Abstract
Observational studies have reported an inverse association between vitamin D intake and breast cancer risk. We examined whether vitamin D supplementation in high-risk premenopausal women reduces mammographic density (MD), an established breast cancer risk factor. We conducted a multicenter randomized double-blind placebo-controlled trial in premenopausal women at high risk for breast cancer (5-year risk≥1.67%, lifetime risk≥20%, lobular carcinoma in situ, prior stage 0-II breast cancer, hereditary breast cancer syndrome, or high MD [heterogeneously/extremely dense]), with a baseline serum 25-hydroxyvitamin D [25(OH)D]≤32 ng/mL. Participants were randomized to 12 months of vitamin D3 20,000IU/week or matching placebo. The primary endpoint was change in MD from baseline to 12 months using the Cumulus technique. Secondary endpoints included serial blood biomarkers (25(OH)D, 1,25(OH)D, insulin-like growth factor (IGF)-1, IGF binding protein-3) and MD change at 24 months. Among 208 women randomized, median age was 44.6 years, 84% were white, 33% had baseline 25(OH)D<20 ng/mL, and 78% had high baseline MD. Comparing the active and placebo groups at 12 months, MD changes were small and did not significantly differ. Mean MD changes at 12 and 24 months were −0.3% and −1.2%, respectively, in the active arm and +1.5% and +1.6% with placebo (p>0.05). We observed a mean change in serum 25(OH)D of +18.9 vs. +2.8 ng/mL (p<.01) and IGF-1 of −9.8 vs. −1.8 ng/mL (p=0.28), respectively. At 12 months, MD was positively correlated with serum IGF-1 and IGF-1/IGFBP-3 (p<0.01). This trial does not support the use of vitamin D supplementation for breast cancer risk reduction.
INTRODUCTION
Vitamin D has diverse biological effects which are potentially relevant to carcinogenesis [1]. Modest amounts of vitamin D come from food sources, but the majority of vitamin D is made when ultraviolet B (UVB) light hits a precursor molecule in the skin [2]. Vitamin D is metabolized in the liver to yield 25-hydroxyvitamin D [25(OH)D], the major circulating form, and in the kidney to produce 1,25(OH)D, the biologically active form which binds to vitamin D receptor (VDR) to locally regulate cell turnover [3, 4]. Cholecalciferol (vitamin D3) is the form which is naturally produced in the body and is also available as a dietary supplement.
Many observational studies support an inverse association between vitamin D status and breast cancer risk, which has resulted in increased interest in the use of vitamin D for breast cancer prevention [5, 6]. Vitamin D deficiency (defined as serum 25(OH)D concentrations below 20 ng/mL) is common, especially in the elderly, blacks, and residents of northern climates [7]. In 2011, the Institute of Medicine (IOM) set the recommended dietary allowance (RDA) of vitamin D at 600 IU/day for women <70 years of age, based on skeletal health and prevention of fractures [8]. Based upon pooled analysis from two large observational studies conducted in the U.S. and U.K., women with serum 25(OH)D concentrations greater than 50 ng/mL had a 50% lower risk of breast cancer compared to women with vitamin D deficiency [9]. Oral daily intake of 1,000 IU of vitamin D increases circulating 25(OH)D by about 10 ng/mL [10]. Given the high prevalence of vitamin D deficiency in the general population, in order to raise serum 25(OH)D above 40–50 ng/mL (the putative target level for breast cancer risk reduction), women would have to consume about 3000–4000 IU/day [10].
Mammographic density (MD) refers to the relative proportions of radiolucent fat and radiodense connective tissue and glandular epithelium within the breast seen on mammogram [11]. Epidemiologic studies have consistently supported that women in the highest quartile of MD demonstrated a 4-to-6-fold increase in breast cancer incidence compared to those in the lowest quartile [12, 13]. MD may be assessed qualitatively by BI-RADS category (1=“almost entirely fat”, 2=“scattered fibroglandular densities”, 3=“heterogeneously dense”, 4=“extremely dense”). However, by using a well-validated computer-assisted method (Cumulus software, University of Toronto) to assess percent density as a continuous measure (0–100% scale), more subtle changes in MD can be detected. Some observational studies demonstrated that increases in dietary vitamin D intake were associated with decreases in MD [14]. In premenopausal women, daily total intakes of 400 IU of vitamin D and 1,000 mg of calcium were associated with an 8.5% lower mean MD [15]. The inverse association of vitamin D with MD was seen primarily in women with high insulin-like growth factor (IGF)-1 or high IGF binding protein (IGFBP)-3 levels [16].
The purpose of this trial was to evaluate the effect of vitamin D supplementation on MD in premenopausal women at high risk for breast cancer. We also evaluated the effects of vitamin D 20,000 IU/week on blood-based biomarkers associated with breast cancer risk (IGF-1, IGFBP-3) and safety. We hypothesized that vitamin D supplementation for 12 months in premenopausal women at high-risk for breast cancer would decrease MD, circulating IGF-1 and IGFBP-3 compared to placebo.
MATERIALS AND METHODS
The study was registered with ClinicalTrials.gov ( NCT01097278). Participants were informed of the investigational nature of the study and signed informed consent. The study was conducted after appropriate approval by individual institutional review boards in compliance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Participant characteristics
Participants were recruited from over 20 sites throughout the U.S. Women were between the ages of 18 and 50 years and premenopausal, defined as <6 months since their last menstrual period, no prior bilateral oophorectomy, and no use of hormone replacement therapy. Women with a prior hysterectomy and intact ovaries had to have serum follicle stimulating hormone (FSH) values consistent with the institutional normal values for the premenopausal state within 28 days prior to registration. Participants had to meet at least one of the following criteria for an elevated risk of breast cancer: 1) 5-year invasive breast cancer risk ≥1.67% or lifetime risk ≥20% according to the Gail, Tyrer-Cuzick, Claus, or BRCAPRO models, 2) atypical ductal or lobular hyperplasia or lobular or ductal carcinoma in situ, 3) prior stage I-II breast cancer and disease-free for at least 5 years, 4) known germline pathogenic variant in BRCA1, BRCA2, PTEN, or TP53, 5) baseline mammographic density (MD) of heterogeneously or extremely dense breasts. Other eligibility criteria included: 1) at least one breast evaluable for imaging without breast implant; 2) baseline serum 25(OH)D ≤32 ng/mL; 3) serum creatinine and serum calcium or corrected calcium below the institutional upper limit of normal (IULN) within 28 days prior to registration; 4) Zubrod performance status of 0 or 1. Women were excluded for: 1) baseline MD of “almost entirely fat”, 2) current use of calcium or vitamin D supplements; 3) tamoxifen use <28 days prior to registration, 4) prior kidney stones; 5) known hypersensitivity to vitamin D or soybean lecithin; 6) pregnant or lactating.
Study Design
We conducted a randomized, double-blind, multi-center trial of oral cholecalciferol (vitamin D3) 2 capsules/week (PRO-PHARMA LLC) versus matching placebo for 12 months. Each active capsule contained 10,000 IU of cholecalciferol in soybean lecithin. Each placebo capsule contained identical ingredients except without the cholecalciferol. Since all participants had insufficient levels of serum 25(OH)D (≤32 ng/mL) at baseline, both groups were given a standard dose of oral vitamin D3 600 IU/day (Solgar) for 12 months, in addition to the active and placebo capsules. Participant randomization was stratified by baseline serum 25(OH)D (<20 versus 20–32 ng/mL) and baseline MD (scattered fibroglandular densities versus heterogeneously/extremely dense). Mammograms were conducted at baseline, 12 and 24 months and timed within 10 days after starting menstrual bleeding (if possible). Fasting serum was collected at baseline, 6 and 12 months, stored at −800C and tested centrally for blood biomarker analyses. Participants had follow-up visits every 3 months for a year and 1-month post-intervention to assess study drug adherence by pill diaries/pill counts and toxicities using CTCAE (NCI Common Terminology Criteria for Adverse Events) Version 4.0. During the 12-month study intervention, clinical blood and urine samples were collected every 3 months to measure serum calcium and albumin (to calculate corrected serum calcium, which was serum calcium + [(4.0 – serum albumin) × 0.8]) and spot urine caclium and creatinine to assess for hypercalcemia (defined as corrected calcium above the institutional upper limit of normal [IULN]) and hypercalciuria (defined as spot urine calcium/creatinine ratio <0.37), respectively. There was also external monitoring of serum 25(OH)D every 3 months by a central laboratory (Quest) to assess for vitamin D toxicity (or 25(OH)D >80 ng/mL, which is defined as the IULN by the central laboratory) and allow for dose reduction to vitamin D3 10,000 IU/week.
Outcome Measures
Mammographic percent density (proportion of the breast with dense tissue) from cranio-caudal views was assessed using semi-automated methods with the Cumulus software [17]. All MD readings were conducted by a trained reader blinded to treatment assignment and the timing of the mammograms (baseline, 12 or 24 months). Baseline and follow-up mammograms from the same women were analyzed within the same batch and we randomized the order of assessment of digitized images. Only baseline mammograms from women with a follow-up 12- or 24-month mammogram were analyzed for MD measurements. We repeated an additional 10% of mammograms in each batch to estimate batch-to-batch variability. For percent density, the overall within-batch correlation coefficient was 0.98, and the between-batch correlation coefficient was 0.94 [18].
Serum 25(OH)D and 1,25(OH)2D were assessed by an LC/MS method that quantitatively provided measurements of vitamin D2 and D3 metabolites. Serum parathyroid hormone (PTH) was measured by a standard 2-site immunoradiometric assay (Scantibodies Laboratory, Santee, CA) that detects only whole PTH,1–84 and does not measure inactive PTH fragment [19]. Serum IGF-1 and IGFBP-3 levels were assayed by ELISA analysis with reagents from Diagnostic Systems Laboratories. The interassay coefficient of variation for serum 25(OH)D and 1,25(OH)2D was <10%. Inter- and intra-assay precision for PTH were 6.3% and 2.8%, respectively. For IGF-1 and IGFBP-3, intra-assay precision were 3.5% and 1.0%, respectively. All blood samples were analyzed in the Biomarkers Core facility at Columbia University Irving Medical Center in batches by personnel blinded to study assignment and time point of blood collection.
Statistical Considerations
The primary endpoint was the absolute difference in change in MD from baseline to 12 months across study arms. Assuming a standard deviation of 4% [20] and a 15% unevaluable rate, a sample size of 200 women (100 per arm) was sufficient for 90% power to detect a 2% absolute difference in change in MD at 12 months between the active and placebo groups. The primary analysis was based on the intent-to-treat principle, comparing mean breast density at 12 months between the active and placebo group in a linear regression model controlling for baseline serum 25(OH)D and MD. Secondary endpoints included change in MD at 24 months, change in serum-based biomarkers (25(OH)D, 1,25(OH)D, PTH, IGF-I, IGFBP-3) at 6 and 12 months, and toxicities. Frequency distributions and summary descriptive statistics were calculated for all biomarkers in the treatment groups. After appropriate transformation, repeated measures analyses were performed on serum biomarker levels collected at baseline and follow-up. If missing values were low (<5%), the primary analyses proceeded under a missing at random assumption. If the rate of missingness was higher, analyses were conducted to determine whether the missingness was correlated with randomization assignment or baseline characteristics. If such associations were found, multiple imputations were used, with the imputation model based on the observed correlation structure. Additional exploratory analyses correlated serum biomarker levels and/or changes in levels from baseline to 12 months with changes in MD at 12 months.
RESULTS
From December 2011 to April 2014, 208 women were accrued (Figure 1). Baseline characteristics of participants evaluable for toxicities by intervention assignment are shown in Table 1. The Supplemental Table includes baseline characteristics for participants evaluable for the primary endpoint of MD at 12 months. No notable imbalances by arm were observed by age, race/ethnicity, body mass index (BMI), or breast cancer risk status. At baseline, about a third of the women had vitamin D deficiency (serum 25(OH)D <20 ng/mL) and nearly 80% had high MD (heterogeneously or extremely dense).
Figure 1.
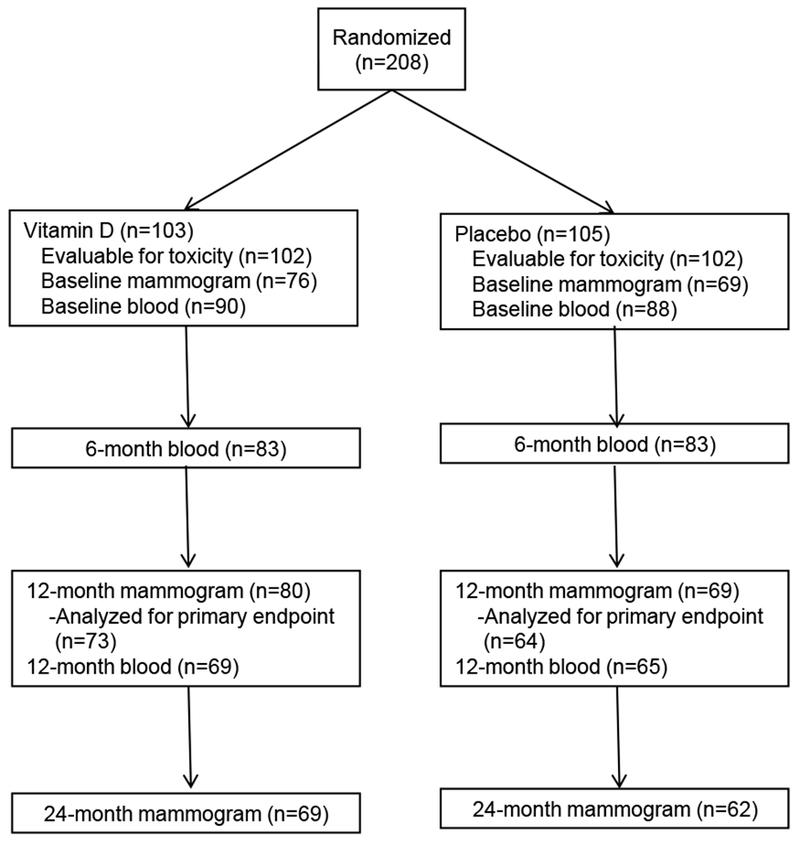
CONSORT Diagram
Table 1:
Baseline Participant Characteristics
| Vitamin D (n=102) | Placebo (n=102) | |
|---|---|---|
| Median age, years (range) | 44.3 (27-49) | 44.9 (21-50) |
| Race, N (%) | ||
| White | 86 (84) | 85 (83) |
| Black | 6 (6) | 6 (6) |
| Asian | 4 (4) | 7 (7) |
| Native American | 1 (1) | 0 |
| Multi-Racial | 4 (4) | 0 |
| Unknown | 1 (1) | 4 (4) |
| Hispanic, N (%) | 11 (11) | 6 (6) |
| Median body mass index, kg/m2 (range) | 25.2 (18.8-42.6) | 26.1 (18.6-45.8) |
| Breast cancer risk status, N (%)* | ||
| 5-year breast cancer risk ≥1.67% | 46 (45) | 41 (40) |
| Lifetime breast cancer risk ≥20% | 26 (25) | 31 (30) |
| Atypical hyperplasia/LCIS | 16 (16) | 20 (20) |
| Prior stage 0-II breast cancer | 7 (7) | 5 (5) |
| High mammographic density** | 80 (78) | 82 (80) |
| Baseline serum 25(OH)D, N (%) | ||
| <20 ng/mL | 33 (32) | 33 (32) |
| 20-32 ng/mL | 69 (68) | 69 (68) |
| Baseline mammographic density, N (%) | ||
| Scattered fibroglandular densities | 22 (22) | 20 (20) |
| Heterogeneously/extremely dense | 80 (78) | 82 (80) |
Abbreviations: 25-hydroxyvitamin D (25(OH)D), lobular carcinoma in situ (LCIS)
Some women met multiple high-risk criteria.
Baseline mammogram with heterogeneously or extremely dense breasts.
Mean baseline MD was comparable between the active and placebo groups (38.6% versus 35.0%, respectively). Change in MD was assessed in participants with available baseline and at least one follow-up (12-month or 24-month) mammogram (Table 2). Comparing the vitamin D and placebo arms, mean MD changes at 12 months were −0.3% (SD 8.0%) and +1.5% (SD 8.8%), respectively, and at 24 months were −1.2% (SD 8.0%) and +1.6% (SD 10.3%), respectively. The differences in MD change between the active and placebo groups were not statistically significant.
Table 2:
Mammographic density and serum biomarkers at baseline and change at 6, 12 and 24 months compared to baseline
| Vitamin D | Placebo | |||||
|---|---|---|---|---|---|---|
| Outcome | Timeframe | N | Mean (SD) | N | Mean (SD) | P-value |
| Mammographic density (%) | Baseline | 76 | 38.6 (18.0) | 69 | 35.0 (19.0) | 0.24 |
| Change at 12 months | 73 | −0.3 (8.0) | 64 | 1.5 (8.8) | 0.22 | |
| Change at 24 months | 63 | −1.2 (8.0) | 57 | 1.6 (10.3) | 0.10 | |
| Serum 25(OH)D, ng/mL | Baseline | 90 | 23.9 (7.2) | 88 | 23.7 (8.4) | 0.93 |
| Change at 6 months | 76 | 19.2 (10.6) | 72 | 5.8 (10.9) | <.01 | |
| Change at 12 months | 64 | 18.9 (8.9) | 62 | 2.8 (8.0) | <.01 | |
| Serum 1,25(OH)D, pg/mL | Baseline | 90 | 51.4 (23.6) | 88 | 50.8 (22.5) | 0.87 |
| Change at 6 months | 76 | 48.8 (33.6) | 72 | 13.0 (27.8) | <.01 | |
| Change at 12 months | 64 | 42.7 (26.0) | 62 | 5.2 (16.7) | <.01 | |
| Serum PTH, pg/mL | Baseline | 87 | 34.0 (18.7) | 88 | 34.0 (19.0) | 0.99 |
| Change at 6 months | 74 | 4.5 (15.7) | 72 | 0.2 (16.6) | 0.09 | |
| Change at 12 months | 61 | −7.2 (15.7) | 61 | 0.5 (17.3) | 0.01 | |
| Serum IGF-I, ng/mL | Baseline | 90 | 158.8 (67.0) | 89 | 140.8 (56.6) | 0.05 |
| Change at 6 months | 77 | −6.2 (42.2) | 73 | 3.9 (30.8) | 0.10 | |
| Change at 12 months | 64 | −9.8 (46.6) | 62 | −1.8 (34.3) | 0.28 | |
| Serum IGFBP-3, ug/mL | Baseline | 90 | 5.1 (1.0) | 89 | 5.0 (1.0) | 0.53 |
| Change at 6 months | 77 | 0.1 (0.7) | 73 | 0.1 (0.6) | 0.72 | |
| Change at 12 months | 64 | −0.2 (0.7) | 62 | 0.04 (0.8) | 0.07 | |
| IGF-I/IGFBP-3 ratio (× 10−3) | Baseline | 90 | 30.9 (11.3) | 89 | 28.3 (10.5) | 0.11 |
| Change at 6 months | 77 | −1.3 (8.6) | 73 | 0.04 (7.1) | 0.32 | |
| Change at 12 months | 64 | −0.2 (9.8) | 62 | −0.9 (7.6) | 0.65 | |
Abbreviations: 1,25-dihydroxyvitamin D (1,25(OH)D), 25-hydroxyvitamin D (25(OH)D), insulin-like growth factor (IGF)-I, IGF binding protein (IGFBP)-3, parathyroid hormone (PTH)
In terms of the blood-based biomarkers (Table 2), there was a significant increase in serum 25(OH)D concentrations at 12 months between the active and placebo arms (+18.9 vs. +2.8 ng/mL, respectively) and increase in serum 1,25(OH)D (+42.7 versus +5.2 pg/mL, respectively). In the vitamin D intervention arm, mean serum 25(OH)D rose from 23.9 ng/mL at baseline to 43.7 ng/mL at 12 months. There were nonsignificant decreases in serum IGF-1 at 12 months between the vitamin D and placebo groups (−9.8 versus −1.8 ng/mL, respectively) and serum IGFBP-3 (−0.20 vs +0.04 ug/mL, respectively). We observed a significant positive correlation between 12-month MD and 12-month serum IGF-1 (correlation coefficient=0.39; p<0.0001) and 12-month IGF-1/IGFBP-3 ratio (correlation coefficient=0.37; p<0.0001) in all participants.
Adherence (defined as taking at least 80% of study agent doses) was comparable between the vitamin D and placebo groups (76% versus 73%, respectively). The study agents were well tolerated with mainly grade 1 and 2 toxicities (Table 3). We observed mainly gastrointestinal toxicities with vitamin D 20,000 IU/week, including abdominal pain and constipation. Only one episode of hypercalcemia and 2 episodes of hypercalciuria occurred in the vitamin D group. The patient who developed hypercalcemia at 3 months did not experience any additional toxicities. There was one episode of vitamin D toxicity (defined as serum 25(OH)D >80 ng/mL) observed during the trial requiring dose reduction of study agent. This patient experienced grade 1 nausea and no other toxicities. The toxicities were comparable in frequency and severity between the active and placebo groups.
Table 3:
Toxicity by treatment arm
| Vitamin D (n=102) | Placebo (n=102) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||
| ADVERSE EVENTS | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Abdominal distension | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Abdominal pain | 97 | 3 | 2 | 0 | 99 | 3 | 0 | 0 |
| Alkaline phosphatase increased | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Anorexia | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Bilirubin increased | 102 | 0 | 0 | 0 | 101 | 0 | 1 | 0 |
| Bloating | 102 | 0 | 0 | 0 | 100 | 1 | 1 | 0 |
| Breast pain | 102 | 0 | 0 | 0 | 100 | 1 | 1 | 0 |
| Constipation | 95 | 6 | 1 | 0 | 101 | 1 | 0 | 0 |
| Dizziness | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Dry mouth | 102 | 0 | 0 | 0 | 101 | 1 | 0 | 0 |
| Dry skin | 102 | 0 | 0 | 0 | 101 | 1 | 0 | 0 |
| Dyspepsia | 102 | 0 | 0 | 0 | 100 | 2 | 0 | 0 |
| Fatigue | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Flatulence | 102 | 0 | 0 | 0 | 100 | 1 | 1 | 0 |
| GI disorders (unspecified) | 102 | 0 | 0 | 0 | 101 | 0 | 1 | 0 |
| Headache | 100 | 0 | 1 | 1 | 102 | 0 | 0 | 0 |
| Hot flashes | 102 | 0 | 0 | 0 | 101 | 0 | 1 | 0 |
| Hypercalcemia | 102 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Hypercalciuria | 100 | 1 | 1 | 0 | 101 | 1 | 0 | 0 |
| Hypertension | 102 | 0 | 0 | 0 | 100 | 1 | 1 | 0 |
| Insomnia | 101 | 0 | 1 | 0 | 102 | 0 | 0 | 0 |
| Nail ridging | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Nausea | 99 | 3 | 0 | 0 | 98 | 4 | 0 | 0 |
| Pain in extremity | 101 | 1 | 0 | 0 | 101 | 1 | 0 | 0 |
| Palpitations | 101 | 1 | 0 | 0 | 102 | 0 | 0 | 0 |
| Vomiting | 101 | 1 | 0 | 0 | 101 | 1 | 0 | 0 |
| Weight loss | 102 | 0 | 0 | 0 | 101 | 1 | 0 | 0 |
| Maximum grade, any adverse event | 76 | 19 | 6 | 1 | 84 | 12 | 6 | 0 |
No grade 4 or 5 adverse events were reported
DISCUSSION
After a year of vitamin D3 20,000 IU/week in premenopausal women at high-risk of breast cancer, changes in mammographic density (MD) at 12 and 24 months were small and did not differ significantly between the active and placebo arms. Compared to standard-dose vitamin D alone, the addition of vitamin D3 20,000 IU/week led to a significant increase in serum 25(OH)D, the main circulating form, and serum 1,25(OH)D, the activated form of vitamin D. There were also non-significant decreases in serum IGF-1 and IGFBP-3 at 12 months with vitamin D supplementation, which correlated with MD at 12 months. Cholecalciferol at a dose of 20,000 IU/week for a year was well-tolerated.
Numerous observational studies have reported an inverse association between various measures of vitamin D status, including sunlight exposure, dietary intake, supplement use, and circulating levels of 25(OH)D [21]. However, most of these studies were based upon a single measurement of serum 25(OH)D and no prospective studies to date have demonstrated that changes in vitamin D status over time alter breast cancer risk. A systematic review of 14 observational studies examining the association between vitamin D status and MD, a strong predictor of breast cancer risk, yielded inconsistent results [22]. However, the association between vitamin D and MD was more pronounced in premenopausal women and those with high serum IGF-1 levels, suggesting that vitamin D may modulate MD via IGF signaling [16].
Our results of the effects of vitamin D on MD are consistent with prior studies. The Women’s Health Initiative (WHI), which randomized postmenopausal women to calcium plus vitamin D 400 IU/day or placebo, found no significant difference in MD after a year of supplementation [23]. However, the ratio of mean MD comparing calcium/vitamin D and placebo was 0.67 (95% CI=0.41–1.07) in those with ≥80% drug compliance and no hormone replacement therapy use. Two prior randomized controlled trials, which examined 1 year of vitamin D3 1000–3000 IU/day in premenopausal women with high baseline MD (>20–25%), found no difference in change in MD at 1 year compared to placebo [24, 25]. In the current trial, we observed a 1.8% absolute difference in change in MD between the active and placebo groups at 12 months and 2.8% at 24 months, however, these differences were not statistically significant. Possible explanations for these null findings include the need for longer exposure of vitamin D or the fact that the potential cancer protective effect of vitamin D may not be mediated by changes in MD.
Observational studies have demonstrated that women with decreases in MD over time were less likely to develop breast cancer compared to those who had no change or an increase in MD [26–29]. In a cohort of women at high-risk for breast cancer, a 1.62% decrease per year in MD as measured by the Cumulus technique was associated with a lower likelihood of breast cancer (p=0.004) [30]. A proven breast cancer chemopreventive agent, tamoxifen, was shown to significantly reduce MD within 12–18 months of initiation compared to placebo [31]. Women who experienced at least a 10% absolute decrease in MD within 12–18 months on tamoxifen had a 63% reduction in breast cancer risk compared to placebo [32], suggesting that MD may serve as a surrogate endpoint for short-term breast cancer risk assessment in early phase chemoprevention trials. However, the effects of non-hormonal agents on MD remain uncertain.
In terms of clinical trials of vitamin D supplementation with cancer incidence as the primary endpoint, two randomized placebo-controlled trials of combined calcium and vitamin D were conducted in average-risk postmenopausal women. In the WHI trial, in which over 36,000 postmenopausal women were randomized to 1000 mg of calcium carbonate and 400 IU of vitamin D3 daily versus placebo, breast and colorectal cancer incidence did not differ between the two groups after a mean follow-up of 7 years [33, 34]. In a nested case-control analysis of the WHI, baseline serum 25(OH)D was inversely associated with breast cancer risk, but this association did not persist after adjustment for BMI and physical activity [33]. In another trial conducted by Lappe et al., 1179 postmenopausal women were randomized to calcium and vitamin D 1100 IU/day for 4 years [35]. The authors found a 60% relative risk reduction in overall cancer incidence with calcium plus vitamin D compared to placebo. However, neither of these trials could distinguish between the effects of calcium and vitamin D. More recent randomized controlled trials are examining the effects of higher doses of vitamin D on cancer incidence [36]. The Vitamin D Assessment (ViDA) study conducted in New Zealand randomized 5110 adults, age 50–84 years, to an oral loading dose of vitamin D3 200,000 IU followed by 100,000 IU/month vs. placebo for a median of 3.3 years (range, 2.5–4.2 years) [37]. There was no difference in all cancer incidence between the vitamin D and placebo groups (6.5% vs. 6.4%, p=0.95). In VITAL (VITamin D and omega-3 triaL), over 25,000 U.S men and women over age 50 years were randomized to vitamin D3 2000 IU/day, omega-3 fatty acids 1 capsule (465mg eicosapentaenoic acid, 375mg docosahexaenoic acid)/day, either alone or in combination compared to placebo with cancer incidence as a primary endpoint [38]. With a median follow-up of 5.3 years, there were no differences between the vitamin D and placebo groups in cancer incidence (hazard ratio [HR], 0.96; 95% confidence interval [CI], 0.88–1.06; p=0.47) or cancer-related deaths (HR, 0.83; 95% CI, 0.67–1.02), including breast cancer.
Concerns for vitamin D toxicities include hypercalcemia, hypercalciuria, and nephrocalcinosis [39]. In addition, the WHI trial reported a 17% relative increase in the incidence of kidney stones with calcium and vitamin D 400 IU/day compared to placebo (2.5% vs. 2.1%, respectively) [40]. In our trial, the active intervention arm received a total dose of vitamin D3 of nearly 3500 IU/day, which was well tolerated in our study population of high-risk premenopausal women. Consistent with our findings, another study found that vitamin D3 4000 IU/day for 5 months given to healthy individuals raised serum 25(OH)D to sufficient levels without causing significant toxicities [41]. In a study which evaluated vitamin D supplement use and serum 25(OH)D among 17,614 healthy adults, doses of vitamin D over 20,000 IU/day did not cause significant vitamin D toxicity based upon serum 25(OH)D levels [42]. In a review of clinical trials on the safety of vitamin D supplementation, the authors concluded that doses of up to 10,000 IU/day are safe [43]. However, some observational studies have found a non-linear U-shaped relationship between cancer incidence rates and serum 25(OH)D concentrations [44, 45].
To our knowledge this is the first randomized controlled trial to assess the effects of vitamin D supplementation in premenopausal women at high-risk for breast cancer. Strengths of our study include the use of a placebo control, prospective follow-up for up to 2 years, collection of serial blood samples and mammograms, and use of a well-validated method for MD measurements which was timed with the women’s menstrual cycles. In contrast to prior trials which examined the effects of vitamin D on MD in premenopausal women [24, 25], we targeted premenopausal women who met high-risk criteria for breast cancer and had insufficient baseline levels of serum 25(OH)D. We also used a higher dose of vitamin D and did not allow personal vitamin D supplement use during the trial (except standard dose vitamin D 600 IU/day which was supplied to both the active and placebo groups). However, our study also had several limitations. We had a higher than anticipated unevaluable rate for MD and blood biomarker analyses. We also had higher variability in MD measurements, which may be due to intra-individual differences due to fluctuations with the menstrual cycle and compression of the breasts with mammography.
Based upon the results of our trial, there is insufficient evidence to support the use of vitamin D supplementation for breast cancer risk reduction among high-risk premenopausal women. Increasing awareness about vitamin D deficiency has led many physicians to routinely test for serum 25(OH)D levels and recommend supplementation for their patients. Despite promising observational data, randomized controlled trials of vitamin D supplementation have not demonstrated a significant decrease in cancer incidence compared to placebo. Similarly, other dietary supplements (e.g., beta carotene, vitamin E, folic acid, and selenium) have not shown a reduction in cancer incidence in randomized controlled trials despite findings of association with reduced risk in observational studies [46–50]. Therefore, we must rely on the results of rigorously conducted randomized controlled trials before making broad recommendations for dietary supplement use for cancer prevention.
Supplementary Material
Acknowledgements
This work supported by the National Institutes of Health, National Cancer Institute awards UG1CA189974, UG1CA190002, UG1CA189830, UG1CA189858, UG1CA189953, UG1CA189960, U10CA180858, U10CA180828, U10CA180819; and by ASCO Conquer Cancer Foundation Career Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Crew KD: Vitamin d: are we ready to supplement for breast cancer prevention and treatment? ISRN Oncol 2013, 2013:483687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webb GP: Dietary Supplements and Functional Foods, 2nd edn Oxford, U.K: Blackwell; 2011. [Google Scholar]
- 3.Eitenmiller RR, Ye L, Landen WO: Vitamin Analysis for the Health and Food Sciences, 2nd edn Boca Raton, FA: CRC Press; 2008. [Google Scholar]
- 4.Hewison M, Adams JS: Extra-renal 1alpha-hydroxylase activity and human disease. In: Vitamin D. 2nd edn Edited by Feldman D, Pike JW, Glorieux FH. San Diego, CA: Elsevier; 2005. [Google Scholar]
- 5.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF: The role of vitamin D in cancer prevention. Am J Public Health 2006, 96(2):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE: Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2005, 14(8):1991–1997. [DOI] [PubMed] [Google Scholar]
- 7.Yetley EA: Assessing the vitamin D status of the US population. Am J Clin Nutr 2008, 88(2):558S–564S. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine, Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C: National Academic Press; 2011. [Google Scholar]
- 9.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC: Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol 2007, 103(3–5):708–711. [DOI] [PubMed] [Google Scholar]
- 10.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ: Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003, 77(1):204–210. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe JN: Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol 1976, 126(6):1130–1137. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ: Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998, 7(12):1133–1144. [PubMed] [Google Scholar]
- 13.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ: Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 1995, 87(9):670–675. [DOI] [PubMed] [Google Scholar]
- 14.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J: Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol Biomarkers Prev 2004, 13(9):1466–1472. [PubMed] [Google Scholar]
- 15.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, Pollak M, Yaffe M, Brisson J: Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev 2005, 14(7):1653–1659. [DOI] [PubMed] [Google Scholar]
- 16.Diorio C, Berube S, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Pollak M, Brisson J: Influence of insulin-like growth factors on the strength of the relation of vitamin D and calcium intakes to mammographic breast density. Cancer Res 2006, 66(1):588–597. [DOI] [PubMed] [Google Scholar]
- 17.Byng JW, Yaffe MJ, Lockwood GA, Little LE, Tritchler DL, Boyd NF: Automated analysis of mammographic densities and breast carcinoma risk. Cancer 1997, 80(1):66–74. [DOI] [PubMed] [Google Scholar]
- 18.Terry MB, Schaefer CA, Flom JD, Wei Y, Tehranifar P, Liao Y, Buka S, Michels KB: Prenatal smoke exposure and mammographic density in mid-life. J Dev Orig Health Dis 2011, 2(6):340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao P, Scheibel S, D’Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL: Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 2001, 16(4):605–614. [DOI] [PubMed] [Google Scholar]
- 20.Reeves KW, Stone RA, Modugno F, Ness RB, Vogel VG, Weissfeld JL, Habel LA, Sternfeld B, Cauley JA: Longitudinal association of anthropometry with mammographic breast density in the Study of Women’s Health Across the Nation. Int J Cancer 2009, 124(5):1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H: Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 2010, 46(12):2196–2205. [DOI] [PubMed] [Google Scholar]
- 22.Yaghjyan L, Colditz GA, Drake B: Vitamin D and mammographic breast density: a systematic review. Cancer Causes Control 2012, 23(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertone-Johnson ER, McTiernan A, Thomson CA, Wactawski-Wende J, Aragaki AK, Rohan TE, Vitolins MZ, Tamimi RM, Johnson KC, Lane D et al. : Vitamin D and calcium supplementation and one-year change in mammographic density in the women’s health initiative calcium and vitamin D trial. Cancer Epidemiol Biomarkers Prev 2012, 21(3):462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brisson J, Berube S, Diorio C, Masse B, Lemieux J, Duchesne T, Delvin E, Vieth R, Yaffe MJ, Chiquette J: A Randomized Double-Blind Placebo-Controlled Trial of the Effect of Vitamin D3 Supplementation on Breast Density in Premenopausal Women. Cancer Epidemiol Biomarkers Prev 2017, 26(8):1233–1241. [DOI] [PubMed] [Google Scholar]
- 25.Wood ME, Seisler DK, Hsieh MK, Kontos D, Ambaye A, Le-Petross HT, Jung SH, Liu H, Zekan P, Cardinal L et al. : Vitamin D and breast cancer biomarkers: CALGB 70806. In: American Society of Clinical Oncology Annual Meeting: 2018; Chicago, IL; 2018. [Google Scholar]
- 26.Salminen T, Hakama M, Heikkila M, Saarenmaa I: Favorable change in mammographic parenchymal patterns and breast cancer risk factors. Int J Cancer 1998, 78(4):410–414. [DOI] [PubMed] [Google Scholar]
- 27.Salminen TM, Saarenmaa IE, Heikkila MM, Hakama M: Risk of breast cancer and changes in mammographic parenchymal patterns over time. Acta Oncol 1998, 37(6):547–551. [DOI] [PubMed] [Google Scholar]
- 28.van Gils CH, Hendriks JH, Holland R, Karssemeijer N, Otten JD, Straatman H, Verbeek AL: Changes in mammographic breast density and concomitant changes in breast cancer risk. Eur J Cancer Prev 1999, 8(6):509–515. [DOI] [PubMed] [Google Scholar]
- 29.Sellers TA, Vierkant RA, Cerhan JR, Gapstur SM, Vachon CM, Olson JE, Pankratz VS, Kushi LH, Folsom AR: Interaction of dietary folate intake, alcohol, and risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2002, 11(10 Pt 1):1104–1107. [PubMed] [Google Scholar]
- 30.Work ME, Reimers LL, Quante AS, Crew KD, Whiffen A, Terry MB: Changes in mammographic density over time in breast cancer cases and women at high risk for breast cancer. Int J Cancer 2014, 135(7):1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW: Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 2004, 96(8):621–628. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM: Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011, 103(9):744–752. [DOI] [PubMed] [Google Scholar]
- 33.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S et al. : Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 2008, 100(22):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L et al. : Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006, 354(7):684–696. [DOI] [PubMed] [Google Scholar]
- 35.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP: Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007, 85(6):1586–1591. [DOI] [PubMed] [Google Scholar]
- 36.Kupferschmidt K: Uncertain verdict as vitamin D goes on trial. Science 2012, 337(6101):1476–1478. [DOI] [PubMed] [Google Scholar]
- 37.Scragg R, Khaw KT, Toop L, Sluyter J, Lawes CMM, Waayer D, Giovannucci E, Camargo CA Jr, Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol 2018:e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D et al. : Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 39.Vieth R: Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999, 69(5):842–856. [DOI] [PubMed] [Google Scholar]
- 40.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P et al. : Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006, 354(7):669–683. [DOI] [PubMed] [Google Scholar]
- 41.Vieth R, Chan PC, MacFarlane GD: Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001, 73(2):288–294. [DOI] [PubMed] [Google Scholar]
- 42.Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ: The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One 2014, 9(11):e111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hathcock JN, Shao A, Vieth R, Heaney R: Risk assessment for vitamin D. Am J Clin Nutr 2007, 85(1):6–18. [DOI] [PubMed] [Google Scholar]
- 44.Melamed ML, Michos ED, Post W, Astor B: 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008, 168(15):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED: Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989, 2(8673):1176–1178. [DOI] [PubMed] [Google Scholar]
- 46.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994, 330(15):1029–1035. [DOI] [PubMed] [Google Scholar]
- 47.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J et al. : Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996, 88(21):1560–1570. [DOI] [PubMed] [Google Scholar]
- 48.Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A et al. : The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res 1994, 54(7 Suppl):2038s–2043s. [PubMed] [Google Scholar]
- 49.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA et al. : Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007, 297(21):2351–2359. [DOI] [PubMed] [Google Scholar]
- 50.Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM et al. : Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306(14):1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


