Abstract
BACKGROUND/OBJECTIVE:
Recent studies indicated that functional outcome after intracranial hemorrhage (ICH) related to direct oral anticoagulation (DOAC-ICH) is similar, if not better, than vitamin K antagonist (VKA)-related ICH (VKA-ICH) due to a smaller initial hematoma volume (HV). However, the association with hematoma expansion (HE) and location is not well understood.
METHODS:
We retrospectively analyzed 102 consecutive patients with acute non-traumatic ICH on oral anticoagulation therapy to determine HV and HE stratified by hematoma location, and the relation to the 90-day outcome.
RESULTS:
DOAC-ICH (n=25) and VKA-ICH (n=77) had a similar admission HV and HE (unadjusted p>0.05, each). Targeted reversal strategies were used in 93.5% of VKA-ICH versus 8% of DOAC-ICH. After adjustment, an unfavorable 90-day functional outcome (modified Rankin scale score 4–6) was independently associated with lower admission Glasgow coma scale score (OR 1.63; 95%-CI 1.26–2.10; p<0.001) and greater HV (OR 1.03; 95%-CI 1.00–1.05; p=0.046). After exclusion of patients without follow-up head CT to allow for adjustment by occurrence of HE, VKA-ICH was associated with an approximately 3.5 times greater odds for a poor 90-day outcome (OR 3.64; 95%-CI 1.01–13.09; p=0.048). However, there was no significant association of the oral anticoagulant strategy with 90-day outcome in the entire cohort (OR 2.85; 95%-CI 0.69–11.86; p=0.15).
CONCLUSIONS:
DOAC use did not relate to worse HE, HV, and functional outcome after ICH, adding to the notion that DOAC are a safe alternative to VKA even in the absence of access to targeted reversal strategies (which are still not universally available).
Keywords: warfarin, direct oral anticoagulants, intracranial hemorrhage
Introduction
Stroke prevention with oral anticoagulation is key to nonvalvular atrial fibrillation treatment1 as clinical trials involving >60,000 patients demonstrated that oral anticoagulation reduces stroke risk by at least 62% over control and 37% over antiplatelet therapy.2 However, clinicians frequently underuse oral anticoagulants particularly due to a perceived risk of major bleeding complications, the most feared being intracranial hemorrhage (ICH).3,4
Several phase III multicenter randomized trials reported similar or superior efficacy of direct oral anticoagulation (DOAC) as compared to vitamin K antagonist (VKA) based anticoagulation in preventing thromboembolic complications including stroke.5 Nevertheless, there has been particular concern for worse outcomes in the setting of DOAC-related ICH (DOAC-ICH) due to lack of universal access to targeted reversal agents for DOAC.6 Yet, recent studies have found no difference in functional outcome and mortality between DOAC-ICH and VKA-related ICH (VKA-ICH).7–9 Furthermore, data from several studies suggested that DOAC-ICH is associated with smaller hematoma volumes (HV) and lower incidence of hematoma expansion (HE) as compared to VKA-ICH.10–14 However, observations from a small case series indicated the difference in HV between DOAC vs. VKA-ICH may be driven by smaller subcortical ICH.13 This may be an important observation because in this study lobar hemorrhages were relatively more frequent among subjects taking VKA.13 Yet, available studies examining the association between HE and HV have not accounted for hematoma location (such as lobar versus non-lobar),8,12 which is a critical determinant of HE and initial functional deficit severity as assessed by the Glasgow Coma scale (GCS).10–13 Lastly, most studies estimated the HV by the ABC/2 method, which has been shown to be less precise than planimetric quantification in the setting of oral anticoagulant related ICH with frequently irregular shapes,15–17 as well as categorized HE as absent versus present or used a semiquantitative approach without accounting for hematoma location.11–13
To address this issue, we therefore sought to determine potential differences in HV and HE in subjects with DOAC-ICH versus VKA-ICH and their relation to the 90-day modified Rankin scale (mRS) score by accounting for the hematoma location as well as by using planimetric quantification of the HE and HV. We hypothesized that VKA-ICH is associated with worse HV, HE, and 90-day outcome as compared to DOAC-ICH.
Methods
Study cohort
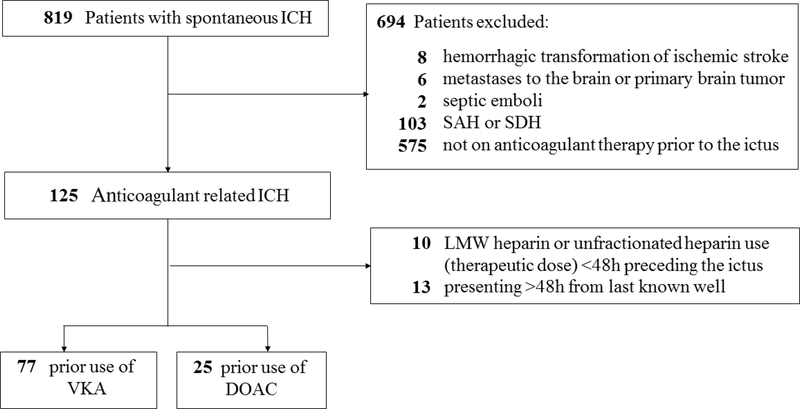
Using local stroke registries, we retrospectively identified adult patients with spontaneous ICH that were consecutively hospitalized at the University of Massachusetts Memorial Medical Center (UMMMC), Worcester, MA (January 2013 to July 2017) and Spectrum Health, Grand Rapids, MI (January 2016 to September 2017). Data were analyzed between March 23rd and June 25th, 2018. A priori defined exclusion criteria were ICH not related to anticoagulant therapy, subdural and subarachnoid hemorrhage, as well as ICH related to vascular malformations, primary brain tumors, metastases, septic emboli, and hemorrhagic transformation of an ischemic stroke. Furthermore, we excluded subjects with ICH related to anticoagulation with unfractionated heparin, low molecular weight heparin, and argatroban. Finally, we excluded subjects who presented more than 48 hours from last known well (LKW) because none demonstrated HE and the time of LKW was uncertain.
Age, gender, GCS score, pre-existing conditions (hypertension, hyperlipidemia, diabetes, history of previous stroke, atrial fibrillation, coronary artery disease), pre-admission medications (including the type of anticoagulation and concomitant antiplatelet or statin therapy), clinical (admission blood pressure, weight, LKW, time to baseline computed tomography [CT] of the brain) and laboratory (International Normalized Ratio [INR], creatinine, glucose, platelet count [PLT]) data on admission, and ICH etiology after completion of diagnostic evaluation were collected for each participant. Members of the stroke team certified in NIHSS-scoring graded the severity of stroke at presentation. Lastly, we collected information on the type of reversal strategy used (idarucizumab, vitamin K, fresh frozen plasma, prothrombin complex concentrates, recombinant activated factor VII [rFVIIa]). We considered idarucizumab targeted reversal for dabigatran (recombinant modified human coagulation factor Xa was not available during the study period) and all other therapies as targeted reversal for VKA.
Image review and analysis
All CT sequences at UMMMC were obtained on a 64-row detector scanner (Philips Medical Systems, Best, the Netherlands) using a non-helical mode at 120 KvP and 200 mA with data reconstruction at 4 or 5 mm axial slices. All CT sequences at Spectrum Health were obtained on a 64-row detector scanner (GE Discovery 750 HD, Schenectady, NY, USA) using a helical mode at 80 KvP and 200 mA with data reconstruction at 5 mm axial slices.
All CT scans were reviewed to determine the ICH location categorized to (i) lobar (cortical and cortico-subcortical and not involving any of the deep gray matter structures) versus non-lobar (i.e. basal ganglia, thalamus, brainstem, cerebellum) and (ii) supra- versus infratentorial. The presence of intraventricular extension was noted. ICH volumes were quantified using manual planimetry by carefully outlining ICH on each CT slice. HE was calculated by subtracting the baseline HV from the first available follow-up CT obtained during hospitalization. If more than one follow-up scan was obtained during hospitalization we used the scan showing the greatest hematoma extent to calculate HE. HV and HE were quantified in “mL” and included as continuous variable in all analyses. In addition to the absolute HE, we also defined substantial HE as HE >33% of the baseline HV.9 The time from last LKW to acquisition of the first and follow-up scan were also noted. All image analyses were done by investigators masked to clinical variables and functional outcome.
Risk factor definitions
We collected information on hypertension (use of antihypertensive medications, or systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg on 2 separate occasions), hypercholesterolemia (use of lipid-lowering agents, or fasting blood total cholesterol level of ≥200 mg/dl, or low density lipoprotein cholesterol [LDLc] of ≥130 mg/dL), coronary artery disease (history of myocardial infarction within the past 20 years, multivessel coronary disease with symptoms or with history of stable or unstable angina, history of percutaneous coronary intervention or multivessel coronary artery bypass graft surgery) and diabetes mellitus (history of fasting glucose ≥126 mg/dL or current use of hypoglycemic drugs) using definitions by the National Diabetes Data Group and World Health Organization.18
Functional outcome
We abstracted the pre-admission, discharge and 90-day mRS from the medical record, which is routinely documented per institutional protocol by a stroke-trained physician or stroke study nurse certified in mRS via structured in-person or telephone interview.19 When the mRS was unavailable, the same observers reconstructed the score from the case description according to the mRS criteria.20 The 90-day outcome was dichotomized to favorable (mRS score 0–3) versus unfavorable (mRS score 4–6).9 Finally, we determined in-hospital death and whether patients’ status was changed to comfort measures only (CMO).
Statistics
Unless otherwise stated, continuous variables are reported as mean ± S.D. or median (25th-75th percentile). Normality of data was examined using Kolmogorov-Smirnov test, review of the distribution graphically (via histograms, boxplots, and Q-Q plots), and assessment of skewness and kurtosis. Categorical variables are reported as proportions. Between-group comparisons for continuous and ordinal variables were made with Mann-Whitney U test and Kruskal–Wallis one-way ANOVA on ranks as appropriate. Categorical variables were compared using the χ2-test. Analysis of covariance (ANCOVA) investigated whether the association between time from LKW to first CT and HV differed between oral anticoagulant treatments. We cube-root transformed the HV to achieve a more suitable distribution for ANCOVA.21,22 After the transformation, the variance was close to the mean (skewness=0.23; kurtosis=−0.85; p=0.15). For ANCOVA, assumptions of homogeneity of variance as well as regression slopes was tested and met. Multivariable logistic regression analysis was performed to determine factors independently associated with an unfavorable 90-day mRS (dependent variable). Analyses were adjusted for oral anticoagulant strategy (VKA versus DOAC), treatment with a targeted reversal strategy, age, sex, pre-admission mRS, admission GCS, HV, HE, lobar hemorrhage location, and presence of an intraventricular hemorrhage. To avoid overfitting, a parsimonious model was created by backward elimination of factors (p>0.1). Collinearity diagnostics were performed (and its presence rejected) for all multivariable regression models. Model calibration was assessed by Hosmer-Lemeshow test and model fit determined by examining the −2 log-likelihood statistic and its associated chi-square statistics.
Additional sensitivity analyses were conducted (i) by excluding patients that did not have a follow-up CT for testing associations of the anticoagulant treatment with HE as well as (ii) by excluding patients that were lost to follow up for testing association with the 90-day outcome.
Two-sided significance tests were used throughout and unless stated otherwise a two-sided p<0.05 was considered statistically significant. All statistical analyses were performed using IBM® SPSS® Statistics Version 22 (IBM®-Armonk, NY).
Results
Study cohort
We identified 819 patients (n=615 at UMMHC; n=204 at Spectrum Health) diagnosed with spontaneous ICH. Of these, 102 patients (77 patients on VKA and 25 patients on DOAC) fulfilled the study criteria and were included for analysis (Figure 1). Data was complete for all variables except for body weight (n=5 [5%] missing), follow up CT (n=32 [31%] had none performed), and the 90-day mRS (n= 28 [27%] lost to follow up). To allow a complete-case analysis, we imputed body weight by using the cohort median, and the 90-day mRS by carrying forward the discharge mRS where missing. For patients without follow up CT we used a conservative approach to impute the HE by defining the HE as zero mL (n=16 were clinically stable and n=16 were made CMO [median ICH volume 70.2 mL; IQR 37.4–98.0 mL]).
Figure 1. Flowchart of study design and patient selection.
DOAC=direct oral anticoagulant, ICH=intracerebral hemorrhage, LMW=low molecular weighted SAH=subarachnoid hemorrhage, SDH=subdural hematoma, VKA=vitamin K antagonist.
Comparison of baseline characteristics of DOAC-ICH versus VKA-ICH
Table 1 summarizes the baseline characteristics of the studied population. Overall, there were no differences in vascular risk factors, baseline laboratory values, and preadmission medications between subjects treated with VKA versus DOAC. Furthermore, there were no differences in the admission GCS, admission NIHSS, as well as systolic and diastolic blood pressure between groups (p>0.05, each). Among subjects treated with VKA, the admission INR was subtherapeutic (INR<2.0) in seven subjects (median 1.7 [IQR 1.1–1.8]), therapeutic (INR 2.0–3.0) in 41 subjects (median 2.4 [IQR 2.2–2.8]), and supratherapeutic (INR >3.0) in 29 subjects (median 4.0 [IQR 3.5–5.1]). Of the 7 patients with subtherapeutic INR, 5 had an INR of 1.6–1.9. The other two subjects were reversed at an outside hospital and arrived with an INR of 1.1 at our institution (the original INR could not be determined).
Table 1.
Baseline characteristics of the studied patient population as stratified by the type of anticoagulant used.
| All patients | VKA-ICH | DOAC-ICH | ||
|---|---|---|---|---|
| Characteristics | (n=102) | (n=77) | (n=25) | P-value |
| Age, years | 79 (71–84) | 80 (72–85) | 78 (68.5–82) | 0.141 |
| Age, >75 years old | 70 (68.6) | 54 (70.1) | 16 (64) | 0.623 |
| Female sex | 42 (41.2) | 33 (42.9) | 9 (36.0) | 0.643 |
| Weight, kg | 82.7 (68.1–113.4) | 80.5 (66.5–93.4) | 89.0 (73.7–104.9) | 0.053 |
| Admission GCS | 13 (7–15) | 12 (6–15) | 14 (10–15) | 0.334 |
| Admission NIHSS | 13 (4–25) | 14 (4–28) | 10 (3–22) | 0.436 |
| SBP on admission, mmHg | 172 (151–203) | 173 (148–204) | 168 (160–203) | 0.966 |
| DBP on admission, mmHg | 91 (73–105) | 91 (71–103) | 89 (75–112) | 0.898 |
| Preexisting risk factors | ||||
| Hypertension | 99 (97.1) | 74 (96.1) | 25 (100.0) | 0.574 |
| Diabetes | 36 (35.3) | 26 (33.8) | 10 (40.0) | 0.633 |
| Prior stroke or TIA | 31 (30.4) | 22 (28.6) | 9 (36.0) | 0.617 |
| Coronary artery disease | 42 (41.2) | 33 (42.9) | 9 (36.0) | 0.643 |
| Hyperlipidemia | 72 (70.6) | 51 (66.2) | 21 (84.0) | 0.129 |
| Atrial fibrillation | 92 (90.2) | 67 (87.0) | 25 (100.0) | 0.114 |
| Preadmission medications | ||||
| Statin | 70 (68.6) | 52 (67.5) | 18 (72.0) | 0.854 |
| Antihypertensive | 97 (95.1) | 73 (94.8) | 24 (96.0) | 1.000 |
| Antiglycemic | 26 (25.5) | 21 (27.3) | 5 (20.0) | 0.601 |
| Antiplatelets | 41 (40.2) | 30 (39.0) | 11 (44.0) | 0.815 |
| Laboratory data | ||||
| Creatinine, mg/dL | 0.9 (0.8–1.4) | 1.0 (0.8–1.4) | 0.94 (0.8–1.2) | 0.747 |
| GFR, ml/min/1.73 m2 | 63 (45–88) | 60 (41–86) | 69 (56–93) | 0.105 |
| GFR <30 ml/min/1.73 m2 | 9 (8.8) | 9 (11.7) | 0 (0.0) | 0.108 |
| PLT, 103cells/μL | 186 (151–233) | 187 (149–236) | 185 (165–233) | 0.481 |
| Random glucose, mg/dL | 138 (109–177) | 138 (111–176) | 137 (105–192) | 0.762 |
| Targeted reversal strategy used | 74 (72.5) | 72 (93.5) | 2 (8.0) | <0.001 |
| Any reversal strategy used | 89 (87.3) | 72 (93.5) | 17 (68.0) | 0.003 |
| Reversal strategies used | ||||
| Vitamin K | 67 (65.7) | 66 (85.7) | 1 (4.0) | <0.001 |
| Fresh frozen plasma | 43 (42.2) | 43 (55.8) | 0 (0.0) | <0.001 |
| Prothrombin complex concentrate | 33 (32.4) | 18 (23.4) | 15 (60.0) | 0.001 |
| Recombinant activated factor VII | 43 (42.2) | 29 (37.7) | 0 (0) | <0.001 |
| Idarucizumab | 2 (2.0) | 0 (0.0) | 2 (8.0) | 0.058 |
| Radiological characteristics | ||||
| Supratentorial hemorrhage | 78 (76.5) | 56 (72.7) | 22 (82.0) | 0.175 |
| Lobar hemorrhage | 47 (46.1) | 34 (44.2) | 13 (52.0) | 0.645 |
| IVH present | 46 (45.1) | 35 (45.5) | 11 (44) | 1.000 |
Data are n (%) or median (25th–75th quartile); DBP=Diastolic blood pressure; DOAC-ICH=direct oral anticoagulation related intracerebral hemorrhage; GCS=Glasgow coma scale, GFR=Glomerular filtration rate; IVH=intraventricular hemorrhage; PLT=platelet count; SBP=Systolic blood pressure; TIA=transient ischemic attack; VKA-ICH=vitamin K antagonist related intracerebral hemorrhage.
Overall, 8 (32%) DOAC patients received no reversal agent and only two patients (8%) received a targeted reversal agent (idarucizumab). 15 (60%) DOAC patients were administered prothrombin complex concentrate of whom one (4%) received vitamin K and prothrombin complex concentrate. Among VKA-treated patients, 72 (93.5%) received at least one of the following reversal treatments: vitamin K in 66 (86%), fresh frozen plasma in 43 (56%), rFVIIa in 29 (38%), and prothrombin complex concentrate in 18 (23.4%) cases. 40 (51.9%) VKA-treated patients received 2 and 22 (28.6%) subjects received 3 reversal therapies. There were no differences in the baseline characteristics of DOAC-patients recruited from UMMMC (n=7) versus Spectrum Health (n=18; data not shown). Furthermore, there were no differences in the admission GCS (p=0.30), time to first CT (p=0.50), HV (p=0.39), presence of HV >60mL (p=0.36), HE (p=0.30), presence of substantial HE (p=0.49), lobar ICH location (p=0.20), CMO status (p=1.00), availability of follow up CT (p=0.60), and 90-day mRS (p=0.53).
Time to first CT and hemorrhage location in VKA versus DOAC treated patients
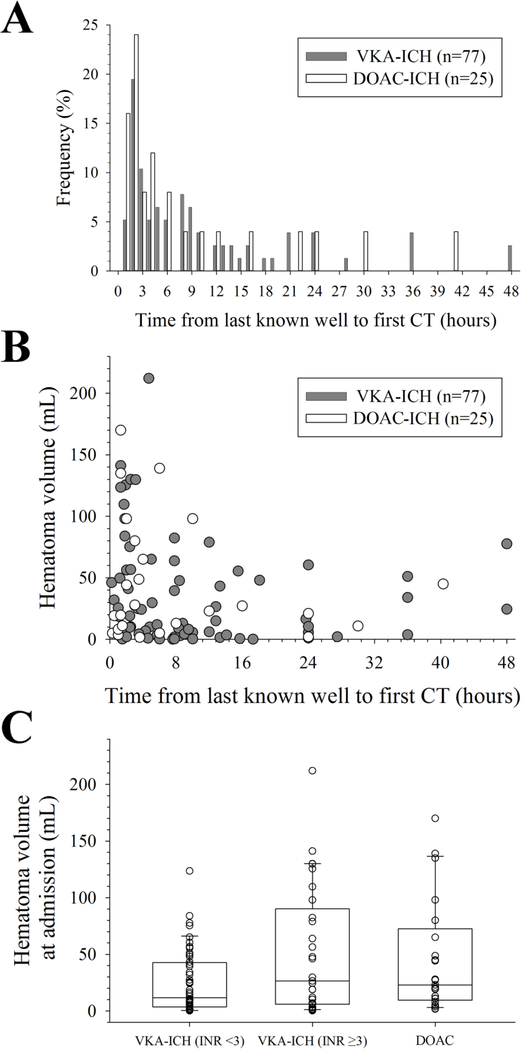
There was no difference in the time from LKW to first CT between VKA versus DOAC groups (5.7 h [IQR 2.1–13.1] versus 3.5 h [IQR 1.3–11.0]; p=0.22). Likewise, there was no difference in the relative frequency of VKA-ICH and DOAC-ICH patients presenting within each hour from LKW (p=0.65; χ2-test, Figure 2A). Lastly, there was no difference in the association between the (cube-root transformed) HV and time from LKW to CT between VKA-ICH and DOAC-ICH (p=0.19; ANCOVA, Figure 2B). The median time from admission CT to follow up CT was 13.5 h (IQR 5.6 to 23.2 h) and there was no difference in the time from admission CT to follow up CT between VKA and DOAC groups (p=0.744). Overall, lobar hemorrhage location (p=0.65), supratentorial hemorrhage location (p=0.18), and intraventricular extension (p=1.00) were similar between VKA-ICH and DOAC-ICH groups (Table 1).
Figure 2. Imaging and hematoma characteristics.
(A) There was no difference in the relative frequency of vitamin K antagonist related intracerebral hemorrhage (VKA-ICH) and direct oral anticoagulation related intracerebral hemorrhage (DOAC-ICH) patients presenting within each hour from last known well (LKW; p=0.65; χ2-test). (B) Similar association between (the cube-root transformed) hematoma volume (HV) and time from LKW to CT between VKA-ICH and DOAC-ICH (p=0.19; ANCOVA). (C) Absent difference in HV on admission between DOAC-ICH and VKA-ICH when stratified according to the INR (p=0.07; ANOVA on ranks).
HV on admission in VKA versus DOAC treated patients
We found no difference in the HV on admission between VKA-ICH and DOAC-ICH (18.5 mL [IQR 3.9–53.3] versus 23.0 mL [IQR 9.45 – 72.5]; p=0.19). Results were similar when we further stratified VKA-ICH according to the INR by <3 versus ≥3 (overall test statistics p=0.07; Figure 2C) and initial HV (≤60 mL versus >60mL; p>0.05 for VKA versus DOAC in each stratum). Lastly, there was no difference in the initial HV between anticoagulant groups for patients who had their head CT within 6 hours (n=57; p=0.57), 3 hours (n=39; p=0.82), and 1 hour (n=8; p=0.06) from LWK.
HE in VKA versus DOAC treated patients
The number of patients that were made CMO or died before a second CT was obtained was similar (p=0.53, χ2-test) between VKA-ICH (n=11 [14.3%]) and DOAC-ICH groups (n=5 [20.0%]). In 16 (20.8%) VKA-treated patients no follow up CT was obtained because the patient remained clinically stable throughout hospitalization (and all survived to the 90-day follow up).
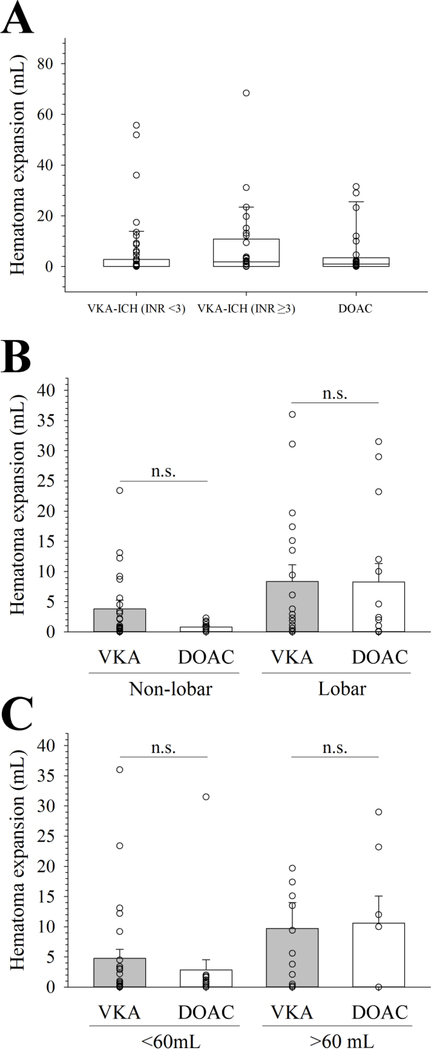
In the complete case analysis (with missing data imputed) there was no difference in median volume of HE between VKA-ICH and DOAC-ICH (0.2 mL [IQR 0.0–4.2] versus 1.0 mL [IQR 0.0–3.5]; p=0.33). Results were similar when we excluded DOAC patients that received idarucizumab (p=0.48) as well as when we stratified VKA-ICH according to the INR by <3 versus ≥3 (Figure 3A), lobar versus non-lobar hemorrhage location (Figure 3B), and the initial HV (≤60 mL versus >60mL; Figure 3C), respectively (p≥0.05 for all multigroup comparisons). There was no difference in the HE between anticoagulant groups for patients who had their head CT within 6 hours (n=57; p=0.91), 3 hours (n=39; p=0.82), and 1 hour (n=8; p=0.69) from LWK. Lastly, there was no statistical difference in the number of patients with substantial HE between VKA-ICH and DOAC-ICH groups (18.2% versus 8.0%; p=0.35).
Figure 3. Hematoma expansion stratified by oral anticoagulant strategy, hematoma location, and initial hematoma volume.
(A) Similar hematoma expansion between DOAC-ICH and VKA-ICH when stratified according to the INR (p=0.05; ANOVA on ranks). (B) There was no difference in hematoma expansion (HE) between vitamin K antagonist (VKA) related intracranial hemorrhage and direct oral anticoagulant (DOAC) related intracranial hemorrhage when stratified by lobar versus non-lobar hematoma location (overall test statistics p=0.27). (C) Similar HE in DOAC related intracranial hemorrhage and VKA related intracranial hemorrhage stratified by the initial HV of ≤60 mL versus >60mL (overall test statistics p=0.19).
To account for the fact that 50% of patients without available follow up CT were made CMO or died we conducted a sensitivity analyses that excluded these patients (n=16). Overall, results did not meaningfully change from the complete case analyses: there was no difference in median HE volume between patients treated with VKA and DOAC (0.7 mL [IQR 0.0–6.8] vs. 1.7 mL [IQR 0.6–8.7]; p>0.05) and the presence of substantial HE was similar between groups (21.2% versus 10.0%; p=0.34). When we further restricted these analyses to patients with available follow up CT (n=70), we found no difference in median HE volume between patients treated with VKA and DOAC (2.3 mL [IQR 0.2–12.2] vs. 1.7 mL [IQR 0.6–7.3]; p=0.79) and the presence of substantial HE was similar between groups (28.0% versus 10.0%; p=0.13). There was no difference in HE between DOAC-ICH and VKA-ICH groups when we further stratified VKA-ICH according to the INR by <3 versus ≥3, lobar versus non-lobar hemorrhage location, and the initial HV (≤60 mL versus >60mL), respectively (p>0.05 for all multigroup comparisons). There was no difference in the HE between anticoagulant groups for patients who had their head CT within 6 hours (n=37; p=0.36), 3 hours (n=26; p=0.46), and 1 hour (n=4; p=0.33) from LWK. Finally, results did not meaningfully change when we further excluded DOAC patients treated with idarucizumab (not shown).
Factors associated with the 90-day outcome
In univariable analyses, patients with an unfavorable 90-day outcome (mRS 4–6; n=59 [58%]) had a lower admission GCS (p<0.001), higher HV at admission (p<0.001), worse HE (p=0.009), earlier admission CT (p=0.012), and more frequently had a HV >60 mL (p<0.001) and presence of intraventricular hemorrhage (p<0.001) than patients with a favorable 90-day outcome (there was no significant difference between patients with a favorable versus unfavorable 90-day outcome in any other baseline and imaging-related variable; p>0.05, each). Results were similar when we restricted our analyses to patients with available follow up information (data not shown).
In unadjusted analyses, there was a shift towards a lower 90-day mRS in DOAC-ICH patients as compared to VKA-ICH patients (p=0.017). After adjustment, a lower admission GCS (p<0.001) and greater HV (p=0.046), but not the oral anticoagulant strategy (p=0.15), were independently associated with an unfavorable 90-day outcome in our cohort (Table 2). After exclusion of subjects that were lost to follow up (n=28) only a lower admission GCS (p=0.001) and the HV (p=0.040) were significantly associated with an unfavorable 90-day outcome (Table 2).
Table 2.
Multivariable logistic regression analysis for factors independently associated with a poor 90-day outcome*
| Independent variable | Crude OR (95% CI) | P-value | Adjusted OR (95%-CI) | P-value |
|---|---|---|---|---|
| All subjects† | ||||
| GCS (per point)** | 1.87 (1.43–2.43) | <0.001 | 1.63 (1.26–2.10) | <0.001 |
| HV (per mL) | 1.05 (1.03–1.07) | <0.001 | 1.03 (1.00–1.05) | 0.046 |
| Vitamin K antagonist treatment | 2.11 (0.84–5.26) | 0.110 | 2.85 (0.69–11.86) | 0.150 |
| Subjects with available follow up mRS (n=74)‡ | ||||
| GCS (per point)** | 1.78 (1.36–2.32) | <0.001 | 1.54 (1.19–1.98) | 0.001 |
| HV (per mL) | 1.05 (1.02–1.08) | <0.001 | 1.03 (1.00–1.05) | 0.040 |
| Vitamin K antagonist treatment | 2.40 (0.90–6.41) | 0.082 | -- | -- |
The 90-day modified Rankin score (mRS) was imputed in n=28 subjects by carrying forward the discharge mRS.
We inverted the Glasgow coma scale (GCS) score for more intuitive interpretation of the odd ratios. Thus, odds ratios indicate the increased odds of vitamin K antagonist treatment, for a 1 point decrease on the Glasgow coma scale (GCS), or a 1 mL increase in the hematoma volume (HV). We used p<0.05 as criteria for backward steps. Blank cells with dashes represent variables that were dropped as non-significant during stepwise selection. GCS and HV were entered into the model as continuous variables; thus the odds ratio for HV represents the estimated change in odds of the outcome for a 1 mL increase in the HV, with all other variables unchanged.
Hosmer-Lemeshow goodness of fit χ2 8.26, P=0.409.
Hosmer-Lemeshow goodness of fit χ2 3.29, P=0.915.
Discussion
The most important finding of our study was that the planimetrically quantified HV at admission as well as HE did not differ between subjects with VKA-ICH and DOAC-ICH, and that these findings were consistent across key hematoma characteristics associated with HE.
Our finding of a similar HV at admission between DOAC-ICH and VKA-ICH are consistent with previous studies.7–9,23 This is an important finding because the initial HV is considered the most powerful predictor of functional outcome and mortality in spontaneous and anticoagulant related ICH.24,25 This observation may in part explain the overall similar functional outcome between the two different anticoagulant strategies in this study, as well as previous studies comparing DOAC-ICH and VKA-ICH.9 This highlights that prediction tools that include information on the HV likely remain valid for outcome prediction after DOAC-ICH.
Early after the introduction of DOAC in clinical practice, a major concern was that DOAC-ICH would result in poor outcomes because of the absence of reversal strategies and thus unmitigated HE.6 However, consistent with recent observations, we found that HE did not differ between patients treated with VKA and DOAC. We further extend prior findings by showing that this observation was consistent across different HE definitions (absolute and relative) as well as when we stratified by key hematoma characteristics that have been associated with outcome after ICH.24–26 Furthermore, because more than 90% of patients treated with DOAC in our cohort did not receive targeted reversal therapies, our data may provide additional reassurance that lack of universal access to targeted reversal strategies,27,28 which is still reality in many rural and community hospitals,29 should not be a key determinant to render decisions regarding the choice of anticoagulant strategy.
In this respect, our data is in line with mounting preclinical and clinical data that even without targeted reversal, rates of HE, as well as overall functional outcome, is similar for DOAC-ICH and VKA-ICH.7,9,14,23,30,31 Consistent with these studies, we found no difference in the presence of an unfavorable 90-day outcome between DOAC-ICH and VKA-ICH groups, after adjustment for confounders. Furthermore, in secondary analyses, we excluded patients without follow-up CT to allow adjustment for the admission HV and rates of HE. In this subgroup analysis, we found that VKA-ICH was associated with an approximately 3.5 times greater odds of a poor 90-day outcome as compared to DOAC-ICH, which is consistent with recent observations.14 However, because this was a sensitivity analysis, the most conservative interpretation of our results is that DOAC-ICH is associated with similar 90-day functional outcomes as VKA-ICH. Further study is required to determine whether severity of DOAC-related ICH and functional outcomes may be further improved32,33 with increasing availability of targeted reversal agents for both dabigatran and direct factor Xa inhibitors,27,28 particularly since the use of rapid reversal strategies have not consistently translated to improvement in overall outcome and mortality in VKA-ICH.34 Moreover, in light of recent improvements in ICH treatment (such as aggressive blood pressure management and rapid OAC reversal),35 it would be interesting to compare patients with non-oral anticoagulant related ICH with DOAC- and VKA-ICH patients as the known differences between these ICH categories may have changed. Given the high cost of DOAC reversal agents, it would be particularly important to understand whether the natural history DOAC-ICH is worse than that of non-OAC. Lastly, although we found no difference in the HV and HE in patients who had their CT done within early time strata (6, 3, and 1 hour from LKW) we were unable to determine the exact time to reversal in treated patients. Therefore, it will be important to understand whether the time to reversal (e.g., early versus delayed) impacts ultimate outcome.
Strengths of our study relate to the detailed characterization of our cohort as well as planimetric assessment of HV and HE, which is less prone to error in the setting of irregularly shaped anticoagulant-related ICH when compared to the more commonly used ABC/2 method.15–17 In addition, we included patients from two different hospital cohorts in different areas of the country, which increases the generalizability of our results. Lastly, we conducted detailed analyses stratified by key hematoma characteristics that have been associated with the outcome after ICH including the ICH location, initial HV, and INR range; and that are frequently not reported.
Limitations of our study include those inherent to its retrospective design, for which reason results should be considered hypothesis generating only. Furthermore, our sample size was modest, which likely reduced the power of our analyses. Nevertheless, the number of included DOAC patients falls within the range of previously reported studies and in fact exceeds those of most.8,10,12 Although admission blood pressures did not differ between DOAC and VKA groups, we were unable to determine the time to adequate blood pressure in our cohort, which may be a source of unmeasured bias. A further limitation relates to the fact that DOAC-specific coagulation assay were not routinely conducted at our institution and we had no reliable information available on the time from last DOAC dose to presentation, which may have biased our results. Lastly, almost one third of patients was lost to follow up. However, this attrition rate is comparable to previous reports. Furthermore, we used a conservative approach to impute missing data and additional sensitivity analyses on patients with available follow up data were consistent with the complete case analysis.
Conclusions
In conclusion, we found no difference in HV, HE, and 90-day functional outcomes after ICH caused by DOAC versus VKA in a cohort of DOAC patients that infrequently received specific DOAC-reversal treatment. Our observations add to mounting data that DOAC are a safe alternative to VKA even in the absence of access to targeted reversal strategies, which are still not universally available.
Acknowledgments
Sources of Funding: Dr. Henninger is supported by NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Dr. Muehlschlegel is supported by 5K23HD080971 from the NIH/National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Dr. Henninger reports grants from NINDS, personal fees from Omniox, Inc, personal fees from Portola Pharmaceuticals, Inc, outside the submitted work. Dr. Muehlschlegel reports grants from NIH/NICHD, other from BARD Inc, outside the submitted work. Dr. Silver reports other from Joint Commission, other from Women’s Health Initiative, other from NIH, personal fees from Medicolegal malpractice reviews, personal fees from Ebix, personal fees from Medlink, personal fees from Medscape, outside the submitted work. Dr. Miller, Dr. Lowe, Dr. Khan, Dr. Azeem, Dr. Jun-O’Connell, Dr. Goddeau, Dr. Moonis, Dr. Gritters has nothing to disclose.
Adherence to ethical guidelines: Our investigation was approved by Institutional Review Boards, and we were granted a Health Insurance Portability and Accountability Act waiver of informed consent at the University of Massachusetts and Spectrum Health.
Use of reporting checklist: We adhere to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (www.strobe-statement.org).
Details Page
This manuscript has been prepared according to the Neurocritical Care Instructions for Authors and has not been published elsewhere and is not under consideration by another publication or electronic medium.
This research was performed at the University of Massachusetts Medical School and Spectrum Health.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use leads to larger intracerebral hematomas. Neurology 2008;71:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45 e4. [DOI] [PubMed] [Google Scholar]
- 5.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 6.Ansell J New oral anticoagulants should not be used as first-line agents to prevent thromboembolism in patients with atrial fibrillation. Circulation 2012;125:165–70; [DOI] [PubMed] [Google Scholar]
- 7.Wilson D, Seiffge DJ, Traenka C, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology 2017;88:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques-Matos C, Alves JN, Marto JP, et al. POST-NOAC: Portuguese observational study of intracranial hemorrhage on non-vitamin K antagonist oral anticoagulants. Int J Stroke 2017;12:623–7. [DOI] [PubMed] [Google Scholar]
- 9.Boulouis G, Morotti A, Pasi M, Goldstein JN, Gurol ME, Charidimou A. Outcome of intracerebral haemorrhage related to non-vitamin K antagonists oral anticoagulants versus vitamin K antagonists: a comprehensive systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2018;89:263–70. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Jimbo Y, Takano H, et al. Intracerebral Hematoma Occurring During Warfarin Versus Non-Vitamin K Antagonist Oral Anticoagulant Therapy. Am J Cardiol 2016;118:222–5. [DOI] [PubMed] [Google Scholar]
- 11.Wilson D, Charidimou A, Shakeshaft C, et al. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology 2016;86:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagii J, Tomita H, Metoki N, et al. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke 2014;45:2805–7. [DOI] [PubMed] [Google Scholar]
- 13.Kawabori M, Niiya Y, Iwasaki M, et al. Characteristics of Symptomatic Intracerebral Hemorrhage in Patient Receiving Direct Oral Anticoagulants: Comparison with Warfarin. J Stroke Cerebrovasc Dis 2018;27:1338–42. [DOI] [PubMed] [Google Scholar]
- 14.Tsivgoulis G, Lioutas VA, Varelas P, et al. Direct oral anticoagulant- vs vitamin K antagonist-related nontraumatic intracerebral hemorrhage. Neurology 2017;89:1142–51. [DOI] [PubMed] [Google Scholar]
- 15.Sheth KN, Cushing TA, Wendell L, et al. Comparison of hematoma shape and volume estimates in warfarin versus non-warfarin-related intracerebral hemorrhage. Neurocrit Care 2010;12:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb AJ, Ullman NL, Morgan TC, et al. Accuracy of the ABC/2 Score for Intracerebral Hemorrhage: Systematic Review and Analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke 2015;46:2470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M, Baird GL, Elias R, et al. Comparison of Intracerebral Hemorrhage Volume Calculation Methods and Their Impact on Scoring Tools. J Neuroimaging 2017;27:144–8. [DOI] [PubMed] [Google Scholar]
- 18.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 19.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified Rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011;42:2276–9. [DOI] [PubMed] [Google Scholar]
- 20.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis 2012;33:525–31. [DOI] [PubMed] [Google Scholar]
- 21.Helenius J, Henninger N. Leukoaraiosis Burden Significantly Modulates the Association Between Infarct Volume and National Institutes of Health Stroke Scale in Ischemic Stroke. Stroke 2015;46:1857–63. [DOI] [PubMed] [Google Scholar]
- 22.Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke 1999;30:2355–9. [DOI] [PubMed] [Google Scholar]
- 23.von der Brelie C, Doukas A, Naumann R, et al. Clinical and radiological course of intracerebral haemorrhage associated with the new non-vitamin K anticoagulants. Acta Neurochir (Wien) 2017;159:101–9. [DOI] [PubMed] [Google Scholar]
- 24.Berwaerts J, Dijkhuizen RS, Robb OJ, Webster J. Prediction of functional outcome and in-hospital mortality after admission with oral anticoagulant-related intracerebral hemorrhage. Stroke 2000;31:2558–62. [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93. [DOI] [PubMed] [Google Scholar]
- 26.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- 27.Pollack CV Jr., Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med 2017;377:431–41. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Milling TJ Jr., Eikelboom JW, et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med 2016;375:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faine BA, Amendola J, Homan J, Ahmed A, Mohr N. Factors associated with availability of anticoagulation reversal agents in rural and community emergency departments. Am J Health Syst Pharm 2018;75:72–7. [DOI] [PubMed] [Google Scholar]
- 30.Lauer A, Cianchetti FA, Van Cott EM, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011;124:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W, Zorn M, Nawroth P, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke 2013;44:771–8. [DOI] [PubMed] [Google Scholar]
- 32.Pollack CV Jr., Bernstein R, Dubiel R, et al. Healthcare resource utilization in patients receiving idarucizumab for reversal of dabigatran anticoagulation due to major bleeding, urgent surgery, or procedural interventions: interim results from the RE-VERSE AD study. J Med Econ 2017;20:435–42. [DOI] [PubMed] [Google Scholar]
- 33.van der Wall SJ, van Rein N, van den Bemt B, et al. Performance of idarucizumab as antidote of dabigatran in daily clinical practice. Europace 2018. [DOI] [PubMed] [Google Scholar]
- 34.Ko D, Razouki Z, Otis J, Marulanda-Londono E, Hylek EM. Anticoagulation reversal in vitamin K antagonist-associated intracerebral hemorrhage: a systematic review. J Thromb Thrombolysis 2018;46:227–37. [DOI] [PubMed] [Google Scholar]
- 35.Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]