Abstract
Background
Peripheral arterial disease (PAD) is known as the greatest risk factor affecting the amputation of diabetic foot. Thus, it is crucial to understand the epidemiology of PAD associated with diabetic foot and the relationship between PTA and amputation in predicting prognosis. However, no such multi-year data are available in Korea. Thus, the purpose of this study was to investigate trends of amputation involving diabetic foot based on vascular interventions for PAD in Korea.
Methods
This study was conducted using six-year data obtained from Health Insurance Review and Assessment Service from January 1, 2011 to December 31, 2016. Our study included data pertaining to diabetic foot, PAD, and vascular intervention codes (percutaneous transluminal angioplasty [PTA, M6597], percutaneous intravascular installation of stent-graft [PIISG, M6605], and percutaneous intravascular atherectomy [PIA, M6620]). We analyzed the number of vascular interventions and minor and major amputations each year. The relationship between annual amputation and vascular intervention was analyzed using χ2 test.
Results
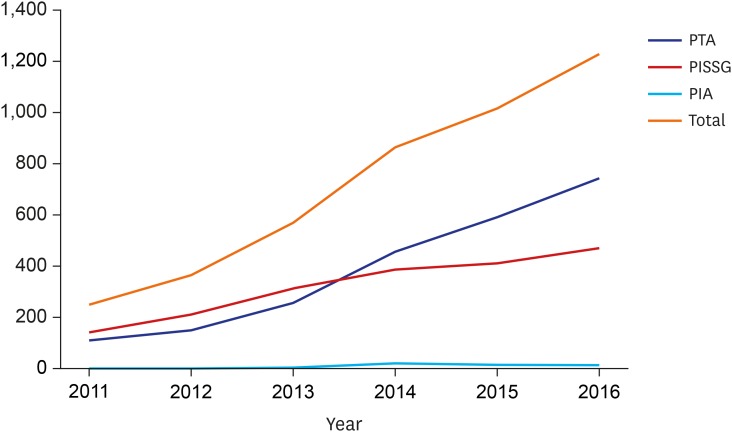
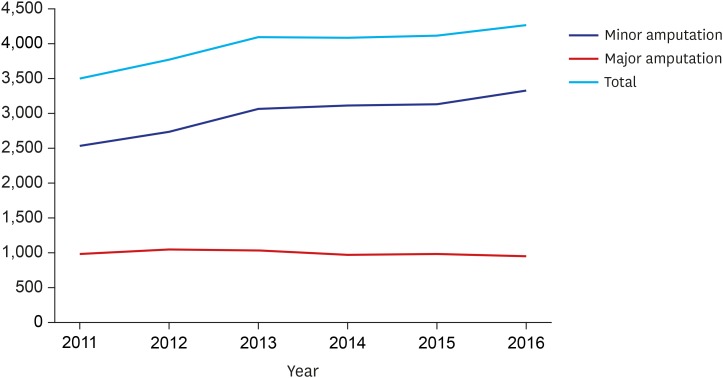
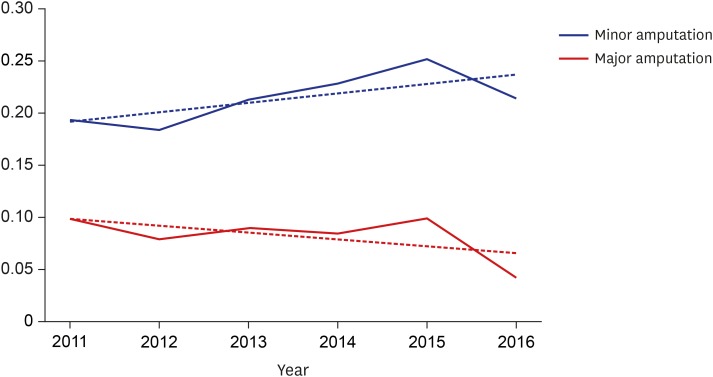
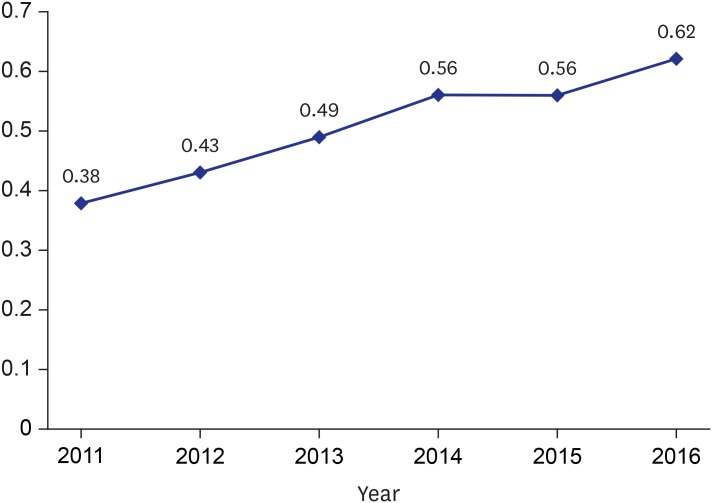
The overall number of vascular interventions increased from 253 (PTA, 111; PIISG, 140; and PIA, 2) in 2011 to 1,230 (PTA, 745; PIISG, 470; and PIA, 15) in 2016. During the same period, the number of minor amputations increased from 2,534 to 3,319 while major amputations decreased from 980 to 956. The proportion of minor amputations among patients who underwent vascular intervention was significantly increased from 19.34% in 2011 to 21.45% in 2016 while the proportion of major amputations among these patients was significantly reduced from 9.88% to 4.27%. In addition, the association between vascular intervention and amputation increased from 0.56 (spearman correlation coefficient) in 2011 to 0.62 in 2016.
Conclusion
In diabetic foot patients, increase in vascular intervention resulted in a change in amputation pattern, showing statistically significant correlation.
Keywords: Diabetic Foot, Peripheral Arterial Disease, Vascular Intervention, Amputation
Graphical Abstract
INTRODUCTION
Diabetic foot is a severe chronic diabetic complication that consists of lesions in deep tissues associated with neurological disorders and peripheral vascular disease in the lower limb.1 Ulceration of diabetic foot is an urgent medical problem. Only two-thirds of foot ulcers eventually heal2,3 and up to 28% may warrant lower extremity amputation.4 It has been reported that every 30 seconds a lower limb is lost somewhere in the world as a consequence of diabetes.5
Peripheral arterial disease (PAD) is known to be a risk factor affecting the amputation of diabetic foot. PAD with the highest severity is associated with a 20-fold higher prevalence of amputation in patients with diabetes.6,7,8 Adequate blood flow is essential for healing and combating severe infections involving diabetic foot. Therefore, appropriate PAD screening and vascular care, especially in patients with diabetes, is important to reduce the risk of amputation.9,10 Percutaneous transluminal angioplasty (PTA) is a commonly used revascularization method with low procedural morbidity and mortality that can reduce the risk.11 In the literature, PTA is routinely indicated as a first-line intervention for revascularization of patients diagnosed with ischemic diabetic ulcers.12 Thus, it is crucial to understand the epidemiology of PAD associated with diabetic foot and the relationship between PTA and amputation in predicting prognosis to establish the health care budget. However, no such multi-year data are available in Korea. Therefore, the purpose of this study was to investigate trends of amputation involving diabetic foot based on vascular interventions for PAD using National Health Insurance Service data provided by the Health Insurance Review and Assessment Service (HIRA) in Korea.
METHODS
Codes that signify diabetic foot directly were started in January 1, 2011 following implementation of the sixth edition of Korean statistical classification of disease and related health problems-6 system (KCD-6). Until year 2010, disease codes indicating diabetic foot gangrene and ulcer were different from diabetes codes. In the absence of appropriate documentation of disease code for foot wound, patients with diabetic foot were missed. Therefore, our study did not investigate data before 2010. We only included data after 2011. Thus, this study was conducted using six-year data obtained from HIRA from January 1, 2011 to December 31, 2016. Additionally, one year of wash-out period was set in order to determine the incidence of diabetic foot and PAD. Accordingly, incidence rate of newly diagnosed diabetic foot and PAD patients since 2012 was determined. Diabetic foot and codes for PAD and amputation included in our study are summarized in Tables 1 and 2. We classified amputations involving thigh (N0572) and tibia (N0573) as major amputations while amputations performed on foot (N0574) and phalanx (N0575) were considered as minor amputations. Vascular intervention codes for percutaneous transluminal angioplasty (PTA, M6597), percutaneous intravascular installation of Stent-Graft (PIISG, M6605), and percutaneous intravascular atherectomy (PIA, M6620) were also included in our study. We analyzed the number of vascular interventions and minor and major amputations by year. The relationship between annual amputation and vascular intervention was analyzed using χ2 test. This study used SAS Enterprise Guide ver. 6.1 M1 (SAS Institute Inc., Cary, NC, USA) for all data analyses.
Table 1. Codes included: diagnosis.
| Diagnosis | |
|---|---|
| Diabetic foot | |
| E105: E1050, E1051, E1058 | |
| E107: E1070, E1071, E1072, E1078 | |
| E115: E1150, E1151, E1158 | |
| E117: E1170, E1171, E1172, E1178 | |
| E125: E1250, E1251, E1258 | |
| E127: E1270, E1271, E1272, E1278 | |
| E135: E1350, E1351, E1358 | |
| E137: E1370, E1371, E1372, E1378 | |
| E145: E1450, E1451, E1458 | |
| E147: E1470, E1471, E1472, E1478 | |
| Peripheral arterial disease | |
| I702, I7022, I7023, I7024, I7025, I7029 | |
Table 2. Codes included: procedures.
| Procedures | ||
|---|---|---|
| Vascular intervention | ||
| PTA (M6597) | ||
| PISSG (M6605) | ||
| PIA (M6620) | ||
| Amputation | ||
| Major amputation | ||
| Amputation, thigh (N0572) | ||
| Amputation, tibia (N0573) | ||
| Minor amputation | ||
| Amputation, foot (N0574) | ||
| Amputation, phalanx (N0575) | ||
PTA = percutaneous transluminal angioplasty, PISSG = percutaneous intravascular installation of Stent-Graft), PIA = percutaneous intravascular atherectomy.
Ethics statement
This retrospective study was approved by the Institutional Review Board of Soonchunhyang University Hospital Seoul (approval No. SCHUH 2018-01-007) and informed consent was waived from Institutional Review Board of Soonchunhyang University Hospital Seoul.
RESULTS
From 2011 to 2016, the overall number of vascular interventions increased from 253 (PTA, 111; PIISG, 140; and PIA, 2) to 1,230 (PTA, 745; PIISG, 470; and PIA, 15) (Fig.1). During the same time period, the total number of amputations increased from 3,514 to 4,275. Types of amputation varied. The number of minor amputations increased from 2,534 to 3,319 while the number of major amputations decreased from 980 to 956 (Fig. 2).
Fig. 1. Overall number of vascular interventions.
PTA = percutaneous transluminal angioplasty, PISSG = percutaneous intravascular installation of Stent-Graft), PIA = percutaneous intravascular atherectomy.
Fig. 2. Overall number of amputations.
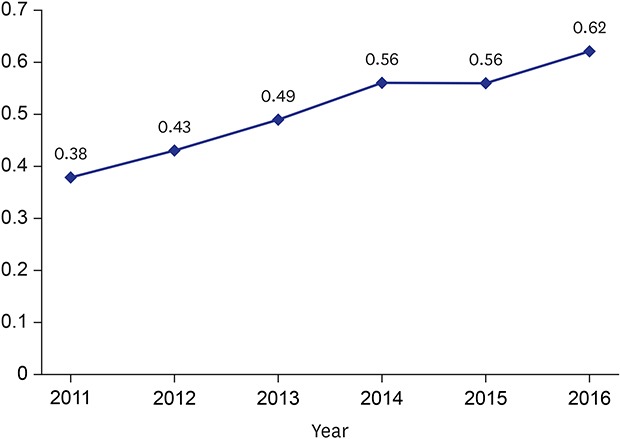
Between 2011 and 2016, the proportion of minor amputations among patients who underwent vascular intervention significantly increased from 19.34% to 21.45% while the proportion of major amputations significantly decreased from 9.88% to 4.27% (Fig. 3). Furthermore, the association between vascular interventions and amputation increased from 0.56 (spearman correlation coefficient) in 2011 to 0.62 in 2016 (Fig. 4).
Fig. 3. Proportions of minor and major amputations among patients who underwent vascular intervention.
Fig. 4. Association between vascular intervention and amputation (Spearman correlation coefficient).
DISCUSSION
To the best of our knowledge, this is the first nationwide analysis focusing on amputation trends in relation to peripheral arterial vascular intervention in Korea. The overall incidence of vascular intervention procedure increased 4.86-fold over a six-year study period. During this time period, a significant increase in minor amputations occurred. It was accompanied by a decrease in major amputation. Similar changes were found in the proportion of amputations among patients who underwent vascular intervention. In addition, the Spearman correlation coefficient demonstrating the association between vascular intervention and amputation was increased.
Previous studies have reported similar results, showing increased use of peripheral endovascular interventions associated with a decrease in the rate of lower limb amputations. For example, from 1996 to 2011, the rate of endovascular interventions increased by 165%, while the rate of lower limb amputations decreased by 45% in the United States.9 Along with increased revascularization rates, major amputation rates decreased from 87.8 to 48.7 while minor amputations increased from 12.3 to 19.6 from 2001 to 2014 in Norway.13 Such results suggest an increase in the rate of endovascular interventions followed by changes in types of amputation, consistent with findings of the current study. Although our observational findings cannot explain causal effect on trends of amputation after PTA, it is clear that PTA has a positive impact on treatment of diabetic foot patients with PAD. Even though PTA in diabetic foot with PAD has progressed to some extent, it may not play a role in preventing amputation itself. However, it plays a role in changing major amputation to minor amputation.
As mentioned earlier, PAD is considered a significant risk factor for diabetic foot. Early screening and vascular care affect disease progression. Pua and Wong14 have reported a healing rate of 66% among patients with pre-existing gangrene and critical limb ischemia after receiving PTA, along with improved hemodynamic parameters. In another study, Gargiulo et al.15 have found that limb salvage and wound healing rates were 92.7% and 74.9%, respectively, during a 12-month follow-up after successful angioplasty of the tibial artery. By improving tissue perfusion, PTA can minimize the range of ischemic tissue, thus affecting the level of amputation. The nationwide trend appears to support such vascular interventions.15
Rapid increase of endovascular interventions is associated with four possibilities.16 First, given the less invasive nature of these newer techniques, the threshold for intervention has decreased. Thus, a group of patients previously untreated with surgical bypass are now candidates for endovascular intervention. Second, due to less durability of endovascular interventions compared to surgical bypass, repeated procedures may be necessary for endovascular interventions to achieve similar results. Third, provider profiles of physicians treating PAD of the lower extremity have increased as vascular surgeons who once only performed surgical therapy now almost universally offer endovascular interventions and bypass surgery. Lastly, competition and variation between treatment providers might also play a role in the expansion of endovascular interventions.
Trends in amputation type discussed in our study played a significant role because minor amputations showed a lower rate of postoperative mortality compared to major amputations. Compared to an overall 5-year mortality rate of 30% to 40% following major amputation, a 27% overall 5-year mortality rate was reported in patients undergoing minor amputation.17 As the majority of patients scheduled for amputation exhibit comorbid conditions such as ischemic heart disease, stroke, and renal impairment, they are vulnerable to operations. Due to its shorter operation time and lower levels of blood loss, a minor amputation may result in positive outcomes postoperatively compared to a major amputation.
This study has a few limitations that need to be addressed. First, diabetic foot and PAD codes are diverse, unclear, and occasionally missing. In addition, there was no defined disease code for diabetic foot until 2010. Therefore, data pertaining to diabetic foot from 2007 to 2010 were excluded. This is considered an inevitable limitation associated with the National Health Insurance Service data provided by HIRA. Diabetic foot codes need more clarification in order to research big data related to diabetic foot in the future for efficient execution of the health care budget. Second, it is common to have at least 2 years of wash-out period when calculating the incidence of chronic diseases such as diabetes and PAD. However, due to limited study period, we used a 1-year wash-out period to calculate the incidence of diabetic foot and PAD. This might have influenced results of this study. Third, we used Spearman correlation to elucidate the direct relationship between trends of amputation and PTA. Although there was a good correlation between trends of amputation and PTA, this study had limitation to explain a causal effect. Future work investigating the causal effect is needed. Fourth, diabetic foot infection was not considered an independent variable in this study because disease code or operational definition for diabetic foot infection was not established in the Big Data. Therefore, in this study, infection was not considered in order to improve quality of the data. Thus, future studies need to consider infection status in diabetic foot through defined operational definition. Nevertheless, the current study is valuable in that it is among the first reports analyzing trends of amputation in relation to peripheral arterial vascular intervention using National Health Insurance Service data provided by the HIRA in Korea.
Trends involving the frequency of peripheral arterial vascular interventions and amputations associated with lower extremities in Korea from 2011 to 2016 were in line with international experience. The Korean population is aging. An increase in patient population with diabetes and peripheral arterial disease is expected. Therefore, evaluation of vascular status of diabetic foot patients represents the first step in minimizing the range of amputations and mobilizing additional treatment resources.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. M20180308941). This work was supported by the Soonchunhyang University Research Fund (2019).
Disclosure: The authors have no potential conflict of interest to disclose.
- Conceptualization: Kim J, Chun DI, Kim S, Won SH.
- Formal analysis: Kim J, Chun DI, Yang HJ, Won SH.
- Investigation: Kim J, Kim S, Cho JH.
- Methodology: Kim S.
- Resources: Yi Y, Kim WJ.
- Software: Kim S.
- Supervision: Chun DI, Yang HJ, Kim JH, Cho JH, Yi Y, Kim WJ, Won SH.
- Visualization: Yang HJ, Kim JH.
- Writing - original draft: Kim J, Won SH.
- Writing - review & editing: Chun DI, Won SH.
References
- 1.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012;41(3):384–397. doi: 10.1007/s12020-012-9619-x. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29(8):1784–1787. doi: 10.2337/dc06-0306. [DOI] [PubMed] [Google Scholar]
- 3.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 7.Won SH, Chung CY, Park MS, Lee T, Sung KH, Lee SY, et al. Risk factors associated with amputation-free survival in patient with diabetic foot ulcers. Yonsei Med J. 2014;55(5):1373–1378. doi: 10.3349/ymj.2014.55.5.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shojaiefard A, Khorgami Z, Larijani B. Independent risk factors for amputation in diabetic foot. Int J Diabetes Dev Ctries. 2008;28(2):32–37. doi: 10.4103/0973-3930.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among medicare patients. JAMA Surg. 2015;150(1):84–86. doi: 10.1001/jamasurg.2014.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolossváry E, Farkas K, Colgan MP, Edmonds M, Fitzgerald HP, Fox M, et al. “No more amputations”: a complex scientific problem and a challenge for effective preventive strategy implementation on vascular field. Int Angiol. 2017;36(2):107–115. doi: 10.23736/S0392-9590.16.03673-7. [DOI] [PubMed] [Google Scholar]
- 11.Papavassiliou VG, Walker SR, Bolia A, Fishwick G, London N. Techniques for the endovascular management of complications following lower limb percutaneous transluminal angioplasty. Eur J Vasc Endovasc Surg. 2003;25(2):125–130. doi: 10.1053/ejvs.2002.1822. [DOI] [PubMed] [Google Scholar]
- 12.Jacqueminet S, Hartemann-Heurtier A, Izzillo R, Cluzel P, Golmard JL, Ha Van G, et al. Percutaneous transluminal angioplasty in severe diabetic foot ischemia: outcomes and prognostic factors. Diabetes Metab. 2005;31(4 Pt 1):370–375. doi: 10.1016/s1262-3636(07)70206-9. [DOI] [PubMed] [Google Scholar]
- 13.Wendt K, Kristiansen R, Krohg-Sørensen K, Gregersen FA, Fosse E. Norwegian trends in numbers of lower extremity revascularisations and amputations including regional trends in endovascular treatments for peripheral arterial disease: a retrospective cross-sectional registry study from 2001 to 2014. BMJ Open. 2017;7(11):e016210. doi: 10.1136/bmjopen-2017-016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pua U, Wong DE. Angioplasty in critical limb ischaemia: one-year limb salvage results. Ann Acad Med Singapore. 2008;37(3):224–229. [PubMed] [Google Scholar]
- 15.Gargiulo M, Maioli F, Ceccacci T, Morselli-Labate AM, Faggioli G, Freyrie A, et al. What's next after optimal infrapopliteal angioplasty? Clinical and ultrasonographic results of a prospective single-center study. J Endovasc Ther. 2008;15(3):363–369. doi: 10.1583/08-2423.1. [DOI] [PubMed] [Google Scholar]
- 16.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50(1):54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Uzzaman MM, Jukaku S, Kambal A, Hussain ST. Assessing the long-term outcomes of minor lower limb amputations: a 5-year study. Angiology. 2011;62(5):365–371. doi: 10.1177/0003319710395558. [DOI] [PubMed] [Google Scholar]