium with 15 µ MEdU for 2 h. After incubation, the cells were washed with 1% BSA in PBS and 100 µl of Click-iT fixative was added for 15 min at room temperature. Following washing, the cells were incubated with the Click-iT Plus reaction cocktail including fluorescent dye (Alexa Fluor 647 picolylazide) for 30 min. The flow cytometric analysis was performed using a BD LSR II analytical flow cytometer (BD Biosciences). MultiCycle AV (Phoenix Flow Systems) DNA analysis software enabled the determination of the phase of cell cycle arrest by comparing the percentages of each cell stage (G1, S and G2/M) between the control and treatment groups.
Cell viability assay
Cell viability was assayed by a RealTimeGlo™ kit containing the MT Cell Viability Substrate and the NanoLuc® Enzyme from Promega Corp. Briefly, confluent KATO-III cells (3×103/well) were seeded on 96-well plates, and then 24 h later in either control or conditional medium with acetyl salicylic acid or inductor media (StemPro®Adipogenesis, Chondrogenesis, Osteogenesis Differentiation Kit and Neurobasal® medium).
Reagents were added directly to the cell culture and the bioluminescence was measured at different time intervals (0, 12, 24, 48, 72 and 96 h) with a Xenius XC spectrofluorometer (SAFAS, Monaco). Cell viability was expressed as the percentage of the absorbance of the drug-treated cells relative to that of the vehicle-treated cells. Each condition was tested in triplicate. The results are representative of 3 independent experiments.
Differentiation of the KATO-III cell line
To induce adipogenic, chondrogenic, osteogenic and neurogenic differentiation, confluent KATO-III cells were incubated for 14 days with the StemPro®Adipogenesis, Chondrogenesis, Osteogenesis Differentiation kit (Gibco; Thermo Fisher Scientific, Inc.) and Neurobasal® medium (Thermo Fisher Scientific, Inc.). All induced cells were fixed for 30 min in 4% paraformaldehyde at room temperature and washed with PBS. For the assessment of calcium deposition in induced osteocytes, cells were treated with 2% Alizarin Red S solution (pH 4.2) for 2–3 min. The induced chondrocyte aggregates were stained with 1% Alcian Blue solution prepared in 0.1 N HCl for 30 min and rinsed with distilled water to neutralize the acidity. The induced adipocytes were incubated with 60% isopropanol for 5 min and then stained with 60% Oil Red O (0.3 g/ml isopropanol) in distilled water for 5 min. The induced neurocytes were stained with cresyl violet solution (0.5 g cresyl violet in 100 ml of 0.6% glacial acetic acid) for 30 min. An inverted microscope was used to image of all stained cells.
Statistical analysis
Data were analyzed using Student's t test and one- or two-way analysis of variance (ANOVA), followed by post-hoc Bonferroni's multiple comparison test when appropriated. Calculations were performed using commercial software (GraphPad Prism version 7.01 for Windows; GraphPad Software, www.graphpad.com). A two-sided P<0.05 was retained for statistical significance.
Results
KATO-III cell line in vitro
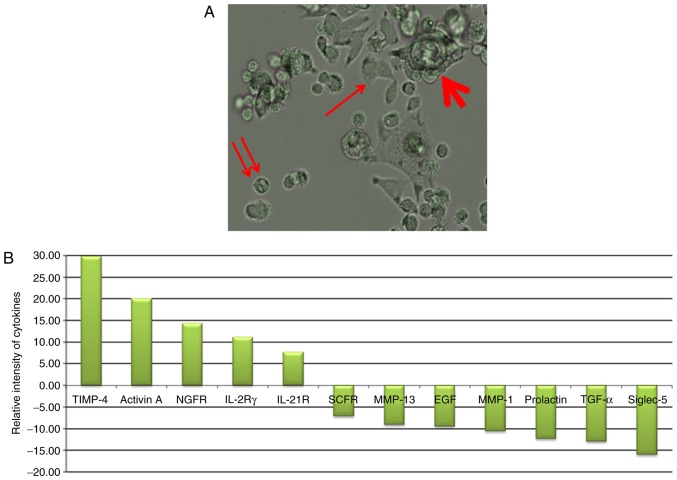
As presented in Fig. 1A, the gastric cancer cell line KATO-III was observed in 3 forms in the culture medium. Adherent cells (indicated by an arrow), non-adherent cells (indicated by a double arrow) and cell clusters (indicated by a bold arrow; some were also non-adherent) were observed. When these cells (adherent, and combined isolated and cluster forms of non-adherent single) were grown separately in culture medium, they subsequently generated other forms. These results are indicative of the internal potential of cell transition in conditional medium. The profile of cytokines secreted under each condition is presented in Fig. 1B. The results revealed the high secretion of tissue inhibitor of metalloproteinase-4 (TIMP-4), Activin A, nerve growth factor receptor (NGFR), interleukin (IL)-2Rγ and IL-21R in adherent cells and stem cell factor receptor (SCFR), matrix metalloproteinase (MMP)-13, epidermal growth factor (EGF), MMP-1, Prolactin, transforming growth factor (TGF)-α, and sialic acid binding immunoglobulin-like lectin-5 (Siglec-5) in non-adherent conditions.
Figure 1.
Spheroid cluster formation and significantly different expression ratio of cytokines (adherent/non-adherent) in the KATO-III cell line (fold-change >5; P<0.05). (A) KATO-III cells form adherent (arrow), non-adherent (double arrow) and cell clusters (bold arrow; some were also non-adherent. (B) Of the 174 proteins proposed in this test only 12 cytokines were reported to be significant in the ratio of cytokine expression in adherent/non-adherent KATO-III cell line. P<0.05.
KATO-III cells present CSC as well as EMT markers
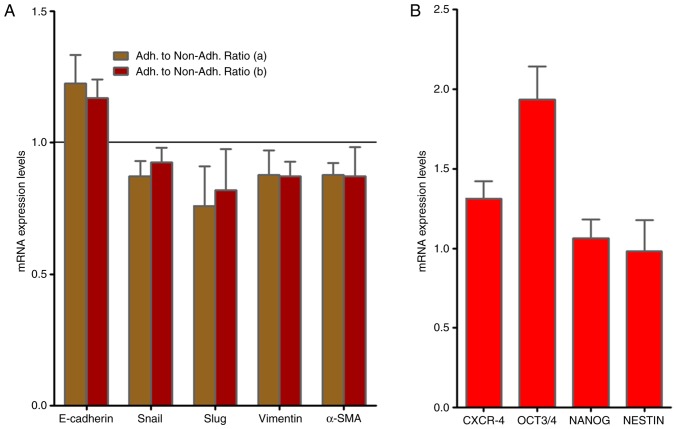
As presented in Fig. 2A, RT-qPCR analysis demonstrated no alterations in the gene expression of the EMT-associated molecules, Slug, vimentin and α-smooth muscle actin (SMA) mRNAs when adherent and non-adherent KATO-III cells were grown separately for 1 week. By contrast, the presence of the CSC markers C-X-C chemokine receptor type 4 (CXCR-4), octamer-binding transcription factor (OCT)-3/4 as well as NANOG and NESTIN were confirmed in KATO-III cells as revealed in Fig. 2B.
Figure 2.
mRNA gene expression of EMT-associated genes and stem cell markers in the KATO-III cell line. (A) RT-qPCR analysis revealed no alterations in the gene expression of EMT-associated molecules when adherent and non-adherent KATO-III cells were grown separately (a) prior to and (b) following 1 week (P<0.05). (B) mRNA gene expression of stem cell markers (CXCR-4, NANOG, OCT3/4 and NESTIN) in the KATO-III cell line via RT-qPCR. EMT, epithelial-mesenchymal transition; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CXCR-4, C-X-C chemokine receptor type 4; OCT3/4, octamer-binding transcription factor 3/4.
Acetyl salicylic acid modifies heparanase, EMT and CSC marker expression in KATO-III cells in vitro
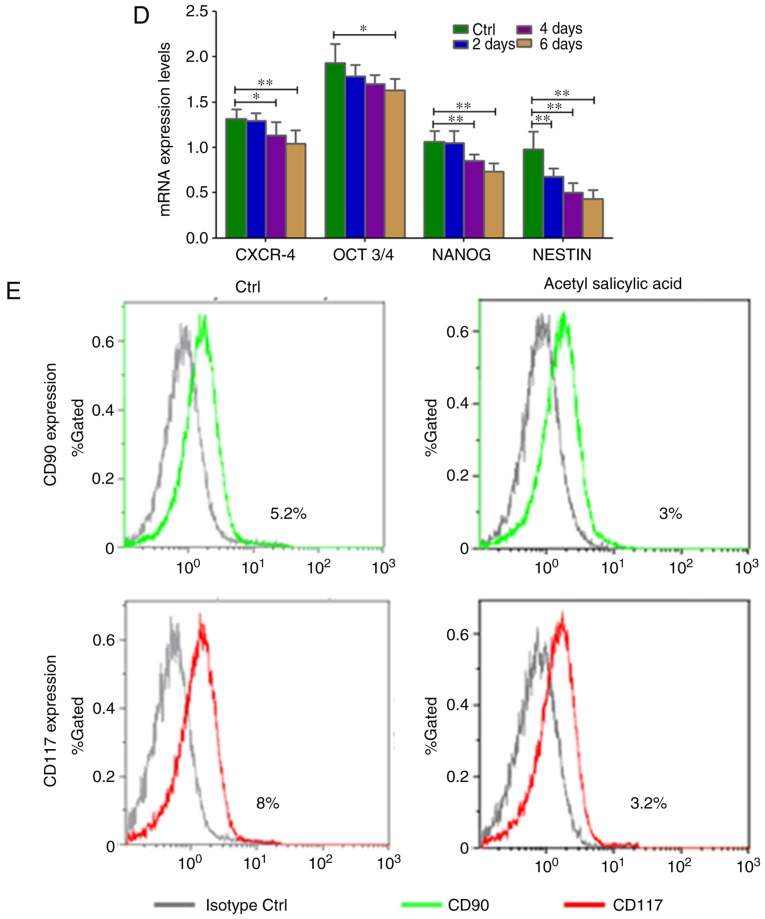
When KATO-III cells were incubated (2, 4 and 6 days) with acetyl salicylic acid (4.5 mM), their morphology gradually changed (Fig. S1). Inhibition of cell proliferation (P=0.002; Fig. 3A) and significant downregulation of heparinase (P=0.0289) expression in the SRCA cell line was observed in a time-dependent manner (Fig. 3B). Mesenchymal markers such as Slug, vimentin and α-SMA (P=0.004) were downregulated while E-cadherin was upregulated (P=0.004; Fig. 3C). Acetyl salicylic acid also decreased the expression of the stem cell markers, CXCR4, OCT3/4, NANOG and NESTIN (P=0.004; Fig. 3D), and CD90 and CD117 (Fig. 3E). This phenomenon was associated with the inhibition of cell proliferation as well as EMT inhibition via the downregulation of heparanase and CSC markers.
Figure 3.
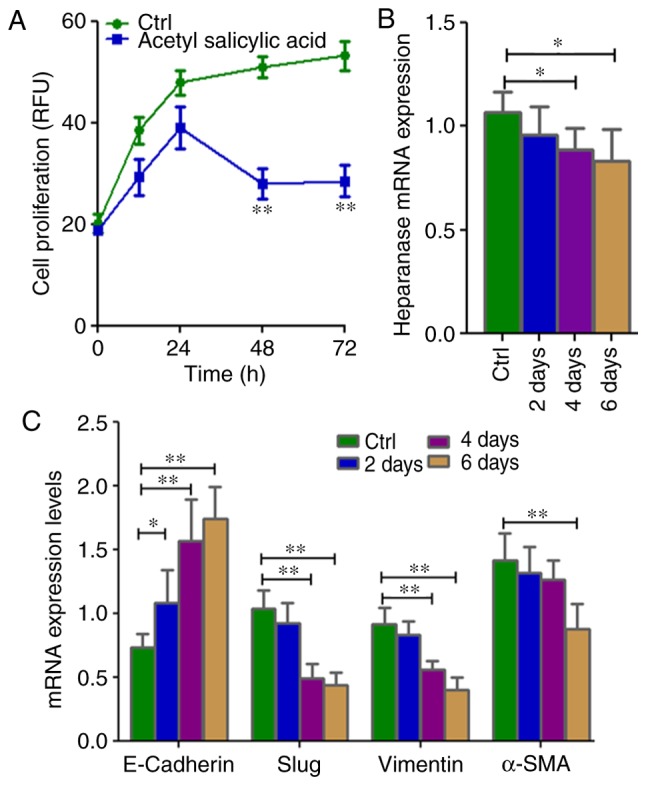
Cell proliferation as well as mRNA expression of EMT-associated genes and stem cell markers in KATO-III following treatment with 4.5 mM acetyl salicylic acid for 6 days. (A) Cell proliferation is inhibited, and (B) the expression of heparanase, (C) mesenchymal markers (Slug, vimentin and α-SMA) and (D) stem cell markers CXCR-4, OCT3/4, NANOG and NESTIN as well as (E) CD90 and CD117 were lower, while those of (C) the epithelial marker E-cadherin was higher in KATO-III cells following treatment with acetyl salicylic acid as determined by reverse transcription-quantitative polymerase chain reaction and flow cytometry. (*P<0.05 and **P<0.01). EMT, epithelial-mesenchymal transition; α-SMA, α-smooth muscle actin; CXCR-4, C-X-C chemokine receptor type 4; OCT3/4, octamer-binding transcription factor 3/4; CD, cluster of differentiation.
Acetyl salicylic acid modifies the cell cycle in KATO-III cells
Flow cytometry was used for cell cycle analysis as revealed in Fig. 4A. The majority of the cells presented an increase in the number of cells in the G0-G1 phase (20%). By contrast, this phenomenon was associated with a reduced number of cells in the S phase (reduction of 38% when compared with the control; Fig. 4B). These results indicated that acetyl salicylic acid via the G0-G1 phase pathway inhibits cancer cell proliferation.
Figure 4.
Cell cycle arrest of the KATO-III cell line via flow cytometry following treatment with 4.5 mM acetyl salicylic acid for 4 days. KATO-III cells were analyzed by flow cytometry for cell cycle distribution following treatment with 4.5 mM acetyl salicylic acid for 4 days. (A) G0/G1, S and G2/M phase cells presented as percentages. (B) Data are expressed as the mean ± standard error of the mean of at least 3 independent experiments.
Cell inducer differentiation media downregulates heparanase and stem cell marker expression as well as the inhibition of cell proliferation
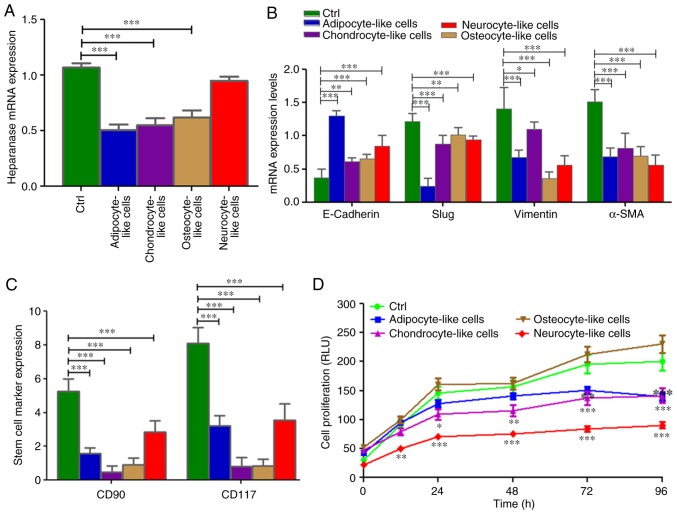
When the SRCA cell line was incubated with different culture media targeting stem cell differentiation in adipocytes, chondrocytes, osteocytes and neuronal cells, the expression of heparanase significantly decreased as observed in adipocytes (P=0.005), chondrocytes (P=0.004) and osteocytes (P=0.004; Fig. 5A). This phenomenon was associated with a high expression of E-cadherin (P=0.004) and the downregulation (P=0.005) of mesenchymal markers such as Slug, vimentin and α-SMA mRNAs (Fig. 5B). By contrast, the conditioned media, except osteocyte-inducer medium, induced the inhibition of KATO-III cell proliferation (P=0.002; Fig. 5D). This inhibition was associated with the downregulation (P=0.004) of the stem cell markers CD90 and CD117 (Fig. 5C).
Figure 5.
mRNA expression of heparanase and EMT markers via reverse transcription-quantitative polymerase chain reaction and stem cell marker expression via flow cytometry as well as cell proliferation in differentiated KATO-III cells following induction of (A) heparanase, (C) stem cell markers (CD90 and CD117) and (B) mesenchymal markers (Slug, Vimentin and α-SMA) were reduced while the epithelial marker (E-cadherin) was increased following the induction of differentiation. (D) Cell proliferation was also significantly inhibited. One- and two-way analysis of variance (ANOVA), followed by post-hoc Bonferroni's multiple comparison test for gene expression and cell proliferation respectively (*P<0.05, **P<0.01 and ***P<0.001). EMT, epithelial-mesenchymal transition; CD, cluster of differentiation; α-SMA, α-smooth muscle actin. ANOVA, analysis of variance.
Cell-inducer differentiation medium induces cancer cell differentiation
Adipogenic, chondrogenic, osteogenic and neurogenic differentiation of the KATO-III cell line was confirmed (Fig. 6) by the visualization of intracytoplasmic lipid drops stained red using Oil Red O (Fig. 6A); a blue coloration was observed due to proteoglycan synthesis using Alcian blue (Fig. 6B), a red coloration was observed due to extracellular calcium deposits using Alizarin Red S (Fig. 6C) and dark black-violet was observed due to the extensive somata-associated accumulation of Nissl bodies, respectively (Fig. 6D). These results confirmed the influence of selective medium on the mesenchymal characteristics of the KATO-III cell line.
Figure 6.
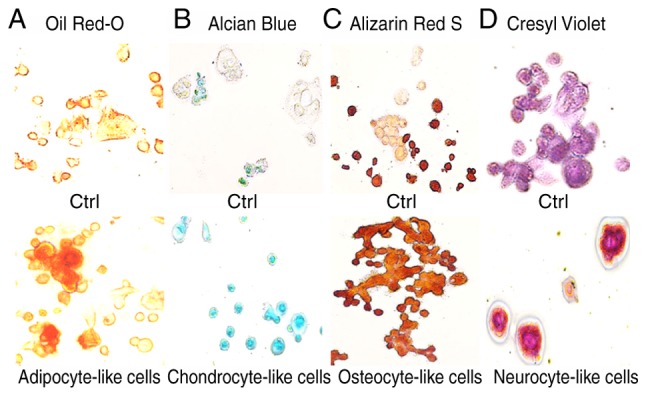
Coloration of differentiated KATO-III cells following induction. (A) Adipogenic, (B) chondrogenic, (C) osteogenic and (D) neurogenic differentiation of KATO-III cells as confirmed by coloration.
Discussion
Apart from elimination therapies that increase the efficacy of cancer treatments, another method to control tumor progression is to induce the differentiation of CSCs (18). In the present study, the differentiation of gastric SRCA cells (KATO-III) were targeted by downregulating EMT and CSC markers. The results revealed that KATO-III cells exhibited 3 phenotypes (adherent, non-adherent and spheroid cluster forms) that are capable of conversion between 2 distinct forms (adherent and non-adherent), a transition we refer to as reversible adaptive plasticity. In addition, no alteration in the RNA expression of EMT-associated molecules was demonstrated via qPCR when grown separately for 1 week. These results are consistent with those of She et al (19) who reported no difference in tumorigenicity in vivo when the side and non-side population of the KATO-III cell line were injected subcutaneously in nude mice.
The present study identified major cytokines significantly secreted by non-adherent cells, including Siglec-5, TGF-α, Prolactin, MMP-1, EGF, MMP-13 and SCFR while those of the adherent cells were TIMP-4, Activin A, NGFR, IL-2Rγ and IL-21R. There is evidence that Siglec-5 is involved in cell-cell interactions and tumor dissemination (20,21), MMP-1 and MMP-13 are implicated in the regulation of extracellular matrix degradation, prolactin was revealed to induce estrogen responsiveness in breast cancer cells (22), TGF-α is implicated in the regulation of cell proliferation and migration through the activation of multiple pathways (23) and EGF and SCFR are thought to be involved in cell differentiation (24,25) indicating that tumor dissemination and cell proliferation may be due to the non-adherent cells of KATO-III. Recent studies have also demonstrated that TIMP-4, as an anti-metalloproteinase, was implicated in cell adhesion (26), Activin A was involved in the regulation of progesterone1 and estradiol production in JEG-3 (27) cells as well as the differentiation of granulosa cells via the activation of steroid hormone receptor (28), while NGFR was a differentiating factor for nerve cells (29) and IL-2 served an adjunctive role in IL-21-induced B-cell differentiation (30), suggesting that cell adhesion and differentiation are characteristics of KATO-III adherent cells. The present study also reported estrogen and progesterone receptors in KATO-III cells (data not shown).
The present study also identified the CSC phenotypes of Slug, Snail, vimentin, NANOG, NESTIN, OCT3/4 and CXCR-4. Recent studies have suggested that these CSCs are long-lived, and display quiescent potentials in a dormant state, and are responsible for angiogenic induction, cell proliferation, apoptotic resistance, self-renewal and differentiation (31,32). In the present study, to the best of our knowledge, it was revealed for the first time in a human SRCA cell line (KATO-III) that gastric cancer stem cells exist in the CD90- and CD117-positive fraction that had potential to differentiate into adipocyte, chondrocyte-, osteocyte- and neurocyte-like cells.
The present results revealed that KATO-III differentiated into adipocyte-, chondrocyte, osteocyte and neurocyte-like cells using inducer media. The inhibitory effect of inducer media on KATO-III proliferation, EMT and stem cell marker expression was observed. This inducing media also significantly downregulated the expression of heparanase. This research is consistent with that of Masola et al (33) on the heparanase-mediated EMT of renal tubular cells. Epidemiological evidence has indicated the chemopreventive effect of acetyl salicylic acid in cancer treatment however the molecular basis for this effect is not fully known (34,35). Consistently, it has been reported that regular acetyl salicylic acid users have a lower risk of breast (36,37), gallbladder (38), prostate (39), gastric (40,41) and non-small cell lung cancer (42). A growing body of evidence has revealed that acetyl salicylic acid is the inhibitor of the enzyme, heparanase (43). The aim of the present study was also to focus on the regulation of EMT by altering the expression of EMT markers by using acetyl salicylic acid. This was investigated in the KATO-III cell line, which revealed the significant inhibitory effect of acetyl salicylic acid on EMT and heparanase expression as well as cell proliferation. The present results are in agreement with those of Masola et al (44) who reported heparanase involvement in EMT and the cell proliferation of renal tubular cells. The results of the present study demonstrated that acetyl salicylic acid induced a marked degree of inhibition of EMT and proliferation in the KATO-III cell line. Similarly, acetyl salicylic acid also induced G0/G1 cell cycle arrest up to 20% and inhibited the S phase by up to 38% of the cell population. The limitation of this study was the inability to isolate single and cluster forms of non-adherent KATO-III cells independently due to unavailability of a technique. Incorporating new technologies in future may potentially isolate such types of non-adherent cells for further studies at the protein level.
In conclusion, the inducer media had the ability to induce the differentiation of KATO-III cells. Acetyl salicylic acid has been revealed to be effective in the prevention and treatment of EMT-associated metastasis. Therefore, it is important to continue efforts to identify therapies that can treat cancers no longer susceptible to current treatments. The results of the present study provide a basis for the development of more effective treatment strategies for controlling gastric cancer in the future.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SS performed the PCR experiments, the KATO III cell treatment and the flow cytometry. MP interpreted the data for the study. MM substantially contributed to the conception of the study and wrote the study. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endo K, Maehara Y, Ichiyoshi Y, Kusumoto T, Sakaguchi Y, Ohno S, Sugimachi K. Multidrug resistance-associated protein expression in clinical gastric carcinoma. Cancer. 1996;77(Suppl 8):1681–1687. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1681::AID-CNCR39>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::AID-CNCR2820690702>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Fourgeaud C, Derieux S, Mirshahi S, Contant G, Pimpie C, Lo Dico R, Soria J, Pocard M, Mirshahi M. The close relationship between heparanase and epithelial mesenchymal transition in gastric signet-ring cell adenocarcinoma. Oncotarget. 2018;9:33778–33787. doi: 10.18632/oncotarget.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YX, Feng JY, Sun MM, Liu BW, Yang G, Bu YN, Zhao M, Wang TJ, Zhang WY, Yuan HF, Zhang XD. Aspirin inhibits the proliferation of hepatoma cells through controlling GLUT1-mediated glucose metabolism. Acta Pharmacol Sin. 2018;40:122–132. doi: 10.1038/s41401-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducros E, Mirshahi S, Azzazene D, Camilleri-Broët S, Mery E, Al Farsi H, Althawadi H, Besbess S, Chidiac J, Pujade-Lauraine E, et al. Endothelial protein C receptor expressed by ovarian cancer cells as a possible biomarker of cancer onset. Int J Oncol. 2012;41:433–440. doi: 10.3892/ijo.2012.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaci R, Shahid S, Réa L, Philipe B, Amu T, Marc P, Massoud M. Neural signature expressed by cells from ovarian carcinoma (A Case Report) Immunochem Immunopathol. 2015;1:2. doi: 10.4172/2469-9756.1000111. [DOI] [Google Scholar]
- 8.Flynn PJ, Miller WJ, Weisdorf DJ, Arthur DC, Brunning R, Branda RF. Retinoic acid treatment of acute promyelocytic leukemia: In vitro and in vivo observations. Blood. 1983;62:1211–1217. [PubMed] [Google Scholar]
- 9.de Thé H Pandolfi PP, Chen Z. Acute promyelocytic leukemia: A paradigm for oncoprotein-targeted cure. Cancer Cell. 2017;32:552–560. doi: 10.1016/j.ccell.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97:1314–1320. doi: 10.1182/blood.V97.5.1314. [DOI] [PubMed] [Google Scholar]
- 11.Werner S, Stenzl A, Pantel K, Todenhöfer T. Expression of epithelial mesenchymal transition and cancer stem cell markers in circulating tumor cells. Adv Exp Med Biol. 2017;994:205–228. doi: 10.1007/978-3-319-55947-6_11. [DOI] [PubMed] [Google Scholar]
- 12.Zabala M, Lobo NA, Qian D, van Weele LJ, Heiser D, Clarke MF. Cancer Stem Cells. Academic Press; 2016. Overview: Cancer stem cell self-renewal; pp. 25–58. [DOI] [Google Scholar]
- 13.Ginestier C, Wicinski J, Cervera N, Monville F, Finetti P, Bertucci F, Wicha MS, Birnbaum D, Charafe-Jauffret E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297–3302. doi: 10.4161/cc.8.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla E, Moro D, Gazzeri S, Brichon PY, Nagy-Mignotte H, Morel F, Jacrot M, Brambilla C. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J Clin Oncol. 1991;9:50–61. doi: 10.1200/JCO.1991.9.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Azzazene D, Al Thawadi H, Al Farsi H, Besbes S, Geyl C, Mirshahi S, Pardo J, Faussat AM, Jeannette S, Therwath A, et al. Plasma endothelial protein C receptor influences innate immune response in ovarian cancer by decreasing the population of natural killer and TH17 helper cells. Int J Oncol. 2013;43:1011–1018. doi: 10.3892/ijo.2013.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 19.She JJ, Zhang PG, Wang X, Che XM, Wang ZM. Side population cells isolated from KATO III human gastric cancer cell line have cancer stem cell-like characteristics. World J Gastroenterol. 2012;18:4610–4617. doi: 10.3748/wjg.v18.i33.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocker PR. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr Opin Struct Biol. 2002;12:609–615. doi: 10.1016/S0959-440X(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 22.Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor α and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Lv X, Jiang C, Cordes CM, Fu L, Lele SM, Davis JS. Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways. PLoS One. 2012;7:e48299. doi: 10.1371/journal.pone.0048299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barberán S, Cebrià F. The role of the EGFR signaling pathway in stem cell differentiation during planarian regeneration and homeostasis. Semin Cell Dev Biol. 2019;87:45–57. doi: 10.1016/j.semcdb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Pierce LJ, Spangrude GJ. Distinct roles of IL-7 and stem cell factor in the OP9-DL1 T-cell differentiation culture system. Exp Hematol. 2006;34:1730–1740. doi: 10.1016/j.exphem.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni X, Luo S, Minegishi T, Peng C. Activin A in JEG-3 cells: Potential role as an autocrine regulator of steroidogenesis in humans. Biol Reprod. 2000;62:1224–1230. doi: 10.1095/biolreprod62.5.1224. [DOI] [PubMed] [Google Scholar]
- 28.Ueno N, Nishimatsu S, Murakami K. Activin as a cell differentiation factor. Prog Growth Factor Res. 1990;2:113–124. doi: 10.1016/0955-2235(90)90027-H. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, Galson DL, Taichman RS, Zhang J. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res. 2008;6:546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 30.Berglund LJ, Avery DT, Ma CS, Moens L, Deenick EK, Bustamante J, Boisson-Dupuis S, Wong M, Adelstein S, Arkwright PD, et al. IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood. 2013;122:3940–3950. doi: 10.1182/blood-2013-06-506865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: Implications for cancer therapy. Curr Protoc Pharmacol. 2013;14:14–25. doi: 10.1002/0471141755.ph1425s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma. 2005;52:435–440. [PubMed] [Google Scholar]
- 33.Masola V, Zaza G, Granata S, Gambaro G, Onisto M, Lupo A. Everolimus-induced epithelial to mesenchymal transition in immortalized human renal proximal tubular epithelial cells: Key role of heparanase. J Transl Med. 2013;11:292. doi: 10.1186/1479-5876-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed I, Musa MO, Umar A. Effects of acetylsalicylic acid and salicylic acid on the growth of HeLa cervical cancer cells line. Am J Pharm Pharmacol. 2014;1:32–44. [Google Scholar]
- 36.Mangiapane S, Blettner M, Schlattmann P. Aspirin use and breast cancer risk: A meta-analysis and meta-regression of observational studies from 2001 to 2005. Pharmacoepidemiol Drug Saf. 2008;17:115–124. doi: 10.1002/pds.1503. [DOI] [PubMed] [Google Scholar]
- 37.Takkouche B, Regueira-Méndez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: A meta-analysis. J Natl Cancer Inst. 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 38.Liu E, Sakoda LC, Gao YT, Rashid A, Shen MC, Wang BS, Deng J, Han TQ, Zhang BH, Fraumeni JF, Jr, Hsing AW. Aspirin use and risk of biliary tract cancer: A population-based study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005;14:1315–1318. doi: 10.1158/1055-9965.EPI-05-0032. [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006;12:130–135. [PubMed] [Google Scholar]
- 40.Jolly K, Cheng KK, Langman MJ. NSAIDs and gastrointestinal cancer prevention. Drugs. 2002;62:945–956. doi: 10.2165/00003495-200262060-00006. [DOI] [PubMed] [Google Scholar]
- 41.Husain SS, Szabo IL, Tarnawski AS. NSAID inhibition of GI cancer growth: Clinical implications and molecular mechanisms of action. Am J Gastroenterol. 2002;97:542–553. doi: 10.1111/j.1572-0241.2002.05528.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17:148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai X, Yan J, Fu X, Pan Q, Sun D, Xu Y, Wang J, Nie L, Tong L, Shen A, et al. Aspirin inhibits cancer metastasis and angiogenesis via targeting heparanase. Clin Cancer Res. 2017;23:6267–6278. doi: 10.1158/1078-0432.CCR-17-0242. [DOI] [PubMed] [Google Scholar]
- 44.Masola V, Gambaro G, Tibaldi E, Brunati AM, Gastaldello A, D'Angelo A, Onisto M, Lupo A. Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem. 2012;287:1478–1488. doi: 10.1074/jbc.M111.279836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.