Abstract
Phosphatidylinositol 4, 5-bisphosphate [PI(4,5)P2] is a multi-functional lipid that regulates several essential sub-cellular processes in eukaryotic cells. In addition to its well-established function as a substrate for receptor activated signalling at the plasma membrane (PM), it is now recognized that distinct PI(4,5)P2 pools are present at other organelle membranes. However, a long-standing question that remains unresolved is the mechanism by which a single lipid species, with an invariant functional head group, delivers numerous functions without loss of fidelity. In this review, we summarize studies that have examined the molecular processes that shape the repertoire of PI(4,5)P2 pools in diverse eukaryotes. Collectively, these studies indicate a conserved role for lipid kinase isoforms in generating functionally distinct pools of PI(4,5)P2 in diverse metazoan species. The sophistication underlying the regulation of multiple functions by PI(4,5)P2 is also shaped by mechanisms that regulate its availability to enzymes involved in its metabolism as well as molecular processes that control its diffusion at nanoscales in the PM. Collectively these mechanisms ensure the specificity of PI(4,5)P2 mediated signalling at eukaryotic membranes.
Keywords: PI(4,5)P2 pools; lipid kinase; Phosphoinositides; lipid microdomains; Cell membranes; PLC signalling
Introduction
The evolution of eukaryotic cells was associated with the development of organelles and entailed the need to define and maintain membrane bound compartments with unique chemical identities. Cell membranes are composed of two key components namely proteins and lipids and both of these have been implicated in specifying compartment identity. The membranes of most cellular compartments contain unique proteins and individual members of some protein families show compartment specific distribution. For example, the numerous members of the Rab family of small GTPases show unique distributions across the compartments of a eukaryotic cell[1]. Likewise there are characteristic distribution patterns of lipids across membrane bound compartments [2]. For example, PI(4,5)P2 is enriched at the PM whereas phosphatidylinositol 4 phosphate (PI4P) is enriched at the Golgi.
Lipids are generally classified into two categories, structural and signalling. Signalling lipids are those whose levels change in the space and time domain; they are regarded as carriers of the information content of stimuli that trigger changes in their levels during cell signalling. The phosphorylated derivatives of phosphatidylinositol (PI) (Fig 1A), phosphoinositides are considered to be critical signalling lipids that regulate a variety of functions. PI can be phosphorylated combinatorially at positions 3, 4 and 5 of the inositol head group to generate seven species, PI 3 phosphate (PI3P), PI4P, PI 5 phosphate (PI5P), PI 3,4- bisphosphate [PI(3,4)P2], PI 3,5-bisphosphate [PI(3,5)P2], PI 4,5-bisphosphate [PI(4,5)P2] and PI 3,4,5-trisphosphate [PI(3,4,5)P3]. Each of these lipids shows a unique distribution across membrane compartments and in some senses, are considered to be lipid markers of distinct cellular compartments.
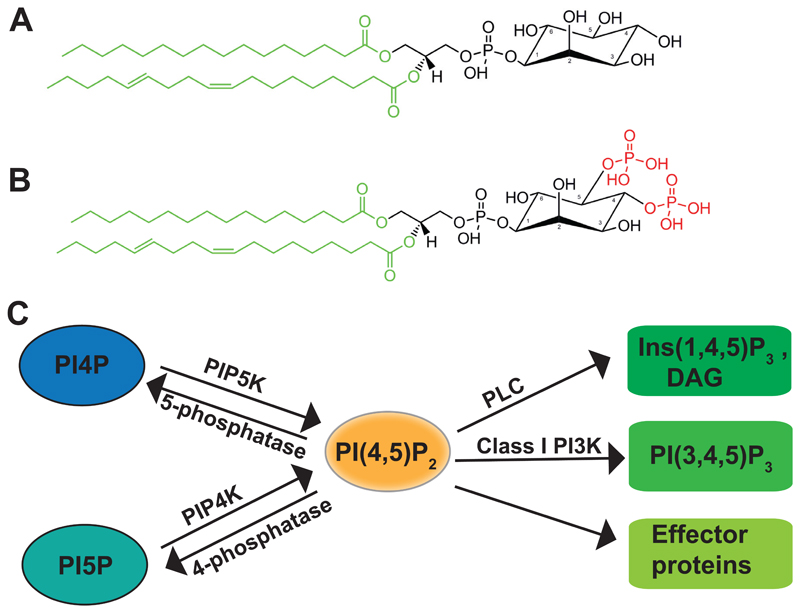
Fig 1. PI(4,5)P2 metabolism pathways.
(A) Chemical structure of a molecule of phosphatidylinositol and (B) its phosphorylated derivative phosphatidylinositol 4,5-bisphosphate. The phosphate groups at positions 4 and 5 of the head-group are indicated in red. The acyl chains at sn-1 and sn-2 position that are potential sources of structural and functional diversity in this lipid species are marked in green. (C) Schematic showing the enzymatic pathways of PI(4,5)P2 metabolism; enzymes with known roles in the metabolism of this lipid are shown. PI4P: phosphatidylinositol 4 phosphate, PI5P: phosphatidylinostiol 5-phosphate, PI(4,5)P2: phosphatidylinositol 4,5-bisphosphate, DAG: diacylglycerol, Ins(1,4,5)P3: inositol 1,4,5-trisphosphate, PI(3,4,5)P3: phosphatidylinositol 3,4,5-trisphosphate. PIP5K: phosphatidylinostiol 4-phosphate 5 kinase, PIP4K: phosphatidylinositol 5-phosphate 4 kinase, PLC: phospholipase C, Class 1 PI3K: Class I phosphatidylinositol 3 kinase.
Of the seven phosphoinositides, PI(4,5)P2 (Fig 1B) is the most abundant species and is thought to be present primarily at the PM. At the PM, PI(4,5)P2 is found on the inner leaflet in relatively low amounts [4000 PI(4,5)P2 molecules/μm2, i.e. 0.1-0.5 % of inner bilayer lipids][3] where it performs numerous functions. These functions can be broadly classified into two groups (a) those that involve the metabolism of PI(4,5)P2 by enzymes such as phospholipase C (PLC), Class I PI 3 kinase (PI3K) and PI(4,5)P2 phosphatase family enzymes (b) other functions that involve the physical interaction of PI(4,5)P2 with PM associated proteins and without breakdown of this lipid. A large number of functions of PI(4,5)P2 at the PM are thought to fall into the latter category; vesicular transport (including both endocytosis and exocytosis) is one such function. Multiple adaptor and accessory proteins required for clathrin mediated endocytosis bind PI(4,5)P2 and mutations which abolish these interactions lead to impairment of endocytosis[4][5][6][7]. The phosphorylated head group of PI(4,5)P2 binds to diverse proteins such as α-actinin, vinculin and profilin[8][9][10] that are known to be regulators of actin dynamics. Additionally, PI(4,5)P2 controls ion channel function; besides regulating channel activation through its derivatives like diacylglycerol (DAG), the binding of PI(4,5)P2 itself to channels like KCNQ, Kir, TRPs as well as the Na+-K+ exchanger is essential for their function [11].
Based on our current understanding of PI turnover, in principle, there are three enzymatic routes by which PI(4,5)P2 can be metabolized in eukaryotic cells (Fig 1C). PI(4,5)P2 is primarily metabolized by the activity of receptor-activated enzymes such as PLC as well as Class I PI3K or dephosphorylated by PI(4,5)P2 phosphatases. Two routes of synthesis are described, namely the kinase activity of PI4P 5 kinase (PIP5K) and PI5P 4 kinase (PIP4K) using PI4P and PI5P respectively as substrates.
PIP4K
PIP4K (also called Type II PIP kinase) was originally cloned as potential isoform of the PIPK that synthesizes PI(4,5)P2 in cells. However in a landmark paper Rameh, et.al showed that in fact, this class of enzyme utilizes PI5P as a substrate to synthesize PI(4,5)P2[12]. This is in contrast to the other, major enzymatic activity (PIP5K) that had been identified as the major source of PI(4,5)P2 synthesis using PI4P as a substrate [13–15]. It is thought that PIP4K activity will most likely generate a quantitatively minor pool of cellular PI(4,5)P2 since both PI4P and PI(4,5)P2 are about 10 times more abundant in cells. Further, in Drosophila [16], C.elegans [17] and mammalian cells [18], loss of PIP4K activity does not result in a reduction in the total cellular PI(4,5)P2 pool but results in an elevation of PI5P levels. Collectively, these observations suggest that a primary function of PIP4Ks might be to regulate intracellular levels of PI5P although they do not exclude a role for these enzymes in regulating a quantitatively minor but functionally important pool of PI(4,5)P2. If PIP4K activity does indeed regulate the generation of a functionally important pool of PI(4,5)P2, it is likely to do so at one of several sub-cellular locations. Irvine and colleagues have studied the localization of tagged versions of each mammalian PIP4K isoform expressed from its endogenous locus. In this setting PIP4K2B is predominantly (>95%) nuclear [19]; PIP4K2A is distributed into a nuclear (40%) and non-nuclear (60%) pool[20] and PIP4K2C is distributed in a vesicular compartment that overlaps with parts of the endosomal system[21]. Gupta et.al have reported that endogenous protein encoded by the single dPIP4K in Drosophila co-fractionates with markers of both the plasma membrane as well as several endosomal compartments [16]. Interestingly biochemical fractionation studies in mammalian cells has suggested that PI5P, the substrate of PIP4K from which it can generate PI(4,5)P2 is mainly present at the plasma membrane but also in the Golgi fraction [22] and also the nucleus [18]. Thus there are multiple locations at which PIP4K can generate a pool of PI(4,5)P2 but the functional significance of the PI(4,5)P2 so produced remains to be established.
PIP5K
It is widely believed that the bulk of cellular PI(4,5)P2 is generated by PIP5K (also called Type I PIPkin). This conclusion is based on the observation that PI4P, the preferred substrate of PIP5K and PI(4,5)P2 are ca.10 times more abundant than PI5P and also early metabolic labeling studies on the route of PI(4,5)P2 synthesis[13–15]. Consistent with this idea, genes encoding PIP5K activity are found in all eukaryotic genomes sequenced to date ranging from unicellular to metazoan organisms. The importance of PIP5K activity in regulating PI(4,5)P2 levels for cellular survival can be judged from genetic analyses in organisms whose genomes contains a single gene for the PIP5K. For example, in the unicellular eukaryote S.cereivisiae, null alleles of Mss4 that encodes the only PIP5K activity are inviable[23]. Likewise, in C.elegans, mutants in the only gene encoding PIP5K activity, ppk-1 are arrested during larval development and the use of RNAi to remove the maternal contribution of ppk-1 in these mutants results in embryos that are not viable[24]. In Drosophila too, null mutants of sktl, a gene encoding a PIP5K that is expressed ubiquitously during development results in lethality during larval stages[25]; presumably development up to this stage is supported by a maternal contribution of sktl RNA as homozygous mutant clones of sktl null alleles at later developmental stages are cell lethal in multiple cell types[25]. These findings imply that the maintenance of PI(4,5)P2 levels in eukaryotic cells is critical for cell survival.
Many subcellular processes that are supported by PI(4,5)P2 operate simultaneously in a typical eukaryotic cell. Even at a single subcellular location such as the PM, there are multiple, ongoing PI(4,5)P2 dependent processes raising the question of how a single lipid species is able to perform these multiple functions simultaneously whilst maintaining signalling fidelity ? The functional consequences of a lack of such specificity is apparent if one asks the question: When cell surface receptors activate PLC (as many do), why does the resulting consumption of PI(4,5)P2 not lead to disruption of ongoing endocytic processes or PM-actin interactions?
Conceptually, a plausible explanation might be that there exist distinct pools of PI(4,5)P2, each of which supports a specific function of this lipid at the PM. In this review, from a functional point of view, a “pool” is defined as a group of lipid molecules that are required to support a specific molecular process in the cell (functional pool). Additionally a “pool” can also refer to a group of molecules that is spatially separated from other molecules of the same class either on another membrane or at a spatially distinct physical location on the same membrane (spatial pool). In the following sections we have discussed the current understanding regarding the generation and maintenance of distinct PI(4,5)P2 pools in cells.
Subcellular localization of PI(4,5)P2 pools
Although it has not been formally demonstrated by biochemical fractionation studies, it is widely accepted that the bulk of cellular PI(4,5)P2 exists at the PM and this idea is supported by the distribution of this lipid as reported by PI(4,5)P2 specific fluorescent reporters[26]. However over the years, several lines of evidence have emerged suggesting that pools of PI(4,5)P2 exist at subcellular locations other than the PM (Fig 2A). Attempts to visualize PI(4,5)P2 using the fluorescent PI(4,5)P2 probe (PLCδ-PH::GFP) and anti-PI(4,5)P2 antibodies have also suggested PI(4,5)P2 pools at non-PM locations. PI(4,5)P2 pools were first visualized using PLCδ-PH::GFP by Varnai et al.[26]; in their study receptor triggered PLC activity was used to deplete PI(4,5)P2 and its resynthesis was followed as a function of time. This study found that the first detectable bright spot of PI(4,5)P2 appears in the perinuclear region and is followed by appearance of PM PI(4,5)P2. In an independent study using immuno-EM labelling of ultrathin sections using the same probe, PI(4,5)P2 was shown to be present in PM, Golgi, rER (rough endoplasmic reticulum), endosomes, mitochondria and nucleus [27]. Another study using an anti-PI(4,5)P2 antibody also suggests that there are non-PM pools of PI(4,5)P2[28]. A second compelling line of evidence is the demonstration that PI phosphate kinases (PIPK), enzymes that mediate the final step of PI(4,5)P2 synthesis are found not only at the PM but also at non-PM locations, suggesting that they mediate synthesis of spatially distinct PI(4,5)P2 pools at the these subcellular locations[29]. Finally, immuno-localization studies have shown that enzymes involved in PI(4,5)P2 degradation such as the PI(4,5)P2 5-phosphatase OCRL (Oculo Cerebro Renal syndrome of Lowe) are reported on internal membrane compartments such as the Golgi [30], endosome [31,32] and lysosomes [33]. Since then, a number of studies have focused on the existence and functions of PI(4,5)P2 in non- PM organelles that are discussed below.
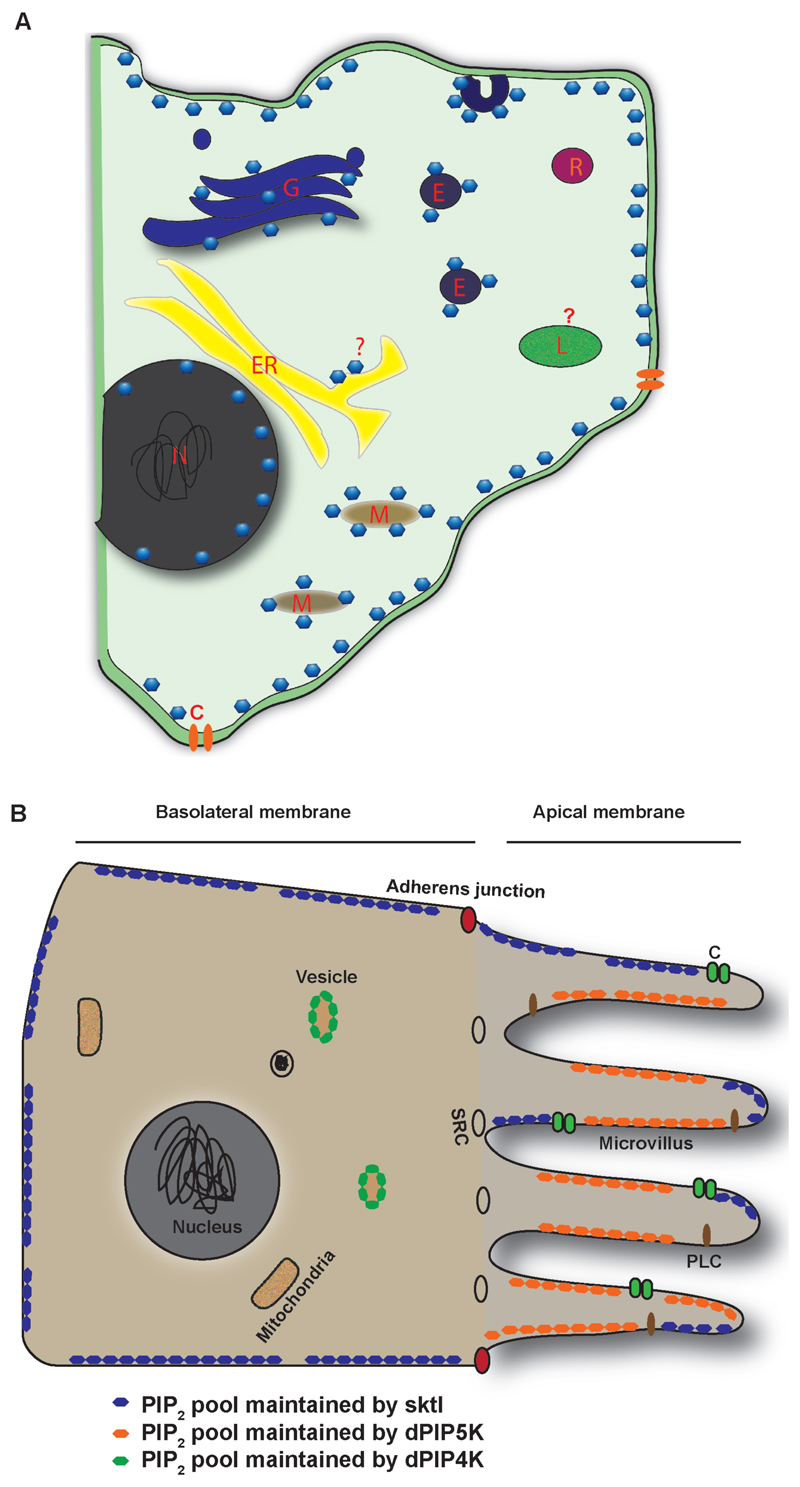
Fig 2. Spatially distributed PI(4,5)2 pools in cells.
A) Schematic diagram of a generic cell showing the distribution of PI(4,5)2 pools in various cellular organelles. PI(4,5)P2 is represented as blue hexagonal structures. Cellular organelles and domains are as marked. N: Nucleus, ER: Endoplasmic reticulum, M: Mitochondria, G: Golgi, L: Lysosome, E: Endosome, R: Recycling endosome, C: Ion channel or transporter.
B) Schematic of the cross section through a Drosophila photoreceptor cell, depicting the pools of PI(4,5)P2 synthesized by distinct PIPKs in various sub-cellular locations. The apical and basolateral domain of the plasma membrane are indicated. The pools of PI(4,5)P2 are represented in different colors. The cellular organelles and domains are as marked. SRC: sub-rhabdomeric cisternae. PLC depict the distribution of the transduction complex and C the channels at the apical plasma membrane of photoreceptors.
Several studies support the existence of nuclear PI(4,5)P2 and an independent PI cycle in nucleus[34]. Biochemical analysis shows that isolated nuclei from rat liver tissue can incorporate γ32P labelled ATP into PIP (phosphatidylinositol phosphate), PI(4,5)P2 and phosphatidic acid (PA) and that a distinct PLC and PIP5K isoform is present in the nucleus[35]. The functions of PI(4,5)P2 in the nucleus have been studied and here this lipid appears to regulate multiple aspects of nuclear function. A recent study has suggested that the transcription factor c-fos can activate nuclear PI(4,5)P2 synthesis via the activity of PIP5K and this in turn regulates transcription[36]. PI(4,5)P2 has also been shown to bind the transcription factor UBF and depletion of PI(4,5)P2 in the nucleus decreases RNA pol I mediated transcription of rRNA[37]. Finally, the specific activity of a novel, non-cannonical polyA RNA polymerase isoform, star-PAP, is shown to be regulated by PI(4,5)P2 [38,39]. Whatever the functions of PI(4,5)P2 within the nucleus might be, a fundamental question that remains to be resolved in the biophysical principles underlying the localization of the lipid within the nucleus. Experiments using conditions that remove the majority of phosphatidylinositol and phosphatidylcholine from nuclei result in ca. 60% of the PI(4,5)P2 being retained in the nucleus[40,41]. Further labeling of nuclei with probes that detect PI(4,5)P2 or immunolocalization of PI4Kβ [42] and PIP5K [38] isoforms suggest that in the nucleus, this lipid is largely localized in nuclear speckles. The mechanism by which PI is made available to enzymes located in nuclear speckles for the synthesis of PI4P and PI(4,5)P2 as well as the biophysical mechanism by which the lipid is retained in speckles remain an interesting and unresolved problem for the future.
PI(4,5)P2 was visualized in Golgi-like perinuclear structures using fluorescent PI(4,5)P2 reporter probes[43] as well as immunogold staining with PI(4,5)P2 antibody. A PIP5K activity that can generate PI(4,5)P2 [44,45] and a Golgi localized PLC that can consume PI(4,5)P2 have also been reported[46]. Blocking PI(4,5)P2 synthesis at the Golgi causes fragmentation of the organelle showing the importance of the PI(4,5)P2 pool at these membranes in maintenance of normal Golgi morphology[47].
The role of PI(4,5)P2 in regulating endocytosis at the PM is well known [48]. However, it is thought that following endocytosis, the PI(4,5)P2 on the early endosome is degraded by the PI(4,5)P2 specific 5-phosphatase OCRL and loss of this enzyme leads to defects in the kinetics of endocytosis [49]. At the PM of synapses, PI(4,5)P2 levels are regulated by a distinct 5-phosphatase synaptojanin that plays a role in regulating endocytosis at the synaptic PM [50]. A recent study by the Anderson group[51] has shown that a unique isoform of PIPK, PIP5Kγi5 co-localizes with early endosome markers in mammalian cells and PI(4,5)P2 produced by PIP5Kγi5 regulates EGFR sorting to lysosomes. Although the PI(4,5)P2 5-phosphatase OCRL has been reported to localize to the lysosome, its function at this location remains unknown [33]. Thus PI(4,5)P2 pools may be present on and function at multiple locations in the endo-lysosomal system.
A limited number of immunogold labeling studies suggest that PI(4,5)P2 is present on mitochondrial membranes[52]. An isoform of the PI(4,5)P2 5-phosphatase synaptojanin has been reported to localize to mitochondria via interaction with a PDZ domain protein in the outer mitochondrial membrane [53] and recently autosomal recessive forms of Parkinson’s disease have been reported to result from mutations in the synaptojanin gene [54–56]. It is also reported that depletion of PI(4,5)P2 in the outer mitochondrial membrane using a phosphatase causes fragmentation and autophagy of mitochondria[57] suggesting that a functional PI(4,5)P2 pool may exist in mitochondria. Although in EM immunogold labelling, PI(4,5)P2 is seen to be present in the rER [52], there is no study that has explored the origin or function of the rER PI(4,5)P2 pool. In summary PI(4,5)P2 pools appear to be present at multiple non-PM locations in cells but their functions remain to be fully understood.
PI(4,5)P2 pools at the PM
Many subcellular processes that require PI(4,5)P2 operate simultaneously at the PM in a typical eukaryotic cell. Thus at this single membrane, PI(4,5)P2 is presumably able to perform multiple functions simultaneously. This raises the question of how fidelity of PI(4,5)P2 dependent functions at the PM is maintained? If distinct pools of PI(4,5)P2 support these functions, how are they generated and maintained in eukaryotic cells ?
The first studies to postulate the existence of multiple pools of PI(4,5)P2 were performed using biochemical approaches [58][59]. Using radiolabelling experiments in human erythrocytes, these authors studied the kinetics of PIP and PI(4,5)P2 labelling following PLC stimulation and postulated that there should be more than one pool of PI(4,5)P2, one that is PLC sensitive and another that is PLC insensitive. From a different perspective, studies across multiple model systems ranging from plants to migrating animal cells report the preferential distribution of PI(4,5)P2 dependent functions to a specific domain of the PM. In plant root cells such specific enrichment of PI(4,5)P2 regulates the localization of PIN proteins, auxin transporters responsible for polarized growth of plant tissue [60], whereas in the developing Drosophila oocyte PI(4,5)P2 regulates the deposition of maternal mRNAs (oskar, bicoid) to specific domain of the cell and controls the positioning of the nucleus in the ovum[61]. In both Drosophila and mammalian cells PI(4,5)P2 has been reported to be enriched at the cytokinesis furrow [62–64]. In addition, studies in MDCK cells have suggested that PI(4,5)P2 is enriched at the apical membrane through the preferential localization of the lipid phosphatase PTEN that converts PI(3,4,5)P3 to PI(4,5)P2 thus actively restricting its substrate from the apical domain [65]. A further study using the polarized cells of the Drosophila trachea as model for tubular organs described PI(4,5)P2 to be specifically enriched in the apical PM[66]. The mechanism of such enrichment is proposed to be the preferential localization of a PIP5K (encoded by skittles) to the apical PM domain[66]. Both these studies provide evidence that spatially restricted transmembrane proteins are mislocalized when this asymmetric PI(4,5)P2 distribution is disturbed. Collectively, such studies suggest that PI(4,5)P2 might not be distributed uniformly at the PM and the domain specific enrichment of PI(4,5)P2 might regulate asymmetric distribution of cellular components and hence their functions.
More recently, several studies have examined the spatial distribution of PI(4,5)P2 at the PM using fluorescent PI(4,5)P2 reporters; these studies suggest a uniform distribution of this lipid along the PM. Thus at the spatial resolution of the light microscope (ca. 250 nm) distinct domains are not apparent; the only reported exception is the case of the T.brucei promastigote where fluorescent reporters of PI(4,5)P2 appear to be concentrated in the flagellar cup[67] with the surrounding PM devoid of labeling; the significance of this observation is unclear. Recently, following technical advances in super resolution microscopy, the distribution of PI(4,5)P2 on the PM (as measured using fluorescent reporters) has been reassessed. Fujita et al.[68] have shown that PI(4,5)P2 is highly enriched at specific domains of the PM. Their study in mouse fibroblast and muscle cells showed that PI(4,5)P2 is enriched at the rim of caveolae and the angiotensin induced reduction in PI(4,5)P2 levels occurred at different rates at distinct domains of the PM. Further in an independent study it was shown that in human monocytes PIP5Kiα is associated with lipid rafts and controls a specific pool of PI(4,5)P2 which is seen as clusters by PI(4,5)P2 antibody labelling[69]. By contrast, a recent study by Ji et al.[70] has suggested that in INS1 cells PI(4,5)P2 is homogeneously distributed at the PM. The reason for these discordant findings is unclear and may be related to varying experimental technique. It should be noted that the distribution of PI(4,5)P2 is very sensitive to the fixation and subsequent processing techniques so the divergent conclusions made by these studies could be the outcome of diverse systems/cell line used and/or distinct processing of samples [28]. In summary, at the resolution of present microscopy techniques, the distribution of PI(4,5)P2 at the PM appears to be largely homogenous with limited evidence for spatially distinct pools. This raises the question of how distinct pools of PI(4,5)P2 that sub-serve multiple functions of this lipid at the PM are defined.
The concept of specialized lipid enriched micro domains in the PM has existed for a number of years. Such domains, referred to in some contexts as lipid rafts have a specific lipid composition and are known to be enriched in sphingolipid and cholesterol[71][72]. One of the many functions attributed to such microdomains is that they represent a platform for the assembly of proteins thus controlling signalling cascades.[73] The presence of saturated sphingolipids makes the raft highly packed and less fluid compared to its surroundings. Many signalling proteins such as G-proteins, MAPK and growth factor receptors are shown to be present in lipid rafts[72]. In the context to this review, PI(4,5)P2, an essential component of many signalling pathways has been shown to be a component of lipid rafts[74][75](Fig 3D). For example, in human NK cells, the presence of PI(4,5)P2 in raft like structures is shown by both imaging approaches and in isolated detergent insoluble membrane fraction[76]. Further, the depletion of cholesterol, an essential component of lipid rafts, is shown to affect the distribution of PI(4,5)P2 and impact receptor activated signalling in cell models[77]. In Drosophila, a study of phototransduction signalling proteins suggests that they are recruited to lipid raft during illumination that triggers PLC based signal transduction in these cells.[78]. However, other studies have suggested that the ability of a receptor to activate PLC mediated PI(4,5)P2 hydrolysis does not depend on its presence in raft like structures.[79] Thus the role of membrane microdomains such as rafts in generating pools of PLC sensitive PI(4,5)P2 remains unresolved and further work is needed to understand the role of microdomains such as rafts in organizing PI(4,5)P2 pools in vivo.
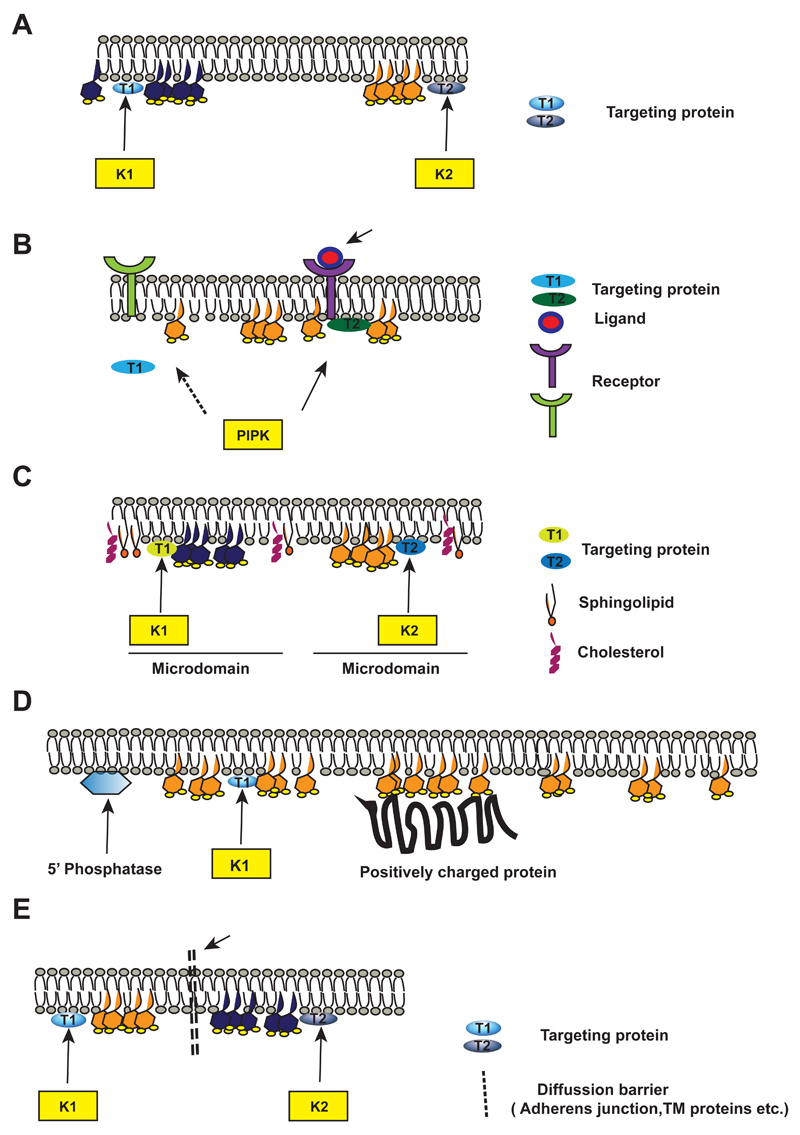
Fig 3. Mechanisms that generate PI(4,5)P2 domains at the plasma membrane.
A) Schematic showing two distinct pools of PI(4,5)P2 (hexagonal structures shaded yellow and grey; generated by two separate lipid kinases (K1 and K2); the size of the PI(4,5)P2 headgroup is not scaled). The lipid kinases generate localized pools of PI(4,5)P2. at the membrane. The PM bilayer lipids are shown. Unique proteins (T1, T2) that target K1 and K2 to the PM are shown.
B) A model showing receptor activated PI(4,5)P2 synthesis. Activation of a receptor through ligand binding recruits PIP5K through a specific effector proteins T2 that mediates the redistribution of PIP5K to the active signalling receptor and localized PI(4,5)P2 synthesis follows.
C) A model depicting two distinct pools of PI(4,5)P2(shaded yellow and grey) generated by K1 and K2 at membrane microdomains such as lipid rafts. The lipid raft region with cholesterol and sphingolipids is shown. The kinases and targeting proteins T1 and T2 are shown.
D) Mechanism to restrict the diffusion of a PI(4,5)P2 pool: A model showing localized PI(4,5)P2 production in the PM by the lipid kinase K1 recruited by effector T1. Positively charged proteins at the adjacent plasma membrane sequester the lipid creating a domain of PI(4,5)P2. The localization of a PI(4,5)P2 5-phosphatase activity that limits the boundary of distribution of PI(4,5)P2 generated by K1 is shown.
E) A model showing two distinct pools of PI(4,5)P2 (shaded yellow and grey) generated by lipid kinases K1 and K2. The kinases are recruited to plasma membrane by targeting proteins T1 and T2. The dotted line represents a diffusion barrier that prevents free mixing of the PI(4,5)P2 pools generated K1 and K2. The diffusion barrier may be formed by for e.g transmembrane proteins or adherens junction proteins.
Mechanisms that maintain distinct PI(4,5)P2 pools
How might PI(4,5)P2 pools be defined at the plasma membrane? In principle, pools of this lipid can be demarcated by a number of overlapping mechanisms: (i) Generating pools of lipid by the activity of distinct lipid kinase isoforms (ii) controlling the accessibility of the lipid to specific PI(4,5)P2 metabolizing enzymes (iii) There might be chemically variant species of PI(4,5)P2 that can be distinguished by cells (iv) spatially confinings the diffusion of the lipid physically to nanoscale domains where a specific function of PI(4,5)P2 occurs. The PM of a single cell may therefore have pools of PI(4,5)P2 that are defined by a combination of these mechanisms, yet experimental approaches to define these pools have been limited.
(i). Role of PIP5K isoforms in generating PI(4,5)P2 pools with distinct functions
In mammalian systems, PIP5K activity is encoded by three distinct genes, each of whom is alternately spliced to generate multiple isoforms. Some of these isoforms are localized to internal membrane compartments [51,80] but a common location at which multiple isoforms are found is the PM[29]. In some cases, such as the splice variants of mouse PIP5Kγ, individual splice variants are also enriched at specific sites such as adherens junctions in epithelial cells and in focal adhesions via interaction with talin [81][82]. All of these splice variants contain the core PIP5K catalytic domain but it is likely that divergent sequences in other domains of the protein may confer unique modes of subcellular localization and regulation. A recent study compared the effect of ablation of all the isoforms of mouse PIP5Kγ against ablation of only one specific isoform [83]. This study found a full range of phenotypes when all isoforms were ablated resulting in perinatal lethality while ablation of the PIPKIγi2 isoform alone results in viable and fertile mice with a restricted phenotype i.e., altered responses of T-cells to LFA mediated adhesion. Likewise a study comparing Ca2+ signalling in mast cells found that while loss of PIP5Kγ affected this process, inhibition of PIP5Kβ did not impact this process[84]. These studies underscore the importance of specific PIP5K isoforms in generating PI(4,5)P2 required to support individual subcellular processes.
A recent study in Drosophila photoreceptors has examined the dependence of multiple PI(4,5)P2 dependent functions in this cell type [85] on specific PIP5K activity. These authors examined the regulation of PI(4,5)P2 required for the robust light-induced PLC activity seen in photoreceptors as well as those PI(4,5)P2 dependent functions that do not require PLC mediated hydrolysis of this lipid. Photoreceptors express PIP5K enzymes encoded by two separate genes, sktl and dPIP5K. While dPIP5K was found localized only to the apical photosensitive PM, SKTL is present on both the apical and basolateral PM (Fig 2B). Thus, in this cell type the apical PM contains two PIP5K enzymes, SKTL and dPIP5K. Loss of sktl results in cell-lethality underscoring the importance of the PI(4,5)P2 pool produced by this enzyme for cell viability. Although sktl null mutants have an intact dPIP5K gene, clearly PI(4,5)P2 generated by dPIP5K activity cannot compensate for or is not available to do so in the absence of the pool produced by SKTL. Conversely, in dPIP5K mutants, while phototransduction is affected, other PI(4,5)P2 dependent functions are normal implying that the pools of lipid used for processes such as endocytosis and cytoskeletal function are not dependent on dPIP5K activity. Conversely although a normal sktl gene is present in dPIP5K mutants, clearly it is unable to fully compensate with regard to providing the PI(4,5)P2 pool required for a normal light response. Collectively, these observations imply that at the microvillar PM of photoreceptors, SKTL and dPIP5K generate functionally distinct pools of PI(4,5)P2. A previous study had found that overexpression of SKTL during pupal development blocked the biogenesis of the apical membrane in photoreceptors in a kinase dependent manner [86] whereas overexpression of dPIP5K did not affect apical domain morphogenesis [Kolay & Padinjat, unpublished]. Interestingly, a previous study in mammalian cells found that depleting PM PI(4,5)P2 using an ectopically expressed PI(4,5)P2 5 - phosphatase domain[87] impacts multiple PI(4,5)P2 functions including Ca2+ influx, TRPM channel activation and receptor internalization. This observation is in sharp contrast to the effect of losing a specific PIP5K and underscores the importance of PIP5Ks in generating PI(4,5)P2 pools dedicated to specific PM functions for this lipid. Interestingly, a recent review has suggested that the plasma membrane might contain a single “megapool” of PI(4,5)P2 with a limited or no role for distinct PIP5K enzymes in generating pools of this lipid that control distinct cellular functions [88]. While this may be true in organisms such as S. cerivisiae that contain a single gene with no splice variants encoding PIP5K activity, the data obtained by analyzing loss of function mutants in individual genes encoding PIP5K activity in Drosophila[85] or mouse models [89,90] are not consistent with the megapool model.
If there are multiple PIP5K enzymes generating functionally distinct pools of PI(4,5)P2 at the PM, what is the mechanism underlying their ability to do this? Multiple studies have indicated that PIP kinases are localized to the PM by interaction with resident proteins and lipids (Fig 3A). For example, Tolias et al. have demonstrated that Rac1 binds to PIP5K and localizes it to PM [91][92]. Another study found that the endocytic adaptor protein AP2 binds to PIPKγ confining it to the site of endocytosis [93][94]. In this context, it has been proposed that effector regulated PI(4,5)P2 synthesis is a key mechanism in generating spatially resolved PI(4,5)P2 pools; i.e. in presence of stimulus PIP5K interacts with specific PI(4,5)P2 effectors and this interaction leads to association of a sub-cellular targeting complex which recruits PIP5K to specific location at the PM. PI(4,5)P2 synthesis in this location would regulate a specific molecular function (Fig 3C). Several such interactions are proposed and have been discussed in detail elsewhere[95]. One group of molecules that has been proposed to regulate the spatiotemporal activity of PIP5K are small G-proteins and their localization at the PM might also contribute to fine-tuning the generation of PI(4,5)P2 pools [96].
The recruitment of PIP5K to a specific location at the PM may also be transient, of a specific isoform of the enzyme and regulated by activation of receptor mediated signalling cascades. For example, in mammalian cells PIPKIα is normally distributed in the cytosol but upon PDGF stimulation it translocates to membrane ruffles and synthesize PI(4,5)P2 at that location[97]. Likewise, in breast cancer cells, EGFR signalling mediates a 2.5 fold increase in PIPKγi1 localization to the apical end of the cell membrane[98] while localized synthesis of PI(4,5)P2 at the leading edge of the cell mediates the formation of cellular protrusions in cancer cell migration[99]. A recent study suggests that upon activation, the T cell CD28 receptors recruit PIP5Kβ to the site of immunological synapse and upon its recruitment PIP5Kβ mediates the formation of lipid rafts through interaction with other proteins and by remodeling the local actin, which is important for raft formation[100]. A similar kind of observation has also been reported by Szymanska et al[69]. The recruitment of PIP5K to a specific location during cell signalling has also been shown to be isoform specific and signal regulated. A well known example is seen during phagocytosis when PIP5K-γ (but not α) is transiently activated to increase PI(4,5)P2 levels which in turn forms phagocytic cup[101]. Together with the reported recruitment of signalling complexes in Drosophila photoreceptors to rafts during PLC signalling[78], these observation suggest that microdomains of PI(4,5)P2 associated with rafts may assemble transiently during signal transduction. Thus collectively, a combination of specific mechanisms will likely operate to define the generation of spatially distinct PI(4,5)P2 pools at nanoscales.
(ii). Regulating accessibility to specific PI(4,5)P2 metabolizing enzymes
A second mechanism by which one might generate functionally distinct PI(4,5)P2 pools is to regulate in a spatially controlled manner the accessibility of this lipid to enzymes such as PLC, Class I PI3K, synaptojanin and OCRL. The idea that regulating the access of PI(4,5)P2 to PLC has been proposed some time ago. Studies in mammalian cells have suggested that proteins such as gelsolin and CapG that can bind PI(4,5)P2 might regulate the access of PLC to its substrate[102]. The mechanism that regulates the pool of PI(4,5)P2 that is accessed by Class I PI3K at the PM also remains to be defined. However one might envisage that protein-protein interactions that localize these enzymes to a specific location at the PM would determine the field of PI(4,5)P2 that is available for their activity
Conceptually, lipid phosphatases that degrade PI(4,5)P2 into PI4P or PI5P and PTEN that generates PI(4,5)P2 from PI(3,4,5)P3 could also help shape the distribution of PI(4,5)P2 pools at the PM (Fig 3E). It is unclear if endogenous lipid phosphatases play a direct role in maintaining distinct PI(4,5)P2 pools. If this was true, the localization of such phosphatases to specific locations on the PM could provide an additional mechanism to help define PI(4,5)P2 pools. As an example, the absence of OCRL gene causes increased level of PI(4,5)P2 in Golgi/endosome like structures resulting in mis-localization of PI(4,5)P2 binding proteins normally localized to PM to mediate cytokinesis; the consequence of this is abnormal cell division[103]. A second 5-phosphatase encoded by the gene synaptojanin(synj) is shown to have role in vesicular transport and mutations in synj cause defect in synaptic vesicle recycling[104]. Over expression of synj in COS 7 cells result in defects in actin stress fibre formation and actin rearrangement[105]. On the other hand knock down of INPP5 (5-phosphatase) results in increased PI3K/AKT signalling resulting in increased cell proliferation, suggesting that INPP5 might be regulating the pool of PIP2 that is responsible for PIP3 synthesis. Interestingly depletion of synj, OCRL or INPP5 does not seem to affect the PLC signalling but in case of synj overexpression, it caused reduced intracellular calcium signalling[106]. These observations hint towards specific regulation of PLC sensitive and insensitive pools of PI(4,5)P2 pools by PI(4,5)P2 phosphatases; it remains poorly understood and is likely to be an area for future studies.
(iii). Chemical heterogeneity and PI(4,5)P2 in distinct pools
Chemical analysis of lipids has suggested that any given lipid class, defined by the presence of a specific head group, may be present as multiple distinct chemical species, each of which is defined by the presence of fatty acids with variant acyl chain length and saturation at the sn-1 and sn-2 position. Such chemical heterogeneity increases the complexity of the lipid composition of cellular membranes[107]. This chemical heterogeneity has also been reported for PI[108][109] and therefore could apply in principle to its derivatives such as PI(4,5)P2. In the context of the current discussion, one can postulate that functionally distinct pools of PI(4,5)P2 might arise from the phosphorylation of PI with variant acyl chain composition but an invariant inositol head group with phosphorylation at position 4 and 5. Interestingly, studies examining the acyl chain variation of PI4P have revealed that the various mammalian PIP5K isoforms have differential affinity towards PI4P in vitro, depending on its acyl chain length[110]. In a separate study, it was shown that the catalytic activity of PIP5Kα varies with the acyl chain composition of PI4P [111]. These observations suggest the potential for generating distinct pools of PI(4,5)P2 based on the acyl chain composition of the precursor lipid PI4P. However, it should also be noted that in mammalian cells, there is considerable remodeling of phospholipids including PI by the Lands cycle[112][113] that involves the sequential action of phospholipase A2 and lysophosphatidic acid acyl transferase; the end result is that most of the PI in mammalian cells is in fact of a unique acyl chain composition, namely 18:0/ 20:4[114]. Additionally to date no known cellular process has been shown to depend on the acyl chain composition of PI(4,5)P2 in vivo although a recent study in Drosophila photoreceptors showed a correlation between the acyl chain composition of phospholipids modulated by diet and the speed and sensitivity of the light response [115], a process that depends on G-protein coupled PI(4,5)P2 hydrolysis.
(iv). Spatial segregation of PI(4,5)P2 pools
Although the localized and regulated generation of PI(4,5)P2 at the PM may be a key contributor to generating PI(4,5)P2 pools with specific functions, how are these pools kept distinct and prevented from mixing with each other, for e.g by diffusion? For example, in S.cerevisiae, there is only a single gene that encodes PIP5K (Mss4) and this gene produces a single isoform of the enzyme with no known splice variants. Yet, at the yeast PM as well, PI(4,5)P2 serves multiple functions including PLC signalling, endocytosis and the actin cytoskeleton. Likewise, in the Drosophila photoreceptor (see previous section, Fig 2B), it is unclear, how PI(4,5)P2 produced by sktl and dPIP5K are segregated for specific functions at the microvillar PM. How is the PI(4,5)P2 required for various processes kept segregated in such settings ?
Studies using externally added fluorescent PI(4,5)P2 coupled with diffusion measurements using fluorescence correlation spectroscopy have shown that the diffusion rate of PI(4,5)P2 in the PM is significantly lower than that of phosphatidylethanolamine[116] under equivalent conditions. Further when diffusion rate of BODIPY-labeled PI(4,5)P2 was compared between inner and outer leaflet of the PM of epithelial cells, it was seen that the diffusion rate in the inner leaflet is around two-fold slower than that in the outer leaflet[116]. These observations suggest that the lateral diffusion of PI(4,5)P2 is restricted in the inner leaflet of the PM and in turn suggest that once made by the activity of a lipid kinase, PI(4,5)P2 could be restricted to a localized domain of the PM.
A number of mechanisms have been proposed to explain how pools of PI(4,5)P2 might be segregated and prevented from mixing by free diffusion [reviewed extensively in [117]]. One major hypothesis is that the PM contains physical barriers that prevent the diffusion and free mixing of lipids [118,119] (Fig 3B). A diverse group of molecules have been proposed to contribute to the structure of molecular fences; these include a range of molecules belonging to the actin cytoskeleton, septins and transmembrane proteins and complexes such as those found at the zonula adherans [120][121]. While the role of such molecules in regulating protein diffusion is better understood, their function in controlling lipid diffusion remains less clear. It remains a possibility that specific PI(4,5)P2 pools may be present in microdomains defined by such fences thus limiting their availability for specific functions.
The PM is composed of molecules that are differentially charged. Highly charged molecules repel others possessing the same charge while attracting oppositely charged molecules to form clusters. It is reported that MARCKS proteins, enriched in positively charged amino acids can bind to PI(4,5)P2 (negatively charged) and sequester it in specific domain of the PM [122]. This may constitute a mechanism by which PI(4,5)P2 pools are formed in a subdomain of the PM (Fig 3E); in this setting the localization of MARCKS like molecules in the molecular neighborhood of specific PI(4,5)P2 dependent process may constitute a mechanism for making a pool of this lipid available for a specific function. Collectively these mechanisms may also contribute to the definition of spatially and functionally demarcated PI(4,5)P2 pools at the PM.
Concluding Remarks
PI(4,5)P2 is a multifunctional lipid with essential roles at the PM that have been conserved during evolution. In the genomes of unicellular eukaryotes such as S.cerevisiae, a single gene encodes PIP5K, the enzyme that synthesizes PI(4,5)P2; loss of this gene being cell lethal. However in mammalian genomes several genes encoding multiple isoforms of PIP5K have evolved. Predictably, as more isoforms of PIP5K appear, the function of PI(4,5)P2 synthesis is distributed over different isoforms; thus ablation of single isoform is not cell lethal. However, if an isoform responsible for cellular housekeeping functions is lost, this would cause cell lethality. In this respect, the timing of duplication and divergence of PIPK genes is interesting. In unicellular eukaryotes, the only PIPK that generates PI(4,5)P2, is a PIP5K. In Dictyostelium, where the state dependent-multicellularity concept is seen, the genome encodes three PIP5Ks. Thus in higher taxa the emergence of more numbers of PIP5Ks, each with multiple splice variants might have been a strategy to deal with increasingly complex needs for PI(4,5)P2 dependent functions in varied cell types. To date, differences in enzymatic activity itself of PIP5K isoforms have not been reported. Reconstitution of specific isoforms in model organisms such as Drosophila with compact genomes may represent a valuable experimental approach to understand the function of PIP5K isoforms. In addition to the regulated activity of PIPK to specific locations at the membrane in space and time, mechanisms that operate to prevent dissipation of a pool of PI(4,5)P2 once it is synthesized will likely be important. While many studies have shown the existence of both spatially distributed and functionally distinct isoforms, it is presently unclear if spatial separation is required for functionally distinct outputs. Understanding the relationship between the functional and spatial domains of PI(4,5)P2 pools will be a challenge for the future. Given that PI(4,5)P2 is involved in a large number of cellular processes and is linked to multiple human diseases(reviewed in [123][124][125]); understanding the control of its synthesis and distribution is important as it may help to define disease mechanisms and define specific therapeutic strategies in clinical settings.
Table 1. Distribution and reported biological functions of lipid kinases that synthesize PI(4,5)P2 in eukaryotes.
| Type I PIP kinase/ Phosphatidylinositol 4 phosphate 5 kinase; Biochemical reaction PI4P → PI(4,5)P2 | ||
| Organism | Gene | Function |
| Yeast (S.cerevisiae) | Mss4 | Cellular growth[126], cell viability and actin cytoskeleton maintenance[127] |
| Worm (C.elegans) | ppk-1 | ppk-1mutant shows larval developmental defect[17] |
| Fly (D.melanogaster) | sktl | Cell viability[25], bristle development[25], Spermatid cell polarity[128] |
| dPIP5K | G-protein coupled PLC mediated signaling in photoreceptors [129] | |
| Zebra fish (D.rerio) | PIP5K α | Role in endocytosis[130], |
| PIP5K β,γ | Function of PIP5K β and γ is not known | |
| Mouse (M.musculus) | PIP5K α | PIP5K α: KO animals survive to adulthood but are less fertile, controls platelet aggregation[131] |
| PIP5K β | PIP5K β: Regulates EGFR endocytosis in cultured fibroblast cells[132] | |
| PIP5K γ | PIP5K γ: KO animals die soon after birth. Synaptic transmission defects in cultured cortical neurons[133] PIPKγ functions in vesicle fusion in chromaffin cells[134] and has role in IP3 signaling in mast cells[90] |
|
| Human (H.sapiens) | PIP5K α | PIP5K α: Role in keratinocyte differentiation[135], FcγRIIIA-mediated natural killer cell cytotoxity[136] |
| PIP5K β | PIP5K β: Regulates actin organization at the site of bacterial invasion in HeLa cells[137] | |
| PIP5K γ | PIP5K γ: Regulates HeLa cell migration[138], controls lymphocyte cell adhesion through ICAM1[139] | |
| Type II PIP kinase/Phosphatidylinositol 5 phosphate 4 kinase; Biochemical reaction PI5P → PI(4,5)P2 | ||
| Yeast (S.cerevisiae) | nil | Not present in yeast genome |
| Worm (C.elegans) | ppk2 | Has role in oxidative stress response[140] |
| Fly (D.melanogaster) | dPIP4K | Larval development and growth; cell size control[16] |
| Zebra fish (D.rerio) | zPIP4K α | Important for post embryonic development[141] |
| Mouse (M.musculus) | PIP4K2A(α) | PIP4K2A and PIP4K2B are important for oxidative stress response[140] and for tumor growth in absence of p53[142] |
| PIP4K2B(β) | PIP4K 2B has role in insulin signaling and glucose metabolism [143] | |
| PIP4K2C(γ) | PIP4K2C has role in mTORC1 mediated signaling [144] | |
| Human (H.sapiens) | PIP4K α | PIP4K α: polymorphisms in this gene variably associated with neuropsychiatric syndromes [145][146][147] |
| PIP4K β | PIP4K β: Regulates CDH1(cell adhesion protein) expression in MCF 7 cells; affects tumor metastasis[148] | |
| PIP4K γ | PIP4K γ: Not known | |
Acknowledgement
We thank laboratory colleagues and Suvrajit Saha for useful comments on this article. Research in the authors’ laboratory is funded by the National Centre for Biological Sciences-TIFR. RP is a recipient of the Wellcome-DBT India Alliance Senior Fellowship. SK and UB are recipients of a Council of Scientific and Industrial Research Fellowship from the Government of India.
References
- 1.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–96. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 2.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu C, Watras J, Loew LM. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161:779–91. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jost M, Simpson F, Kavran JM, Lemmon Ma, Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol. 8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 5.Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson a, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, Koshiba S, Kigawa T, Kikuchi a, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Carroll S, Kaksonen M, Toshima JY, Drubin DG. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J Cell Biol. 2007;177:355–367. doi: 10.1083/jcb.200611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukami K. Requirement of PIP2 for a-actinin function. Nature. 1992;359:150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- 9.Hüttelmaier S, Mayboroda O, Harbeck B, Jarchau T, Jockusch BM, Rüdiger M. The interaction of the cell-contact proteins VASP and vinculin is regulated by phosphatidylinositol-4,5-bisphosphate. Curr Biol. 1998;8:479–88. doi: 10.1016/s0960-9822(98)70199-x. [DOI] [PubMed] [Google Scholar]
- 10.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 11.Hilgemann DW, Feng S, Nasuhoglu C. The Complex and Intriguing Lives of PIP2 with Ion Channels and Transporters. Sci STKE. 2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 12.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 13.King CE, Stephens LR, Hawkins PT, Guy GR, Michell RH. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987;244:209–217. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King CE, Hawkins PT, Stephens LR, Michell RH. Determination of the steady-state turnover rates of the metabolically active pools of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in human erythrocytes. Biochem J. 1989;259:893–6. doi: 10.1042/bj2590893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–9. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Toscano S, Trivedi D, Jones DR, Mathre S, Clarke JH, Divecha N, Raghu P. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc Natl Acad Sci U S A. 2013;110:5963–5968. doi: 10.1073/pnas.1219333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinkove D, Bastiani M, Chessa TaM, Joshi D, Hauth L, Cooke FT, Divecha N, Schuske K. Overexpression of PPK-1, the Caenorhabditis elegans Type I PIP kinase, inhibits growth cone collapse in the developing nervous system and causes axonal degeneration in adults. Dev Biol. 2008;313:384–397. doi: 10.1016/j.ydbio.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, Heck AJ, D’Santos CS, Divecha N. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell. 2006;23:685–695. doi: 10.1016/j.molcel.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Richardson JP, Wang M, Clarke JH, Patel KJ, Irvine RF. Genomic tagging of endogenous type IIbeta phosphatidylinositol 5-phosphate 4-kinase in DT40 cells reveals a nuclear localisation. Cell Signal. 2007;19:1309–14. doi: 10.1016/j.cellsig.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Bond NJ, Letcher AJ, Richardson JP, Lilley KS, Irvine RF, Clarke JH. Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIalpha and IIbeta and a partial nuclear localization of the IIalpha isoform. Biochem J. 2010;430:215–21. doi: 10.1042/BJ20100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke JH, Emson PC, Irvine RF. Localization of phosphatidylinositol phosphate kinase IIgamma in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am J Physiol Renal Physiol. 2008;295:F1422–30. doi: 10.1152/ajprenal.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkes D, Rameh LE. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J. 2010;428:375–84. doi: 10.1042/BJ20100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desrivières S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273:15787–93. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- 24.Weinkove D, Bastiani M, Chessa TaM, Joshi D, Hauth L, Cooke FT, Divecha N, Schuske K. Overexpression of PPK-1, the Caenorhabditis elegans Type I PIP kinase, inhibits growth cone collapse in the developing nervous system and causes axonal degeneration in adults. Dev Biol. 2008;313:384–397. doi: 10.1016/j.ydbio.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan BA, Prokopenko SN, Breuer S, Zhang B, Paululat A, Bellen HJ. skittles, a Drosophila phosphatidylinositol 4-phosphate 5-kinase, is required for cell viability, germline development and bristle morphology, but not for neurotransmitter release. Genetics. 1998;150:1527–1537. doi: 10.1093/genetics/150.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watt Sa, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J. 2002;363:657–666. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond GRV, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2) Biochem J. 2009;422:23–35. doi: 10.1042/BJ20090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schramp M, Hedman A, Li W, Tan X, Anderson RA. PIP Kinases from the Cell Membrane to the Nucleus. Phosphoinositides I: Enzymes of Synthesis and Degradation. 2012:25–59. doi: 10.1007/978-94-007-3012-0_2. [DOI] [PubMed] [Google Scholar]
- 30.Olivos-Glander IM, Jänne PA, Nussbaum RL. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am J Hum Genet. 1995;57:817–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Ungewickell A, Ward ME, Ungewickell E, Majerus PW. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci U S A. 2004;101:13501–6. doi: 10.1073/pnas.0405664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–90. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Hartz PA, Philip E, Racusen LC, Majerus PW. Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5-phosphatase and accumulate phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1998;273:1574–82. doi: 10.1074/jbc.273.3.1574. [DOI] [PubMed] [Google Scholar]
- 34.Divecha N, Banfic H, Irvine RF. The nuclear phosphoinositide cycle--does it play a role in nuclear Ca2+ homoeostasis? Cell. 1994;16:297–300. doi: 10.1016/0143-4160(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 35.Divecha N, Rhee SG, Letcher AJ, Irvine RF. Phosphoinositide signalling enzymes in rat liver nuclei: phosphoinositidase C isoform beta 1 is specifically, but not predominantly, located in the nucleus. Biochem J. 1993;289:617–20. doi: 10.1042/bj2890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrero GO, Renner ML, Gil GA, Rodríguez-Berdini L, Caputto BL. c-Fos-activated synthesis of nuclear phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] promotes global transcriptional changes. Biochem J. 2014;461:521–30. doi: 10.1042/BJ20131376. [DOI] [PubMed] [Google Scholar]
- 37.Yildirim S, Castano E, Sobol M, Philimonenko VV, Dzijak R, Venit T, Hozák P. Involvement of phosphatidylinositol 4,5- bisphosphate in RNA polymerase I transcription. J Cell Sci. 2013;126:2730–9. doi: 10.1242/jcs.123661. [DOI] [PubMed] [Google Scholar]
- 38.Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, Anderson RA. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451:1013–7. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Laishram RS, Anderson RA. The novel poly(A) polymerase Star-PAP is a signal-regulated switch at the 3’-end of mRNAs. Adv Biol Regul. 2013;53:64–76. doi: 10.1016/j.jbior.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payrastre B, Nievers M, Boonstra J, Breton M, Verkleij AJ, Van Bergen en Henegouwen PM. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J Biol Chem. 1992;267:5078–84. [PubMed] [Google Scholar]
- 41.Vann LR, Wooding FB, Irvine RF, Divecha N. Metabolism and possible compartmentalization of inositol lipids in isolated rat-liver nuclei. Biochem J. 1997;327(Pt 2):569–76. doi: 10.1042/bj3270569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Graaf P, Klapisz EE, Schulz TKF, Cremers AFM, Verkleij AJ, van Bergen en Henegouwen PMP. Nuclear localization of phosphatidylinositol 4-kinase beta. J Cell Sci. 2002;115:1769–75. doi: 10.1242/jcs.115.8.1769. [DOI] [PubMed] [Google Scholar]
- 43.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–10. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- 45.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 46.Díaz Añel AM. Phospholipase C β3 is a key component in the Gβγ/PKCη/PKD-mediated regulation of trans -Golgi network to plasma membrane transport. Biochem J. 2007;406:157–165. doi: 10.1042/BJ20070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddhanta A, Backer JM, Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J Biol Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- 48.Posor Y, Eichhorn-Grünig M, Haucke V. Phosphoinositides in endocytosis. Biochim Biophys Acta. 2015;1851:794–804. doi: 10.1016/j.bbalip.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Nández R, Balkin DM, Messa M, Liang L, Paradise S, Czapla H, Hein MY, Duncan JS, Mann M, De Camilli P. A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. Elife. 2014;3:e02975. doi: 10.7554/eLife.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Hedman AC, Tan X, Schill NJ, Anderson Ra. Dev Cell. Vol. 25. Elsevier Inc; 2013. Endosomal type Iγ PIP 5-kinase controls EGF receptor lysosomal sorting; pp. 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J. 2002;363:657–66. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemoto Y, De Camilli P. Recruitment of an alternatively spliced form of synaptojanin 2 to mitochondria by the interaction with the PDZ domain of a mitochondrial outer membrane protein. EMBO J. 1999;18:2991–3006. doi: 10.1093/emboj/18.11.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat. 2013;34:1208–15. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- 55.Chen K-H, Wu R-M, Lin H-I, Tai C-H, Lin C-H. Mutational analysis of SYNJ1 gene (PARK20) in Parkinson’s disease in a Taiwanese population. Neurobiol Aging. 2015;36:2905.e7–8. doi: 10.1016/j.neurobiolaging.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Drouet V, Lesage S. Synaptojanin 1 mutation in Parkinson’s disease brings further insight into the neuropathological mechanisms. Biomed Res Int. 2014 doi: 10.1155/2014/289728. 289728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosivatz E, Woscholski R. Cell Signal. Vol. 23. Elsevier Inc; 2011. Removal or masking of phosphatidylinositol(4,5)bisphosphate from the outer mitochondrial membrane causes mitochondrial fragmentation; pp. 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vickers JD, Mustard JF. The phosphoinositides exist in multiple metabolic pools in rabbit platelets. Biochem J. 1986;238:411–417. doi: 10.1042/bj2380411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King CE, Stephens LR, Hawkins PT, Guy GR, Michell RH. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987;244:209–17. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ischebeck T, Werner S, Krishnamoorthy P, Lerche J, Meijon M, Stenzel I, Lofke C, Wiessner T, Im YJ, Perera IY, et al. Phosphatidylinositol 4,5-Bisphosphate Influences PIN Polarization by Controlling Clathrin-Mediated Membrane Trafficking in Arabidopsis. Plant Cell. 2013;25:4894–4911. doi: 10.1105/tpc.113.116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gervais L, Claret S, Januschke J, Roth S, Guichet A. PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Development. 2008;135:3829–3838. doi: 10.1242/dev.029009. [DOI] [PubMed] [Google Scholar]
- 62.Field SJ, Madson N, Kerr ML, Galbraith KAA, Kennedy CE, Tahiliani M, Wilkins A, Cantley LC. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr Biol. 2005;15:1407–12. doi: 10.1016/j.cub.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 63.Wong R, Hadjiyanni I, Wei H-C, Polevoy G, McBride R, Sem K-P, Brill JA. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15:1401–6. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 64.Wong R, Fabian L, Forer A, Brill JA. Phospholipase C and myosin light chain kinase inhibition define a common step in actin regulation during cytokinesis. BMC Cell Biol. 2007;8:15. doi: 10.1186/1471-2121-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–97. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rousso T, Shewan AM, Mostov KE, Schejter ED, Shilo B-Z. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. Elife. 2013;2:e00666. doi: 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demmel L, Schmidt K, Lucast L, Havlicek K, Zankel A, Koestler T, Reithofer V, de Camilli P, Warren G. The endocytic activity of the flagellar pocket in Trypanosoma brucei is regulated by an adjacent phosphatidylinositol phosphate kinase. J Cell Sci. 2014;127:2351–64. doi: 10.1242/jcs.146894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A. 2009;106:9256–9261. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szymańska E, Korzeniowski M, Raynal P, Sobota A, Kwiatkowska K. Contribution of PIP-5 kinase Iα to raft-based FcγRIIA signaling. Exp Cell Res. 2009;315:981–995. doi: 10.1016/j.yexcr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 70.Ji C, Zhang Y, Xu P, Xu T, Lou X. Nanoscale Landscape of Phosphoinositides Revealed by the Specific PH-domains Using Single-molecule Super-resolution Imaging in the Plasma Membrane. J Biol Chem. 2015 doi: 10.1074/jbc.M115.663013. jbc.M115.663013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 72.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 74.Pike LJ, Casey L. Localization and Turnover of PIP2 in caveolin enriched Membrane Domains. J Biol Chem. 1996;271:26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–690. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- 76.Capuano C, Paolini R, Molfetta R, Frati L, Santoni A, Galandrini R. PIP2-dependent regulation of Munc13-4 endocytic recycling: impact on the cytolytic secretory pathway. Blood. 2012;119:2252–62. doi: 10.1182/blood-2010-12-324160. [DOI] [PubMed] [Google Scholar]
- 77.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 78.Sanxaridis PD, Cronin MA, Rawat SS, Waro G, Acharya U, Tsunoda S. Light-induced recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila photoreceptors. Mol Cell Neurosci. 2007;36:36–46. doi: 10.1016/j.mcn.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Rheenen J. PIP2 as local second messenger: a critical re-evaluation. EMBO J. 2005;24:1664–73. doi: 10.1038/sj.emboj.7600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan X, Sun Y, Thapa N, Liao Y, Hedman AC, Anderson RA. LAPTM4B is a PtdIns(4,5)P2 effector that regulates EGFR signaling, lysosomal sorting, and degradation. EMBO J. 2015;34:475–90. doi: 10.15252/embj.201489425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson Ra. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 82.Goñi GM, Epifano C, Boskovic J, Camacho-Artacho M, Zhou J, Bronowska A, Martín MT, Eck MJ, Kremer L, Gräter F, et al. Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc Natl Acad Sci U S A. 2014;111:E3177–3186. doi: 10.1073/pnas.1317022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legate KR, Montag D, Bottcher RT, Takahashi S, Fassler R. Comparative phenotypic analysis of the two major splice isoforms of PIP kinase Type I in vivo. J Cell Sci. 2012:5636–5646. doi: 10.1242/jcs.102145. [DOI] [PubMed] [Google Scholar]
- 84.Vasudevan L, Jeromin A, Volpicelli-Daley L, De Camilli P, Holowka D, Baird B. The beta- and gamma-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J Cell Sci. 2009;122:2567–2574. doi: 10.1242/jcs.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakrabarti P, Kolay S, Yadav S, Kumari K, Nair A, Trivedi D, Raghu P. A dPIP5K dependent pool of phosphatidylinositol 4,5 bisphosphate (PIP2) is required for G-protein coupled signal transduction in Drosophila photoreceptors. PLoS Genet. 2015;11:e1004948. doi: 10.1371/journal.pgen.1004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raghu P, Coessens E, Manifava M, Georgiev P, Pettitt T, Wood E, Garcia-Murillas I, Okkenhaug H, Trivedi D, Zhang Q, et al. Rhabdomere biogenesis in Drosophila photoreceptors is acutely sensitive to phosphatidic acid levels. J Cell Biol. 2009;185:129–145. doi: 10.1083/jcb.200807027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hammond GRV. Does PtdIns(4,5)P2 concentrate so it can multi-task? Biochem Soc Trans. 2016;44:228–33. doi: 10.1042/BST20150211. [DOI] [PubMed] [Google Scholar]
- 89.Legate KR, Montag D, Böttcher RT, Takahashi S, Fässler R. Comparative phenotypic analysis of the two major splice isoforms of phosphatidylinositol phosphate kinase type Iγ in vivo. J Cell Sci. 2012;125:5636–46. doi: 10.1242/jcs.102145. [DOI] [PubMed] [Google Scholar]
- 90.Vasudevan L, Jeromin A, Volpicelli-Daley L, De Camilli P, Holowka D, Baird B. The beta- and gamma-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J Cell Sci. 2009;122:2567–2574. doi: 10.1242/jcs.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type I?? phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 92.Weernink PaO, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of Type I Phosphatidylinositol 4-Phosphate 5-Kinase Isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- 93.Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci U S A. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kahlfeldt N, Vahedi-Faridi A, Koo SJ, Schäfer JG, Krainer G, Keller S, Saenger W, Krauss M, Haucke V. Molecular basis for association of PIPKIγ-p90 with clathrin adaptor AP-2. J Biol Chem. 2010;285:2734–2749. doi: 10.1074/jbc.M109.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi S, Thapa N, Tan X, Hedman AC, Anderson RA. PIP kinases define PI4,5P2 signaling specificity by association with effectors. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbalip.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santarius M, Lee CH, Anderson Ra. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doughman RL, Firestone AJ, Wojtasiak ML, Bunce MW, Anderson RA. Membrane ruffling requires coordination between type Ialpha phosphatidylinositol phosphate kinase and Rac signaling. J Biol Chem. 2003;278:23036–23045. doi: 10.1074/jbc.M211397200. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y, Turbin DA, Ling K, Thapa N, Leung S, Huntsman DG, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase modulates invasion and proliferation and its expression correlates with poor prognosis in breast cancer. Breast Cancer Res. 2010:1–11. doi: 10.1186/bcr2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi S, Thapa N, Hedman AC, Li Z, Sacks DB, Anderson Ra. IQGAP1 is a novel phosphatidylinositol 4,5 bisphosphate effector in regulation of directional cell migration. EMBO J Nature Publishing Group. 2013;32:2617–30. doi: 10.1038/emboj.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kallikourdis M, Trovato AE, Roselli G, Muscolini M, Porciello N, Tuosto L, Viola A. Phosphatidylinositol 4-Phosphate 5-Kinase β Controls Recruitment of Lipid Rafts into the Immunological Synapse. J Immunol. 2016 Jan 15; doi: 10.4049/jimmunol.1501788. 1501788. [DOI] [PubMed] [Google Scholar]
- 101.Mao YS, Yamaga M, Zhu X, Wei Y, Sun H-Q, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, et al. Essential and unique roles of PIP5K-gamma and -alpha in Fcgamma receptor-mediated phagocytosis. J Cell Biol. 2009;184:281–96. doi: 10.1083/jcb.200806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun HQ, Kwiatkowska K, Wooten DC, Yin HL. Effects of CapG overexpression on agonist-induced motility and second messenger generation. J Cell Biol. 1995;129:147–56. doi: 10.1083/jcb.129.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben El Kadhi K, Roubinet C, Solinet S, Emery G, Carréno S. The inositol 5-phosphatase dOCRL controls PI(4,5)P2 homeostasis and is necessary for cytokinesis. Curr Biol. 2011;21:1074–9. doi: 10.1016/j.cub.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 104.Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–599. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakisaka T, Itoh T, Miura K, Takenawa T. Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol Cell Biol. 1997;17:3841–3849. doi: 10.1128/mcb.17.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johenning FW, Wenk MR, Uhlén P, Degray B, Lee E, De Camilli P, Ehrlich BE. InsP3-mediated intracellular calcium signalling is altered by expression of synaptojanin-1. Biochem J. 2004;382:687–694. doi: 10.1042/BJ20040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–65. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Souza K, Epand RM. Enrichment of phosphatidylinositols with specific acyl chains. Biochim Biophys Acta. 2014;1838:1501–8. doi: 10.1016/j.bbamem.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 109.König S, Mosblech A, Heilmann I. Stress-inducible and constitutive phosphoinositide pools have distinctive fatty acid patterns in Arabidopsis thaliana. FASEB J. 2007;21:1958–67. doi: 10.1096/fj.06-7887com. [DOI] [PubMed] [Google Scholar]
- 110.Shulga YV, Anderson RA, Topham MK, Epand RM. Phosphatidylinositol-4-phosphate 5-kinase isoforms exhibit acyl chain selectivity for both substrate and lipid activator. J Biol Chem. 2012;287:35953–63. doi: 10.1074/jbc.M112.370155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shulga YV, Topham MK, Epand RM. J Mol Biol. Vol. 409. Elsevier Ltd; 2011. Study of arachidonoyl specificity in two enzymes of the PI cycle; pp. 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LANDS WE. Metabolism of glycerolipids: a comparisn of Lecithin and Triglyceride synthesis. J Biol Chem. 1957:883–889. [PubMed] [Google Scholar]
- 113.Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc Natl Acad Sci U S A. 2008;105:10–15. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson KE, Kielkowska A, Durrant TN, Juvin V, Clark J, Stephens LR, Hawkins PT. Lysophosphatidylinositol-acyltransferase-1 (LPIAT1) is required to maintain physiological levels of PtdIns and PtdInsP(2) in the mouse. PLoS One. 2013;8:e58425. doi: 10.1371/journal.pone.0058425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Randall AS, Liu C-H, Chu B, Zhang Q, Dongre SA, Juusola M, Franze K, Wakelam MJO, Hardie RC. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J Neurosci. 2015;35:2731–46. doi: 10.1523/JNEUROSCI.1150-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol Biol Cell. 2008;19:1663–9. doi: 10.1091/mbc.E07-12-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trimble WS, Grinstein S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. J Cell Biol. 2015;208:259–71. doi: 10.1083/jcb.201410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A. Rapid hop diffusion of a G-protein-coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys J. 2005;88:3659–80. doi: 10.1529/biophysj.104.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–78. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 120.Beise N, Trimble W. Septins at a glance. J Cell Sci. 2011;124:4141–4146. doi: 10.1242/jcs.087007. [DOI] [PubMed] [Google Scholar]
- 121.Trimble WS, Grinstein S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. J Cell Biol. 2015;208:259–271. doi: 10.1083/jcb.201410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gambhir A, Hangyás-Mihályné G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arancio O. PIP2: a new key player in Alzheimer’s disease. Cellscience. 2008;5:27–28. [PMC free article] [PubMed] [Google Scholar]
- 124.Halstead JR, Jalink K, Divecha N. An emerging role for PtdIns(4,5)P2-mediated signalling in human disease. Trends Pharmacol Sci. 2005;26:654–660. doi: 10.1016/j.tips.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 125.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Isao uno, Lipscomb JD, Weber PC. Essential role for phosphatidylinositol 4,5-biphosphate in yeast cell proliferation. Nature. 1988;336:403–405. [Google Scholar]
- 127.Desrivières S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- 128.Fabian L, Wei HC, Rollins J, Noguchi T, Blankenship JT, Bellamkonda K, Polevoy G, Gervais L, Guichet A, Fuller MT, et al. Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila. Mol Biol Cell. 2010;21:1546–1555. doi: 10.1091/mbc.E09-07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chakrabarti P. A dPIP5K Dependent Pool of Is Required for G-Protein Coupled Signal Transduction in Drosophila Photoreceptors. PLoS Genet. 2015:1–24. doi: 10.1371/journal.pgen.1004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oltrabella F, Pietka G, Ramirez IB-R, Mironov A, Starborg T, Drummond Ia, Hinchliffe Ka, Lowe M. The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule. PLOS Genet. 2015;11:e1005058. doi: 10.1371/journal.pgen.1005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, Petrich BG, Monkley SJ, Critchley DR, Sasaki T, Birnbaum MJ, et al. Loss of PIP5KIγ, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barbieri MA, Heath CM, Peters EM, Wells A, Davis JN, Stahl PD. Phosphatidylinositol-4-phosphate 5-Kinase-1?? Is Essential for Epidermal Growth Factor Receptor-mediated Endocytosis. J Biol Chem. 2001;276:47212–47216. doi: 10.1074/jbc.C100490200. [DOI] [PubMed] [Google Scholar]
- 133.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan Ta, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 134.Gong L-W, Di Paolo G, Diaz E, Cestra G, Diaz M-E, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc Natl Acad Sci U S A. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie Z, Chang SM, Pennypacker SD, Liao E, Bikle DD. Extracellular Calcium-induced Keratinocyte Differentiation. Mol Biol Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galandrini R, Micucci F, Tassi I, Cifone MG, Cinque B, Piccoli M, Frati L, Santoni A, Riiia IF. Arf6 : a new player in Fcg RIIIA lymphocyte-mediated cytotoxicity. Blood. 2016;106:577–584. doi: 10.1182/blood-2004-10-4100. [DOI] [PubMed] [Google Scholar]
- 137.Balañá ME, Niedergang F, Subtil A, Alcover A, Chavrier P, Dautry-varsat A. ARF6 GTPase controls bacterial invasion by actin remodelling. J Cell Sci. 2005;118:2201–2210. doi: 10.1242/jcs.02351. [DOI] [PubMed] [Google Scholar]
- 138.Sun Y, Ling K, Wagoner MP, Anderson RA. type I phosphatidylinositol phosphate kinase is required for EGF-stimulated directional cell migration. J Cell Biol. 2007;178:297–308. doi: 10.1083/jcb.200701078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bolomini-vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 140.Fiume R, Stijf-Bultsma Y, Shah ZH, Keune WJ, Jones DR, Jude JG, Divecha N. Biochim Biophys Acta - Mol Cell Biol Lipids. Vol. 1851. Elsevier B V; 2015. PIP4K and the role of nuclear phosphoinositides in tumour suppression; pp. 898–910. [DOI] [PubMed] [Google Scholar]
- 141.Elouarrat D, Van Der Velden YU, Jones DR, Moolenaar WH, Divecha N, Haramis APG. Int J Biochem Cell Biol. Vol. 45. Elsevier Ltd; 2013. Role of phosphatidylinositol 5-phosphate 4-kinase α in zebrafish development; pp. 1293–1301. [DOI] [PubMed] [Google Scholar]
- 142.Emerling BM, Hurov JB, Poulogiannis G, Tsukazawa KS, Choo-Wing R, Wulf GM, Bell EL, Shim HS, Lamia KA, Rameh LE, et al. Cell. Vol. 155. Elsevier; 2013. XDepletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-Null tumors; pp. 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta-/- mice. 2004;24:5080–5087. doi: 10.1128/MCB.24.11.5080-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mackey AM, Sarkes DA, Bettencourt I, Asara JM, Rameh LE. PIP4kgamma is a substrate for mTORC1 that maintains basal mTORC1 signaling during starvation. Sci Signal. 2014;7 doi: 10.1126/scisignal.2005191. ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stopkova P, Saito T, Fann CSJ, Papolos DF, Vevera J, Paclt I, Zukov I, Stryjer R, Strous RD, Lachman HM. Am J Med Genet Part B Neuropsychiatr Genet. 123B. Wiley Subscription Services, inc., A Wiley Company; 2003. Polymorphism Screening of PIP5K2A: A Candidate Gene for Chromosome 10p-Linked Psychiatric Disorders; pp. 50–58. [DOI] [PubMed] [Google Scholar]
- 146.Réthelyi JM, Bakker SC, Polgár P, Czobor P, Strengman E, Pásztor PI, Kahn RS, Bitter I. Association study of NRG1, DTNBP1, RGS4, G72/G30, and PIP5K2A with schizophrenia and symptom severity in a Hungarian sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:792–801. doi: 10.1002/ajmg.b.31049. [DOI] [PubMed] [Google Scholar]
- 147.Kaur H, Jajodia A, Grover S, Baghel R, Gupta M, Jain S, Kukreti R. Genetic variations of PIP4K2A confer vulnerability to poor antipsychotic response in severely ill schizophrenia patients. PLoS One. 2014;9:e102556. doi: 10.1371/journal.pone.0102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keune W, Sims AH, Jones DR, Bultsma Y, Lynch JT. Low PIP4K2B Expression in Human Breast Tumors Correlates with Reduced Patient Survival : A Role for PIP4K2B in the Regulation of E-Cadherin Expression. Cancer Res. 2013;73:6913–6926. doi: 10.1158/0008-5472.CAN-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]